Abstract
Candida biofilm infections pose an increasing threat in the health care setting due to the drug resistance associated with this lifestyle. Several mechanisms underlie the resistance phenomenon. In Candida albicans, one mechanism involves drug impedance by the biofilm matrix linked to β-1,3 glucan. Here, we show this is important for other Candida spp. We identified β-1,3 glucan in the matrix, found that the matrix sequesters antifungal drug, and enhanced antifungal susceptibility with matrix β-1,3 glucan hydrolysis.
TEXT
Similar to many microbes, Candida species exhibit a propensity to grow as biofilms on implanted medical devices such as a central venous catheter (1, 2). Among biofilm-forming pathogens, infection due to Candida spp. is associated with the highest nosocomial mortality (3). Treating these infections proves challenging due to high levels of drug resistance (4, 5). Compared to their planktonic counterparts, biofilm cells exhibit up to a 1,000-fold increase in resistance (6, 7). For most patients, removal of the medical device is the only viable treatment option (1).
A number of factors contribute to Candida albicans biofilm resistance (8–11). The extracellular matrix that enmeshes the biofilm cells accounts for a large percentage of this phenotype by sequestering antifungal drugs. The matrix polysaccharide β-1,3 glucan has been strongly linked to this mechanism (12–14).
While C. albicans remains the most frequently isolated Candida species, other members of the genus are increasingly common. The most recent surveillance data in the United States (15) found C. albicans comprised far less than 50% of isolates. Candida glabrata (29%), Candida parapsilosis (17%), and Candida tropicalis (10%) as a group represented the majority of infections. Each of these species has been shown to form biofilms with comparable levels of antifungal resistance to C. albicans (16–18).
The increasing prevalence of non-albicans Candida species and their role in biofilm device infections prompted us to ask if they also exhibit a β-1,3 glucan matrix resistance mechanism. The purpose of this study was to determine if β-1,3 glucan was present in the matrix of these species and, if so, did it play a role in drug resistance similar to that described for C. albicans. Specifically, three experiments with three non-albicans Candida species were undertaken: (i) determination of matrix β-1,3 glucan content, (ii) assessment of the ability of the extracellular matrix to sequester the antifungal fluconazole, and (iii) examination of the impact of β-1,3 glucan disruption on biofilm antifungal drug susceptibility.
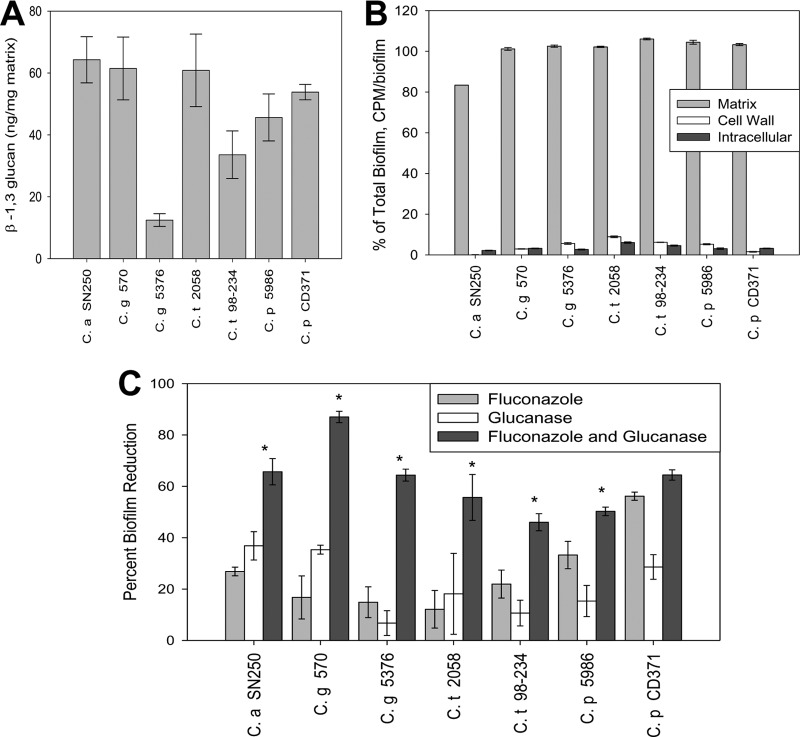
C. glabrata, C. parapsilosis, and C. tropicalis were chosen for study based upon relative incidence in clinical surveillance and demonstrated propensity for device biofilm formation. With the exception of the C. albicans isolate (strain SN250), all strains were clinical isolates from cases of invasive candidiasis (C. glabrata strains 570 and 5376, C. parapsilosis strains 5986 and CD371, and C. tropicalis strains 2058 and 98-234). Biofilms were grown in RPMI-MOPS (morpholinepropanesulfonic acid) medium on a polystyrene substrate for all experiments. Each of the strains formed robust biofilms with an average XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide inner salt] optical density (OD) of 1.42 for C. albicans and 1.40 for the non-albicans group after 24 h of incubation. For matrix composition analysis, biofilms were grown for 48 h using 1-liter roller bottles. Matrix was isolated using water bath sonication and vortexing as previously described (19). A β-1,3 glucan enzyme-linked immunosorbent assay (ELISA) was performed on three biologic replicates, and assays were completed in triplicate for each strain as previously detailed. Matrix β-1,3 glucan was normalized by matrix dry weight and expressed as ng/mg matrix. As shown in Fig. 1A, the β-1,3 glucan polymer was identified in the biofilm matrix of each Candida strain tested. The concentrations of this polysaccharide among the species were relatively similar.
Fig 1.
(A) The amount of β-1,3 glucan in a 1-mg/ml sample of purified in vitro biofilm matrix, measured by ELISA. Samples are measured in triplicate. Standard errors are shown. (B) [H3]fluconazole in the matrix, cell wall, and cytoplasm of in vitro biofilms. Shown as a percentage of [H3]fluconazole in the total biofilm, measured as CPM/biofilm. Standard errors are shown. (C) Biofilm susceptibility to fluconazole and/or β-1,3 glucanase as measured by the XTT reduction assay. Fluconazole dosed at 1 mg/ml and β-1,3 glucanase dosed at 0.7 U/ml. Data shown are representative examples, each read in triplicate. *, P < 0.05, comparing the combined drug values to either of the single-drug values using a one-way ANOVA.
We utilized a 6-well plate format for assessment of antifungal drug biofilm penetration using [H3]fluconazole as described previously (13, 19). Briefly, mature biofilms (24 h of incubation) were washed twice with sterile water followed by exposure to a total of 8.48 × 105 cpm of [H3]fluconazole in RPMI-MOPS medium. Biofilms were incubated for 30 min at 37°C and then chased with 20 μM unlabeled fluconazole in medium. The fluconazole content was measured in intact biofilms, isolated matrix, cell wall, and cell cytoplasm by scintillation counting. Assays were performed in triplicate for each Candida isolate. Consistent with previous findings in C. albicans (13, 19), the majority of [H3]fluconazole is present in the extracellular matrix for each of these species, with very little or no drug found intracellularly or in the cell wall (Fig. 1B).
We next determined the effect of matrix β-1,3 glucan hydrolysis on biofilm susceptibility to fluconazole. Using a 96-well plate format, biofilm cell metabolic activity was assayed following exposure to fluconazole and β-1,3 glucanase alone and in combination using a tetrazolium salt XTT reduction assay (20–23). Briefly, after 24 h of biofilm growth, medium was replaced by fresh RPMI-MOPS with dilutions of fluconazole at 1 mg/ml, β-1,3 glucanase (Zymolyase 20T; MP Biomedicals) at 0.7 U/ml, or a combination of the two. The β-1,3 glucanase concentration was chosen based upon our previous studies with C. albicans demonstrating synergy with fluconazole and no effect on cell viability for the enzyme alone (12, 19). Experiments were performed in triplicate. Drug effect is expressed at the percent biofilm reduction relative to growth of untreated controls. The statistical significance of differences among therapies was determined using analysis of variance (ANOVA). Similar to previous reports, fluconazole alone exhibited minimal activity against biofilms for each strain and species (12, 19). The low concentration of β-1,3 glucanase also produced little change in cell metabolic activity. However, fluconazole caused marked biofilm reduction in the presence of the β-1,3 glucan-hydrolyzing enzyme. This effect was observed for all strains tested (Fig. 1C).
The results of the present study with non-albicans Candida species are similar to those from C. albicans, which demonstrate the contribution of biofilm matrix β-1,3 glucan for the antifungal drug resistance phenomenon linked to this common infection lifestyle (12–14). The relative impact of the mechanism for these other common Candida species appears congruent with that shown for C. albicans based upon comparable concentrations of matrix β-1,3 glucan, antifungal drug sequestration, and influence of β-1,3 glucanase treatment on fluconazole efficacy. The prevalence of these non-albicans Candida species continues to rise. Insight to the mechanisms responsible for resistance to therapy is critical for design of new treatment strategies. The present study suggests that drug development targeting matrix β-1,3 glucan may potentiate the activity of the currently available antifungal option.
ACKNOWLEDGMENT
D.R.A. was funded by NIH R01 AI073289-01.
Footnotes
Published ahead of print 14 January 2013
REFERENCES
- 1. Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE; Infectious Diseases Society of America 2004. Clinical infectious disease guidelines for treatment of candidiasis. Clin. Infect. Dis. 38:161–189 [DOI] [PubMed] [Google Scholar]
- 2. Kojic EM, Darouiche RO. 2004. Candida infections of medical devices. Clin. Microbiol. Rev. 17:255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J. Clin. Microbiol. 45:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramage G, Rajendran R, Sherry L, Williams C. 2012. Fungal biofilm resistance. Int. J. Microbiol. 2012:528521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baillie GS, Douglas LJ. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644–656 [DOI] [PubMed] [Google Scholar]
- 7. LaFleur MD, Kumamoto CA, Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973–980 [DOI] [PubMed] [Google Scholar]
- 9. Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J. Antimicrob. Chemother. 46:397–403 [DOI] [PubMed] [Google Scholar]
- 11. Jabra-Rizk MA, Falkler WA, Meiller TF. 2004. Fungal biofilms and drug resistance. Emerg. Infect. Dis. 10:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nett JE, Sanchez H, Cain MT, Andes DR. 2010. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J. Infect. Dis. 202:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vediyappan G, Rossignol T, d'Enfert C. 2010. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans. Antimicrob. Agents Chemother. 54:2096–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J. Clin. Microbiol. 50:3435–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. 2012. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 36:288–305 [DOI] [PubMed] [Google Scholar]
- 17. Melo AS, Bizerra FC, Freymüller E, Arthington-Skaggs BA, Colombo AL. 2011. Biofilm production and evaluation of antifungal susceptibility amongst clinical Candida spp. isolates, including strains of the Candida parapsilosis complex. Med. Mycol. 49:253–262 [DOI] [PubMed] [Google Scholar]
- 18. Al-Fattani MA, Douglas LJ. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55(Part 8):999–1008 [DOI] [PubMed] [Google Scholar]
- 19. Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, Hamaker J, Mitchell AP, Andes DR. 2012. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 8:e1002848 doi:10.1371/journal.ppat.1002848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nett JE, Cain MT, Crawford K, Andes DR. 2011. Optimizing a Candida biofilm microtiter plate model for measurement of antifungal susceptibility by tetrazolium salt assay. J. Clin. Microbiol. 49:1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taff HT, Nett JE, Andes DR. 2012. Comparative analysis of Candida biofilm quantitation assays. Med. Mycol. 50:214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramage G, Vande Walle K, Wickes BL, López-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. da Silva WJ, Seneviratne J, Parahitiyawa N, Rosa EA, Samaranayake LP, Del Bel Cury AA. 2008. Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz. Dent. J. 19:364–369 [DOI] [PubMed] [Google Scholar]