Abstract
P-873 is a novel compound in the RX-04 pyrrolocytosine series of protein synthesis inhibitors currently under development by Rib-X Pharmaceuticals. We evaluated the pharmacodynamic and pharmacokinetic properties of this compound against Klebsiella pneumoniae using a murine neutropenic thigh infection model. P-873 demonstrated potent and rapid in vivo activity against this organism with enhanced penetration and duration of exposure in thigh tissue.
TEXT
The increase in resistance to currently available antibiotics underscores the need to develop new therapies, particularly against Gram-negative organisms, including Klebsiella pneumoniae (1, 2). To address this unmet medical need, Rib-X Pharmaceuticals has designed and developed novel antibacterial scaffolds that inhibit protein synthesis by binding to the 50S ribosomal subunit (RX-04 program). The pyrrolocytosine (P) series has been shown to have broad-spectrum activity against clinically relevant organisms, including K. pneumoniae (3, 4, 5, 6). The pharmacodynamic and pharmacokinetic properties of P-873 were evaluated against K. pneumoniae using a murine neutropenic thigh infection model.
P-873 (Rib-X Pharmaceuticals, New Haven, CT) was used throughout these studies. Prior to each experiment, P-873 was reconstituted in 10% Captisol-50 mM sodium phosphate buffer, pH 7.2 (vehicle), and further diluted to achieve the desired concentrations. Dosing solutions were held on ice throughout the dosing period. Specific-pathogen-free female ICR (CD-1) mice (25 to 30 g) were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). All mice were provided food and water ad libitum. The study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee.
A previously described murine neutropenic thigh infection model was used for both bacterial density and pharmacokinetic experiments (7). Briefly, mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL) at 150 mg/kg and 100 mg/kg of body weight given 4 days and 1 day, respectively, prior to inoculation. Two hours prior to the initiation of antimicrobial therapy, each thigh was inoculated intramuscularly with 0.1 ml of Klebsiella pneumoniae 457 (P-873 MIC, 0.06 μg/ml by broth microdilution [8]) containing approximately 107 CFU/ml in normal saline. Groups of 3 animals were administered total daily doses of P-873 ranging from 0.625 to 15 mg/kg subcutaneously as 0.2-ml injections. P-873 dose fractionation studies were conducted using total daily doses of 0.625, 1.25, 2.5, 5, and 10 mg/kg. Doses were administered 1, 2 (every 12 h [q12h]), or 4 (q6h) times over a 24-h period. Control mice received vehicle as sham treatment. A group of untreated control mice (n = 3) was euthanized just prior to antibiotic treatment initiation to establish the initial bacterial burden (0 h). At 24 h postinfection, sham-treated control mice and all treated mice were euthanized. Both thighs were harvested aseptically and individually homogenized in 5 ml sterile normal saline. Serial dilutions of thigh homogenate were subcultured onto Trypticase soy agar with 5% sheep blood for bacterial density determinations. Antibacterial efficacy was calculated as the change in average bacterial density in treated or control animals after 24 h compared to the average in the control animals at the initiation of dosing (0 h).
For pharmacokinetic studies, single doses of P-873 at 5, 1.25, or 0.3125 mg/kg were administered 2 h after bacterial inoculation. Terminal blood samples were collected via cardiac puncture into lithium heparin tubes (Terumo, Elkton, MD) at 0.25, 0.5, 1, 2, 3, 4, 6, and 8 h postdose. Plasma was separated by centrifugation. In conjunction with the plasma pharmacokinetic studies, time-kill studies were conducted in thigh tissue at 1, 2, 4, and 8 h postdose. Thigh tissue was processed as described above for bacterial density determination, and an aliquot of the thigh homogenate was reserved to evaluate P-873 pharmacokinetic distribution into the site of infection. While tissue homogenates can provide an early look into drug concentrations at the site of infection, it should be noted that their use does not allow differentiation between interstitial, intracellular, and vascular compartments. Plasma and thigh homogenate samples were stored at −80°C until analyzed. Drug concentrations in plasma and thigh homogenate samples were analyzed by a validated liquid chromatography-tandem mass spectrophotometry assay.
The average (±standard deviation [SD]) bacterial density at the onset of therapy was 6.00 ± 0.18 log10 CFU and increased to 8.83 ± 0.27 log10 CFU at 24 h in untreated mice. Single daily doses of P-873 of ≥5 mg/kg resulted in reductions of thigh bacterial burden of ≥0.5 log10 CFU.
During dose fractionation studies, a general trend toward greater activity was noted with increases in dose fractionation, suggesting the importance of duration of exposure for P-873 activity.
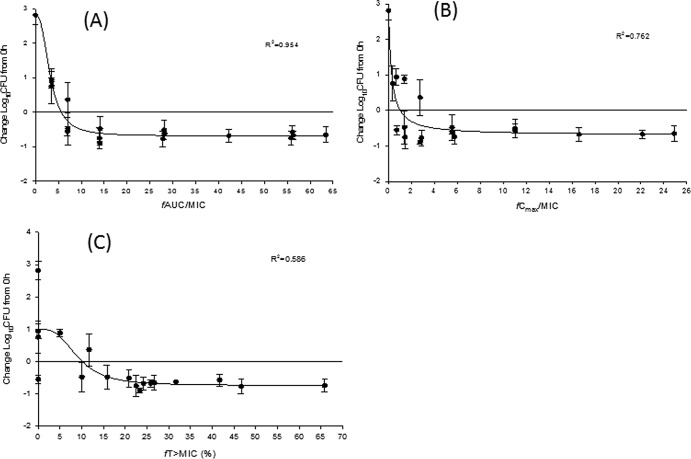
For pharmacodynamic analyses, plots of the change in log10 CFU versus the free drug (P-873, 95% protein bound) pharmacodynamic parameters (fAUC/MIC [ratio of the free drug area under the concentration-time profile to MIC], fCmax/MIC [ratio of the free peak drug concentration to the MIC], and fT>MIC [percentage of the dosing interval in which free drug concentrations remained above the MIC]) were constructed and analyzed using the sigmoidal Emax model: E = E0 − (E0 − Emax) × [Iγ/(Iγ + EI50γ)], where Emax is maximum efficacy, I is pharmacodynamic index of interest, γ is the slope of the inhibitory-effect curve, and EI50 is the value of the pharmacodynamic index associated with 50% of maximal efficacy (WinNonlin version 5.0.1; Pharsight, Mountain View, CA). As noted in Fig. 1A to C, the respective R2 values for fAUC/MIC, fCmax/MIC, and fT>MIC were 0.954, 0.762, and 0.586, suggesting that fAUC/MIC was the predominant driver of in vivo efficacy. The calculated exposure index values required for stasis, 50% of maximal efficacy (EI50), and 80% of maximal efficacy (EI80) for fAUC/MIC were determined to be 5.6, 3.2, and 5.5, respectively.
Fig 1.
Free drug pharmacodynamic profiles of P-873 versus Klebsiella pneumoniae 457 (MIC, 0.06 μg/ml) in the neutropenic thigh infection model. (A) fAUC/MIC; (B) fCmax/MIC; (C) fT>MIC. Symbols represent means ± standard deviations.
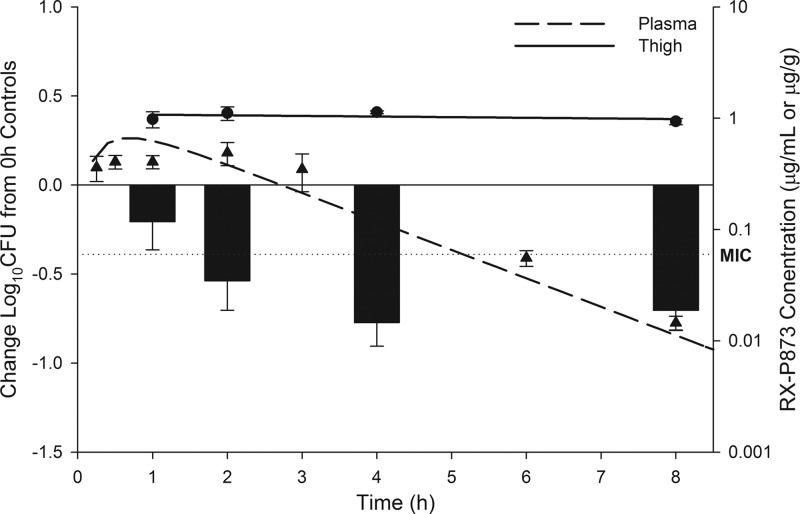
Over the time course tested, thigh tissue concentrations (μg/g tissue) of P-873 for all doses exceeded the MIC (0.06 μg/ml) of the infecting K. pneumoniae isolate for the 8-h sampling period. When comparing relative 8-hour AUCs, the penetration of P-873 was ∼4 to 6 times higher in thigh tissue than in free plasma. The efficacy of a single 5-mg/kg dose of P-873 as determined during time-kill experiments, compared with the free plasma and thigh tissue concentrations over the same time period, is shown in Fig. 2. Of note, reductions in thigh bacterial burden were observed at 1 h postdose and reached maximal activity at 4 h postdose. Further, while free plasma concentrations of P-873 exceeded the MIC for 5 h postdose, thigh tissue concentrations not only exceeded those found in plasma but remained above the MIC for the duration of the experiment.
Fig 2.
Efficacy of a single 5-mg/kg dose of P-873 (bars) compared with tissue homogenate (circles, solid line) and free drug plasma concentrations (triangles, dashed line). Klebsiella pneumoniae 457 MIC, 0.06 μg/ml (dotted line).
P-873 was shown to exhibit potent and rapid in vivo activity against K. pneumoniae 457 in a murine neutropenic thigh infection model. While dose fractionation studies showed a trend toward enhanced activity with increasing duration of exposure, plasma Emax profiles found fAUC/MIC to be the parameter most predictive of in vivo activity. This discordance in the plasma-defined pharmacodynamic profile appears to be explained by the enhanced penetration and duration of exposure in tissue.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Amira Bhalodi, Henry Christensen, Mao Hagihara, Jennifer Hull, Debora Santini, Christina Sutherland, Pam Tessier, and Lindsay Tuttle for their assistance with the conduct of the animal experimentation and Hongwu Jing for bioanalytical support (Rib-X Pharmaceuticals).
This work was supported by a grant from Rib-X Pharmaceuticals, New Haven, CT.
Footnotes
Published ahead of print 28 January 2013
REFERENCES
- 1. Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J. 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Disease Society of America. Clin. Infect. Dis. 46:155–164 [DOI] [PubMed] [Google Scholar]
- 2. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Disease Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharjee A, Chen S, Collin F, Dalton J, DeVito J, Kanyo Z, Leggio M, Lou R, Martynow J, O'Dowd H, Paik I, Remy J, Scheideman M, Sinishtaj S, Tang Y, Wimberly B, Wu Y, Duffy E. 2011. Completely novel antibiotics for treating multidrug-resistant Gram-negative infections: the pyrrolocytosines, abstr F1-1846. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 4. Marra A, Bortolon E, Molstad D, Wu Y, Jing H, Duffy E. 2011. Novel ribosome inhibitors are efficacious in a murine skin and soft tissue infection model caused by Klebsiella pneumoniae, abstr F1-1853. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 5. Jacobs MR, Bajaksouzian S, Windau AR, Foster AN, Hujer KM, Bonomo RA. 2012. Activity of novel pyrrolocytosine agents against multidrug and carbapenem resistant Klebsiella pneumoniae, abstr F-2059. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 6. Bortolon E, Molstad D, DelGorge C, Rey A, Duffy E. 2012. Novel ribosome inhibitors are efficacious in a murine respiratory tract infection model caused by Streptococcus pneumoniae, abstr F-1523. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 7. Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4112–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI publication M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]