Abstract
Growth as a biofilm facilitates the emergence of antibiotic resistance by mutation in Staphylococcus aureus. Here we demonstrate that biofilm growth of this species also dramatically increases horizontal transfer of plasmid-borne antibiotic resistance determinants by conjugation/mobilization and that standard laboratory practices to induce conjugation in staphylococci achieve optimal efficiency owing to the presence of a biofilm.
TEXT
The important human pathogen Staphylococcus aureus often forms biofilms on biological and inert surfaces during infection. Biofilms present considerable challenges to the successful eradication of staphylococcal infection in patients since they act to protect bacteria from the effects of both the host immune system and antibacterial drugs (1). We have recently established that staphylococci resident in biofilms exhibit increased mutability, thereby accelerating the emergence of heritable antibiotic resistance through spontaneous mutation (2). Here we show that the biofilm mode of growth also dramatically increases the ability of S. aureus to acquire/disseminate plasmid-borne antibiotic resistance determinants by horizontal gene transfer.
Our decision to investigate resistance plasmid transfer in staphylococcal biofilms stems from two published observations. First, there is evidence that biofilms cause some promotion of horizontal plasmid transfer in other bacterial species (3, 4), although this phenomenon has to our knowledge not been documented in the staphylococci. Second, it is well established that conjugal transfer in S. aureus is optimal when the organism is applied to a surface (5, 6); since this represents a situation under which a staphylococcal biofilm is likely to form, we reasoned that the high frequencies of horizontal plasmid transfer observed under these conditions might be attributable in part to biofilm formation. Throughout these studies, we employed the prototypical conjugative multidrug resistance plasmid pGO1 (7) and the mobilizable plasmid pC223 (8). Donor strains (Table 1, strains with the suffix “D”) were generated by transforming S. aureus SH1000 (9, 10) and UAMS-1 (11) with these plasmids by electroporation. To produce recipient strains (Table 1, strains with the suffix “R”), rifampin- and novobiocin-resistant mutants of S. aureus SH1000, SH1000ΔsigB, and UAMS-1 were generated. Table 1 provides a list of strains and selection conditions.
Table 1.
S. aureus strains used in this studya
| Strain | Comments | Selection | Source |
|---|---|---|---|
| SH1000 | Standard laboratory strain | None | 9 |
| SH1000-D | SH1000 strain containing plasmid pGO1 | GEN (5 μg/ml) | This study |
| SH1000-R | SH1000 strain resistant to NOV and RIF | NOV/RIF (5 μg/ml) | This study |
| SH1000ΔsigB | Spontaneous sigB mutant of SH1000, deleted for nucleotides 650–770 of sigB; unable to form a biofilm. | None | Our unpublished data |
| SH1000ΔsigB-R | SH1000ΔsigB strain resistant to NOV and RIF | NOV/RIF (2/5 μg/ml) | This study |
| SH1000-MD | SH1000 strain containing plasmids pGO1 and pC223 | GEN/CHL (5/10 μg/ml) | This study |
| UAMS-1 | Prolific biofilm-forming strain | None | 11 |
| UAMS-1-D | UAMS-1 strain containing plasmid pGO1 | GEN (5 μg/ml) | This study |
| UAMS-1-R | UAMS-1 strain resistant to NOV | NOV (2 μg/ml) | This study |
Abbreviations: D, donor; R, recipient; MD, mobilization donor; GEN, gentamicin; NOV, novobiocin; RIF, rifampin; CHL, chloramphenicol.
Cultures for conjugation or mobilization studies comprised equal numbers (3 × 108 CFU) of donor and recipient strains in brain heart infusion broth (BHB) at 30°C. Conjugation experiments in planktonic culture were conducted without shaking. For standard filter mating, donors and recipients were mixed, applied to nylon membranes (diameter, 13 mm; pore size, 0.2 μm; Whatman, Maidstone, United Kingdom) using Swinnex syringe-driven filter holders (Millipore, MA), and incubated on brain heart infusion agar (BHA). Postincubation, bacteria were harvested by vigorous agitation of filters in phosphate-buffered saline by vortex mixing. Biofilm cultures were generated using the cellulose disk static biofilm model (2). Briefly, mixtures of donor and recipient bacteria were applied to cellulose disks presoaked in human plasma (4% [vol/vol] in 500 μM carbonate-bicarbonate buffer) and incubated on BHA. During long-term growth of biofilms, human plasma was reapplied to the filters every 48 h. Postincubation, bacteria were detached from the disks using buffered cellulase (1 mg/ml in 0.05 M citrate buffer). To determine conjugation and mobilization frequencies, transconjugants, recipients, and donors were individually enumerated by plating onto Mueller-Hinton agar (MHA) containing appropriate selective agents (Table 1). Conjugation frequency was defined as the number of pGO1 transconjugants per donor, mobilization frequency was defined as the number of pC223 transconjugants per donor, and mobilization efficiency was defined as the number of transconjugants containing both pGO1 and pC223 per pGO1 transconjugant. Statistical analysis was performed by determining 95% confidence intervals (12).
Consistent with previous work, planktonic growth was not found to be conducive for conjugation/mobilization (13); there was no detectable transfer of either plasmid between strains of S. aureus SH1000 in static broth culture (frequencies of <5 × 10−9). In contrast, under optimal standard filter mating conditions, we recorded conjugation and mobilization frequencies of up to 6.1 × 10−5 and 4.5 × 10−6, respectively (Fig. 1A). Conjugation frequencies in SH1000 biofilms were somewhat reduced (5.5 × 10−6) compared with those of standard filter mating, while mobilization frequencies were not significantly different (2.6 × 10−6) (Fig. 1A). Mobilization efficiencies were 0.03 and 0.14 for standard filter mating and biofilm cultures, respectively, suggesting that biofilm growth conditions, compared to standard filter mating, are more likely to permit simultaneous transfer of both pGO1 and pC223. To confirm that our findings were not strain specific, we also determined conjugation frequencies for transfer of pGO1 in planktonic, biofilm, and standard filter mating cultures of the prolific biofilm-forming strain S. aureus UAMS-1. For this strain, conjugation was found to occur at a low, but detectable, frequency in planktonic culture (2.7 × 10−8) (Fig. 1B). However, conjugation frequencies achieved by standard filter mating or in biofilms were considerably higher (up to ∼16,000-fold) than those seen with planktonic cultures (Fig. 1B) and were comparable (filter mating, 4.4 × 10−4; biofilm, 1.9 × 10−4). Our results clearly demonstrate that transfer of antibiotic resistance plasmids by conjugation and mobilization is dramatically enhanced in biofilms compared with planktonic cultures.
Fig 1.
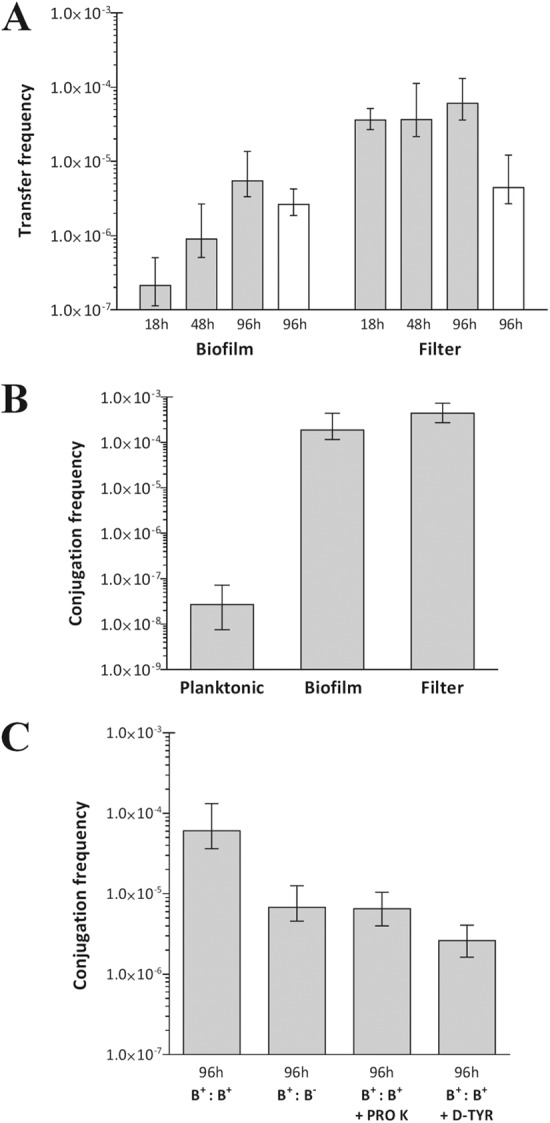
Frequency of plasmid transfer between staphylococci under different culture conditions. (A) Conjugation frequencies of pGO1 from S. aureus SH1000-D to SH1000-R (gray bars) and mobilization frequencies of pC223 from S. aureus SH1000-MD to SH1000-R (white bars). (B) Conjugation frequencies of pGO1 from S. aureus UAMS-1-D to UAMS-1-R. (C) Conjugal transfer of pGO1 between strains of S. aureus by standard filter mating under conditions that reduce biofilm growth. Abbreviations: PRO K, proteinase K; D-TYR, d-tyrosine, B+:B+, biofilm-proficient donor and recipient; B+:B−, biofilm-proficient donor and biofilm-deficient recipient. Error bars indicate 95% confidence intervals. Data are the means of six experimental replicates.
Since plasmid transfer occurred at similar frequencies under standard filter mating conditions and in the static biofilm model, we investigated whether standard filter mating involves the formation of a biofilm. We first assessed conjugal transfer of pGO1 from S. aureus SH1000-D to a recipient strain incapable of forming a biofilm (S. aureus SH1000ΔsigB-R); transfer of pGO1 from SH1000-D was reduced ca. 9-fold compared to the wild-type recipient (Fig. 1C). To further examine a potential role for biofilms in standard filter mating, we evaluated the impact on conjugation of applying biofilm-degrading agents (proteinase K and d-tyrosine [2, 14–16]) to filter mating experiments. Proteinase K digests proteins that are crucial for the structural integrity of the SH1000 biofilm (15), while d-amino acids appear to act as signaling molecules that trigger dispersal of bacterial biofilms (16). Subinhibitory concentrations of proteinase K (100 μg/ml in 20 mM Tris HCl, 100 mM NaCl) or d-tyrosine (100 μM in water) were applied dropwise (40 μl) to the filter cultures immediately after inoculation and then every 24 h. These agents reduced pGO1 transfer by 9-fold (proteinase K) and 23-fold (d-tyrosine) in SH1000-D/SH1000-R filter-based cultures (Fig. 1C). In contrast, neither of these agents reduced the frequency of conjugation in planktonic cultures of S. aureus UAMS-1 (data not shown). These observations suggest that standard filter mating achieves optimal conjugation in part as a consequence of biofilm formation on the filters.
In summary, S. aureus biofilms dramatically increase the frequency of plasmid transfer events by both conjugation and mobilization, thereby promoting horizontal spread of antibiotic resistance determinants. This phenomenon probably results, in part, from the close cell-to-cell contact occurring in the biofilm and the fact that the biofilm matrix may act to stabilize contacts between neighboring bacteria. Together with the fact that staphylococci resident in biofilms show elevated mutation frequencies to antibiotic resistance (2), this observation identifies biofilms as a privileged environment for the emergence and spread of antibiotic resistance in S. aureus. Given the importance of improving the treatment of biofilm-related infections (e.g., device-related infections, chronic wounds), the development of therapeutic approaches to prevent and eradicate biofilms is an area of active research (17). Our findings suggest that the development of effective antibiofilm approaches would not only enable improved treatment of biofilm-related infections but may also potentially offer benefits in slowing the rate at which antibiotic resistance emerges and spreads in patients.
ACKNOWLEDGMENT
We thank Jamie Caryl for technical advice.
Footnotes
Published ahead of print 28 January 2013
REFERENCES
- 1. Davies D. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114–122 [DOI] [PubMed] [Google Scholar]
- 2. Ryder VJ, Chopra I, O'Neill AJ. 2012. Increased mutability of staphylococci in biofilms as a consequence of oxidative stress. PLoS One 7:e47695 doi:10.1371/journal.pone.0047695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ehlers LJ, Bouwer EJ. 1999. RP4 plasmid transfer among species of Pseudomonas in a biofilm reactor. Water Sci. Technol. 39:163–171 [Google Scholar]
- 4. Angles ML, Marshall KC, Goodman AE. 1993. Plasmid transfer between marine bacteria in the aqueous phase and biofilms in reactor microcosms. Appl. Environ. Microbiol. 59:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Archer GL, Johnston JL. 1983. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob. Agents Chemother. 24:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Archer GL, Coughter JP, Johnston JL. 1986. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob. Agents Chemother. 29:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caryl JA, O'Neill AJ. 2009. Complete nucleotide sequence of pGO1, the prototype conjugative plasmid from the staphylococci. Plasmid 62:35–38 [DOI] [PubMed] [Google Scholar]
- 8. Caryl JA, Thomas CD. 2006. Investigating the basis of substrate recognition in the pC221 relaxosome. Mol. Microbiol. 60:1302–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Neill AJ. 2010. Staphylococcus aureus SH1000 and 8325-4: comparative genome sequences of key laboratory strains in staphylococcal research. Lett. Appl. Microbiol. 51:358–361 [DOI] [PubMed] [Google Scholar]
- 11. Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cumming G. 2009. Inference by eye: reading the overlap of independent confidence intervals. Stat. Med. 28:205–220 [DOI] [PubMed] [Google Scholar]
- 13. Forbes BA, Schaberg DR. 1983. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J. Bacteriol. 153:627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. 2010. D-amino acids trigger biofilm disassembly. Science 328:627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boles BR, Horswill AR. 2008. agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052 doi:10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. 2011. Inhibitory effects of d-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 193:5616–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Neill AJ. 2011. Bacterial phenotypes refractory to antibiotic-mediated killing: mechanisms and mitigation, p 195–210 In Miller AA, Miller PF. (ed), Emerging trends in antibacterial discovery: answering the call to arms. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]


