Abstract
We compare long-term outcomes in patients with node negative early stage breast cancer treated with breast radiotherapy (RT) without the axillary RT field after sentinel lymph node dissection (SLND) or axillary lymph node dissection (ALND). We hypothesize that though tangential RT was delivered to the breast tissue, it at least partially sterilized occult axillary nodal metastases thus providing low nodal failure rates. Between 1995 and 2001, 265 patients with AJCC stages I–II breast cancer were treated with lumpectomy and either SLND (cohort SLND) or SLND and ALND (cohort ALND). Median follow-up was 9.9 years (range 8.3–15.3 years). RT was administered to the whole breast to the median dose of 48.2 Gy (range 46.0–50.4 Gy) plus boost without axillary RT. Chi-square tests were employed in comparing outcomes of two groups for axillary and supraclavicular failure rates, ipsilateral in-breast tumor recurrence (IBTR), distant metastases (DM), and chronic complications. Progression-free survival (PFS) was compared using log-rank test. There were 136/265 (51%) and 129/265 (49%) patients in the SLND and ALND cohorts, respectively. The median number of axillary lymph nodes assessed was 2 (range 1–5) in cohort SLND and 18 (range 7–36) in cohort ALND (P < 0.0001). Incidence of AFR and SFR in both cohorts was 0%. The rates of IBTR and DM in both cohorts were not significantly different. Median PFS in the SLND cohort is 14.6 years and 10-year PFS is 88.2%. Median PFS in the ALND group is 15.0 years and 10-year PFS is 85.7%. At a 10-year follow-up chronic lymphedema occurred in 5/108 (4.6%) and 40/115 (34.8%) in cohorts SLND and ALND, respectively (P = 0.0001). This study provides mature evidence that patients with negative nodes, treated with tangential breast RT and SLND alone, experience low AFR or SFR. Our findings, while awaiting mature long-term data from NSABP B-32, support that in patients with negative axillary nodal status such treatment provides excellent long-term cure rates while avoiding morbidities associated with ALND or addition of axillary RT field.
Keywords: Sentinel lymph node sampling, Axillary lymph node dissection, Breast cancer, Tangential breast radiotherapy, Radiotherapy
Introduction
Breast conserving therapy (BCT) has redefined the approach to breast cancer in the 1980s, with lumpectomy and adjuvant tangential breast radiotherapy (RT) achieving excellent local control [1, 2]. Though axillary failure rates (AFR) in patients treated with BCT, axillary lymph node dissection (ALND), and tangential breast radiation therapy (RT) is low, in 4–7% of patients with negative sentinel nodes (SLN), the remaining axillary lymph nodes (ALN) may harbor occult malignant cells [3]. The benefit of ALND or axillary clearance as a treatment is to reduce the risk of axillary recurrence. However, the risk of considerable morbidity and complications from such therapeutic method has compelled exploration of other treatment options [4–9]. An alternative to ALND may be a biopsy of SLN or irradiation of the axillary contents. Treatment of the axilla has evolved since the early 1990s as a minimally invasive method to assess the status of the ALN in the setting of breast cancer. Such a minimally aggressive surgical approach has allowed to avoid ALND if the status of the SLN is negative [10–12].
Adjuvant tangential RT fields have become the standard of care after breast-conserving surgery. Irradiating a patient in a supine position will typically not only involve the ipsilateral breast tissue but will also include the lower aspect of the axillary contents, in particular the majority of the level I of lower echelon and partially level II of the ALN [13]. Thus, while targeting the breast tissue, RT may inadvertently treat, at least partially, the same ALN which are subject to dissection during ALND thus eradicating microscopic nodal disease [2, 14, 15]. Though the risks of regional nodal failure after tangential breast RT are not well understood [16], such a localized radiotherapeutic approach may sterilize occult malignant cells in the remaining axilla even if the SLN is negative.
The purpose of this study is to compare long-term outcomes (regional nodal failure rates, in-breast tumor recurrences, distant metastases) and morbidity at mature follow-up in patients with early stage breast cancer with negative SLN-treated BCT and adjuvant breast RT following sentinel lymph node dissection (SLND) alone or ALND. In the absence of the long-term follow-up from the NSABP B-32 randomized prospective trial, the only trial of sufficient size to provide definitive information related to the primary outcome measures of survival and regional control, such mature follow-up warrants careful consideration.
Materials and methods
After obtaining institutional review board (IRB) approval, we identified 1,396 women with AJCC stages I–II breast cancer treated between 1995 and 2001 at Thomas Jefferson University Hospital (TJUH), Philadelphia, PA and Saint Barnabas Medical Center (SBMC), Livingston, NJ with the BCT approach. Of these, we selected 265 (24%) node negative patients—200 from TJUH and 65 from SBMC—who underwent lumpectomy and either a SLND or SLND followed by ALND. The standard ALND involved at least dissection of levels I–II ALN, based on the arbitrarily set anatomic principles [17]. The group of patients who underwent a combination approach of SLND- and ALND-sustained axillary clearance during the same surgery. More than five lymph nodes dissected during SLND were defined as ALND rather than SLND.
Identification of the sentinel lymph node
Assessment of the sentinel lymph node was largely performed by a combined technique of technetium (99mTc)-sulphur colloid (Nanocoll; GE Healthcare, USA) and isosulfan blue dye (1% lymphazurin; Hirsch Industries Inc, Richmond, VA). A 2-ml 99mTc was injected peritumorally (doses 30–50 MBq) on the day prior to surgery or (dose 20 MBq) on the day of surgery, and static scintigraphic images in anterior and oblique projections were obtained 3 h after injection a dynamic gamma camera. The sites of axillary and nonaxillary sentinel lymph nodes were marked on the patient’s skin.
A vial of blue dye (2 ml) was diluted to a total volume of 5 ml with normal saline and was injected peritumorally. The blue dye was injected after the gamma detector identified a hot spot (if a hot spot was not identified, the blue dye was injected after a 40-ml saline bolus). All blue-stained nodes and nodes with radioactive counts more than ten times the background count, as measured with the gamma probe, were defined as sentinel lymph nodes. Standard hematoxylin–eosin (H&E) staining was used to examine ALN. The institutional policy was to section every node above 5 mm. All lymph nodes larger than 5 mm were sectioned into 2–3-mm sections, and each section was stained with H&E. Lymph nodes smaller than 5 mm were bisected and stained with H&E.
Adjuvant breast radiotherapy and systemic therapy
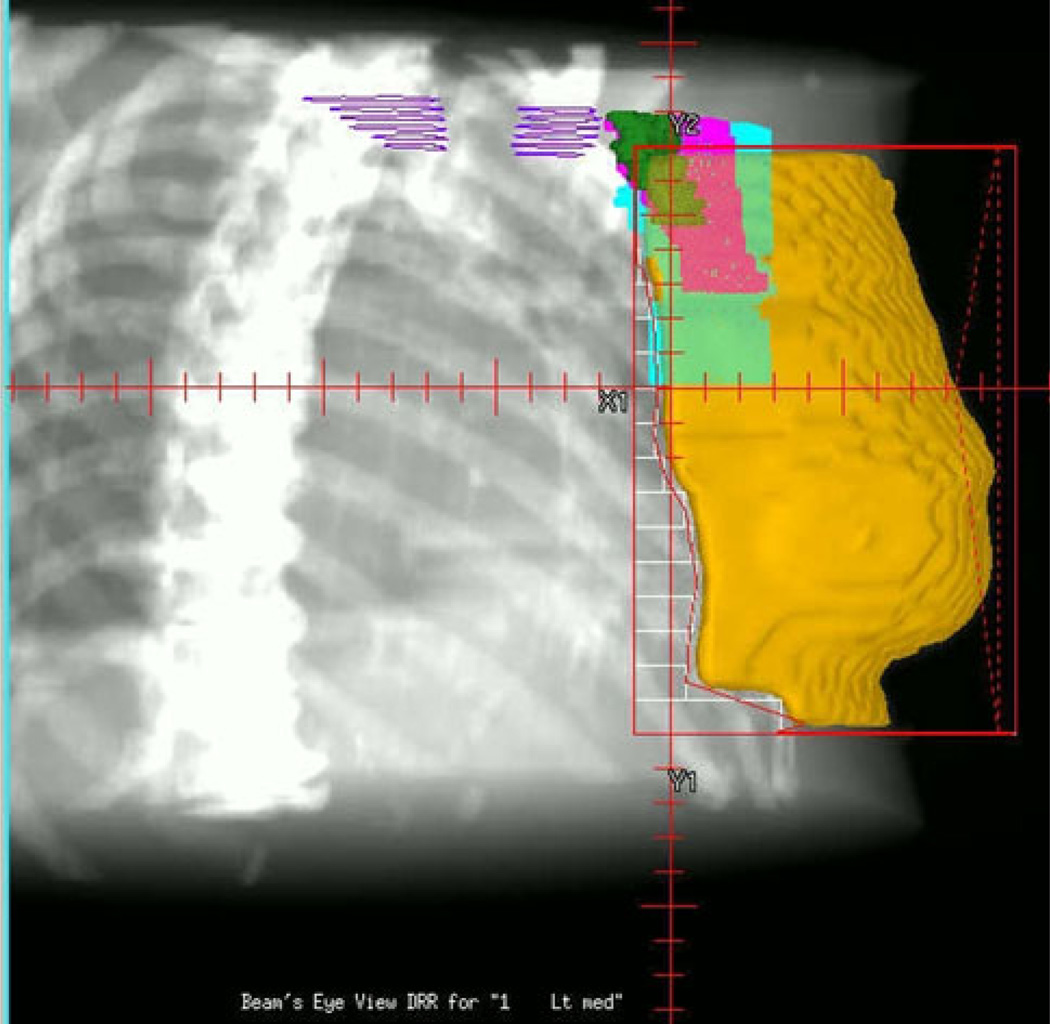
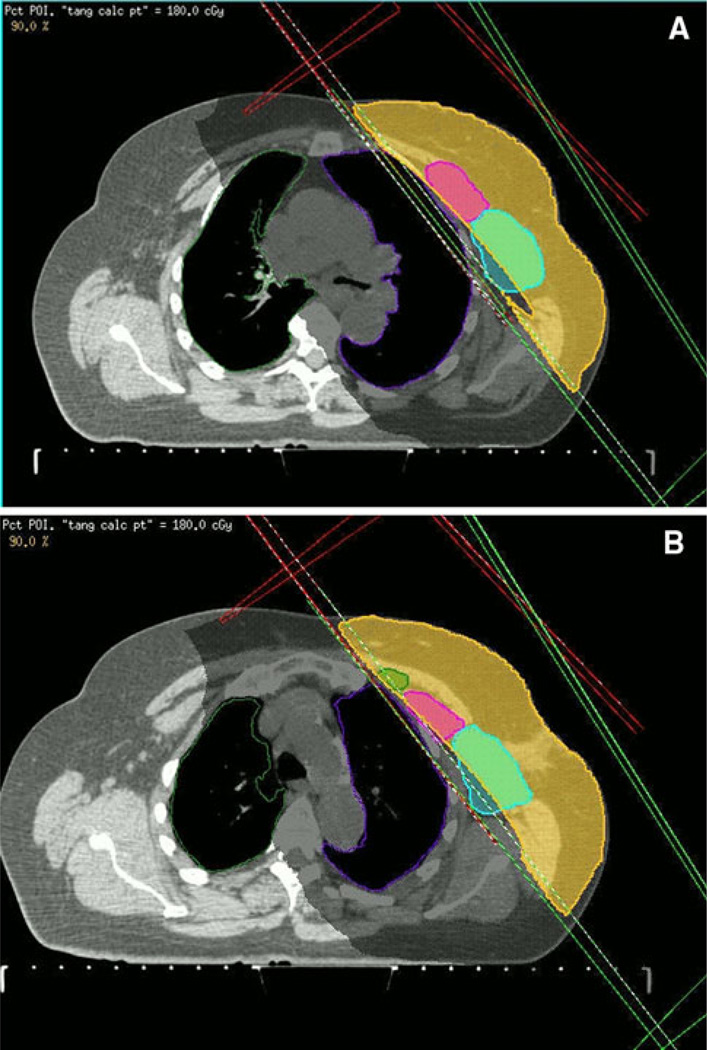
All patients were treated with adjuvant RT with high-energy photons targeting the ipsilateral breast tissue alone. Tangential RT fields were set up and the physics calculations were carried out using supine CT simulation with 3–5-mm interval slices for treatment planning. Whole-breast RT was delivered by means of tangential portals to a median dose of 48.2 Gy (range 46.0–50.4 Gy) administered at 1.8–2.0-Gy fractions, five times a week. An electron boost was subsequently administered to the lumpectomy bed at 2-Gy fractions as prescribed to 90% isodose line, bringing the total median dose to the tumor bed to 63.2 Gy (range 60.4–66.0 Gy). The superior border of the tangential fields was clinically placed at the inferior edge of the clavicular head (Fig. 1). The lateral border typically was set in the mid-axillary line, thus having standard tangential RT fields encompass the majority of the level I of lower echelon, at least partially level II, and occasionally level III of the ALN (Fig. 2a, b). No axillary RT was employed to treat ALN.
Fig. 1.
A relationship between local breast tangent radiation field and regional lymph nodes as depicted in the lateral beam’s eye view with a tangential portal. The superior border of the radiation portal is set below the heads of the clavicles (navy). Level I (blue), level II (purple), and level III (dark green) are partially covered by the 90% isodose area covering the breast tissue (orange). (Color figure online)
Fig. 2.
A relationship between local breast tangent radiation fields and regional lymph nodes as depicted in the axial view on a CT slice. a Level I (blue) and level II (purple) ALN are included in the 90% isodose area covering the breast tissue (orange). b A small portion of the 90% isodose area covers all three echelons of ALN (level III is in dark green). (Color figure online)
Adjuvant systemic therapy was determined and administered by the medical oncologist, based on the patients’ risk factors, tumor characteristics, receptor status, and patient preference.
Complications and morbidity assessments
Chronic long-term complications are defined as the ones which persist to date at the last follow-up in the evaluable patients. Lymphedema was evaluated at each follow-up visit by a patient self-assessment as present or absent and in the present category as none, mild, moderate, or severe. The institutional policy was to assess lymphedema by medical professionals. Clinical examination of lymphedema was measured by clinical professionals (nursing staff in radiation, medical oncology, or surgical oncology clinics) and consisted of objective measurements at baseline and at each follow-up visit in both arms at the antecubital fossa, 10-cm superior, 10-cm inferior, and at the wrists. Arm lymphedema was defined as the difference in the operated arm and the opposite arm by 1 cm. Sensory neuropathy was defined as numbness and was recorded as reported by patients. Decreased range of motion of the shoulder as compared to the contralateral shoulder was defined as reduced abduction and measured my medical professionals. Wound infection and seroma were recorded by the medical professionals and obtained from the clinic records.
Statistical considerations
Descriptive statistics (including mean, range, frequency, percent), stratified by patient cohort (SLND and ALND), are presented for demographic and clinical/treatment factors of interest. The chi-square test or Fisher’s exact test was used, as appropriate, to compare categorical clinical outcomes between the two cohorts. The two-sample t test or Wilcoxon rank-sum test was used, as appropriate, to compare continuous demographic/clinical outcomes between the two cohorts. Progression-free survival (PFS) for the two cohorts (from date of diagnosis) was evaluated by Kaplan–Meier survival analysis and the log-rank test was employed to compare PFS between patients in the SLND and ALND cohorts. Median follow-up time for both cohorts was based on surviving patients. Ninety-five percent confidence intervals (95% CI) for median PFS-time and 10-year PFS probability estimates were calculated to assess the precision of the obtained estimates. All analyses were performed in SAS Version 9.2 (SAS Institute, Inc., Cary, NC), SPSS Version 18.0 (SPSS Inc., Chicago, IL), and STATA Version 11.0 (StataCorp, College Station, TX).
Results
The baseline characteristics of the patients, tumors, and systemic therapy in two cohorts are listed in Table 1. The mean age for cohort SLND and cohort ALND is 57.8 years (range 36.0–86.0 years) and 56.0 years (range 24.0–85.0 years). The vast majority of tumors had favorable characteristics: smaller size lesions (T1), of low-to-intermediate nuclear grade, estrogen (ER) and/or progesterone receptors (PR) expressing, and without lymphovascular invasion (Table 1).
Table 1.
Characteristics of the patients and tumors, and systemic therapy in the two cohorts
| Cohorts | SLND (N = 136) | ALND (N = 129) |
|---|---|---|
| Mean age (year) | 57.8 (range, 36–86) | 56.0 (range, 24–85) |
| T stage (%) | ||
| T1 | 82 | 81 |
| T2 | 18 | 19 |
| Nuclear grade (%) | ||
| 1 | 16 | 18 |
| 2 | 63 | 59 |
| 3 | 21 | 23 |
| Tumor location (%) | ||
| Outer quadrants | 71 | 72 |
| Inner/central quadrants | 29 | 28 |
| Histological tumor type (%) | ||
| Ductal | 82 | 81 |
| Lobular | 7 | 6 |
| Other | 11 | 13 |
| Receptor status (%) | ||
| ER/PR+ | 69 | 73 |
| ER/PR− | 15 | 12 |
| ER +/PR− | 13 | 12 |
| ER−/PR+ | 2 | 1 |
| Unknown | 1 | 2 |
| Lymphovascular invasion (%) | ||
| Yes | 19 | 21 |
| No | 75 | 74 |
| Unknown | 6 | 5 |
| Chemotherapy (%) | ||
| Yes | 25 | 15 |
| No | 75 | 85 |
| Hormonal therapy (%) | ||
| Yes | 54 | 56 |
| No | 46 | 44 |
Table 2 points to the number of ALN assessed in each cohort and method of their assessment. There were 136/265 (51%) patients whose axilla was assessed by means of SLND alone and 129/265 (49%) women who underwent a combined surgical approach of SLND followed by ALND. The vast majority of SLN were determined by utilization of the combined 99mTc and isosulfan blue dye technique. The median number of ALN assessed in the cohort SLND was 2 (range 1–5) and in cohort ALND was 18 (range 7–36) (P < 0.0001).
Table 2.
Method of sentinel lymph nodes assessment and the median number ALN removed in each cohort
| SLND | ALND | P value | |
|---|---|---|---|
| Number of patients undergoing SLN assessment | 136/265 (51.3%) | 129/265 (48.7%) | N/A |
| Method of SLN assessment | |||
| Technetium 99 (99mTc) | 4/136 (2.9%) | 5/129 (3.9%) | 0.91 |
| Isosulfan blue dye | 3/136 (2.2%) | 4/129 (3.1%) | 0.90 |
| Combination of 99mTc and isosulfan blue dye | 129/136 (94.9%) | 120/129 (93.0%) | 0.67 |
| Median number of ALN assessed | 2 (range 1–5) | 18 (range 7–36) | <0.0001 |
The median follow-up time for all patients was 9.9 years (range 8.3–15.3 years), and based on survivors (N = 233) it was 9.4 years (range 8.6–15.2 years) in the SLND cohort and 9.9 years (range 8.3–15.3 years) in the ALND cohort. To date, there were no axillary or supraclavicular failures in either SLND or ALND cohorts (Table 3). The incidence of IBTR was similar in both the groups, 7/136 (5.1%) and 6/129 (4.7%) in the SLND and ALND groups, respectively. The patients who sustained IBTR were all treated with salvage mastectomy. The rate of DM was also similar 11/136 (8.1%) and 11/129 (8.5%) for the SLND and the ALND cohorts, respectively. The treatment of DM included systemic chemotherapy, hormonal therapy, surgery, and/or palliative RT. To date, follow-up on patients with chronic complications was available on 108/136 (80%) in the SLND and on 115/129 (89%) in the ALND cohort. The rate of these chronic complications, predominantly lymphedema as assessed by the medical professionals, varied in a statistically significant manner: 5/108 (4.6%) for SLND and 40/115 (34.8%) for ALND groups, respectively (P < 0.0001) (Table 4). Other complications recorded by medical personnel were more statistically significant in the ALND cohort as compared to the SLND cohort for sensory neuropathy (P = 0.0001) and decreased range of motion in the ipsilateral shoulder (P < 0.0001).
Table 3.
Unfavorable events, patients alive with breast cancer and deaths in the two cohorts
| SLND 136/265 (51%) |
ALND 129/265 (49%) |
P value | |
|---|---|---|---|
| Unfavorable events | |||
| Axillary failures | 0/136 (0%) | 0/129 (0%) | N/A |
| Supraclavicular failures | 0/136 (0%) | 0/129 (0%) | N/A |
| In-breast tumor recurrences | 7/136 (5.1%) | 6/129 (4.7%) | >0.99 |
| Distant metastases | 11/136 (8.1%) | 11/129 (8.5%) | >0.99 |
| Total number of events | 18/136 (13.2%) | 17/129 (13.2%) | >0.99 |
| Patients alive with disease after unfavorable events | 6/136 (4.4%) | 10/129 (7.8%) | 0.37 |
| Deaths | |||
| Deaths from breast cancer causes | 7/136 (5.1%) | 5/129 (3.9%) | 0.86 |
| Deaths from other causes | 12/136 (8.8%) | 8/129 (6.2%) | 0.57 |
| Total number of deaths | 19/136 (14.0%) | 13/129 (10.1%) | 0.43 |
Table 4.
Long-term complication rates in the two cohorts as assessed at 10 years
| SLND | ALND | P value | |
|---|---|---|---|
| Number of patients assessed for complications at 10 years | 108/136 (79.4%) | 115/129 (89.1%) | 0.05 |
| Complication rates as assessed by medical professionals at 10-years (ipsilateral arm lymphedema) |
5/108 (4.6%) | 40/115 (34.8%) | <0.0001 |
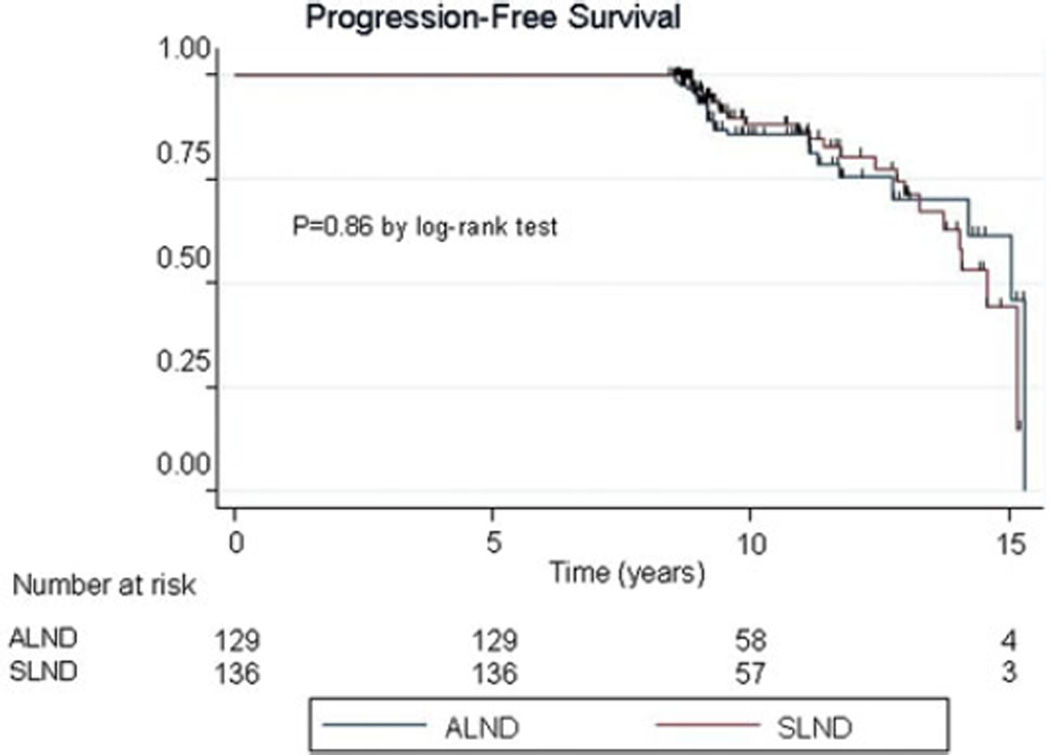
Of the 136 patients treated with SLND and breast tangential RT, 7 patients succumbed to the metastatic disease, 12 died of other unrelated causes (stroke, cardiovascular disease, etc.), and 6 remain alive with disease. Median PFS in this SLND cohort is 14.6 years (95% CI 13.7–15.2 years), and a 10-year PFS is 88.2% (95% CI 79.5%, 93.4%) (Fig. 3). In the cohort of combined SLND and ALND approach followed by tangential RT to the breast, of 129 patients, 5 died of disease, 8 died due to other unrelated causes, and 10 are still alive with disease. Median PFS in this ALND cohort is 15.0 years (95% CI 14.2–15.3 years), and the 10-year PFS is 85.7% (95% CI 77.3%, 91.2%) (Fig. 2). Of total, 12 patients have died of breast cancer, 5 in the ALND cohort, and 7 in the SLND group (log-rank, P = 0.86; Table 4), and 16 women remain alive with breast cancer, 10 in the ALND arm and 6 in the SLND cohort (log-rank, P = 0.37; Table 4), lymphovascular invasion was present in 20/28 (71%) of patients, tumor size was ≥2.0 cm in 11/28 (39%) cases, and 4/28 (14%) were younger than 35 years of age. Histology, site of primary carcinoma, receptor status, nuclear grade, and type of systemic therapy were not significantly related to being alive or dead of breast cancer as demonstrated in multivariate analysis.
Fig. 3.
Progression-free survival for the cohort stratified by the type surgery addressing ALN status: sentinel lymph node sampling alone (SLND) and sentinel lymph node sampling followed by the full axillary lymph node dissection (ALND)
Discussion
Adjuvant RT in the setting of BCT confers an absolute benefit of 19% in 5-year local rate of recurrence and 5% in 15-year cancer mortality for patients with breast cancer [18]. ALN are frequently the first site of regional metastatic disease in the setting of breast cancer [19]. Thus, ALN clearance does not only provide important information for prognosis and staging based on either the presence or the absence of dissemination to the ALN [20] but it also ensures local tumor control, decreased AFR and may improve survival [21]. Despite the clear advantages of ALND, morbidity, including lymphedema and decreased arm and shoulder function, is seen in 5–39% of the patients [5–9]. Our study corroborates the incidence of complications associated with ALND provided in the literature and provides a mature 10-year follow up of the rates, which are statistically significantly worse in the ALND cohort.
The SLN biopsy was introduced with the objective to minimize the morbidity, i.e., in the setting of negative SLND, ALND can be avoided [11, 12, 20, 22–26]. However, with the notion that 4–7% of patients with negative SLN harbor occult metastases in the remaining ALN [3], several critical questions require long-term follow-up data: (1) Is the incidence of AFR low enough in patients with negative SLN treated with BCT, consisting of lumpectomy and ipsilateral local breast RT not warrant completion ALND or axillary RT? (2) Is their equivalence in survival between the two cohorts: SLND and ALND? (3) Is there a difference in the morbidity profile between the two groups of patients? (4) Could tangential breast RT in the setting of negative SLN at least partially sterilize the undissected ALN potentially harboring metastatic deposits?
This study presents 10-year outcomes of two cohorts of patients with negative SLN treated with lumpectomy and breast RT with tangential ports: one cohort treated with SLND alone and the other one with SLND followed by a completion ALND (axillary clearance).
The cohorts were well matched for age, tumor size, nuclear grade, presence or absence of lymphovascular invasion, receptor status, and systemic therapy. At a 10-year follow-up, there are no axillary or supraclavicular failures in either SLND or ALND cohorts, and the incidence of IBTR and DM was similar in both the groups (Table 3). This provided clear evidence that in patients with negative SLN, completion ALND or axillary RT are not warranted. As corroborated by prior reports in the literature, we report that the most important predictors of being alive or dead of breast cancer were presence of lymphovascular invasion, larger tumor size, and younger age [1, 19, 27, 28]. A 10-year progression-free survival was similar in both the groups of patients, further confirming that foregoing of axillary clearance in the setting of BCT and adjuvant tangential RT to the breast when the SLN biopsy is negative is as effective as the once gold standard treatment of ALND.
The mature results of our study are analogous to the general outcomes and morbidity of a few European prospective randomized trials which were reported at a rather short follow-up interval (Table 4) [5, 29–31]. These prospective studies have a considerable variability in the inclusion criteria with respect to the tumor size (ranging from <2.0 to >5.0 cm), method of SLN detection (99mTc, blue dye, or combination of the two), and type of surgery, ranging from various forms of breast conservation (using wide excision, lumpectomy, quadrantectomy) to mastectomy. Our study is akin to the American prospective randomized trial NSABP B-32 with respect to the inclusion criteria of tumor size for breast conservation, method of SLN assessment, and tangential breast RT. To date, only the technical outcomes are available from NSABP B-32 [32, 33] and no more than a 3-year follow-up of morbidity [34, 35], our report provides clinically important information not only with regard to the outcomes but also the rates of complications at 10 years. In fact, our study may render the only 10-year long follow-up of chronic complications rates, firmly pointing at a statistical difference in the morbidity between two surgical approaches of the axilla, undisputedly favoring SLND alone (Table 5).
Table 5.
A summary of outcomes and morbidities from the published prospective studies
| Prospective studies | Median follow-up (mo) |
N | Inclusion criteria | SLN localization | AFR (%) |
SFR (%) |
IBTR (%) |
DM (%) |
DFR (%) |
Complications (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Milan [29, 30] (1998–1999) (negative SLN in 341 patients (167 SLN + 174 ALND) | 36 | 516 | ≤2 cm, L only (wide excision or quadrantectomy) | 99mTc-radioactive albumin | 0a | 0a | 1 | 2 | 87.6a | At 2 years available on 200/516 patient |
| 95 updated | 0b | 1.1b | 1 | 4 | 89.9b | Pain | ||||
| 1.2a | 0a | 1.5a | 6.6a | 93.5a | (0.08%)a (0.39%)b | |||||
| 0b | 1.1b | 1.6b | 7.8b | 89.7b | Numbness/paresthesia | |||||
| (0.01%)a (0.68%)b | ||||||||||
| Lymphedema | ||||||||||
| (0.07%)a (0.75%)b | ||||||||||
| Sentinella/GIVOM [31] (1999–2004) | 56 | 749 | ≤3 cm, L, MRM | 99mTc-nanocolloidal albumin | 0.3a | 0.9a | 2.3a | 3.2a | 87.6a | Women in the SLN group had significantly less lymphedema (P = 0.01), restrictions of shoulder mobility (P = 0.016), and numbness (P < 0.0001) compared the ALND group |
| 0b | 0.b | 0.6b | 4.5b | 89.9b | ||||||
| ALMANAC-UK [5] (1999–2003) | 12 | 1031 | <2, 2–5, >5 cm, L, MRM, axillary RT | 99mTc-albumin colloid | low | n/a | n/a | n/a | n/a | There was no difference in lymphedema, sensory loss, intercostobrachial nerve division rates, or impairment of shoulder movement, between the two groups |
| NSABP B-32 [32–35] (2001–2004) (data available of 3,983) | 36 | 5,611 | ≤2 cm, 2.1–4, ≥4.1 cm, L, MRM | 99mTc-sulphur colloid and isosulfan blue | n/a | n/a | n/a | n/a | n/a | Arm volume differences for the ALND (14%) and SLND (8%) |
| TJUH/SBMC (1995–2001) | 120 | 265 | <5.0 cm, L | 99mTc-sulphur colloid and isosulfan blue | 0a | 0a | 5.1a | 8.1a | 88.2a | 4.6a |
| 0b | 0b | 4.7b | 8.5b | 85.7b | 34.8b lymphedema |
L lumpectomy, MRM modified radical mastectomy, RT radiotherapy
Sentinel lymph node dissection (SLND)
Axillary lymph node dissection (ALND)
Though our study provides similarly low rates of axillary and supraclavicular failures to most studies, our rates of IBTR and DM are higher than the equivalently long 10-year follow-up of the Milan trial [30]. The disparity may lie in the surgical approach, i.e., our patients underwent a lumpectomy whereas the overwhelming majority of cases in the Milan trial were treated with quadrantectomy. Furthermore, while the main bulk of tumors in our study were T1 and <20% patients had T2 tumors size (<5.0 cm), the maximum size of the tumor in the Milan trial was 2.0 cm. Our subset of patients with a larger tumor size may also reflect the higher rates of DM in each of the two cohorts as compared to the numbers reported by Veronesi et al.
There are several hypotheses addressing the reason for the low rate of axillary metastases after SLND alone in the setting of negative SLN biopsy. One of the theories of why the occult metastases never become clinically evident may be related to maintaining immunologic barrier in the remaining ALN. In fact, such observations have been made not only in cancer of the breast [36–38] but also in primary cancers involving other organs such as prostate, thyroid, bone marrow, etc. [39]. This hypothesis was tested with the primary objective of improving survival. However, no difference in survival outcomes between the dissected axilla with the ALND technique and the undissected but irradiated axilla in a prospective British trial appears to undermine this hypothesis [40].
In our minds, one of the more compelling justifications for maintaining low incidence of ALN failures lies in sterilization of the occult metastases in the ALN with the conventional breast tangential ports delivering RT to a patient in a supine position. Goodman et al. [13] reported that with the standard radiation tangents 90% of the Berg level I axilla and up to 70% of level II ALN received 95% of the prescribed dose to the breast. The vast majority of literature, with only a few negative studies, supports the fact that modern 3-D tangential port RT of the breast administered in supine position will at least partially irradiate the undissected ALN stations [13, 41–50]. For instance, Schlembach et al. [45] reports that tangential breast field border encompassed an average of 80% of cranial/caudal extent of axillary level I and II dissections and that the RT fields used to treat the breast can include the sentinel lymph node region and most of axillary levels I and II. All our patients were treated with 3-D RT in the supine position (Fig. 1) with the standard tangential RT fields targeting the breast tissue and inadvertently providing at least partial coverage for at least two of the Berg axillary levels. This technique appears to contrast the RT delivery method of the Milan trial where “most patients received conformational radiotherapy to the breast only, including the axillary tail but excluding the first Berg level of the axilla” [29].
Limitations
We want to note the limitations of this study, beginning with the fact that this is a retrospective review which is inherently prone to bias. While the overwhelming bulk of patients was treated at a single institution, a small portion of women was treated at another facility. Nevertheless, the mode of detection of SLN, type of breast surgery, and methodology of delivering breast RT were largely uniform. Another criticism we may face is that even though the mean number of SLN biopsied was 2, the maximum number sampled was 5 which may be too extensive for a SLND. For the purposes of this report, complications are reported as assessed by the medical professionals and not patients. It will be important to report complications based on the patients’ experiences. With the advent of prone breast radiotherapy technique, our conclusions regarding radiation axillary nodal coverage will need to be subject to further dosimetric examination. We do not know whether such coverage is equivalent to the one obtained with the patients treated with the prone technique, as all of our patients were treated in supine position.
Conclusion
We have found that the long-term breast cancer related progression-free survival in the SLND cohort was similar to that in the ALND group, re-affirming equivalent outcomes of SLND alone to SLND followed by ALND. Further, SLND as means of assessment of axilla in a woman with early stage breast cancer spares the patient from a significant and long-term risk of morbidity associated with axillary clearance. This study provides mature evidence that patients with negative nodes, treated with breast tangential RT and SLND alone, experience low AFR or SFR. Several hypotheses underpin the reason why the potentially occult metastatic ALN do not manifest clinically if the SLN is negative. While we favor the reasonable explanation of at least partial sterilization of ALN with traditional tangential RT ports targeting the breast tissue, no definitive proof has yet been rendered. Our findings, while awaiting long-term follow-up from NSABP B-32, support that in patients with negative axillary nodal status SLND alone provides excellent long-term cure rates while avoiding morbidities associated with ALND or addition of axillary RT field.
Acknowledgments
Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996).
Abbreviations
- AFR
Axillary failure rates
- SLN
Sentinel lymph node
- ALN
Axillary lymph node
- SLND
Sentinel lymph node dissection
- ALND
Axillary lymph node dissection
- IBTR
Ipsilateral breast tumor recurrence
- DM
Distant metastases
- BCT
Breast conserving therapy
- SFR
Supraclavicular failure rates
- RT
Radiotherapy
- ER
Estrogen receptor
- PR
Progesterone receptor
- L
Lumpectomy
- MRM
Modified radical mastectomy
Footnotes
Preliminary results of this study were presented at the 25th Annual San Antonio Breast Cancer Symposium in 2002 in San Antonio, USA and the long-term follow up was presented as an oral presentation at the American Radium Society (ARS) in 2009 in Vancouver, Canada.
Conflict of interest None.
Contributor Information
A. Gabriella Wernicke, Department of Radiation Oncology, Stich Radiation Oncology, Weil Cornell Medical College of Cornell University, 525 East 68th Street, New York, NY 10065, USA.
Robert L. Goodman, Department of Radiation Oncology, Saint Barnabas Medical Center, Livingston, NJ, USA
Bruce C. Turner, Department of Radiation Oncology, Thomas Jefferson University Hospital, Philadelphia, PA, USA
Lydia T. Komarnicky, Department of Radiation Oncology, Drexel University Hospital, Philadelphia, PA, USA
Walter J. Curran, Department of Radiation Oncology, Emory University School of Medicine, Atlanta, GA, USA
Paul J. Christos, Division of Biostatistics and Epidemiology, Department of Public Health, Weill Cornell Medical College of Cornell University, New York, NY, USA
Imraan Khan, Department of Biological Sciences, State University of New York, Stony Brook, NY, USA.
Katherine Vandris, Division of Hematology/Medical Oncology, Department of Medicine, Weill Cornell Medical College of Cornell University, New York, NY, USA.
Bhupesh Parashar, Department of Radiation Oncology, Stich Radiation Oncology, Weil Cornell Medical College of Cornell University, 525 East 68th Street, New York, NY 10065, USA.
Dattatreyudu Nori, Department of Radiation Oncology, Stich Radiation Oncology, Weil Cornell Medical College of Cornell University, 525 East 68th Street, New York, NY 10065, USA.
K. S. Clifford Chao, Department of Radiation Oncology, Stich Radiation Oncology, Weil Cornell Medical College of Cornell University, 525 East 68th Street, New York, NY 10065, USA.
References
- 1.Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347(8):567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Galimberti V, Paganelli G, Maisonneuve P, Viale G, Orecchia R, Luini A, Intra M, Veronesi P, Caldarella P, Renne G, Rotmensz N, Sangalli C, De Brito Lima L, Tullii M, Zurrida S. Axillary metastases in breast cancer patients with negative sentinel nodes: a follow-up of 3548 cases. Eur J Cancer. 2009;45(8):1381–1388. doi: 10.1016/j.ejca.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Larson D, Weinstein M, Goldberg I, Silver B, Recht A, Cady B, Silen W, Harris JR. Edema of the arm as a function of the extent of the axillary surgery inpatients with Stage I-II carcinoma of the breast treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1575–1583. doi: 10.1016/0360-3016(86)90280-4. [DOI] [PubMed] [Google Scholar]
- 5.Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, England D, Sibbering M, Abdullah TI, Barr L, Chetty U, Sinnett DH, Fleissig A, Clarke D, Ell PJ. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 6.Olson JA, Jr, McCall LM, Beitsch P, Whitworth PW, Reintgen DS, Blumencranz PW, Leitch AM, Saha S, Hunt KK. Giuliano AE: Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 7.Petrek JA, Heelan MC. Incidence of breast carcinomarelated lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 8.Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencanz PW, Leitch AM, Saha S, Hunt KK, Giuliano A. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 9.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Morbidity in breast cancer patients with sentinel node metastases undergoing delayed axillary lymph node dissection (ALND) compared with immediate ALND. Ann Surg Oncol. 2008;15(1):262–267. doi: 10.1245/s10434-007-9593-3. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. 398–401. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel lymph node in breast cancer using a gamma probe. Surg Oncol. 1993;2(6):335–339. doi: 10.1016/0960-7404(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, Costa A, de Cicco C, Geraghty JG, Luini A, Sacchini V, Veronesi P. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349(9069):1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- 13.Goodman RL, Grann A, Saracco P, Needham MF. The relationship between radiation fields and regional lymph nodes in carcinoma of the breast. Int J Radiat Oncol Biol Phys. 2001;50(1):99–105. doi: 10.1016/s0360-3016(00)01581-9. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro G, Magee B, Swindell R, Harris M, Banerjee SS. The Christie Hospital breast conservation trial: an update at 8 years from inception. Clin Oncol. 1993;5:278–283. doi: 10.1016/s0936-6555(05)80900-8. [DOI] [PubMed] [Google Scholar]
- 15.Wong J, Recht A, Beard CJ, Busse PM, Cady B, Chaffey JT, Come S, Fam S, Kaelin C, Lingos TI, Nixon AJ, Shulman LN, Troyan S, Silver B, Harris JR. Treatment outcome after tangential radiation therapy without axillary dissection in patients with early-stage breast cancer and clinically negative axillary nodes. Int J Radiat Oncol Biol Phys. 1997;39:915–920. doi: 10.1016/s0360-3016(97)00456-2. [DOI] [PubMed] [Google Scholar]
- 16.Recht A, Houlihan MJ. Axillary lymph nodes and breast cancer. Cancer. 1995;76(9):1491–1512. doi: 10.1002/1097-0142(19951101)76:9<1491::aid-cncr2820760902>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Berg JW. The significance of axillary node levels in the study of breast carcinoma. Cancer. 1955;8(4):776–778. doi: 10.1002/1097-0142(1955)8:4<776::aid-cncr2820080421>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, Luini A, Merson M, Saccozzi R, Rilke F. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24, 740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Orr RK. The impact of prophylactic axillary node dissection on breast cancer survival: a Bayesian meta-analysis. Ann Surg Oncol. 1999;6:109–116. doi: 10.1007/s10434-999-0109-1. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albertini JJ, Lyman GH, Cox C, Yeatman T, Balducci L, Ku N, Shivers S, Berman C, Wells K, Rapaport D, Shons A, Horton J, Greenberg H, Nicosia S, Clark R, Cantor A, Reintgen DS. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 24.Suami H, Taylor GI, Pan WR. The lymphatic territories of the upper limb: anatomical study and clinical implications. Plast Reconstr Surg. 2007;119(6):1813–1822. doi: 10.1097/01.prs.0000246516.64780.61. [DOI] [PubMed] [Google Scholar]
- 25.Suami H, Pan W-R, Mann B, Taylor GI. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. Ann Surg Oncol. 2008;15(3):863–871. doi: 10.1245/s10434-007-9709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olivier JB, Verhaeghe JL, Butarelli M, Marchal F, Houvenaeghel G. Functional anatomy of the lymphatic drainage of the breast: contribution of sentinel lymph node biopsy. Ann Chir. 2006;131(10):608–615. doi: 10.1016/j.anchir.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer III. Vascular invasion: relationship with recurrence and survival in a large study with long-term follow-up. Histopathology. 1994;24:41–47. doi: 10.1111/j.1365-2559.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 28.de Bock GH, van der Hage JA, Putter H, Bonnema J, Bartelink H, van de Velde CJ. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organization for Research and Treatment of Cancer studies. Eur J Cancer. 2006;42(3):351–356. doi: 10.1016/j.ejca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–553. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 30.Veronesi U, Viale G, Paganelli G, Zurrida S, Luini A, Galimberti V, Veronesi P, Intra M, Maisonneuve P, Zucca F, Gatt G, Mazzaro G, De Cicco C. Sentinel lymph node biopsy in breast cancer ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 31.Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del Bianco P, Renier M, Racano C, Carraro P, Nitti D. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the sentinella/GIVOM trial. Ann Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 32.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, Weaver DL, Miller BJ, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Mammolito DM, McCready DR, Mamounas EP, Costantino JP, Wolmark N. National Surgical Adjuvant Breast and Bowel Project. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 33.Harlow SP, Krag DN, Julian TB, Ashikaga T, Weaver DL, Feldman SA, Klimberg VS, Kusminsky R, Moffat FL, Jr, Noyes RD, Beitsch PD. Prerandomization Surgical Training for the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial: a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005;241(1):48–54. doi: 10.1097/01.sla.0000149429.39656.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, Skelly JM, Harlow SP, Weaver DL, Mamounas EP, Costantino JP, Wolmark N. National Surgical Adjuvant Breast Bowel Project. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010;102(2):111–118. doi: 10.1002/jso.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Land SR, Kopec JA, Julian TB, Brown AM, Anderson SJ, Krag DN, Christian NJ, Costantino JP, Wolmark N, Ganz PA. Patient-Reported Outcomes in Sentinel-Node Negative Adjuvant Breast Cancer Patients Receiving Sentinel-Node Biopsy or Axillary Dissection: National Surgical Adjuvant Breast and Bowel Project Phase III Protocol B-32. J Clin Oncol. 2010;28(25):3929–3936. doi: 10.1200/JCO.2010.28.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC. Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet. 1999;354:197–202. doi: 10.1016/s0140-6736(98)10175-7. [DOI] [PubMed] [Google Scholar]
- 37.Veronesi U, Orecchia R, Zurrida S, Galimberti V, Luini A, Veronesi P, Gatti G, D’Aiuto G, Cataliotti L, Paolucci R, Piccolo P, Massaioli N, Sismondi P, Rulli A, Lo Sardo F, Recalcati A, Terribile D, Acerbi A, Rotmensz N, Maisonneuve P. Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16:259–262. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 38.Veronesi U, Galimberti V, Mariani L, Gatti G, Paganelli G, Viale G, Zurrida S, Veronesi P, Intra M, Gennari R, Rita Vento A, Luini A, Tullii M, Bassani G, Rotmensz N. Sentinel node biopsy in breast cancer: early results in 953 patients with negative sentinel node biopsy and no axillary dissection. Eur J Cancer. 2005;41:231–237. doi: 10.1016/j.ejca.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayward JL. New surgical and radiotherapeutic techniques. In: Harris I, et al., editors. Conservative management of breast cancer. Philadelphia: Lippincott Company; 1983. [Google Scholar]
- 41.Smitt M, Goffinet D. Utility of three-dimensional planning for axillary node coverage with breast-conserving radiation therapy: early experience. Radiology. 1999;210:221–226. doi: 10.1148/radiology.210.1.r99ja24221. [DOI] [PubMed] [Google Scholar]
- 42.Takeda A, Shigematsu N, Kondo M, Amemiya A, Kawaguchi O, Sato M, Kutsuki S, Toya K, Ishibashi R, Kawase T, Tsukamoto N, Kubo A. The modified tangential irradiation technique for breast cancer: how to cover the entire axillary region. Int J Radiat Oncol Biol Phys. 2000;46:815–822. doi: 10.1016/s0360-3016(99)00463-0. [DOI] [PubMed] [Google Scholar]
- 43.Krasin M, McCall A, King S, Olson M, Emami B. Evaluation of a standard breast tangent technique: a dose-volume analysis of tangential irradiation using three-dimensional tools. Int J Radiat Oncol Biol Phys. 2000;47:327–333. doi: 10.1016/s0360-3016(00)00449-1. [DOI] [PubMed] [Google Scholar]
- 44.Aristei C, Chionne F, Marsella A, Alessandro M, Rulli A, Lemmi A, Perrucci E, Latini P. Evaluation of level I and II axillary nodes included in the standard breast tangential fields and calculation of the administered dose: results of a prospective study. Int J Radiat Oncol Biol Phys. 2001;51:69–73. doi: 10.1016/s0360-3016(01)01595-4. [DOI] [PubMed] [Google Scholar]
- 45.Schlembach PJ, Buchholz TA, Ross MI, Kirsner SM, Salas GJ, Strom EA, McNeese MD, Perkins GH, Hunt KK. Relationship of sentinel and axillary level I-II lymph nodes to tangential fields used in breast irradiation. Int J Radiat Oncol Biol Phys. 2001;51:671–678. doi: 10.1016/s0360-3016(01)01684-4. [DOI] [PubMed] [Google Scholar]
- 46.Takeda A, Shigematsu N, Ikeda T, Kawaguchi O, Kutsuki S, Ishibashi R, Kunieda E, Takemasa K, Ito H, Uno T, Jinno H, Kubo A. Evaluation of novel modified tangential irradiation technique for breast cancer patients using dose-volume histograms. Int J Radiat Oncol Biol Phys. 2004;58(4):1280–1288. doi: 10.1016/j.ijrobp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Orecchia R, Huscher A, Leonardi MC, Gennari R, Galimberti C, Garibaldi C, Rondi E, Bianchi LC, Zurrida S, Franzetti S. Irradiation with standard tangential breast fields in patients treated with conservative surgery and sentinel node biopsy: using a three-dimensional tool to evaluate the first level coverage of the axillary nodes. Br J Radiol. 2005;78:51–54. doi: 10.1259/bjr/29242407. [DOI] [PubMed] [Google Scholar]
- 48.Wong JS, Taghian AG, Bellon JR, Keshaviah A, Smith BL, Winer EP, Silver B, Harris JR. Tangential radiotherapy without axillary surgery in early-stage breast cancer: results of a prospective trial. Int J Radiat Oncol Biol Phys. 2008;72(3):866–870. doi: 10.1016/j.ijrobp.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Rabinovitch R, Ballonoff A, Newman F, Finlayson C. Evaluation of breast sentinel lymph node coverage by standard radiation therapy fields. Int J Radiat Oncol Biol Phys. 2008;70(5):1468–1471. doi: 10.1016/j.ijrobp.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 50.Ohashi T, Takeda A, Shigematsu N, Fukada J, Sanuki N, Amemiya A, Kubo A. Dose distribution analysis of axillary lymph nodes for three-dimensional conformal radiotherapy with a field-in-field technique for breast cancer. Int J Radiat Oncol Biol Phys. 2009;73(1):80–87. doi: 10.1016/j.ijrobp.2008.04.003. [DOI] [PubMed] [Google Scholar]