Abstract
Ceftolozane is a new cephalosporin with potent activity against Pseudomonas aeruginosa and Enterobacteriaceae. A neutropenic murine thigh infection model was used to determine which pharmacokinetic/pharmacodynamic index and magnitude drives the efficacy of ceftolozane with Gram-negative bacilli, to compare the rates of in vivo killing of P. aeruginosa by ceftolozane and ceftazidime, and to determine the impact of different ratios of ceftolozane plus tazobactam on Enterobacteriaceae containing extended-spectrum β-lactamases (ESBLs). Neutropenic mice had 106.2-7.1 CFU/thigh when treated with ceftolozane for 24 h with (i) various doses (3.12 to 1,600 mg/kg) and dosage intervals (3, 6, 12, and 24 h) against two Enterobacteriaceae strains, (ii) 0.39 to 800 mg/kg every 6 h for four Enterobacteriaceae and four P. aeruginosa strains, and (iii) 400 or 800 mg/kg with 2:1. 4:1, and 8:1 ratios of tazobactam against five Enterobacteriaceae strains with ESBLs. The pharmacokinetics of ceftolozane at 25, 100, and 400 mg/kg were linear with peak/dose values of 1.0 to 1.4 and half-lives of 12 to 14 min. T>MIC was the primary index driving efficacy. For stasis (1 log kill), T>MIC was 26.3% ± 2.1% (31.6% ± 1.6%) for wild-type Enterobacteriaceae, 31.1% ± 4.9% (34.8% ± 4.4%) for Enterobacteriaceae with ESBLs, and 24.0% ± 3.3% (31.5% ± 3.9%) for P. aeruginosa. At 200 mg/kg every 3 h, the rate of in vivo killing of P. aeruginosa was faster with ceftolozane than with ceftazidime (−0.34 to −0.41 log10 CFU/thigh/h versus −0.21 to −0.24 log10 CFU/thigh/h). The 2:1 ratio of ceftolozane with tazobactam was the most potent combination studied. The T>MIC required for ceftolozane is less than with other cephalosporins and may be due to more rapid killing.
INTRODUCTION
Enterobacteriaceae and Pseudomonas aeruginosa have been causing increasing numbers of nosocomial infections, especially in intensive care units (1, 2). Ceftolozane (CXA-101) is a new cephalosporin antibiotic with potent activity against Gram-negative bacilli, including P. aeruginosa (3, 4). Against various strains of P. aeruginosa, including multiple-drug-resistant strains, ceftolozane had an MIC90 of 2.0 μg/ml and was 8- to 16-fold more potent than cefepime and ceftazidime (5, 6). Ceftolozane appears to have less affinity for hydrolysis by Amp C β-lactamases and is also a weak substrate for efflux (5–9). The drug also shows an at least 2-fold-greater affinity for penicillin-binding proteins 1b, 1c, 2, and 3 than ceftazidime (10). Ceftolozane is also active against Enterobacteriaceae, including some with extended-spectrum β-lactamases (ESBLs) (3, 11). For example, in one study of 59 ESBL-producing Enterobacteriaceae, 20% were susceptible to ceftolozane at 8 mg/liter (11). However, the addition of the β-lactamase inhibitor tazobactam at 4 mg/liter (a 2:1 ratio of ceftolozane to tazobactam increased the susceptibility of these resistant Enterobacteriaceae to 76%. At 8 mg/liter for both ceftolozane and tazobactam, the percentage of susceptible strains increased to 93%.
We used the neutropenic mouse thigh infection model to characterize the pharmacodynamic activity of ceftolozane against various strains of P. aeruginosa and Enterobacteriaceae (12). Recent in vitro studies have suggested that ceftolozane has better killing kinetics of P. aeruginosa than ceftazidime (13). Because of these in vitro studies, we also measured and compared the rate of in vivo killing of P. aeruginosa over 12 h by these two cephalosporins in the thighs of neutropenic mice. Lastly, we studied the activity of ceftolozane alone and in combination with tazobactam at 2:1, 4:1, and 8:1 ratios against various Enterobacteriaceae producing known ESBLs in the same neutropenic mouse thigh model.
MATERIALS AND METHODS
Organisms.
The following strains were used for these experiments: P. aeruginosa strains ATCC 27853, 4304A, PO2, and 313, Escherichia coli strains ATCC 25922, NIH-J, and 6042, Klebsiella pneumoniae strains ATCC 43816, 216, 4105, 4110, and 81-1260A, and Enterobacter cloacae strain 81-1260A. Five of the Enterobacteriaceae strains produced ESBLs (TEM 10, TEM 10 + SHV 1, TEM 26, CTX-M3 + Amp C, and SHV 5).
Pharmacokinetics.
The pharmacokinetics of ceftolozane at doses of 25, 100, and 400 mg/kg were determined in infected neutropenic mice by microbiological assay using E. coli NIH-J as the test organism. The lower limit of quantification was 1.0 mg/liter. Plasma samples were collected at 15, 30, 45, 60, 90, and 120 min and stored at −70°C until assay. The assay was linear from 1.0 to 128 mg/liter. Concentrations higher than 128 mg/liter were diluted 1:5 or 1:10 in pooled mouse plasma for assay. The intraday variation was <15%. Protein binding was performed by ultrafiltration at ceftolozane concentrations of 10 and 100 mg/liter. The pharmacokinetics of tazobactam were not determined.
MICs.
The MICs for ceftolozane against the wild-type Enterobacteriaceae and P. aeruginosa isolates and for ceftazidime against two isolates of P. aeruginosa were determined by standard Clinical and Laboratory Standards Institute (CLSI) microdilution methods (14). The MICs for ceftolozane alone and in combination with fixed concentrations (4 and 8 mg/liter) of tazobactam and 2:1, 4:1, and 8:1 ratios with tazobactam for five strains of Enterobacteriaceae with ESBLs were also determined by microdilution methods. The CLSI approved method has a fixed concentration of tazobactam at 4 mg/liter. MICs were determined in quadruplicate; the MIC used was the value obtained on three or four determinations. When different MIC determinations were equally split between two 2-fold dilutions, the mean log2 value was used.
Neutropenic murine thigh infection model.
These studies and protocols were approved by the Animal Study and Research Committees of the William S. Middleton Memorial VA Hospital. Six-week-old specific-pathogen-free, female ICR/Swiss mice weighing 23 to 27 g were made neutropenic (neutrophils < 100 mm3) by two intraperitoneal injections of cyclophosphamide (150 mg/kg 4 days and 100 mg/kg 1 day before infection). Thigh infection with various organisms was produced by the injection of ∼105-6 CFU in the thighs of neutropenic mice. Two hours later, mice were treated for 24 h with ceftolozane administered sc in 0.2-ml volumes as multiple dosing regimens (varying the total dose from 25 to 1,600 mg/kg and using dosage intervals from 3, 6, 12, and 24 h for two strains of Enterobacteriaceae) or as doses of 0.39 to 800 mg/kg every 6 h (for all strains). With studies comparing the rate of in vivo killing for ceftolozane and ceftazidime against two strains of P. aeruginosa, mice were treated with either drug at 200 mg/kg every 3 h for 12 h; the mice were euthanized at 2, 4, 6, 9, and 12 h. For strains of Enterobacteriaceae producing ESBLs, neutropenic mice were treated with ceftolozane at 400 and 800 mg/kg alone or in combination with tazobactam at ratios of 2:1 (200 and 400 mg of tazobactam/kg), 4:1 (100 and 200 mg of tazobactam/kg), and 8:1 (50 and 100 mg of tazobactam/kg). For one strain (K. pneumoniae 4105), lung and thigh infections were produced in the same mice. The lung was infected 4 h before treatment by intranasal inoculation of 108 bacteria in a 0.05-ml volume. The thighs of the same neutropenic mice were infected 2 h later as stated previously.
Three mice were used for each sample time point with each dosage, organism, and drug studied. Saline treated control mice were included in each study and were euthanized at 0 and 24 h in the efficacy studies and at 0, 2, 6, and 12 h in the in vivo kill-rate studies. Treated mice were euthanized at 24 h in the efficacy studies and at 2, 4, 6, 9, and 12 h in the in vivo kill-rate studies. Thighs and lungs were removed and homogenized in saline. Aliquots of serial 10-fold dilutions were plated on agar plates for CFU determinations. The data were expressed as the mean log10 CFU/thigh or lung ± the standard deviation (SD).
Data analysis.
The results were analyzed by using a sigmoid dose-effect model derived from the Hill equation. The Emax, 50% effective dose, and N were calculated from the relationship between different doses and resulting log10 CFU/thigh by using nonlinear least-squares regression. The static and 1-log kill dose for each organism was calculated. The same nonlinear regression was used to analyze the relationship between the magnitude of different pharmacokinetic/pharmacodynamic (PK/PD) indices and the log10 CFU/thigh. The PK/PD index values for doses not specifically studied were linearly extrapolated from the pharmacokinetic parameters for doses that were studied. The coefficient of determination (or R2) represents the percentage of the variance in bacterial numbers that can be attributed to each PK/PD index. Because of the limited data points with ceftolozane alone and in combination with tazobactam against ESBL-producing Enterobacteriaceae, linear regression was used to examine the relationship between the MIC for ceftolozane (alone or in combination with tazobactam) and the log10 CFU/thigh. Lastly, the values for the different groups of organisms were compared by one-way analysis of variance and t test.
RESULTS
MICs.
The MICs for ceftolozane alone and in combination with tazobactam against the study organisms are shown in Table 1. The type of ESBL enzyme is also listed. For the Enterobacteriaceae the MICs varied from 0.06 to 1.4 mg/liter for wild-type strains and from 11.2 to >64 mg/liter for ESBL-producing strains. An 8:1 ratio of ceftolozane and tazobactam reduced the MICs of ESBL-producing strains ∼4-fold. A 2:1 ratio of ceftolozane and tazobactam reduced the MICs of these same strains from 8- to 16-fold. Fixed concentrations of tazobactam at 4 and 8 mg/liter also reduced the ceftolozane MICs 8- to 16-fold. The ceftolozane MICs for the four P. aeruginosa strains varied only from 0.5 to 1 mg/liter. The MICs for ceftazidime against P. aeruginosa ATCC 27853 and PO2 were both 2 mg/liter.
Table 1.
Ceftolozane MICs alone and in combination with tazobactam
| Organism | Ceftolozane MIC (mg/liter) | Ceftolozane MIC (mg/liter) + tazobactam |
ESBL(s) | ||||
|---|---|---|---|---|---|---|---|
| 2:1 | 4:1 | 8:1 | 4 | 8 | |||
| E. coli ATCC 25922 | 0.5 | None | |||||
| E. coli NIH-J | 0.06 | None | |||||
| K. pneumoniae ATCC 43816 | 1.4 | None | |||||
| K. pneumoniae 216 | 1 | None | |||||
| P. aeruginosa ATCC 27853 | 0.5 | None | |||||
| P. aeruginosa 4304A | 0.7 | None | |||||
| P. aeruginosa PO2 | 0.5 | None | |||||
| P. aeruginosa 313 | 1 | None | |||||
| E. coli 6042 | 22.4 | 2 | 4 | 8 | 2 | 1 | TEM 10 |
| K. pneumoniae 4105 | >64 | 11.2 | 16 | 22.4 | 16 | 11.2 | TEM 26, SHV-1 |
| K. pneumoniae 4110 | >64 | 5.6 | 11.2 | 32 | 8 | 4 | TEM 10, SHV-1 |
| K. pneumoniae 81-1260A | 44.8 | 2 | 4 | 8 | 2 | 1 | CTX-M3, AmpC |
| E. cloacae 81-1291A | 11.2 | 2 | 1.4 | 4 | 1 | 0.7 | SHV-5 |
Pharmacokinetics.
The plasma concentrations over time for ceftolozane following subcutaneous injection of 25, 100, and 400 mg/kg in thigh-infected neutropenic mice are shown in Fig. 1. These concentrations were used to calculate the various pharmacokinetic parameters listed in Table 2. The kinetics at these doses were linear for both Cmax and AUC. The half-life varied from 12 to 14 min. Protein binding values at 10 and 100 mg/liter were <5%.
Fig 1.
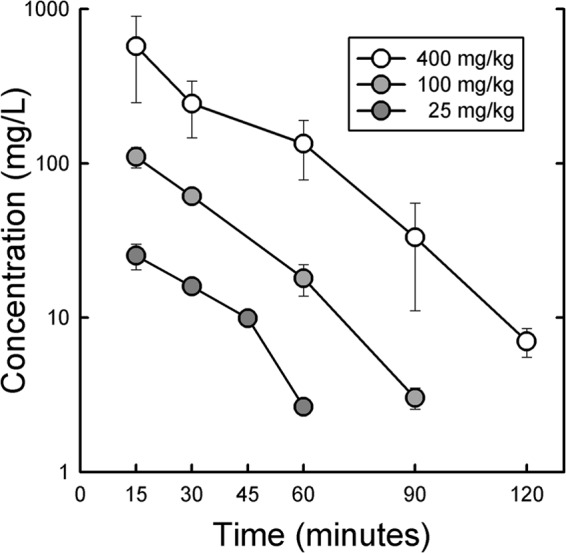
Plasma concentrations of ceftolozane in infected neutropenic mice. Each point is the mean for three mice, and the error bar represents the SD.
Table 2.
Pharmacokinetic parameters for ceftolozane in infected neutropenic mice
| Dose (mg/kg) | Tmax (h) | Mean Cmax ± SEM (μg/ml) | AUC0-24 (mg h/liter) | Half-life (min) |
|---|---|---|---|---|
| 25 | 0.25 | 25.2 ± 4.80 | 13.3 | 11.6 |
| 100 | 0.25 | 110 ± 16.7 | 55.5 | 13.8 |
| 400 | 0.25 | 574 ± 326 | 299 | 14.1 |
Animal studies.
The number of organisms in the thighs of neutropenic mice at the start of therapy in these studies varied from 106.25 to 107.05 CFU/thigh for the various organisms. The saline-treated mice had bacterial numbers that increased 1.57 to 3.15 log10 CFU/thigh over 24 h. K. pneumoniae 4105, an ESBL-producing organism, was infected in both the thigh and lung of the same mice. The lung was infected 4 h before treatment, whereas the thigh was infected 2 h later. The starting inoculum in the lung was 6.52 log10 CFU/lung and increased by 2.12 log10 CFU/lung in saline-treated mice over 24 h.
Dose-fractionation studies.
In these studies, multiple dosing regimens varying the dose and dosage interval were administered subcutaneously in 0.2-ml volumes to groups of mice for 24 h. The dosing intervals chosen were 3, 6, 12, and 24 h. The total 24-h doses ranged from 25 to 1,600 mg/kg. The total 24-h doses resulting in stasis with E. coli ATCC 25922 were 60, 154, 382, and 914 mg/kg for 3, 6, 12, and 24 h dosing regimens, respectively. Similar values for K. pneumoniae ATCC 43816 were 86, 244, 528, and >1,600 mg/kg with 3-, 6-, 12-, and 24-h dosing intervals, respectively. This pattern suggests that time above MIC is the important PK/PD index for the activity of ceftolozane.
We determined which PK/PD index correlated best with efficacy by relating the number of bacteria in the thigh at the end of 24 h of therapy with (i) the Cmax/MIC ratio, (ii) AUC0-24/MIC ratio, and (iii) the percentage of the dosing interval that plasma levels exceed the MIC (T>MIC) for each of the dosage regimens studied. Because of the low binding of the drug, total drug concentrations were used to calculate the magnitude of the various PK/PD indices. The relationships between efficacy (change in log10 CFU/thigh over 24 h) and the magnitude of the Cmax/MIC ratio, the AUC0-24/MIC ratio, and the percent T>MIC for ceftolozane with K. pneumoniae ATCC 43816 are shown in Fig. 2. R2 values were highest for T>MIC and lowest for the Cmax/MIC ratio. Similar values of 62.2% for T>MIC, 48.7% for the AUC0-24/MIC ratio, and 27.3% for the Cmax/MIC ratio were obtained with ceftolozane against E. coli ATCC 25922.
Fig 2.
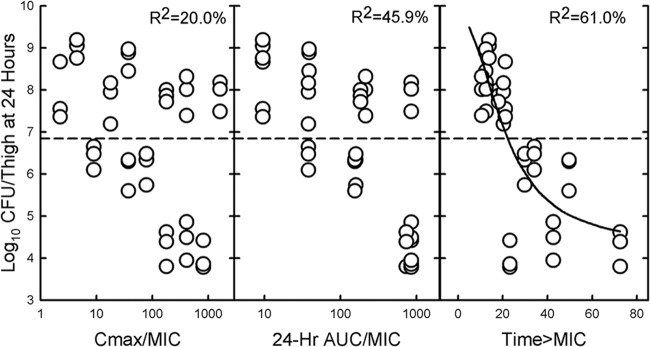
Relationship between the CFU/thigh for K. pneumoniae ATCC 43816 and Cmax/MIC ratio (left panel), the AUC0-24/MIC ratio (center panel), and the percent T>MIC (right panel) after 24 h of treatment with ceftolozane administered at different doses and dosing intervals. Each point represents one mouse. R2 represents the percentage of variance in CFU/thigh that can be attributed to each PK/PD index.
Magnitude of T>MIC index for efficacy.
The dose-response relationships with 6-hourly dosing of ceftolozane for the four strains of wild-type Enterobacteriaceae and the four strains of P. aeruginosa are shown in Fig. 3. The calculated 6-hourly doses for stasis and 1-log kill and the percent T>MIC with ceftolozane required for the static and 1-log kill doses are listed in Table 3. Although the static and 1-log kill doses varied by ∼10-fold, the percent T>MIC varied only from 21.4 to 28.5% for stasis and 26.7 to 35.3% for a 1-log kill. The maximum killing observed for the wild-type Enterobacteriaceae and P. aeruginosa were −2.60 ± 0.24 and −2.44 ± 0.46 log10 CFU/thigh, respectively.
Fig 3.
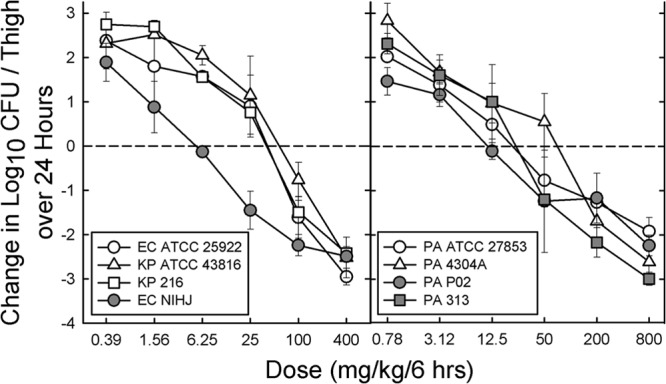
Dose-response relationships for 6-hourly dosing of ceftolozane against four wild-type strains of Enterobacteriaceae (left panel) and four strains of P. aeruginosa (right panel). Each point represents the mean log10 CFU/thigh for three mice, and the error bar represents the SD.
Table 3.
Dose and T>MIC values for stasis and 1-log kill and the maximum extent of killing with 6-hourly dosing of ceftolozane against four wild-type Enterobacteriaceae strains and four P. aeruginosa strainsa
| Organism | Static dose (mg/kg/6 h) | T>MIC (%) | 1-Log kill dose (mg/kg/6 h) | T>MIC (%) | Maximal killing (log10 CFU/thigh) |
|---|---|---|---|---|---|
| Wild-type Enterobacteriaceae strains | |||||
| E. coli ATCC 25922 | 38.7 | 28.1 | 75.6 | 32.8 | –2.95 |
| E. coli NIH-J | 5.69 | 28.0 | 14.3 | 32.3 | –2.49 |
| K. pneumoniae ATCC 43816 | 61.2 | 25.2 | 127 | 32.0 | –2.52 |
| K. pneumoniae 216 | 36.0 | 24.0 | 76.6 | 29.2 | –2.42 |
| Mean | 26.3 ± 2.1 | 31.6 ± 1.6 | –2.60 ± 0.24 | ||
| P. aeruginosa strains | |||||
| P. aeruginosa ATCC 27853 | 21.3 | 24.3 | 88.5 | 33.9 | –1.92 |
| P. aeruginosa 4034A | 41.5 | 28.5 | 119 | 35.3 | –2.61 |
| P. aeruginosa PO2 | 12.2 | 21.7 | 50.5 | 30.1 | –2.24 |
| P. aeruginosa 313 | 21.4 | 21.4 | 51.9 | 26.7 | –2.99 |
| Mean | 24.0 ± 3.3 | 31.5 ± 3.9 | –2.44 ± 0.46 | ||
| Mean for all strains | 25.2 ± 2.8 | 31.5 ± 2.8 | –2.52 ± 0.35 |
Mean values are expressed as means ± the standard errors of the mean.
In vivo killing rates for ceftolozane and ceftazidime with P. aeruginosa.
The time course of bacterial killing of ceftolozane and ceftazidime against P. aeruginosa ATCC 27853 and PO2 are shown in Fig. 4. All samples over 12 h had lower log10 CFU/thigh values in animals receiving ceftolozane than in those receiving ceftazidime. With P. aeruginosa ATCC 27853, the differences between the two drugs became significant after 4 h. With P. aeruginosa PO2 the differences became significant earlier at 2 h. After 6 h, the rate of killing of both organisms became slower. The slopes of in vivo killing over the first 6 h were −0.34 and −0.41 log10 CFU/thigh/h for ceftolozane and −0.24 and −0.21 log10 CFU/thigh/h for ceftazidime for P. aeruginosa ATCC 27358 and PO2, respectively.
Fig 4.
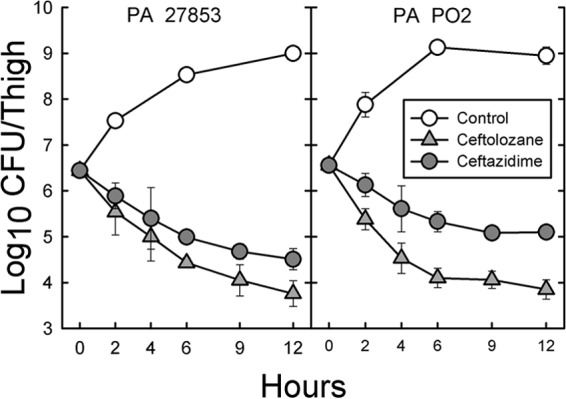
Time course of bactericidal activity of ceftolozane (gray filled triangles) and ceftazidime (gray filled circles) with two strains of P. aeruginosa (ATCC 23815 and PO2) in the thighs of neutropenic mice. Saline-treated controls (open circles) were also examined at various times. Each point represents the mean of three mice, and the error bar represents the SD. Both drugs were administered at doses of 200 mg/kg every 3 h for 12 h.
Impact of tazobactam on in vivo activity of ceftolozane against ESBL-producing Enterobacteriaceae.
The change in log10 CFU/thigh after 24 h with saline treatment and with 6-hourly dosing of 400 or 800 mg of ceftolozane alone/kg and in combination with tazobactam at 2:1, 4:1, and 8:1 ratios for five strains of Enterobacteriaceae producing ESBLs are shown in Table 4. All treatment groups with ceftolozane alone or in combination with tazobactam were significantly less (P < 0.05) than the values for controls treated only with saline. Only mice receiving ceftolozane with tazobactam at a 2:1 ratio were significantly different (P = 0.048) than animals treated with ceftolozane alone. This 2:1 ratio of ceftolozane to tazobactam was the most potent combination regimen for these strains. One strain (K. pneumoniae 4105) was studied in the thighs and lungs of the same mice. The change in log10 CFU/thigh or lung was slightly greater in the lung than in the thigh, but the differences were not statistically significant.
Table 4.
Change in log10 CFU/thigh (and lung) after 24 h of therapy with ceftolozane alone or in combination with tazobactam at ratios of 2:1, 4:1, and 8:1 against Enterobacteriaceae producing various ESBLs in neutropenic micea
| Organism | Control | Ceftolozane dose (mg/kg) | Change in log10 CFU/thigh after 24 h of therapy |
|||
|---|---|---|---|---|---|---|
| Ceftolozane | Ceftolozane + TZB (2:1) | Ceftolozane + TZB (4:1) | Ceftolozane + TZB (8:1) | |||
| E. coli 6042 | 2.22 ± 0.17 | 400 | –0.32 ± 0.56 | –1.91 ± 0.63 | –1.31 ± 0.21 | –1.15 ± 0.37 |
| K. pneumoniae 4105 | 3.07 ± 0.10 | 400 | 1.76 ± 0.30 | –0.21 ± 0.30 | 0.58 ± 0.35 | 1.28 ± 0.10 |
| 2.63 ± 0.37 | 800 | 0.87 ± 0.77 | –1.44 ± 0.40 | –1.01 ± 0.25 | 0.53 ± 0.82 | |
| Lung | 2.22 ± 0.50 | 400 | 1.09 ± 0.85 | –0.60 ± 0.45 | –0.09 ± 0.22 | 0.41 ± 0.81 |
| K. pneumoniae 4110 | 1.82 ± 0.10 | 400 | 1.05 ± 0.07 | 1.03 ± 0.04 | 0.91 ± 0.08 | 1.01 ± 0.05 |
| 1.57 ± 0.24 | 800 | 0.29 ± 0.12 | –0.53 ± 0.10 | –0.41 ± 0.23 | 0.33 ± 0.92 | |
| K. pneumoniae 81-1260A | 3.15 ± 0.07 | 400 | –0.87 ± 0.41 | –2.33 ± 0.50 | –1.59 ± 0.86 | –1.08 ± 0.57 |
| E. cloacae 81-1291A | 2.04 ± 0.09 | 400 | –1.06 ± 0.30 | –3.05 ± 0.37 | –3.29 ± 0.57 | –2.78 ± 0.30 |
Values are expressed as means ± the standard error of the mean where applicable.
Significant linear regressions were obtained with the change in log10 CFU/thigh values listed in Table 4 with the MIC values in Table 1. The R2 values were 70% for ceftolozane alone, 77% for ceftolozane + tazobactam at a 2:1 ratio, 81% for ceftolozane + tazobactam at a 4:1 ratio, and 94% with ceftolozane + tazobactam at a 8:1 ratio. Combining all of the changes in log10 CFU/thigh obtained at the different ratios of ceftolozane + tazobactam the linear correlation was slightly higher (R2 = 67%) with individual MICs at the different ratios than with MICs with fixed tazobactam concentrations of 4 mg/liter (R2 = 63%) and 8 mg/liter (R2 = 54%). However, all three correlations were highly significant (P < 0.01). Figure 5 shows the relationship between the change in log10 CFU/thigh and the percent T>MIC observed with the treatment regimens of ceftolozane with tazobactam at the three different ratios. The line of best fit of the data had a R2 value of 69%. The regression resulted in stasis at a T>MIC of 31.1% ± 4.9% and 1-log kill at a T>MIC of 34.8% ±4.4. These values are slightly higher than the mean values shown in Table 3 for stasis and 1-log kill with ceftolozane against wild-type Enterobacteriaceae.
Fig 5.
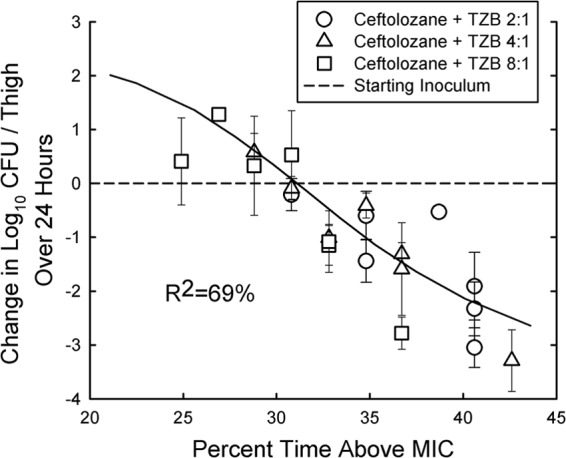
Relationship between the percent T>MIC and the change in CFU/thigh over 24 h for five ESBL-producing Enterobacteriaceae after 6-hourly therapy with 400 and 800 of ceftolozane mg/kg in combination with tazobactam (TZB) at 2:1, 4:1, and 8:1 ratios. Each point represents the mean of three mice, and the error bar represents the SD. R2 represents the percentage of variance in CFU/thigh that can be attributed to the percent T>MIC.
DISCUSSION
As with other cephalosporins, T>MIC is the major PK/PD index driving the efficacy of ceftolozane. The mean percent T>MIC values required for stasis and 1-log kill against the eight susceptible Gram-negative bacilli were 25.2 ± 2.8 and 31.5 ± 2.8, respectively. For Enterobacteriaceae with ESBLs, stasis and 1-log kill was observed at T>MIC values of 31.1% ± 4.4% and 34.8% ± 4.9%, respectively. These values are lower than observed in our previous studies with other cephalosporins against various Enterobacteriaceae strains (15–20). Those studies showed mean values of 35 to 43% for stasis with cephalosporins such as cefotaxime, ceftazidime, cefazolin, cefepime, cefpirome, ceftriaxone (free drug), and ceftobiprole. There are several factors that could explain these differences. The half-lives of ceftolozane in the infected neutropenic mice were 12 to 14 min. Our previous studies with other cephalosporins have reported half-lives of 14 to 25 min (16, 19, 20). It is possible that our microbiologic assay was in error and resulted in a faster half-life than would be observed with a direct chemical assay and at lower drug concentrations. Using a liquid chromatography-tandem mass spectroscopy assay, ceftolozane had a half-life of ∼20 min in infected non-neutropenic mice (21). This 6- to 8-min difference in the drug's half-life could explain some, but not all, of the lower T>MIC values for ceftolozane with Enterobacteriaceae in our studies.
For most cephalosporins, the T>MIC required for stasis with P. aeruginosa is even longer than observed Enterobacteriaceae (18, 19). With ceftazidime against three strains of P. aeruginosa, the percent T>MIC to produce a net static effect over 24 h ranged from 40 to 45% (19). However, our current studies demonstrate that ceftolozane required a much lower percent T>MIC (24.0% ± 3.3%). One group of researchers suggested that the major factor determining the actual percent T>MIC appears to be the relationship between the growth rate of the organism and the bacterial killing rate of the antibiotic (10). These researchers studied the kinetics of growth and bacterial killing of P. aeruginosa ATCC 25783 in vitro and used in vivo pharmacokinetics in mice to predict the percent T>MIC for stasis. The T>MIC predictions for stasis with ceftazidime were ca. 40%. In vitro studies with ceftolozane and ceftazidime against P. aeruginosa PAO1 suggested that ceftolozane has better killing kinetics than ceftazidime (13). In time-kill studies with fixed concentrations, ceftolozane initiated killing at 2- to 4-fold lower multiples of the MIC than ceftazidime. Because of these in vitro studies, we decided to compare the rate of killing of two strains of P. aeruginosa by ceftolozane and ceftazidime. Ceftolozane clearly had faster killing of both strains than ceftazidime, which could account for some of the difference in T>MIC required for stasis by these two drugs. Faster killing by ceftolozane could also explain some of the difference in percent T>MIC with other cephalosporins for Enterobacteriaceae, but such studies was not performed.
Pharmacokinetics of ceftolozane at 1 g every 8 h in healthy subjects has demonstrated serum concentrations of >8 mg/liter, respectively, for >50% of the dosing interval (22). Most clinical trials will use ceftolozane combined with tazobactam at a 2:1 ratio. Tazobactam is considered a “suicide inhibitor” for β-lactamases and has inhibitory effects that last after the initial exposure (23). The half-life of ceftolozane and tazobactam are relatively similar in mice (21), whereas in humans the half-life of ceftolozane is ∼2.5-fold longer than tazobactam (24). The human pharmacokinetics of 1 g of ceftolozane plus 500 mg of tazobactam was simulated in the murine thigh infection model (21). All organisms with MICs of ≤8 mg/liter had a T>MIC of 56% or higher and showed significant reductions of CFU in the thighs of mice over 24 h. One strain of P. aeruginosa for which the MIC was 16 mg/liter was also reduced with this simulated treatment even though the T>MIC was only 32.5%. On the other hand, for a strain of K. pneumoniae producing an ESBL the MIC was 32 mg/liter and actually grew in the thigh with this therapy, which resulted in only 21% T>MIC. These results are very similar to the data reported in the present study using murine pharmacokinetics.
In summary, ceftolozane is a potent cephalosporin resulting in over a 2-log kill over 24 h in the thighs of neutropenic mice for most organisms. The 2:1 ratio of ceftolozane with tazobactam was the most potent formulation studied both in efficacy and MIC reduction. The T>MIC required for stasis and 1-log kill was less with ceftolozane for both Enterobacteriaceae and P. aeruginosa than observed with other cephalosporins. Although some of the difference could be due to differences in assay accuracy, ceftolozane did result in faster killing of P. aeruginosa than observed with ceftazidime in the same animal model.
ACKNOWLEDGMENTS
This study was funded by a research grant from Calixa Therapeutic, Inc., San Diego, CA, to the Department of Medicine, University of Wisconsin School of Medicine and Public Health.
We thank Karen Bush for three strains (E. coli 6042 and K. pneumoniae 4105 and 4110) and Ron Jones for two strains (K. pneumoniae 81-1260A and E. cloacae 81-1291A) used in these studies.
Footnotes
Published ahead of print 28 December 2012
REFERENCES
- 1. Rosenthal VD, Maki DG, Mehta A, Alvarez-Moreno B, Leblebiciglu H, Higuera F. 2008. International nosocomial infection control consortium report, data summary of 2002–2007, issued January 2009. Am. J. Infect. Control 36:627–637 [DOI] [PubMed] [Google Scholar]
- 2. Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J. 2009. International study of the prevalence and outcomes of infection in intensive care units. JAMA 302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 3. Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob. Agents Chemother. 55:2390–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takeda S, Nakai T, Wakai Y, Ikeda F, Hatano K. 2007. In vitro and in vivo activities of a new cephalosporin, FR264205, against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 51:826–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bulik CC, Christensen H, Nicolau DP. 2010. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob. Agents Chemother. 54:557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int. J. Antimicrob. Agents 34:402–406 [DOI] [PubMed] [Google Scholar]
- 7. Juan C, Zamoramo L, Perez JL, Ge Y, Oliver A, Spanish Group for the Study of Pseudomonas (Spanish Network for Research in Infectious Diseases) 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 54:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moya B, Zamoramo L, Juan C, Perez JL, Ge Y, Oliver A. 2010. Activity of a new cephalosporin, CXA-101 (FR264295), against beta-lactamase-resistant Pseudomonas aeruginosa mutants selected in vitro after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeda S, Ishii Y, Hatano K, Teteda K, Yamaguchi K. 2007. Stability of FR264205 against AmpC β-lactamase of Pseudomonas aeruginosa. Int. J. Antimicrob. Chemother. 30:443–445 [DOI] [PubMed] [Google Scholar]
- 10. Mouton JW, Punt N, Vinks AA. 2007. Concentration effect relationship of ceftazidime explains why the time above MIC is 40% for a static effect in vivo. Antimicrob. Agents Chemother. 51:3449–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Livermore DM, Mushtag S, Ge Y. 2010. Chequerboard titration of cephalosporin CXA-101 (FR264205) and tazobactam versus beta-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 65:1972–1974 [DOI] [PubMed] [Google Scholar]
- 12. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–12 [DOI] [PubMed] [Google Scholar]
- 13. Moya B, Zamorano L, Juan C, Ge Y, Oliver A. 2010. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3933–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed CLSI document M7-A7 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15. Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 [DOI] [PubMed] [Google Scholar]
- 16. Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96 [DOI] [PubMed] [Google Scholar]
- 17. Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479–501 [DOI] [PubMed] [Google Scholar]
- 18. Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh- and lung-infection models. Antimicrob. Agents Chemother. 52:3492–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fantin B, Leggett J, Ebert S, Craig WA. 1991. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob. Agents Chemother. 35:1413–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leggett JE, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig WA. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh infection models. J. Infect. Dis. 159:281–292 [DOI] [PubMed] [Google Scholar]
- 21. Bulik CC, Tessier PR, Keel RA, Sutherland CA, Nicolau DP. 2012. In vivo comparison of CXA-101 (FR264205) with or without tazobactam versus piperacillin-tazobactam using human simulated exposures against phenotypically diverse gram-negative organisms. Antimicrob. Agents Chemother. 56:544–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ge Y, Whitehouse MJ, Friedland I, Talbot GH. 2010. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob. Agents Chemother. 54:3427–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strayer AH, Gilbert DH, Pivarnik P, Medeiros AA, Zinner SH, Dudley MN. 1994. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob. Agents Chemother. 38:2351–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob. Agents Chemother. 56:3086–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]


