Abstract
SUMMARY
Acute apical abscess is the most common form of dental abscess and is caused by infection of the root canal of the tooth. It is usually localized intraorally, but in some cases the apical abscess may spread and result in severe complications or even mortality. The reasons why dental root canal infections can become symptomatic and evolve to severe spreading and sometimes life-threatening abscesses remain elusive. Studies using culture and advanced molecular microbiology methods for microbial identification in apical abscesses have demonstrated a multispecies community conspicuously dominated by anaerobic bacteria. Species/phylotypes commonly found in these infections belong to the genera Fusobacterium, Parvimonas, Prevotella, Porphyromonas, Dialister, Streptococcus, and Treponema. Advances in DNA sequencing technologies and computational biology have substantially enhanced the knowledge of the microbiota associated with acute apical abscesses and shed some light on the etiopathogeny of this disease. Species richness and abundance and the resulting network of interactions among community members may affect the collective pathogenicity and contribute to the development of acute infections. Disease modifiers, including transient or permanent host-related factors, may also influence the development and severity of acute abscesses. This review focuses on the current evidence about the etiology and treatment of acute apical abscesses and how the process is influenced by host-related factors and proposes future directions in research, diagnosis, and therapeutic approaches to deal with this disease.
INTRODUCTION
Apical periodontitis is an inflammatory disease affecting the tissues surrounding the root end of a tooth and is caused by root canal (endodontic) infection. The disease can manifest itself in different clinical ways, including the development of an acute abscess (1). The factors influencing the development of the acute form of the disease have been the subject of continuous interest (2–4). A recurrent theme in this regard is the association of certain bacterial species with clinical signs and symptoms. However, the search for a single or even a small group of species to be considered the major pathogen involved with acute endodontic infections has proven fruitless. Recent studies in the fields of molecular and cellular microbiology and immunology have provided information to implicate a multitude of factors in the pathogenesis of symptomatic apical periodontitis, including its most severe form, the acute apical abscess. Understanding the factors that make a chronic asymptomatic endodontic infection evolve to an acute abscess, sometimes with severe complications, may help establish better strategies to prevent and deal with these conditions. This review focuses on the microbiology and treatment of acute apical abscesses and how the disease development is influenced by host-related factors. Future directions in research and therapeutic approaches to deal with this disease are also discussed.
THE DISEASE PROCESS
An abscess consists of a collection of pus into a cavity formed by tissue liquefaction. The terms dental abscess, dentoalveolar abscess, and odontogenic abscess are often used synonymously to describe abscesses formed in the tissues around the tooth. The cause may be an endodontic infection (acute apical abscess) or a periodontal infection (periodontal abscess and pericoronitis). The acute apical abscess is the most common form of dental abscesses and is the subject of this review.
Endodontic infection develops only in root canals of teeth devoid of a vital pulp. This may be due to necrosis of the dental pulp as a consequence of caries or trauma to the tooth or to removal of the pulp tissue for previous root canal treatment. Once the infection is established in the root canal, bacteria may contact the periradicular tissues via apical and lateral foramina or root perforations and induce a chronic or acute inflammatory response (5). The chronic response is usually asymptomatic and almost invariably leads to bone resorption around the root apex, which is the typical radiographic feature of apical periodontitis. Acute periradicular inflammation in turn usually gives rise to signs and/or symptoms, including pain and swelling. The acute (symptomatic) process may develop without previous chronic inflammation or may be the result of exacerbation of a previously chronic asymptomatic lesion. It has been estimated that the incidence of exacerbations of apical periodontitis (i.e., asymptomatic lesions becoming symptomatic) is about 5% per year (6).
The acute abscess can be regarded as an advanced stage of the symptomatic form of apical periodontitis. In acute endodontic infections, not only are the involved bacteria located in the root canal, but they invade the periradicular tissues and have the potential to spread to other anatomical spaces of head and neck to form a cellulitis or phlegmon, which is a disseminating diffuse inflammatory process with pus formation (7).
Clinically, the patient with acute apical abscess experiences mild to severe pain and swelling (Fig. 1). Trismus may occur. Systemic manifestations may also develop, including fever, lymphadenopathy, malaise, headache, and nausea. Because the acute reaction to endodontic infection may develop very quickly, the involved tooth may not show radiographic evidence of periradicular bone destruction. When a periradicular radiolucency is radiographically observed, the abscess is usually the result of exacerbation of a previous chronic asymptomatic condition (Fig. 1). In most cases, the tooth is extremely sensitive to percussion.
Fig 1.
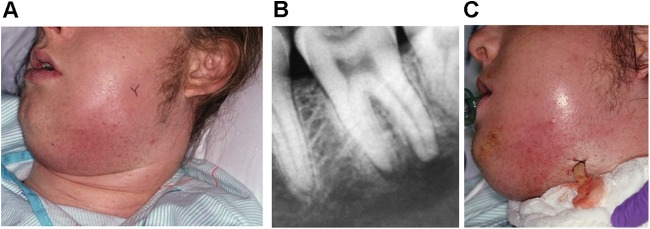
(A) An individual with spread acute apical abscess. (B) A bone radiolucent lesion is visible around the root apexes of the mandibular second molar, which is the source of infection. (C) Incisional drainage is essential for management of these conditions. In this complicated case, it was performed extraorally. (Courtesy of Craig Baumgartner.)
The purulent exudate formed in response to root canal infection spreads through the medullary bone to perforate the cortical bone and discharge into the submucous or subcutaneous soft tissue. In many cases, swelling develops only intraorally (8). In the maxilla, acute apical abscesses drain through the buccal or palatal bone into the oral cavity or occasionally into the maxillary sinus or the nasal cavity. Apical abscesses of mandibular teeth may drain through the buccal or lingual bone into the oral cavity. However, the infectious process may also extend into fascial spaces of the head and neck and result in cellulitis and systemic signs and symptoms, with consequent complications.
Complications Stemming from Acute Apical Abscesses
Almost 60% of all nontraumatic dental emergencies are associated with acute apical abscesses and toothaches (9). Acute dental abscesses have been reported to cause severe complications and even mortality (10–12). Mortality is more likely a result of sepsis or airway obstruction (13, 14), but death due to a spreading infection leading to massive hemorrhage from the subclavian vein into the pleural cavity has been reported (10). The spread of bacteria from endodontic abscesses to other tissues may give rise to fascial plane infections (15). The most commonly affected fascial spaces are the sublingual, submandibular, buccal and pterygomandibular spaces, but others such as the temporal, masseteric, lateral pharyngeal, and retropharyngeal spaces can be occasionally involved (8).
The spread of infections of endodontic origin into the fascial spaces of the head and neck is determined by the location of the root end of the involved tooth in relation to its overlying buccal or lingual cortical plate, the thickness of the overlying bone, and the relationship of the apex to the attachment of a muscle. For example, if a mandibular molar is affected and its root apices lie closer to the lingual cortical plate and above the attachment of the mylohyoid muscle, the purulent exudate can break through the lingual cortical plate into the sublingual space. If the root apices instead lie below the attachment of the mylohyoid muscle, the infection can spread into the submandibular space. If infection affects the sublingual and submandibular spaces bilaterally as well as the submental space, a condition known as Ludwig's angina is diagnosed. Swelling from Ludwig's angina can give rise to difficulty breathing and potentially lethal airway obstruction (16–18).
Another example of abscess complications involves infections of the midface, which can be very dangerous and result in cavernous sinus thrombosis. This is also a life-threatening infection, in which a thrombus formed in the cavernous sinus breaks free and leads to spread of the infection. Under normal conditions, the angular and ophthalmic veins and the pterygoid plexus of veins flow into the facial and external jugular veins. If an infection has spread into the midfacial area, however, edema and the resultant increased pressure cause the blood to back up into the cavernous sinus. Once in the sinus, the blood can stagnate and clot. The resultant infected thrombi remain in the cavernous sinus or escape into the circulation (19, 20).
Other reported complications of disseminating dental infections include brain abscess (21–24), septicemia in a patient with multiple myeloma (25), deep neck infection (26, 27), mediastinitis (14, 28–30), necrotizing fasciitis (31–33), orbital abscess (34–36), and cervical spondylodiscitis with spinal epidural abscess (37). It has been suggested that some host-related factors may contribute toward increased morbidity and mortality associated with acute dental abscesses, including diabetes, chronic alcohol and tobacco consumption, malnourishment, and the use of illicit substances (26, 27, 33).
Complications of dental abscesses can be severe enough to require hospitalization. A large number of hospitalizations due to oral abscesses/cellulitis with a resulting substantial economic burden have been recorded (38). For instance, in 2007, 7,886 hospitalizations were attributed primarily to abscesses of endodontic origin, with total hospital charges on the order of $100 million (39).
MICROBIOLOGY OF ACUTE APICAL ABSCESSES
Microbiology Diagnostic Methods
Culture.
Culture methods have been traditionally used to investigate the microbiota of acute apical abscesses and have provided a substantial body of information about the bacterial etiology and the species involved. However, some important limitations of culture make it difficult to achieve a comprehensive analysis of the apical abscess microbiota. Because anaerobic bacteria are dominant in apical abscesses (40–49), samples for research or clinical diagnosis using culture should be collected and transported to the laboratory under conditions that favor the survival of these bacteria. The laboratory that will analyze the samples has to be properly prepared and equipped to isolate, cultivate, and identify anaerobes. The procedures for isolation and identification can be laborious and time-consuming, and many anaerobic species may require multiple phenotype-based tests for reliable identification (50).
More important, the difficulties in culturing a large number of oral bacteria or even in identifying many species are of special concern (51). As early estimated by correlative microscopic and culturing analyses (52) and further elegantly demonstrated and specified by molecular biology techniques (53–58), 40% to 70% of the oral bacterial species remain to be cultivated and phenotypically characterized. The most possible reasons for the fact that a large number of oral bacteria still have to be cultivated include (i) lack of essential nutrients or growth factors in the culture medium, (ii) overfeeding conditions during cultivation, (iii) toxicity of the culture medium itself, (iv) inhibition by other species present in the sample, (v) metabolic dependence on other species, (vi) disruption of natural bacterial quorum-sensing systems, and (vii) cells in a “viable-but-noncultivable” state (51, 59, 60). Efforts have been expended toward the development of approaches that allow cultivation of as-yet-uncultivated bacteria (61–64). This is of great importance for description of novel species and study of their ecological and pathogenic potential, as well as the patterns of susceptibility to antimicrobial drugs.
Successful cultivation does not ensure successful identification. Culture-dependent identification is based on phenotypic traits reported for reference strains, with predictable biochemical and physical properties under optimal growth conditions. However, there are several phenotype-related factors that can lead to difficulties in identification and even to misidentification. They include (i) strains of the same species showing a divergent phenotypic behavior (65, 66), (ii) strains of different species showing a convergent phenotypic behavior (51, 65), (iii) an altered phenotype in response to conditions such as stress (67, 68), (iv) strains showing different results after repeated tests (69), (v) incomplete databases not including newly named species and as-yet-uncharacterized species, (vi) the fact that even small alterations in the assay may lead to false results (70), and (vii) the fact that test results rely on individual interpretation and expertise (70).
Molecular methods.
Tools and procedures based on molecular biology have become available to sidestep the limitations of culture and have been substantially improved to achieve a more realistic description of the microbial communities of different environments without the need for cultivation. One gene that has been widely used for rapid identification of known and unknown bacterial species is the one encoding the 16S rRNA (71). There are a myriad of molecular methods for the study of bacteria in abscess samples, and the choice of a particular approach depends on the questions to be answered. Molecular methods for diagnostic microbiology can be used for specific detection of target species (species-specific or closed-ended analysis), identification of all or the most dominant species in a sample (broad-range or open-ended analysis), or profiling of the microbial community structure (community analysis) (72). Broad-range PCR followed by cloning and sequencing and more recently the massive parallel 454 pyrosequencing approach can be used to unravel the breadth of bacterial diversity in a site. DNA hybridization arrays (e.g., checkerboard arrays and microarrays), species-specific PCR, nested PCR, multiplex PCR, and quantitative real-time PCR can be used to survey large numbers of samples for the presence of target species. Bacterial community structures can be analyzed via the pyrosequencing technology and by fingerprinting techniques, such as the denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (T-RFLP) assays. As with any other technology, molecular methods have their own limitations. However, variations in virtually every technique have emerged to circumvent or minimize limitations, or sometimes more than one approach is required for reliable information to be obtained.
The chronology of the microbiological study of acute apical abscesses can be didactically divided into five generations of studies based on different strategic diagnostic approaches (73). The first generation involves studies of the abscess microbiota conducted using open-ended (or broad-range) culture methods, which disclosed many cultivable species in association with the disease (41–49). The second generation consisted of studies employing closed-ended molecular detection methods, such as species-specific PCR and its derivatives as well as the original checkerboard hybridization assay, to target cultivable bacteria (74–82). These methods allowed the inclusion of some difficult-to-culture species in the set of candidate abscess pathogens. Next, a third generation of studies adopted open-ended molecular methods, such as broad-range PCR followed by cloning and sequencing or T-RFLP, which allowed an even more comprehensive investigation of the bacterial diversity in abscesses (83–87). By these approaches, not only cultivable species but also as-yet-uncultivated and uncharacterized bacteria have been identified. Technical hurdles make it difficult to analyze a large number of samples by cloning and sequencing, but cataloguing bacterial species in the oral cavity by this approach provided 16S rRNA gene sequence data that could be used to design primers or oligonucleotide probes to target both cultivable and as-yet-uncultivated bacteria. The fourth generation of studies involved closed-ended molecular analyses with PCR and DNA hybridization assays (e.g., reverse-capture checkerboard) in large-scale clinical studies to investigate the prevalence and association of cultivable and as-yet-uncultivated bacteria with abscesses (88–90). A fifth generation has been possibly heralded by the use of pyrosequencing technology for a deep-coverage open-ended analysis of abscess samples (91, 92) (see below).
In general, culture analyses of abscesses resulted in the establishment of a set of species thought to play an important role in the pathogenesis of the disease. Not only have molecular methods confirmed and even strengthened the association of many cultivable bacterial species with abscesses, but they also revealed new suspected pathogens (93). The list of candidate pathogens has expanded to include difficult-to-culture species or even as-yet-uncultivated bacteria that had never been previously found in abscesses by culturing approaches (Fig. 2). Consequently, the microbiota of apical abscesses has been refined and redefined by molecular methods (93). Although molecular methods have been widely used for research purposes, they still have yet to be implemented for clinical diagnosis. There is a great potential for these methods to be used for rapid identification of potential pathogens concomitantly with specific antibiotic susceptibility, which would allow immediate and more appropriate patient care with possibly reduced morbidity or mortality (87, 94). This application will be further discussed in the section Future Directions.
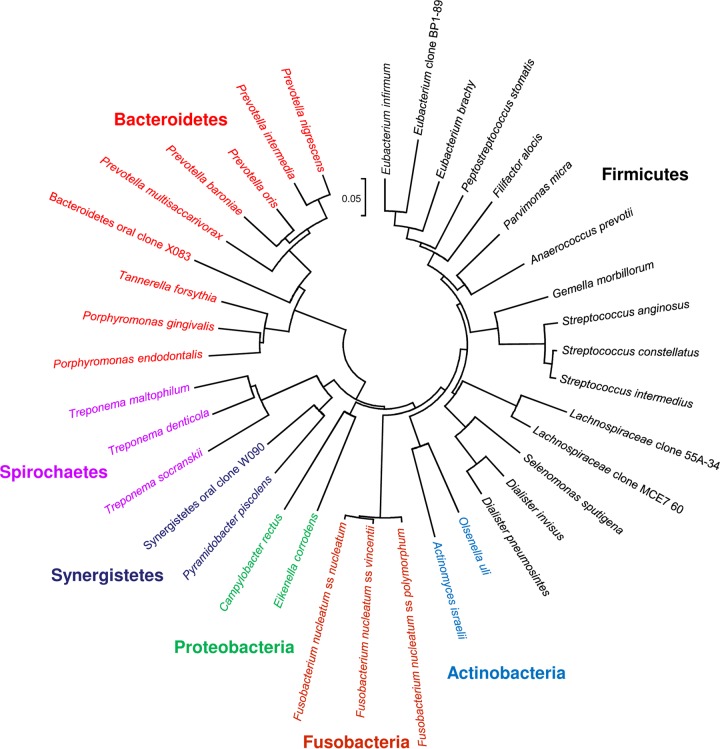
Fig 2.
Bacterial species/phylotypes frequently detected in acute apical abscesses. A phylogenetic tree based on 16S rRNA gene comparisons, showing several candidate pathogens and their respective phyla, is shown. The scale bar shows the number of nucleotide substitutions per site.
Microbial Diversity in Acute Apical Abscesses
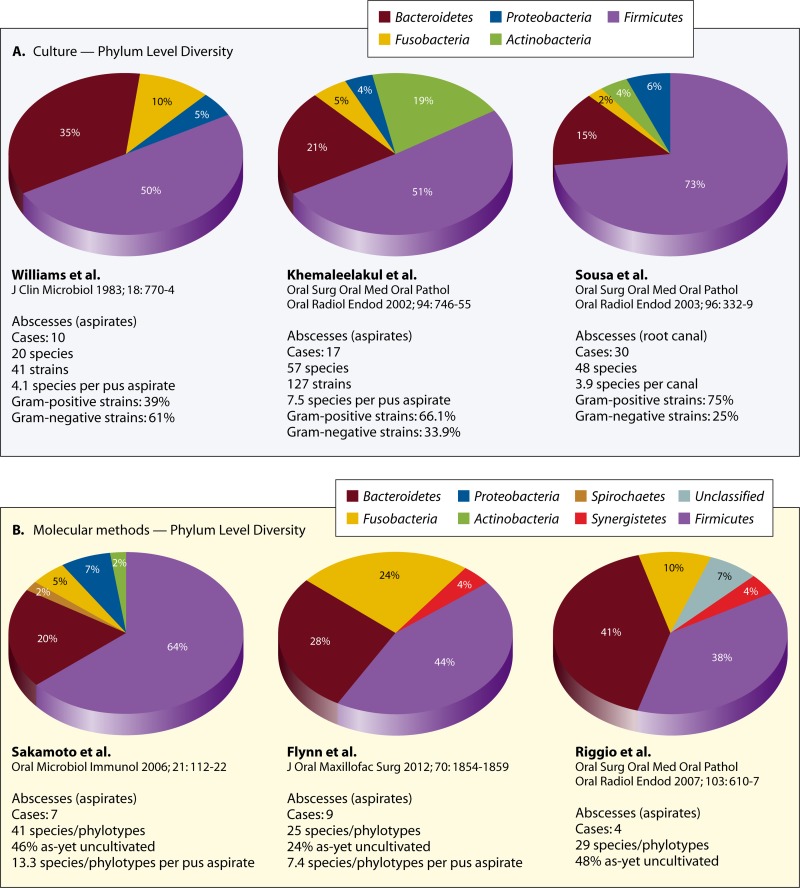
Samples for microbiological analyses of abscesses can be taken either from the root canals of affected teeth or by aspiration of the purulent exudate from the swollen mucosa/skin. Culture and molecular microbiology studies have clearly demonstrated that the apical abscess microbiota is mixed and conspicuously dominated by anaerobic bacteria (42, 43, 49, 83, 87, 95–96). Table 1 provides a compilation of the main microbiological findings from most of these studies. It is noteworthy that while some bacterial species or groups are reported in many studies, the most prevalent species vary from study to study.
Table 1.
Microbiological findings for acute apical abscesses
| Study type and authors (reference) | No. of abscess cases examined | Method | Mean no. of species per abscess sample | Most prevalent species (%) |
|---|---|---|---|---|
| Culture (open ended) | ||||
| Heimdahl et al. (40) | 58 | Culture | 3.4 | Fusobacterium nucleatum (45), “Streptococcus milleri” (31), Parvimonas micra (29), Prevotella ruminicola (29), Prevotella melaninogenica (26) |
| Sabiston et al. (44) | 58 | Culture | 3.8 | Streptococcus spp. (71), Fusobacterium nucleatum (24), Parvimonas micra (22), Actinomyces spp. (17) |
| Williams et al. (45) | 10 | Culture | 4 | Fusobacterium nucleatum (60), Bacteroides spp. (60), Parvimonas micra (50) |
| Lewis et al. (41) | 50 | Culture | 3.3 | Peptostreptococcus spp. (64), Peptococcus spp. (64), Streptococcus “milleri” (50), Prevotella oralis (40) |
| Sundqvist et al. (42) | 17 | Culture | 8.5 | Fusobacterium nucleatum (71), Lactobacillus spp. (65), Prevotella intermedia/nigrescens (59), Parvimonas micra (53), Peptostreptococcus anaerobius (53), Eggerthella lenta (47) |
| Brook et al. (46) | 32 | Culture | 2.4 | Alpha-hemolytic streptococci (34), Porphyromonas gingivalis (22), Parvimonas micra (19), Fusobacterium nucleatum (16) |
| Kulekçi et al. (47) | 13 | Culture | 5.4 | Peptostreptococcus spp. (92), alpha-hemolytic streptococci (69), Prevotella intermedia/nigrescens (69), Fusobacterium nucleatum (38) |
| Sakamoto et al. (48) | 23 | Culture | 4.9 | Peptostreptococcus spp. (52), Fusobacterium spp. (43), Prevotella oris (39), Streptococcus constellatus (35), Streptococcus intermedius (35) |
| de Sousa et al. (49) | 30 | Culture | 3.9 | Anaerococcus prevotii (43), Parvimonas micra (30), Gemella morbillorum (30), Fusobacterium necrophorum (23), Streptococcus constellatus (20) |
| Khemaleelakul et al. (43) | 17 | Culture | 7.5 | Prevotella intermedia (35), Prevotella baroniae (35), Streptococcus constellatus (29), Prevotella buccae (29), Prevotella melaninogenica (29) |
| Molecular | ||||
| Closed ended, several target species | ||||
| Siqueira et al. (74) | 27 | Conventional checkerboard (49 target species) | Not applicable | Tannerella forsythia (30), Porphyromonas gingivalis (30), Streptococcus constellatus (26), Prevotella intermedia (22), Prevotella nigrescens (22) |
| Siqueira and Rôças (90) | 42 | Reverse-capture checkerboard (81 target species) | Not applicable | Fusobacterium nucleatum (64), Parvimonas micra (52), Porphyromonas endodontalis (48), Olsenella uli (45), Streptococcus spp. (38), Eikenella corrodens (38), Bacteroidetes clone X083 (36), Prevotella baroniae (36), Treponema denticola (36) |
| Siqueira and Rôças (230) | 22 | Nested PCR (40 target species) | Not applicable | Treponema denticola (77), Porphyromonas endodontalis (68), Dialister pneumosintes (64), Tannerella forsythia (64), Porphyromonas gingivalis (59), Dialister invisus (53), Filifactor alocis (42), Fusobacterium nucleatum (41), Streptococcus spp. (41) |
| Open ended | ||||
| Sakamoto et al. (83) | 7 | T-RFLP; PCR, cloning, and sequencing | 13.3 | Fusobacterium nucleatum (86), Parvimonas micra (57), Lachnospiracea clone 55A-34 (57), Prevotella intermedia (43), Prevotella baroniae (43), Dialister pneumosintes (43), Eubacterium clone BP1-89 (43), Lachnospiracea clone MCE7_60 (43) |
| Flynn et al. (86) | 9 | PCR, cloning, and sequencing | 7.4 | Parvimonas micra (78), Dialister pneumosintes (78), Prevotella oris (67), Eubacterium brachy (56), Fusobacterium nucleatum subsp. nucleatum (44) |
| Open ended, next-generation sequencing | ||||
| Santos et al. (91) | 9 | Pyrosequencing | 114 | Fusobacterium spp. (89), Parvimonas spp. (78), Dialister spp. (78), Atopobium spp. (78), Eubacterium spp. (67), Porphyromonas spp. (67), Prevotella spp. (67) |
| Hsiao et al. (92) | 8 | Pyrosequencing | 77 (genera) | Fusobacterium spp. (100), Streptococcus spp. (100), Phocaeicola spp. (100), Prevotella spp. (100), Porphyromonas spp. (100) |
At a broader taxonomic level, the large majority of the frequently detected bacterial species belong to seven different bacterial phyla, namely, the Firmicutes (e.g., genera Streptococcus, Dialister, Filifactor, and Pseudoramibacter), Bacteroidetes (e.g., genera Porphyromonas, Prevotella, and Tannerella), Fusobacteria (e.g., genera Fusobacterium and Leptotrichia), Actinobacteria (e.g., genera Actinomyces and Propionibacterium), Spirochaetes (e.g., genus Treponema), Synergistetes (e.g., genus Pyramidobacter and some as-yet-uncultivated phylotypes), and Proteobacteria (e.g., genera Campylobacter and Eikenella) (Fig. 2). Regardless of the study and method of identification, the phyla Firmicutes and Bacteroidetes together contribute to more than 70% of the species found in abscesses (Fig. 3). Representatives of Spirochaetes and Synergistetes have been revealed only by culture-independent molecular methods (Fig. 3). Diverse groups of Gram-negative and Gram-positive bacteria have been identified, and the most frequent genera and species identified in abscesses and regarded as putative pathogens are described next. The fact that many genera have undergone reclassifications over the years makes interpretation of “old” studies difficult, especially when identifications were restricted to the genus level.
Fig 3.
Bacterial phyla with representatives in acute apical abscesses as revealed by studies using either culture (A) or molecular (B) open-ended methods. Data refer to the number of different taxa found in each phylum. Note that regardless of the study and method, the majority of species detected belong to the phyla Firmicutes and Bacteroidetes. Representatives of the phyla Synergistetes and Spirochaetes were revealed only by molecular methods. (Reference citations correspond to references 43, 45, 49, 83, 86, and 87.)
Gram-negative bacteria.
Dark-pigmented anaerobic bacteria have been closely associated with acute symptoms of endodontic infections, including abscesses (41, 47, 96–100). Two culture studies (42, 101) found that virtually all abscesses of endodontic origin harbored one or more species of this group. Dark-pigmented anaerobic bacteria are usually found in mixed infection, which may be required for their optimal growth and contributes to a significant increase in their pathogenicity (102–106). This bacterial group comprises two genera: Prevotella (containing saccharolytic species) and Porphyromonas (containing asaccharolytic species). The genus Prevotella also includes some nonpigmented species. The Prevotella species frequently found in apical abscess samples include P. intermedia, P. nigrescens, P. baroniae, and P. oris, and in most studies they are among the most prevalent and/or dominant species (40, 42, 43, 75, 83, 86, 87, 101, 107). Of the Porphyromonas species, P. endodontalis was first isolated from endodontic infections (108) and has been consistently encountered in abscess samples, with increased prevalence in molecular studies (42, 77, 90, 101, 109, 110). Porphyromonas gingivalis is one of the most important periodontal pathogens (111, 112) and has also been detected in association with endodontic abscesses (74, 77, 101, 113). P. gingivalis fimA genotype variants II, III, and IV and type I have been reported in abscess aspirates (114).
Fusobacterium nucleatum is an anaerobic spindle-shaped rod that is one of the most commonly detected Gram-negative species in the large majority of culture and molecular studies of acute apical abscesses (40, 45, 85, 90), reaching prevalence values as high as approximately 70% (42, 75) or even 86% (83) of the samples. This species induces severe abscess lesions in animals in both pure and mixed cultures (115–118). Four subspecies (F. nucleatum subsp. nucleatum, F. nucleatum subsp. polymorphum, F. nucleatum subsp. vincentii, and F. nucleatum subsp. animalis) have been identified in apical abscesses (74, 86), but the frequency of each one has yet to be accurately determined. The species Fusobacterium periodonticum has also been detected in abscess aspirates by a study using the checkerboard hybridization assay (74).
In a seminal study of endodontic infections published in 1894 (119), Willoughby Dayton Miller suggested that spirochetes could play a role in the etiology of abscesses. Nevertheless, it was not until the introduction of molecular methods in endodontic microbiology research that the potential involvement of spirochetes with this disease was confirmed. Oral spirochetes fall within the genus Treponema and have been linked to several oral diseases (120–123). Molecular methods have revealed the occurrence of treponemes in 61% to 89% of endodontic abscess samples (79, 80). Of the 10 cultivable and validly named oral Treponema species, the asaccharolytic species T. denticola and T. medium as well as the saccharolytic species T. socranskii and T. maltophilum have been most frequently detected (80, 109, 124), with prevalence values in studies using species-specific nested PCR of up to 75% for T. socranskii (125) and 79% for T. denticola (79). Oral treponemes have been demonstrated to induce abscesses and disseminating infections when inoculated in animals (126–128).
Association of other Gram-negative bacterial species with abscesses has also been suggested based on molecular studies. One example is Tannerella forsythia, a fastidious obligate anaerobic rod, which is an important periodontal pathogen and was never previously detected in apical abscesses by culture (74, 78, 90, 109). Another example of bacteria that have been consistently detected in apical abscesses only after the advent of molecular methods is the asaccharolytic anaerobic Gram-negative Dialister species, especially D. pneumosintes and D. invisus (76, 83, 86, 88).
Other Gram-negative bacteria that have been detected in abscessed samples in some culture or molecular studies include Campylobacter spp. (42), Catonella morbi (129), Veillonella parvula (48, 130), and Eikenella corrodens (90, 130).
Gram-positive bacteria.
Several Gram-positive bacteria have also been frequently detected in acute apical abscesses by culture and molecular methods. Along with the Gram-negative bacteria of the genera Prevotella, Porphyromonas, and Fusobacterium, Gram-positive cocci, specifically peptostreptococci and streptococci, comprise the most prevalent bacteria in most studies. Peptostreptococci have been subjected to several recent taxonomic reclassifications, and new genera have emerged, such as Parvimonas and Anaerococcus. Parvimonas micra (formerly Peptostreptococcus micros) is an asaccharolytic anaerobic small coccus that has been isolated from or detected in a high number of apical abscess samples (40, 42, 44, 45, 49, 83, 90), reaching up to 78% of the cases (86). This species has been revealed to be pathogenic in animal studies, especially in mixed infections (115, 116, 131, 132).
The predominant streptococci associated with abscesses belong to the Streptococcus anginosus group (also referred to as the “S. milleri” group in some studies) (40, 41, 43, 48, 49, 74, 133). This group is composed of the microaerophilic or anaerobic species S. anginosus, S. constellatus, and S. intermedius, which have been shown to cause purulent infections in animal models (104, 115, 117, 134, 135). Members of the S. anginosus group have been often reported in a variety of abscesses in other body sites (94, 136, 137).
Other Gram-positive bacteria that have also been detected in apical abscesses included Filifactor alocis (78, 89, 90, 138–139), Actinomyces species (44, 90, 140), Pseudoramibacter alactolyticus (42, 90), Olsenella uli (88, 90), Mogibacterium timidum (42), Granulicatella adiacens (88), Eubacterium species (E. brachy and E. infirmum) (86, 90), Gemella morbillorum (49), and anaerobic lactobacilli (42).
As-yet-uncultivated phylotypes.
In 1894, Miller (119) wrote, “It must strike every one that the results of the culture experiments do not tally with those of the microscopical examination. While a careful microscopical examination of the diseased pulp almost invariably revealed a mixed infection, the pure cultures show, in the majority of cases, either only cocci or only bacilli.” One of Miller's possible explanations for this finding was that “many species of bacteria occurring in the diseased pulp, vibriones, spirochaetes, the stiff pointed bacilli and threads, have not been found cultivable on artificial media anyway; and possibly there are still other uncultivable pulp-bacteria.” The development of methods for anaerobic cultivation showed that the bacterial diversity in endodontic infections was underestimated by previous culture studies by excluding a high number of species that compose the largest proportion of the bacterial community in these infections. Further breakthroughs in microbial identification represented by molecular technologies revealed that even advances in anaerobic culturing left a large proportion of the microbiota undisclosed.
Indeed, molecular investigations of the bacteria involved in abscesses unveiled a far more complex picture than anticipated by culture studies. Noteworthy is the common occurrence of as-yet-uncultivated bacterial phylotypes, which can be regarded as species-level bacteria known only by a 16S rRNA gene sequence. Open-ended molecular analysis of acute apical abscesses revealed that, in terms of richness, as-yet-uncultivated phylotypes encompass approximately 24% to 46% of the taxa found (83, 86), while in terms of abundance, they collectively represent from 6% to more than 30% of the clones sequenced (83, 87).
Several of the as-yet-uncultivated phylotypes are suspected pathogens based on association data. For instance, a phylotype of the Bacteroidetes phylum known as oral clone X083 has been found in 14% to 36% of apical abscess aspirates (90, 107). Oral Synergistetes phylotypes, which had been originally assigned to the Flexistipes or Deferribacteres groups, are another example of as-yet-uncultivated bacteria that have been frequently encountered in abscesses (88–90). The great majority of Synergistetes bacteria remain uncultivated (141), and this can be the primary reason for the fact that their presence in abscesses has been overlooked by culture studies. Some Synergistetes phylotypes have been cultivated and given a species name; one of them, Pyramidobacter piscolens (previously oral clone BA121), is probably the most prevalent representative of the Synergistetes phylum in abscess cases (88–90). Phylotypes from the family Lachnospiraceae or the genera Eubacterium, Megasphaera, Leptotrichia, Oribacterium, Peptostreptococcus, Prevotella, Selenomonas, and Solobacterium have been disclosed in pus samples from apical abscesses (83, 86, 87). There is no reason to believe that these previously unrecognized and overlooked bacteria do not play a role in the pathogenesis of the disease.
Pyrosequencing analysis of abscess samples.
Advances in DNA sequencing technologies and computational biology have substantially enhanced molecular phylogenetic surveys of human-associated microbial communities in health and disease, offering a great depth of coverage at a very high analytical speed (142, 143). The 454 pyrosequencing method is one of these advanced DNA sequencing techniques that has been widely used in medical microbiology. This technology is a sequencing-by-synthesis method that involves a combination of emulsion PCR and pyrosequencing. Pyrosequencing relies on the detection of pyrophosphate release and consequent light generation as nucleotides are incorporated in a growing chain of DNA (144, 145). One of the greatest advantages of the pyrosequencing approach over the conventional Sanger sequencing method is that hundreds of thousands of sequence reads can be obtained in a single run, generating sequence information data that are orders of magnitude larger (146).
A recent study used 454 pyrosequencing to compare the microbiota of endodontic infections associated with acute abscesses and asymptomatic chronic apical periodontitis, and it found operational taxonomic units (at 3% divergence) belonging to 13 phyla (91). The most abundant phyla in acute infections were Firmicutes (52%), Fusobacteria (17%), and Bacteroidetes (13%), while the dominant phyla in asymptomatic infections were Firmicutes (59%), Bacteroidetes (14%), and Actinobacteria (10%). Members of Fusobacteria were much more prevalent in acute (89%) than in chronic (50%) infections. Of the 49 genera detected in acute cases, the most abundant were Fusobacterium, Parvimonas, and Peptostreptococcus. Fusobacterium was also the most prevalent, followed by Parvimonas, Dialister, and Atopobium. The bacterial communities in abscesses were significantly more diverse than those in chronic infections, and a pattern related to the presence of symptoms was apparently evident through community analysis. The overall diversity of abscesses as revealed by pyrosequencing was much higher than previously reported. Most of these findings were confirmed by another study using pyrosequencing, which revealed representatives of 11 phyla and Fusobacterium as the most abundant genus (92).
Bacterial Species and Acute Infections: Is There a Single Culprit?
A matter of intense debate and investigation is why only some selected cases evolve to acute infections with severe symptoms and potential complications. In this context, the desire to find a single species or at least a group of major species that is associated with acute symptoms is an ever recurrent topic in the study of endodontic infections. In his milestone study, Miller (119) was the first one to suggest the involvement of a specific group of bacteria with endodontic symptoms: spirochetes were associated with acute abscesses. However, the first consistent report on the association of a given species with endodontic symptoms came from the classic Ph.D. thesis of Goran Sundqvist (99), who found dark-pigmented bacteria (formerly Bacteroides melaninogenicus) in association with acute symptoms. Further culture and molecular studies reported on the association with symptoms of several species, with the most frequent belonging to the genera Porphyromonas, Fusobacterium, Parvimonas, and Prevotella (Table 2). Nevertheless, these findings have not been consistently confirmed by other studies, because basically the same species can also be found in the root canals of teeth with asymptomatic apical periodontitis in similar prevalences (74, 93, 100, 147–151).
Table 2.
Bacterial species associated with signs and symptoms of acute endodontic infections
| Species | Signs and symptoms | Method | Study author(s) (reference) |
|---|---|---|---|
| Spirochetes | Pain, purulent exudate | Microscopy | Miller (119) |
| Prevotella intermedia, Porphyromonas endodontalis | Pain | Culture | Sundqvist (99) |
| Prevotella spp., Porphyromonas spp. | Pain | Culture | Griffee et al. (231) |
| Prevotella spp., Porphyromonas endodontalis | Pain | Culture | Sundqvist et al. (42) |
| Porphyromonas endodontalis, Porphyromonas gingivalis | Pain | Culture | Haapasalo et al. (100) |
| Finegoldia magna | Pain | Culture | Yoshida et al. (232) |
| Peptostreptococcus spp., Eubacterium spp., Porphyromonas spp. | Pain | Culture | Hashioka et al. (233) |
| Parvimonas micra, Prevotella spp. | Pain, swelling | Culture | Gomes et al. (234) |
| Fusobacterium necrophorum, Prevotella loescheii, Streptococcus constellatus | Purulent exudate | Culture | Gomes et al. (235) |
| Streptococcus spp. | Pain | PCR | Fouad et al. (150) |
| Streptococcus constellatus | Pain, swelling, purulent exudate | Checkerboard | Siqueira et al. (236) |
| Prevotella loescheii, Peptostreptococcus spp., Anaerococcus prevotii | Pain | Culture | Jacinto et al. (207) |
| Bifidobacterium spp., Actinomyces spp., Streptococcus constellatus | Tenderness to percussion | Culture | Jacinto et al. (207) |
| Fusobacterium nucleatum | Swelling | Culture | Jacinto et al. (207) |
| Parvimonas micra, Prevotella intermedia/nigrescens, Eubacterium spp. | Pain | Culture | Gomes et al. (237) |
| Porphyromonas spp., Fusobacterium spp., Peptostreptococcus spp. | Tenderness to percussion, purulent exudate | Culture | Gomes et al. (237) |
| Porphyromonas spp., Peptostreptococcus spp., Enterococcus spp. | Swelling | Culture | Gomes et al. (237) |
| Treponema denticola | Pain | PCR | Foschi et al. (238) |
| Filifactor alocis | Pain, swelling, purulent exudate | PCR | Gomes et al. (78) |
| Tannerella forsythia | Tenderness to percussion, purulent exudate | PCR | Gomes et al. (78) |
| Fusobacterium nucleatum, Prevotella intermedia, Dialister pneumosintes, Prevotella clone E9_42, Prevotella baroniae, Eubacterium clone BP1-89, Lachnospiraceae clone MCE7_60 | Pain, swelling, purulent exudate | PCR, cloning, and sequencing | Sakamoto et al. (83) |
| Tannerella forsythia | Pain | Checkerboard | Sassone et al. (188) |
| Porphyromonas gingivalis | Pain, swelling, purulent exudate | PCR | Siqueira et al. (113) |
| Selenomonas sputigena | Pain | Checkerboard | Rôças et al. (187) |
| Fusobacterium spp., Parvimonas spp., Atopobium spp., Dialister spp., Porphyromonas spp., Prevotella spp. | Pain, swelling, purulent exudate | Pyrosequencing | Santos et al. (91) |
A significant limitation of virtually all the microbiological studies of acute endodontic infections is their cross-sectional nature. Restriction is mostly because of obvious ethical reasons and precludes any strong conclusion about involvement of certain species present in a mixed consortium in causation of acute symptoms. As a consequence, only association with symptoms, instead of a cause-and-effect relationship, can be inferred from these human studies. This makes the species associated with acute infection be referred to as “candidate,” “suspected,” or “putative” pathogens. Animal studies have given some support to the potential role in abscess causation. For instance, the ability to cause purulent infections in animal models strengthens the possible causative role of certain species clinically associated with symptomatic disease. Many candidate endodontic pathogens, alone or in combinations, have been shown to cause abscesses in animal models (104, 105, 116, 126, 152). One of the main problems with the animal model is that only cultivable bacteria have been tested, either in single culture or in combinations of two or a few species. Thus, any speculation about extension of these results to a clinical condition where there is a network of interacting species (10 or more), including as-yet-uncultivated phylotypes, may lead to oversimplification.
The Community-as-Pathogen Concept
Pathogenicity has traditionally been assumed on the basis of “guilt by association,” and some classic diseases, such as tetanus, gonorrhea, cholera, and syphilis, have been determined as having a “single-species etiology.” Unlike these diseases, endodontic infections are similar to several other human endogenous infections in that no single pathogen but a set of species, usually organized in multispecies biofilm communities, is involved (83, 99, 153–155). Mounting evidence indicates that while there is little specificity as to the involvement of single named species in the etiology of apical periodontitis, specificity becomes more evident when bacterial community profiles are taken into account. This is because while associations of any specific species with any form of apical periodontitis is seldom, if ever, confirmed, the bacterial community profiles seem to follow some patterns related to the different presentations of apical periodontitis (156).
The concept of the community as pathogen is based on the principle that teamwork is what eventually counts. The behavior of the community and the outcome of the host/bacterial community interaction will depend on the species composing the community and how the myriad of associations that can occur within the community affect and modulate the virulence of involved species. The virulence of a given species is allegedly different when it is in pure culture, in pairs, or as part of a large bacterial “society” (community). In mixed communities, a broad spectrum of relationships may arise between the component species, ranging from no effect (rare) or reduced pathogenicity to additive or synergistic pathogenic effects. Acute apical abscesses are examples of polymicrobial infections whereby bacterial species that individually may have low virulence and are unable to cause disease can do so when in association with others as part of a mixed consortium (pathogenic synergism) (105, 157, 158).
Bacterial community patterns related to acute infections.
Bacterial community profiles are essentially determined by species richness and abundance and have been recently investigated in the different types of endodontic infections. Community profiling analyses of the endodontic microbiota have disclosed a great interindividual variability in endodontic communities associated with the same clinical disease (95, 159); i.e., no two root canal infections are the same in terms of species richness and abundance. This indicates that the etiology of apical periodontitis, including the acute forms, is heterogeneous (95, 160).
Of great interest is that bacterial communities seem to follow a specific pattern according to the clinical condition (e.g., asymptomatic versus symptomatic disease) (95). Therefore, the severity of apical periodontitis may be related to the overall bacterial community composition. In other words, from the perspective of the single-pathogen concept, apical periodontitis can be considered to be of no specific microbial etiology. However, based on the community-as-pathogen concept, it is possible to infer that some communities are more related to certain forms of the disease, such as abscesses (83, 95). A challenge that arises from this notion is the need to unravel the specific genotypic and phenotypic characteristics of these abscess-related bacterial communities.
Acute apical abscesses are characterized by a concomitant infection of the root canal and the periradicular tissues, as the latter is an extension of the former. Even so, studies have reported some discrepancies between bacterial community profiles for matched samples taken from the root canal and abscess aspirates (92, 161). The differences observed suggest that some selection of species occurs as the infection advances from the canal to the periradicular tissues. This “filtering” process is highly likely to be a result of the different environments faced by the infecting bacteria: from a site relatively protected from host defenses (necrotic root canal) to a site with an exuberant acute inflammatory response (highly vascularized periradicular tissues). Moreover, differences in the tissue invasion ability of the involved species may contribute to the selective process of species present in pus aspirates.
Geographic Differences in the Abscess Microbiota
A curious observation when analyzing separate studies performed in different countries is the different prevalences of species involved in abscesses. Variations in microbiological diagnostic methodologies may account for most of these differences, but a geographical variation in bacterial composition cannot be disregarded, as it also occurs for other human body sites (162–164). Molecular studies have been conducted to directly compare the bacterial community structures and prevalences of some target species in abscesses from patients residing in different geographic locations. In two studies comparing samples from Portland, OR, and Rio de Janeiro, Brazil (75, 89), species-specific PCR analyses revealed that there was a significant difference in prevalence between the two geographical locations for P. intermedia, P. nigrescens, Prevotella tannerae, F. nucleatum, and P. gingivalis, all of which more frequent in Portland samples, while T. denticola and T. forsythia were more prevalent in Rio de Janeiro samples. Open-ended DGGE analysis of the bacterial community profiles in acute apical abscesses from the same two locations also disclosed a geography-related pattern (160). Several species were exclusive for each location, and others shared by the two locations showed great differences in prevalence. The occurrence of geographical variations was confirmed when comparing abscess samples from Rio de Janeiro and Seoul, South Korea (110). These differences have the potential to translate into relevant therapeutic implications, specifically in cases requiring systemic antibiotic therapy.
THE HOST SIDE OF THE STORY
Given the microbial etiology of apical periodontitis, development of acute symptoms has been traditionally searched for candidate microbial risk factors. Although there is a clear implication of microbiological factors, the possibility certainly exists that host-related factors (disease modifiers) can influence the severity of apical periodontitis lesions. Examples of disease modifiers include systemic conditions (e.g., diabetes, herpesvirus infection, stress, autoimmune diseases, and diseases that weaken the immune response) and the genetic background (e.g., gene polymorphism).
Diabetic individuals have been shown to develop complications from abscesses more frequently and to have a longer duration of hospital stay than nondiabetics (27, 33). Similarly, diabetic patients can exhibit about twice the rate of interappointment exacerbations (flare-ups) following endodontic intervention (165, 166). Furthermore, diabetic animals develop apical periodontitis lesions that are larger and more severe than those in nondiabetic controls (167). Type 2 diabetes mellitus has been found to be significantly associated with increased prevalence of apical periodontitis (168, 169). Although diabetic patients are apparently more prone to develop severe forms of apical periodontitis, no study so far has reported on the prevalence of endodontic abscesses in diabetic individuals.
Potentially, genetic polymorphism is another factor that can make individuals more susceptible to develop acute infections. De Sá et al. (170) investigated the association of acute apical abscesses with polymorphisms in the genes for interleukin-1β (IL-1β), IL-6, IL-10, tumor necrosis factor alpha (TNF-α), and CD14. Individuals with asymptomatic apical periodontitis, without previous exacerbation, were included as controls. A significant association was observed between the occurrence of the GG genotype or the G allele expression of the IL-6 gene and acute abscesses in women and in individuals younger than 35 years. The G allele is associated with high levels of IL-6 production compared with the C allele (171). Intermediate- and high-producer IL1B genotypes and low-producer TNFA genotype also showed some association with abscesses, although not as strong as observed for IL-6. Other studies are needed to confirm and expand these findings to analyze other potential inflammation-related genes.
It has been hypothesized that herpesviruses, especially human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV), may be implicated in the pathogenesis of apical periodontitis as a direct result of virus infection and replication or as a result of virally induced impairment of local host defenses, which might give rise to overgrowth of pathogenic bacteria in the very apical part of the root canal system (172). Infection by HCMV and EBV has been more frequently observed in symptomatic lesions (173, 174), and an association has been suggested. However, data related to the occurrence of herpesviruses in acute apical abscesses are rather inconclusive. Chen et al. (175) found herpesviruses in low prevalences and low copy numbers in abscess samples and concluded that herpesviruses may be present but are not required for the development of abscesses and cellulitis of endodontic origin. Ferreira et al. (176) evaluated the presence of human herpesviruses 1 to 8 and human papillomavirus in acute apical abscesses and reported that about 60% of the samples were positive for at least one target virus. Human herpesvirus 8 (HHV-8) occurred at a high prevalence (48%), followed by human papillomavirus (13%) and varicella-zoster virus and HHV-6 (9%). Viral coinfection occurred in some cases. In another study, the same group (109) found positive (but weak) associations between candidate endodontic bacterial pathogens and human viruses in samples from acute apical abscesses. Although these findings may suggest a role for viruses in the etiology of endodontic abscesses, the possibility also exists that the presence of viruses in the purulent exudate is merely a consequence of the bacterially induced inflammatory disease process.
There are many other host-related factors that have the potential to influence the resistance to infection, including age, stress, drug abuse, malnutrition, and other systemic disorders. Future research should focus on these potential candidate disease modifiers and their influence on the development of acute endodontic symptoms.
FACTORS INFLUENCING THE DEVELOPMENT OF ACUTE INFECTION
It has been suggested that although the microbial etiology of abscesses is characterized by low specificity, certain species have been more frequently detected than others and then might be considered more decisive in the outcome of the infectious process (158). However, based on the discussion above, in addition to the presence of some potentially pathogenic species, a multitude of other factors can be regarded as influential to the development of acute endodontic infections. These factors can be summarized as follows: (i) difference in virulence among clonal types of the same species, (ii) bacterial interactions resulting in collective pathogenicity, (iii) bacterial load, (iv) environment-regulated expression of virulence factors, and (v) host resistance and disease modifiers.
Difference in Virulence among Clonal Types of the Same Species
Clonal types of a given pathogenic bacterial species can significantly diverge in their virulence (177–180). A disease attributed to a given species is in fact caused by specific virulent clonal types of that species (181). Therefore, the possibility exists that the presence of virulent clonal types of candidate endodontic pathogens in the root canal may be a predisposing factor for abscess formation. This would help explain why the same species can be found in both symptomatic and asymptomatic infections. So far, there is no comparative study typing bacterial strains isolated from patients with symptomatic and asymptomatic infections.
Bacterial Interactions Resulting in Collective Pathogenicity
Bacterial combinations contribute to the development of more virulent communities due to synergism (104, 105, 116, 118, 126, 152). Abscesses are polymicrobial infections for which culture studies have reported a mean number of species ranging from 2 to 8.5 per pus specimen (41–43, 45, 46, 48, 49, 96, 97, 182), while molecular studies have revealed a mean of 12 to 18 species/phylotypes per case (83, 95). Findings from pyrosequencing studies suggest that these numbers can be much higher (91, 92). What has become evident from most studies comparing acute apical abscesses and asymptomatic endodontic infections is that there are different dominant species in the communities, and the former are usually characterized by a significantly higher species richness than that of the latter (83, 91, 95, 159). Increased diversity may be an important aspect of acute infections and collective pathogenicity, which is expected to be a result of the incalculable synergistic interactions among the community members and summation of virulence factors produced. It has been shown that every component of a polymicrobial infection, even species regarded as avirulent and/or in low numbers in the consortium, may somewhat affect the virulence of other members of the community (183–186). Therefore, communication between bacterial community members can alter the production of virulence factors by certain pathogenic species and affect the collective pathogenicity of the consortium (185).
Indeed, it has been shown that different species combinations may result in different outcomes because of the network of interactions. Rôças et al. (187) compared the prevalences of 50 bacterial species or phylotypes in samples from symptomatic and asymptomatic endodontic infections and found that none of the most prevalent taxa were significantly associated with symptoms; i.e., most species were as prevalent in symptomatic cases as they were in asymptomatic cases. However, cluster analyses revealed that the highly prevalent species formed different partnerships and associations according to the presence of symptoms. For example, D. invisus and P. endodontalis were found in both symptomatic and asymptomatic cases, but their common partners in the former were different from those in the latter. Therefore, the possibility exists that bacterial interactions result in communities that are more or less aggressive and consequently can cause host responses of corresponding intensity.
Bacterial Load
The bacterial load can be a decisive factor in acute disease causation. Here two aspects need to be considered: the total bacterial load (nonspecific load) and the levels of certain species (specific load). Total bacterial loads per abscess case have been reported to range from 104 to 109 cells (41, 43, 45). The overall number of bacterial cells in the community, regardless of the species, may be important because it may result in a heavy bioburden to the host, given the concentration of virulence factors released from large masses of bacteria colonizing the canal and invading the periradicular tissues. Clinical approaches such as chemomechanical debridement of the infected dental root canal or tooth extraction and incision of the mucosa/skin for drainage of pus act primarily and in a nonspecific way on the total bacterial load, reducing the infectious bioburden.
As for the second aspect, i.e., the specific load, the possibility exists that the number of cells of a given species found in both symptomatic and asymptomatic infections is larger in the former. For instance, T. forsythia has been detected at significantly higher levels in symptomatic than in asymptomatic endodontic infections (188). The presence of a potentially virulent pathogen in high counts may increase the virulence of the whole community and lead to symptomatic infection. Although logical, this assumption still has to be confirmed by more studies comparing the total and specific bacterial counts in symptomatic and asymptomatic infections.
Environment-Regulated Expression of Virulence Factors
A virulent clonal type of a given pathogenic species does not always express its virulence factors throughout its lifetime. The environment exerts an important role in inducing the turning on or the turning off of virulence genes (189–192). This is a crucial part of the process of bacterial adaptation to the environment and allows for establishment, optimal growth, and survival (193). Throughout the different stages of the infectious process, different sets of virulence genes are expressed in response to different environmental cues (193). Studies have demonstrated that environmental factors can influence gene/protein expression and consequently the behavior of some oral species commonly associated with abscesses, including P. gingivalis, F. nucleatum, P. intermedia, and treponemes (194–198). Changes in the environment, induced by the physiology of the microbial community itself (interactions and arrival of latecomers), by treatment procedures (imbalance), or by the host (hormonal changes, disease modifiers, etc.), may create conditions conducive to the turning on of virulence genes, enhancing the collective pathogenicity and leading to emergence of symptoms.
Host Resistance and Disease Modifiers
As aforementioned, although not being the cause of acute inflammation, disease modifiers, such as diabetes, genetic polymorphisms, and herpesvirus infection, may influence the host response to infection and predispose to more severe forms of apical periodontitis. Whether the overlap of any of these disease modifiers with the microbiological factors listed above is required for the development of acute forms of the disease remains to be determined.
TREATMENT OF THE ACUTE APICAL ABSCESS
Treatment of acute apical abscesses involves incision for drainage and root canal treatment or extraction of the involved tooth to remove the source of infection (199, 200). In some cases, drainage can be obtained through the root canal, but when swelling is present, incision for drainage should also be performed whenever possible, since this approach has been shown to produce a quicker improvement than drainage only by opening of the root canal (200). Adjunctive systemic antibiotics are not necessary in most cases of localized and uncomplicated apical abscesses (201–205). Analgesics may be prescribed for pain control.
The selective occasions when antibiotics are indicated in cases of acute apical abscesses include the following: abscesses associated with systemic involvement, including fever, malaise, and lymphadenopathy; disseminating infections resulting in cellulitis, progressive diffuse swelling, and/or trismus; and abscesses in medically compromised patients who are at increased risk of a secondary (focal) infection following bacteremia.
Therefore, in complicated cases, in addition to prompt and aggressive surgical drainage for treatment (8, 26), initiation of empirical therapy with antibiotics is highly recommended. If necessary, it can be adjusted according to the results of antibiotic sensitivity tests. The combination of early diagnosis, initiation of empirical antibiotic therapy, and timely surgical intervention can be regarded as the decisive triad for the successful management of complications of acute dental abscesses (26).
The selection of antibiotics in clinical practice is either empirical or based on the results of microbial susceptibility testing. For diseases with known microbial causes for which the probable microbiota has been established in the literature, empirical therapy may be used. This is especially applicable to acute dental abscesses, because culture-dependent antimicrobial tests of anaerobic bacteria can take too long to provide results about antibiotic susceptibility (around 7 to 14 days). Therefore, it is preferable to opt for an antimicrobial agent whose spectrum of action includes the most commonly detected bacteria.
Most of the bacterial species involved with endodontic infections, including abscesses, are susceptible to penicillins (43, 206–208). This makes these drugs the first choice for treatment of endodontic infections when allergy of the patient to penicillin has been ruled out. Penicillin V or amoxicillin has been commonly prescribed. Since the use of antibiotics is restricted to severe and complicated abscess infections, it seems prudent to use amoxicillin, a semisynthetic penicillin with a broader spectrum of antimicrobial activity than penicillin V. In addition, amoxicillin may provide more rapid improvement in pain or swelling, and patient compliance with the prescribed regimen may be better because of the longer dosage interval of amoxicillin (209). In even more serious cases, including life-threatening conditions, association of amoxicillin with either clavulanic acid or metronidazole may be required to achieve optimum antimicrobial effects as a result of the spectrum of action being extended to include penicillin-resistant strains (208, 210). A randomized clinical trial (199) compared the efficacy of adjunct therapy with amoxicillin-clavulanic acid to that with penicillin V in the treatment of acute apical abscess following surgical drainage and either tooth extraction or root canal intervention and reported that, even though symptoms improved in all patients, those receiving amoxicillin-clavulanic acid recorded a significantly greater decrease in symptoms during the second and third days. Another study (211) observed less swelling in patients in the amoxicillin group than in patients taking penicillin.
Clindamycin has strong antimicrobial activity against oral anaerobes (96, 208, 212) and has been shown to produce good clinical results similar to those with penicillin for treatment of acute dental abscesses (213, 214). However, a higher rate of adverse gastrointestinal effects and diarrhea has been reported in association with clindamycin treatment (214), leaving this drug as an effective alternative in patients allergic to penicillin or when treatment with amoxicillin resulted in failure.
Moxifloxacin is a fluoroquinolone that has emerged as a potential drug for the treatment of abscesses, given its good antibacterial activity against Gram-positive and Gram-negative aerobic and anaerobic bacteria isolated from odontogenic infections (215). A clinical trial showed that moxifloxacin resulted in significantly better pain reduction and overall clinical response than clindamycin for patients with dental abscesses (216).
Emerging resistance to commonly used antibiotics has been reported for bacteria found in dental abscesses. A systematic review revealed that the overall results of laboratory studies indicate that no single antibiotic is effective in vitro against all species found in dental abscesses (209). Prevotella species have been regarded as prominent sources of resistance to beta-lactam agents in the oral cavity due to production of beta-lactamase (98, 217–220). Kuriyama et al. (221) revealed that beta-lactamase was detected in 36% of the dark-pigmented Prevotella and 32% of the nonpigmented Prevotella species isolated from pus samples of oral abscesses. Other enzyme-producing oral anaerobic species include strains of the Gram-negative bacteria F. nucleatum, E. corrodens, and T. forsythia and the Gram-positive bacteria P. acnes, Actinomyces species, and Peptostreptococcus species (98, 217–219, 221–223). Bacteria that produce beta-lactamases not only may protect themselves from penicillins but also can protect other penicillin-susceptible bacteria present in a mixed community by releasing free beta-lactamase into the environment (224).
Susceptibility of Prevotella strains to several cephalosporins, erythromycin, and azithromycin has been found to correlate with amoxicillin susceptibility; i.e., amoxicillin-resistant strains can be similarly resistant to these other antibiotics (208). Moreover, macrolides (erythromycin and azithromycin) have presented decreased activity against Fusobacterium and nonpigmented Prevotella species (40, 208, 221). Therefore, it seems to be of little value to use oral cephalosporins and macrolides for management of dental abscesses as an alternative to amoxicillin.
FUTURE DIRECTIONS
There are many microbial and host-related aspects that need to be elucidated to refine and expand the knowledge of the etiology of acute apical abscesses and therefore contribute to enhancing preventive and therapeutic measures for this morbid and sometimes life-threatening condition. This article places endodontic abscesses into a context of complex etiology, with many aspects still remaining to be unearthed.
Evidence is growing that, like in other polymicrobial infections, the community is the unit of pathogenicity in apical periodontitis, including its most severe forms. Endodontic microbial communities are composed of several different species, which interact one with the other to give rise to disease. The fact that there is no single pathogen to blame for acute abscesses does not preclude the possibility of some species playing an important role in influencing the development of symptoms. Like a team, the bacterial community may have specific players that can be decisive in making it more aggressive (virulent). If this is proven true, it remains to be clarified which are the key species in making a community be more virulent. It is probable that there are not only one but several species in each community that contribute to increasing the community aggressiveness. What key species do and produce to cause acute infections should be elucidated. Techniques with potential to shed light on this issue include proteomic and transcriptomic analyses of samples from abscesses and asymptomatic infections as well as experimental infection models using mixed consortia of known species.
It would be interesting to evaluate whether the clonal types of the same species found in both acute abscesses and asymptomatic dental root canal infections are really different and whether this difference translates into increased virulence in the ones found in abscesses. Also, it is important to use quantitative diagnostic techniques to compare the total bacterial loads in symptomatic and asymptomatic infections. Comparative quantitative analysis of species suspected to participate in acute infections, such as F. nucleatum, P. micra, Porphyromonas species, Prevotella species, and streptococci of the S. anginosus group, is also important. There is also a need to look for some potential pathogens in the as-yet-uncultivated portion of the abscess microbiota.
How the bacterial species interact in the multispecies community is another focus of future research. Different partnerships and associations between community members may influence the development of symptoms. Some bacterial associations can result in a more virulent multispecies community, therefore giving rise to acute periradicular inflammation. Knowledge of the species involved, as well as the nature and outcome of their associations, needs to be expanded. How the environment changed by disease contributes to such an increased community virulence and how the clinician may interfere with the whole process are other areas that need further consideration.
Whether more-virulent communities develop right from the beginning of the infection process or are a result of a shift in the community composition due to environmental changes remains to be determined. It is difficult or even impossible to look for this answer in longitudinal studies in humans, because of obvious ethical reasons, but animal studies using combined histopathological and microbiological approaches might help clarify this issue.
Virtually all community profiling studies demonstrate a high interindividual variation in the bacterial community composition. This implies that treatment either should target the broad spectrum of species involved (nonspecific) or should be personalized for the individual (specific). Personalized treatment is a tendency in medicine and exploits information on each person's unique clinical, genetic, and environmental aspects, not only to predict disease risk but also to devise customized interventions and improve treatment outcome (225, 226). In the future, personalized management of acute abscesses may be related to identifying bacterial or host-related biomarkers of disease progression and using this knowledge to establish tailored strategies to properly prevent or mitigate complicated infections. Personalized treatment in the case of acute apical abscesses might also be related to adjunct antimicrobial therapy dictated by microbiological findings. Acute abscesses may progress to life-threatening complications, and, as a consequence, rapid microbiological identification results should be valuable for proper management. Antibiotics are usually prescribed based on the empirical knowledge of endodontic infections. However, patients with abscesses rapidly disseminating to facial and/or neck anatomic spaces may benefit from rapid molecular diagnosis of the bacteria involved in the abscess/cellulitis as well as of antibiotic resistance genes, allowing clinicians to manage this infectious condition proactively. It has been envisioned that in the near future the pyrosequencing technology may be routinely used for effective and rapid microbiological diagnosis of polymicrobial infections and the prediction of antibiotic susceptibility (94, 227).
Fast, accurate, and highly sensitive molecular assays can provide microbial diagnostic results in a matter of minutes to a few hours. Using these methods to rapidly detect the presence of antibiotic resistance genes in a sample seems to be an attractive way of guiding antibiotic treatment. Although the presence of a resistance gene in a sample does not necessarily imply phenotypic resistance, its absence does imply a lack of resistance through that particular genetic mechanism (228). The potential exists for molecular methods, such as a multiplex PCR approach or DNA microarray technology, to simultaneously identify the presence of multiple resistance genes in just a few hours (93). Alternatively to the detection of resistance genes, a universal 16S rRNA gene- and rpoB-based real-time quantitative PCR assay for susceptibility testing of bacteria has been devised to decrease the time needed for antimicrobial testing results (229).
Given the network of interactions in polymicrobial infections, it is highly likely that not all species in the consortium need to be targeted with antibiotic therapy. However, it remains to be determined which species should be targeted; this might include the most pathogenic species, the most dominant species or even the most important keystone species for the community ecology, considering that an imbalance might lead to catastrophic effects for community survival. These aspects should be the focus of future research, as this piece of information will favor proper analysis of data from microbial diagnostic techniques and permit rapid, proactive, and effective therapeutic intervention.
Interindividual differences in the abscess microbiota are even more pronounced when individuals from different countries are evaluated (160). It remains to be elucidated whether the observed differences in bacterial community profiles influence the outcome of standard local and systemic therapeutic procedures. For instance, a drug shown to be effective against pathogens identified in a given location will not necessarily be effective to treat the same infection in another location, given the differences in the composition of the polymicrobial community. Adding complexity to this issue, geographical differences in the antibiotic susceptibility profiles of oral bacterial isolates have been reported (223).
The host side of the history has not been subject of great scrutiny. It remains to be determined whether transient (e.g., stress, active herpesvirus infection, malnutrition, or use of corticosteroids) or permanent (e.g., genetic polymorphism, diabetes, or HIV infection) systemic conditions may predispose to exacerbations and abscess complications. If this is proven so, the next focus of future research is to evaluate how the clinician can target specific host processes to effectively treat patients and prevent or minimize potential sequelae of acute apical abscesses.
Biographies

José F. Siqueira, Jr., Ph.D., is the Chairman of Endodontics and Director of the Postgraduate Program in Endodontics at Estácio de Sá University in Rio de Janeiro, Brazil. He received his D.D.S. from Gama Filho University, Rio de Janeiro, in 1989, a master's degree in microbiology and immunology from Federal University of Rio de Janeiro in 1996, and a Ph.D. in microbiology and immunology from the same university in 1998. Dr. Siqueira has authored 6 textbooks and several book chapters for leading international endodontic textbooks as well as more than 200 peer-reviewed scientific papers in the areas of endodontics, microbiology, and infection control. Ongoing research includes studies on the composition of the endodontic microbiota in different forms of apical periodontitis, development of clinical strategies to deal with endodontic infections, and the influence of disease modifiers on the patient response to treatment. He is actively lecturing worldwide.

Isabela N. Rôças, Ph.D., is Professor of Endodontics and head of the molecular microbiology laboratory at Estácio de Sá University in Rio de Janeiro, Brazil. She received her D.D.S. from State University of Rio de Janeiro in 1992 and her endodontic training at Gama Filho University in 1996. In 2002, she earned a master's degree in clinical bacteriology from State University of Rio de Janeiro. She concluded her Ph.D. in microbiology and immunology at Federal University of Rio de Janeiro in 2004. Dr. Rôças has authored and coauthored several chapters for leading international endodontic textbooks and more than 150 peer-reviewed scientific articles. Her primary research interests are in the areas of endodontic microbiology and infection control. Ongoing research from her lab includes studies on diversity of the endodontic microbiota using molecular techniques, control of biofilm infection, and disease modifiers of apical periodontitis.
REFERENCES
- 1. Torabinejad M, Shabahang S. 2009. Pulp and periapical pathosis, p 49–67 In Torabinejad M, Walton RE. (ed), Endodontics. Principles and practice, 4th ed Saunders/Elsevier, St. Louis, MO [Google Scholar]
- 2. Seltzer S, Farber PA. 1994. Microbiologic factors in endodontology. Oral Surg. Oral Med. Oral Pathol. 78:634–645 [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner JC. 1991. Microbiologic and pathologic aspects of endodontics. Curr. Opin. Dent. 1:737–743 [PubMed] [Google Scholar]
- 4. Siqueira JF., Jr 2002. Endodontic infections: concepts, paradigms, and perspectives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94:281–293 [DOI] [PubMed] [Google Scholar]
- 5. Sasaki H, Stashenko P. 2012. Interrelationship of the pulp and apical periodontitis, p 277–299 In Hargreaves KM, Goodis HE, Tay FR. (ed), Seltzer and Bender's dental pulp, 2nd ed Quintessence Publishing, Chicago, IL [Google Scholar]
- 6. Eriksen HM. 2008. Epidemiology of apical periodontitis, p 262–274 In Orstavik D, Pitt Ford T. (ed), Essential endodontology, 2nd ed Blackwell Science Ltd, Oxford, United Kingdom [Google Scholar]
- 7. Baumgartner JC, Siqueira JF, Jr, Sedgley CM, Kishen A. 2008. Microbiology of endodontic disease, p 221–308 In Ingle JI, Bakland LK, Baumgartner JC. (ed), Ingle's endodontics, 6th ed BC Decker, Hamilton, Canada [Google Scholar]
- 8. Gill Y, Scully C. 1990. Orofacial odontogenic infections: review of microbiology and current treatment. Oral Surg. Oral Med. Oral Pathol. 70:155–158 [DOI] [PubMed] [Google Scholar]
- 9. Quinonez C, Gibson D, Jokovic A, Locker D. 2009. Emergency department visits for dental care of nontraumatic origin. Community Dent. Oral Epidemiol. 37:366–371 [DOI] [PubMed] [Google Scholar]
- 10. Green AW, Flower EA, New NE. 2001. Mortality associated with odontogenic infection! Br. Dent. J. 190:529–530 [DOI] [PubMed] [Google Scholar]
- 11. Robertson D, Smith AJ. 2009. The microbiology of the acute dental abscess. J. Med. Microbiol. 58:155–162 [DOI] [PubMed] [Google Scholar]
- 12. Flynn TR. 2000. The swollen face. Severe odontogenic infections. Emerg. Med. Clin. North Am. 18:481–519 [DOI] [PubMed] [Google Scholar]
- 13. Wong TY. 1999. A nationwide survey of deaths from oral and maxillofacial infections: the Taiwanese experience. J. Oral Maxillofac. Surg. 57:1297–1299 [DOI] [PubMed] [Google Scholar]
- 14. Baqain ZH, Newman L, Hyde N. 2004. How serious are oral infections? J. Laryngol Otol. 118:561–565 [DOI] [PubMed] [Google Scholar]
- 15. Gendron R, Grenier D, Maheu-Robert L. 2000. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2:897–906 [DOI] [PubMed] [Google Scholar]
- 16. Marcus BJ, Kaplan J, Collins KA. 2008. A case of Ludwig angina: a case report and review of the literature. Am. J. Forensic Med. Pathol. 29:255–259 [DOI] [PubMed] [Google Scholar]
- 17. Boscolo-Rizzo P, Da Mosto MC. 2009. Submandibular space infection: a potentially lethal infection. Int. J. Infect. Dis. 13:327–333 [DOI] [PubMed] [Google Scholar]
- 18. Larawin V, Naipao J, Dubey SP. 2006. Head and neck space infections. Otolaryngol. Head Neck Surg. 135:889–893 [DOI] [PubMed] [Google Scholar]
- 19. Yun MW, Hwang CF, Lui CC. 1991. Cavernous sinus thrombosis following odontogenic and cervicofacial infection. Eur. Arch. Otorhinolaryngol. 248:422–424 [DOI] [PubMed] [Google Scholar]
- 20. Ogundiya DA, Keith DA, Mirowski J. 1989. Cavernous sinus thrombosis and blindness as complications of an odontogenic infection: report of a case and review of literature. J. Oral Maxillofac. Surg. 47:1317–1321 [DOI] [PubMed] [Google Scholar]
- 21. Corson MA, Postlethwaite KP, Seymour RA. 2001. Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis. 7:61–65 [PubMed] [Google Scholar]
- 22. Ewald C, Kuhn S, Kalff R. 2006. Pyogenic infections of the central nervous system secondary to dental affections—a report of six cases. Neurosurg. Rev. 29:163–166 [DOI] [PubMed] [Google Scholar]
- 23. Al Masalma M, Lonjon M, Richet H, Dufour H, Roche PH, Drancourt M, Raoult D, Fournier PE. 2012. Metagenomic analysis of brain abscesses identifies specific bacterial associations. Clin. Infect. Dis. 54:202–210 [DOI] [PubMed] [Google Scholar]
- 24. Marques DA, Silva R, Caugant DA, Josefsen R, Tronstad L, Olsen I. 2004. Characterization of Streptococcus constellatus strains recovered from a brain abscess and periodontal pockets in an immunocompromised patient. J. Periodontol. 75:1720–1723 [DOI] [PubMed] [Google Scholar]
- 25. Detry G, Pierard D, Vandoorslaer K, Wauters G, Avesani V, Glupczynski Y. 2006. Septicemia due to Solobacterium moorei in a patient with multiple myeloma. Anaerobe 12:160–162 [DOI] [PubMed] [Google Scholar]
- 26. Daramola OO, Flanagan CE, Maisel RH, Odland RM. 2009. Diagnosis and treatment of deep neck space abscesses. Otolaryngol. Head Neck Surg. 141:123–130 [DOI] [PubMed] [Google Scholar]
- 27. Huang TT, Tseng FY, Liu TC, Hsu CJ, Chen YS. 2005. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol. Head Neck Surg. 132:943–947 [DOI] [PubMed] [Google Scholar]
- 28. Bonapart IE, Stevens HP, Kerver AJ, Rietveld AP. 1995. Rare complications of an odontogenic abscess: mediastinitis, thoracic empyema and cardiac tamponade. J. Oral Maxillofac. Surg. 53:610–613 [DOI] [PubMed] [Google Scholar]
- 29. Pappa H, Jones DC. 2005. Mediastinitis from odontogenic infection. A case report. Br. Dent. J. 198:547–548 [DOI] [PubMed] [Google Scholar]
- 30. Garatea-Crelgo J, Gay-Escoda C. 1991. Mediastinitis from odontogenic infection. Report of three cases and review of the literature. Int. J. Oral Maxillofac. Surg. 20:65–68 [DOI] [PubMed] [Google Scholar]
- 31. Umeda M, Minamikawa T, Komatsubara H, Shibuya Y, Yokoo S, Komori T. 2003. Necrotizing fasciitis caused by dental infection: a retrospective analysis of 9 cases and a review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 95:283–290 [DOI] [PubMed] [Google Scholar]
- 32. Stoykewych AA, Beecroft WA, Cogan AG. 1992. Fatal necrotizing fasciitis of dental origin. J. Can. Dent. Assoc. 58:59–62 [PubMed] [Google Scholar]
- 33. Tung-Yiu W, Jehn-Shyun H, Ching-Hung C, Hung-An C. 2000. Cervical necrotizing fasciitis of odontogenic origin: a report of 11 cases. J. Oral Maxillofac. Surg. 58:1347–1352 [DOI] [PubMed] [Google Scholar]
- 34. Udaondo P, Garcia-Delpech S, Diaz-Llopis M, Salom D, Garcia-Pous M, Strottmann JM. 2008. Bilateral intraorbital abscesses and cavernous sinus thromboses secondary to Streptococcus milleri with a favorable outcome. Ophthal. Plast. Reconstr. Surg. 24:408–410 [DOI] [PubMed] [Google Scholar]
- 35. Kim IK, Kim JR, Jang KS, Moon YS, Park SW. 2007. Orbital abscess from an odontogenic infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 103:e1–e6 [DOI] [PubMed] [Google Scholar]
- 36. Koch F, Breil P, Marroquin BB, Gawehn J, Kunkel M. 2006. Abscess of the orbit arising 48 h after root canal treatment of a maxillary first molar. Int. Endod. J. 39:657–664 [DOI] [PubMed] [Google Scholar]
- 37. Pasqualini L, Mencacci A, Scarponi AM, Leli C, Fabbriciani G, Callarelli L, Schillaci G, Bistoni F, Mannarino E. 2008. Cervical spondylodiscitis with spinal epidural abscess caused by Aggregatibacter aphrophilus. J. Med. Microbiol. 57:652–655 [DOI] [PubMed] [Google Scholar]
- 38. Kim MK, Nalliah RP, Lee MK, Allareddy V. 2012. Factors associated with length of stay and hospital charges for patients hospitalized with mouth cellulitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 113:21–28 [DOI] [PubMed] [Google Scholar]
- 39. Allareddy V, Lin CY, Shah A, Lee MK, Nalliah R, Elangovan S, Karimbux NY. 2010. Outcomes in patients hospitalized for periapical abscess in the United States: an analysis involving the use of a nationwide inpatient sample. J. Am. Dent. Assoc. 141:1107–1116 [PubMed] [Google Scholar]
- 40. Heimdahl A, von Konow L, Satoh T, Nord CE. 1985. Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings. J. Clin. Microbiol. 22:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewis MA, MacFarlane TW, McGowan DA. 1986. Quantitative bacteriology of acute dento-alveolar abscesses. J. Med. Microbiol. 21:101–104 [DOI] [PubMed] [Google Scholar]
- 42. Sundqvist G, Johansson E, Sjogren U. 1989. Prevalence of black-pigmented bacteroides species in root canal infections. J. Endod. 15:13–19 [DOI] [PubMed] [Google Scholar]
- 43. Khemaleelakul S, Baumgartner JC, Pruksakorn S. 2002. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 94:746–755 [DOI] [PubMed] [Google Scholar]
- 44. Sabiston CB, Jr, Grigsby WR, Segerstrom N. 1976. Bacterial study of pyogenic infections of dental origin. Oral Surg. Oral Med. Oral Pathol. 41:430–435 [DOI] [PubMed] [Google Scholar]
- 45. Williams BL, McCann GF, Schoenknecht FD. 1983. Bacteriology of dental abscesses of endodontic origin. J. Clin. Microbiol. 18:770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brook I, Frazier EH, Gher ME. 1991. Aerobic and anaerobic microbiology of periapical abscess. Oral Microbiol. Immunol. 6:123–125 [DOI] [PubMed] [Google Scholar]
- 47. Kulekci G, Inanc D, Kocak H, Kasapoglu C, Gumru OZ. 1996. Bacteriology of dentoalveolar abscesses in patients who have received empirical antibiotic therapy. Clin. Infect. Dis. 23(Suppl. 1):S51–S53 [DOI] [PubMed] [Google Scholar]
- 48. Sakamoto H, Kato H, Sato T, Sasaki J. 1998. Semiquantitative bacteriology of closed odontogenic abscesses. Bull. Tokyo Dent. Coll. 39:103–107 [PubMed] [Google Scholar]
- 49. de Sousa EL, Ferraz CC, Gomes BP, Pinheiro ET, Teixeira FB, de Souza-Filho FJ. 2003. Bacteriological study of root canals associated with periapical abscesses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 96:332–339 [DOI] [PubMed] [Google Scholar]
- 50. Engelkirk PG, Duben-Engelkirk J, Dowell VR., Jr 1992. Principles and practice of clinical anaerobic bacteriology. Star Publishing Company, Belmont, CA [Google Scholar]
- 51. Siqueira JF, Jr, Rôças IN. 2005. Exploiting molecular methods to explore endodontic infections. 1. Current molecular technologies for microbiological diagnosis. J. Endod. 31:411–423 [DOI] [PubMed] [Google Scholar]
- 52. Socransky SS, Gibbons RJ, Dale AC, Bortnick L, Rosenthal E, MacDonald JB. 1963. The microbiota of the gingival crevice in man. 1. Total microscopic and viable counts and counts of specific organisms. Arch. Oral Biol. 8:275–280 [DOI] [PubMed] [Google Scholar]
- 53. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J. Bacteriol. 192:5002–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, Sahasrabudhe A, Dewhirst FE. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kroes I, Lepp PW, Relman DA. 1999. Bacterial diversity within the human subgingival crevice. Proc. Natl. Acad. Sci. U. S. A. 96:14547–14552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. 2005. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 43:3944–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, Wade WG. 2006. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol. Immunol. 21:61–68 [DOI] [PubMed] [Google Scholar]
- 59. Wade W. 2002. Unculturable bacteria—the uncharacterized organisms that cause oral infections. J. R. Soc. Med. 95:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kell DB, Young M. 2000. Bacterial dormancy and culturability: the role of autocrine growth factors. Curr. Opin. Microbiol. 3:238–243 [DOI] [PubMed] [Google Scholar]
- 61. Breznak JA. 2002. A need to retrieve the not-yet-cultured majority. Environ. Microbiol. 4:4–5 [DOI] [PubMed] [Google Scholar]
- 62. Stevenson BS, Eichorst SA, Wertz JT, Schmidt TM, Breznak JA. 2004. New strategies for cultivation and detection of previously uncultured microbes. Appl. Environ. Microbiol. 70:4748–4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Renesto P, Crapoulet N, Ogata H, La Scola B, Vestris G, Claverie JM, Raoult D. 2003. Genome-based design of a cell-free culture medium for Tropheryma whipplei. Lancet 362:447–449 [DOI] [PubMed] [Google Scholar]
- 64. Vartoukian SR, Palmer RM, Wade WG. 2010. Strategies for culture of ‘unculturable’ bacteria. FEMS Microbiol. Lett. 309:1–7 [DOI] [PubMed] [Google Scholar]
- 65. Tanner A, Lai Maiden C-HM. 1992. Characteristics of oral gram-negative species, p 299–341 In Slots J, Taubman MA. (ed), Contemporary oral microbiology and immunology. Mosby, St Louis, MO [Google Scholar]
- 66. Beighton D, Hardie JM, Whiley RA. 1991. A scheme for the identification of viridans streptococci. J. Med. Microbiol. 35:367–372 [DOI] [PubMed] [Google Scholar]
- 67. Petti CA, Polage CR, Schreckenberger P. 2005. The role of 16S rRNA gene sequencing in identification of microorganisms misidentified by conventional methods. J. Clin. Microbiol. 43:6123–6125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ochman H, Lerat E, Daubin V. 2005. Examining bacterial species under the specter of gene transfer and exchange. Proc. Natl. Acad. Sci. U. S. A. 102:6595–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tardif G, Sulavik MC, Jones GW, Clewell DB. 1989. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect. Immun. 57:3945–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bosshard PP, Abels S, Altwegg M, Bottger EC, Zbinden R. 2004. Comparison of conventional and molecular methods for identification of aerobic catalase-negative gram-positive cocci in the clinical laboratory. J. Clin. Microbiol. 42:2065–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clarridge JE., III 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17:840–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sibley CD, Peirano G, Church DL. 2012. Molecular methods for pathogen and microbial community detection and characterization: current and potential application in diagnostic microbiology. Infect. Genet. Evol. 12:505–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Siqueira JF, Jr, Rôças IN. 2009. Diversity of endodontic microbiota revisited. J. Dent. Res. 88:969–981 [DOI] [PubMed] [Google Scholar]
- 74. Siqueira JF, Jr, Rôças IN, Souto R, Uzeda M, Colombo AP. 2001. Microbiological evaluation of acute periradicular abscesses by DNA-DNA hybridization. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 92:451–457 [DOI] [PubMed] [Google Scholar]
- 75. Baumgartner JC, Siqueira JF, Jr, Xia T, Rôças IN. 2004. Geographical differences in bacteria detected in endodontic infections using polymerase chain reaction. J. Endod. 30:141–144 [DOI] [PubMed] [Google Scholar]
- 76. Rôças IN, Siqueira JF., Jr 2002. Identification of Dialister pneumosintes in acute periradicular abscesses of humans by nested PCR. Anaerobe 8:75–78 [Google Scholar]
- 77. Siqueira JF, Jr, Rôças IN, Oliveira JC, Santos KR. 2001. Molecular detection of black-pigmented bacteria in infections of endodontic origin. J. Endod. 27:563–566 [DOI] [PubMed] [Google Scholar]
- 78. Gomes BP, Jacinto RC, Pinheiro ET, Sousa EL, Zaia AA, Ferraz CC, Souza-Filho FJ. 2006. Molecular analysis of Filifactor alocis, Tannerella forsythia, and Treponema denticola associated with primary endodontic infections and failed endodontic treatment. J. Endod. 32:937–940 [DOI] [PubMed] [Google Scholar]
- 79. Siqueira JF, Jr, Rôças IN. 2004. Treponema species associated with abscesses of endodontic origin. Oral Microbiol. Immunol. 19:336–339 [DOI] [PubMed] [Google Scholar]
- 80. Baumgartner JC, Khemaleelakul SU, Xia T. 2003. Identification of spirochetes (treponemes) in endodontic infections. J. Endod. 29:794–797 [DOI] [PubMed] [Google Scholar]
- 81. Riggio MP, Lennon A, Smith A. 2001. Detection of Peptostreptococcus micros DNA in clinical samples by PCR. J. Med. Microbiol. 50:249–254 [DOI] [PubMed] [Google Scholar]
- 82. Riggio MP, Lennon A. 2007. Development of a novel PCR assay for detection of Prevotella oris in clinical specimens. FEMS Microbiol. Lett. 276:123–128 [DOI] [PubMed] [Google Scholar]
- 83. Sakamoto M, Rôças IN, Siqueira JF, Jr, Benno Y. 2006. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol. Immunol. 21:112–122 [DOI] [PubMed] [Google Scholar]
- 84. Wade WG, Spratt DA, Dymock D, Weightman AJ. 1997. Molecular detection of novel anaerobic species in dentoalveolar abscesses. Clin. Infect. Dis. 25(Suppl. 2):S235–S236 [DOI] [PubMed] [Google Scholar]
- 85. Dymock D, Weightman AJ, Scully C, Wade WG. 1996. Molecular analysis of microflora associated with dentoalveolar abscesses. J. Clin. Microbiol. 34:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Flynn TR, Paster BJ, Stokes LN, Susarla SM, Shanti RM. 2012. Molecular methods for diagnosis of odontogenic infections. J. Oral Maxillofac. Surg. 70:1854–1859 [DOI] [PubMed] [Google Scholar]
- 87. Riggio MP, Aga H, Murray CA, Jackson MS, Lennon A, Hammersley N, Bagg J. 2007. Identification of bacteria associated with spreading odontogenic infections by 16S rRNA gene sequencing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 103:610–617 [DOI] [PubMed] [Google Scholar]
- 88. Rôças IN, Siqueira JF., Jr 2005. Detection of novel oral species and phylotypes in symptomatic endodontic infections including abscesses. FEMS Microbiol. Lett. 250:279–285 [DOI] [PubMed] [Google Scholar]
- 89. Rôças IN, Baumgartner JC, Xia T, Siqueira JF., Jr 2006. Prevalence of selected bacterial named species and uncultivated phylotypes in endodontic abscesses from two geographic locations. J. Endod. 32:1135–1138 [DOI] [PubMed] [Google Scholar]
- 90. Siqueira JF, Jr, Rôças IN. 2009. The microbiota of acute apical abscesses. J. Dent. Res. 88:61–65 [DOI] [PubMed] [Google Scholar]
- 91. Santos AL, Siqueira JF, Jr, Rôças IN, Jesus EC, Rosado AS, Tiedje JM. 2011. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS One 6:e28088 doi:10.1371/journal.pone.0028088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF. 2012. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 13:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Siqueira JF, Jr, Rôças IN. 2005. Exploiting molecular methods to explore endodontic infections. 2. Redefining the endodontic microbiota. J. Endod. 31:488–498 [DOI] [PubMed] [Google Scholar]
- 94. Sibley CD, Church DL, Surette MG, Dowd SE, Parkins MD. 2012. Pyrosequencing reveals the complex polymicrobial nature of invasive pyogenic infections: microbial constituents of empyema, liver abscess, and intracerebral abscess. Eur. J. Clin. Microbiol. Infect. Dis. 31:2679–2691 [DOI] [PubMed] [Google Scholar]
- 95. Siqueira JF, Jr, Rôças IN, Rosado AS. 2004. Investigation of bacterial communities associated with asymptomatic and symptomatic endodontic infections by denaturing gradient gel electrophoresis fingerprinting approach. Oral Microbiol. Immunol. 19:363–370 [DOI] [PubMed] [Google Scholar]
- 96. Kuriyama T, Karasawa T, Nakagawa K, Saiki Y, Yamamoto E, Nakamura S. 2000. Bacteriologic features and antimicrobial susceptibility in isolates from orofacial odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90:600–608 [DOI] [PubMed] [Google Scholar]
- 97. Wasfy MO, McMahon KT, Minah GE, Falkler WA., Jr 1992. Microbiological evaluation of periapical infections in Egypt. Oral Microbiol. Immunol. 7:100–105 [DOI] [PubMed] [Google Scholar]
- 98. Brook I, Frazier EH, Gher ME., Jr 1996. Microbiology of periapical abscesses and associated maxillary sinusitis. J. Periodontol. 67:608–610 [DOI] [PubMed] [Google Scholar]
- 99. Sundqvist G. 1976. Bacteriological studies of necrotic dental pulps. Odontological dissertation no. 7. University of Umea, Umea, Sweden [Google Scholar]
- 100. Haapasalo M, Ranta H, Ranta K, Shah H. 1986. Black-pigmented Bacteroides spp. in human apical periodontitis. Infect. Immun. 53:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Winkelhoff AJ, Carlee AW, de Graaff J. 1985. Bacteroides endodontalis and others black-pigmented Bacteroides species in odontogenic abscesses. Infect. Immun. 49:494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Socransky SS, Gibbons RJ. 1965. Required role of Bacteroides melaninogenicus in mixed anaerobic infections. J. Infect. Dis. 115:247–253 [DOI] [PubMed] [Google Scholar]
- 103. MacDonald JB, Socransky SS, Gibbons RJ. 1963. Aspects of the pathogenesis of mixed anaerobic infection of mucous membranes. J. Dent. Res. 42:529–544 [Google Scholar]
- 104. Siqueira JF, Jr, Magalhaes FA, Lima KC, de Uzeda M. 1998. Pathogenicity of facultative and obligate anaerobic bacteria in monoculture and combined with either Prevotella intermedia or Prevotella nigrescens. Oral Microbiol. Immunol. 13:368–372 [DOI] [PubMed] [Google Scholar]
- 105. Sundqvist GK, Eckerbom MI, Larsson AP, Sjogren UT. 1979. Capacity of anaerobic bacteria from necrotic dental pulps to induce purulent infections. Infect. Immun. 25:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hafstrom C, Dahlen G. 1997. Pathogenicity of Prevotella intermedia and Prevotella nigrescens isolates in a wound chamber model in rabbits. Oral Microbiol. Immunol. 12:148–154 [DOI] [PubMed] [Google Scholar]
- 107. Rôças IN, Siqueira JF., Jr 2009. Prevalence of new candidate pathogens Prevotella baroniae, Prevotella multisaccharivorax and as-yet-uncultivated Bacteroidetes clone X083 in primary endodontic infections. J. Endod. 35:1359–1362 [DOI] [PubMed] [Google Scholar]
- 108. van Steenbergen TJM, van Winkelhoff AJ, Mayrand D, Grenier D, de Graaff J. 1984. Bacteroides endodontalis sp. nov., an asaccharolytic black-pigmented Bacteroides species from infected dental root canals. Int. J. Syst. Bacteriol. 34:118–120 [Google Scholar]
- 109. Ferreira DC, Rôças IN, Paiva SS, Carmo FL, Cavalcante FS, Rosado AS, Santos KR, Siqueira JF., Jr 2011. Viral-bacterial associations in acute apical abscesses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 112:264–271 [DOI] [PubMed] [Google Scholar]
- 110. Siqueira JF, Jr, Jung IY, Rôças IN, Lee CY. 2005. Differences in prevalence of selected bacterial species in primary endodontic infections from two distinct geographic locations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 99:641–647 [DOI] [PubMed] [Google Scholar]
- 111. Bostanci N, Belibasakis GN. 2012. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333:1–9 [DOI] [PubMed] [Google Scholar]
- 112. Lamont RJ, Jenkinson HF. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Siqueira JF, Jr, Rôças IN, Silva MG. 2008. Prevalence and clonal analysis of Porphyromonas gingivalis in primary endodontic infections. J. Endod. 34:1332–1336 [DOI] [PubMed] [Google Scholar]
- 114. Rôças IN, Siqueira JF., Jr 2010. Distribution of Porphyromonas gingivalis fimA genotypes in primary endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109:474–478 [DOI] [PubMed] [Google Scholar]
- 115. Kuriyama T, Karasawa T, Nakagawa K, Kawashiri S, Nakanishi I, Nakamura S, Yamamoto E. 2000. Characterization of bacterial orofacial infections using a new murine model. Microb. Pathog. 29:115–120 [DOI] [PubMed] [Google Scholar]
- 116. Baumgartner JC, Falkler WA, Jr, Beckerman T. 1992. Experimentally induced infection by oral anaerobic microorganisms in a mouse model. Oral Microbiol. Immunol. 7:253–256 [DOI] [PubMed] [Google Scholar]
- 117. Lewis MA, MacFarlane TW, McGowan DA, MacDonald DG. 1988. Assessment of the pathogenicity of bacterial species isolated from acute dentoalveolar abscesses. J. Med. Microbiol. 27:109–116 [DOI] [PubMed] [Google Scholar]
- 118. Feuille F, Ebersole JL, Kesavalu L, Steffen MJ, Holt SC. 1996. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infect. Immun. 64:2095–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Miller WD. 1894. An introduction to the study of the bacterio-pathology of the dental pulp. Dent. Cosmos 36:505–528 [Google Scholar]
- 120. Dewhirst FE, Tamer MA, Ericson RE, Lau CN, Levanos VA, Boches SK, Galvin JL, Paster BJ. 2000. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol. Immunol. 15:196–202 [DOI] [PubMed] [Google Scholar]
- 121. Dahle UR, Titterud Sunde P, Tronstad L. 2003. Treponemes and endodontic infections. Endod. Topics 6:160–170 [Google Scholar]
- 122. Edwards AM, Dymock D, Jenkinson HF. 2003. From tooth to hoof: treponemes in tissue-destructive diseases. J. Appl. Microbiol. 94:767–780 [DOI] [PubMed] [Google Scholar]
- 123. Ellen RP, Galimanas VB. 2005. Spirochetes at the forefront of periodontal infections. Periodontol. 2000 38:13–32 [DOI] [PubMed] [Google Scholar]
- 124. Sakamoto M, Siqueira JF, Jr, Rôças IN, Benno Y. 2009. Diversity of spirochetes in endodontic infections. J. Clin. Microbiol. 47:1352–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Montagner F, Jacinto RC, Signoretti FG, Gomes BP. 2010. Treponema species detected in infected root canals and acute apical abscess exudates. J. Endod. 36:1796–1799 [DOI] [PubMed] [Google Scholar]
- 126. Kesavalu L, Holt SC, Ebersole JL. 1998. Virulence of a polymicrobic complex, Treponema denticola and Porphyromonas gingivalis, in a murine model. Oral Microbiol. Immunol. 13:373–377 [DOI] [PubMed] [Google Scholar]
- 127. Foschi F, Izard J, Sasaki H, Sambri V, Prati C, Müller R, Stashenko P. 2006. Treponema denticola in disseminating endodontic infections. J. Dent. Res. 85:761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kesavalu L, Walker SG, Holt SC, Crawley RR, Ebersole JL. 1997. Virulence characteristics of oral treponemes in a murine model. Infect. Immun. 65:5096–5102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Siqueira JF, Jr, Rôças IN. 2006. Catonella morbi and Granulicatella adiacens: new species in endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102:259–264 [DOI] [PubMed] [Google Scholar]
- 130. Rôças IN, Siqueira JF., Jr 2006. Culture-independent detection of Eikenella corrodens and Veillonella parvula in primary endodontic infections. J. Endod. 32:509–512 [DOI] [PubMed] [Google Scholar]
- 131. Araki H, Kuriyama T, Nakagawa K, Karasawa T. 2004. The microbial synergy of Peptostreptococcus micros and Prevotella intermedia in a murine abscess model. Oral Microbiol. Immunol. 19:177–181 [DOI] [PubMed] [Google Scholar]
- 132. van Dalen PJ, van Deutekom-Mulder EC, de Graaff J, van Steenbergen TJ. 1998. Pathogenicity of Peptostreptococcus micros morphotypes and Prevotella species in pure and mixed culture. J. Med. Microbiol. 47:135–140 [DOI] [PubMed] [Google Scholar]
- 133. Fisher LE, Russell RR. 1993. The isolation and characterization of milleri group streptococci from dental periapical abscesses. J. Dent. Res. 72:1191–1193 [DOI] [PubMed] [Google Scholar]
- 134. Kuriyama T, Nakagawa K, Kawashiri S, Yamamoto E, Nakamura S, Karasawa T. 2000. The virulence of mixed infection with Streptococcus constellatus and Fusobacterium nucleatum in a murine orofacial infection model. Microbes Infect. 2:1425–1430 [DOI] [PubMed] [Google Scholar]
- 135. Takahashi Y, Yoshida A, Nagata E, Hoshino T, Oho T, Awano S, Takehara T, Ansai T. 2011. Streptococcus anginosus l-cysteine desulfhydrase gene expression is associated with abscess formation in BALB/c mice. Mol. Oral Microbiol. 26:221–227 [DOI] [PubMed] [Google Scholar]
- 136. Han JK, Kerschner JE. 2001. Streptococcus milleri: an organism for head and neck infections and abscess. Arch. Otolaryngol. Head Neck Surg. 127:650–654 [DOI] [PubMed] [Google Scholar]
- 137. Corredoira J, Casariego E, Moreno C, Villanueva L, Lopez Varela J, Rodriguez A, Alonso P, Coira A. 1998. Prospective study of Streptococcus milleri hepatic abscess. Eur. J. Clin. Microbiol. Infect. Dis. 17:556–560 [DOI] [PubMed] [Google Scholar]
- 138. Montagner F, Jacinto RC, Signoretti FG, Sanches PF, Gomes BP. 2012. Clustering behavior in microbial communities from acute endodontic infections. J. Endod. 38:158–162 [DOI] [PubMed] [Google Scholar]
- 139. Siqueira JF, Jr, Rôças IN. 2003. Detection of Filifactor alocis in endodontic infections associated with different forms of periradicular diseases. Oral Microbiol. Immunol. 18:263–265 [DOI] [PubMed] [Google Scholar]
- 140. Xia T, Baumgartner JC. 2003. Occurrence of Actinomyces in infections of endodontic origin. J. Endod. 29:549–552 [DOI] [PubMed] [Google Scholar]
- 141. Vartoukian SR, Palmer RM, Wade WG. 2007. The division “Synergistes”. Anaerobe 13:99–106 [DOI] [PubMed] [Google Scholar]
- 142. Siqueira JF, Jr, Fouad AF, Rôças IN. 2012. Pyrosequencing as a tool for better understanding of human microbiomes. J. Oral Microbiol. doi:10.3402/jom.v3404i3400.10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Relman DA. 2012. The human microbiome: ecosystem resilience and health. Nutr. Rev. 70(Suppl. 1):S2–S9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Varshney RK, Nayak SN, May GD, Jackson SA. 2009. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 27:522–530 [DOI] [PubMed] [Google Scholar]
- 145. Shendure J, Ji H. 2008. Next-generation DNA sequencing. Nat. Biotechnol. 26:1135–1145 [DOI] [PubMed] [Google Scholar]
- 146. Engelbrektson A, Kunin V, Wrighton KC, Zvenigorodsky N, Chen F, Ochman H, Hugenholtz P. 2010. Experimental factors affecting PCR-based estimates of microbial species richness and evenness. ISME J. 4:642–647 [DOI] [PubMed] [Google Scholar]
- 147. Baumgartner JC, Watkins BJ, Bae KS, Xia T. 1999. Association of black-pigmented bacteria with endodontic infections. J. Endod. 25:413–415 [DOI] [PubMed] [Google Scholar]
- 148. Jung IY, Choi BK, Kum KY, Roh BD, Lee SJ, Lee CY, Park DS. 2000. Molecular epidemiology and association of putative pathogens in root canal infection. J. Endod. 26:599–604 [DOI] [PubMed] [Google Scholar]
- 149. Siqueira JF, Jr, Rôças IN, Souto R, de Uzeda M, Colombo AP. 2000. Checkerboard DNA-DNA hybridization analysis of endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 89:744–748 [DOI] [PubMed] [Google Scholar]
- 150. Fouad AF, Barry J, Caimano M, Clawson M, Zhu Q, Carver R, Hazlett K, Radolf JD. 2002. PCR-based identification of bacteria associated with endodontic infections. J. Clin. Microbiol. 40:3223–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rôças IN, Siqueira JF., Jr 2008. Root canal microbiota of teeth with chronic apical periodontitis. J. Clin. Microbiol. 46:3599–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yoneda M, Hirofuji T, Anan H, Matsumoto A, Hamachi T, Nakayama K, Maeda K. 2001. Mixed infection of Porphyromonas gingivalis and Bacteroides forsythus in a murine abscess model: involvement of gingipains in a synergistic effect. J. Periodontal Res. 36:237–243 [DOI] [PubMed] [Google Scholar]
- 153. Sundqvist G. 1992. Associations between microbial species in dental root canal infections. Oral Microbiol. Immunol. 7:257–262 [DOI] [PubMed] [Google Scholar]
- 154. Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. 2002. Molecular and cultural analysis of the microflora associated with endodontic infections. J. Dent. Res. 81:761–766 [DOI] [PubMed] [Google Scholar]
- 155. Baumgartner JC, Falkler WA., Jr 1991. Bacteria in the apical 5 mm of infected root canals. J. Endod. 17:380–383 [DOI] [PubMed] [Google Scholar]
- 156. Siqueira JF, Jr, Rôças IN. 2009. Community as the unit of pathogenicity: an emerging concept as to the microbial pathogenesis of apical periodontitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 107:870–878 [DOI] [PubMed] [Google Scholar]
- 157. Brook I. 1986. Encapsulated anaerobic bacteria in synergistic infections. Microbiol. Rev. 50:452–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Dahlen G. 2002. Microbiology and treatment of dental abscesses and periodontal-endodontic lesions. Periodontol. 2000 28:206–239 [DOI] [PubMed] [Google Scholar]
- 159. Saito D, Marsh TL, de Souza Cannavan F, Hofling JF, Goncalves RB. 2009. Assessment of intraradicular bacterial composition by terminal restriction fragment length polymorphism analysis. Oral Microbiol. Immunol. 24:369–376 [DOI] [PubMed] [Google Scholar]
- 160. Machado de Oliveira JC, Siqueira JF, Jr, Rôças IN, Baumgartner JC, Xia T, Peixoto RS, Rosado AS. 2007. Bacterial community profiles of endodontic abscesses from Brazilian and USA subjects as compared by denaturing gradient gel electrophoresis analysis. Oral Microbiol. Immunol. 22:14–18 [DOI] [PubMed] [Google Scholar]
- 161. Montagner F, Gomes BP, Kumar PS. 2010. Molecular fingerprinting reveals the presence of unique communities associated with paired samples of root canals and acute apical abscesses. J. Endod. 36:1475–1479 [DOI] [PubMed] [Google Scholar]
- 162. Herrera D, Contreras A, Gamonal J, Oteo A, Jaramillo A, Silva N, Sanz M, Botero JE, Leon R. 2008. Subgingival microbial profiles in chronic periodontitis patients from Chile, Colombia and Spain. J. Clin. Periodontol. 35:106–113 [DOI] [PubMed] [Google Scholar]
- 163. Haffajee AD, Bogren A, Hasturk H, Feres M, Lopez NJ, Socransky SS. 2004. Subgingival microbiota of chronic periodontitis subjects from different geographic locations. J. Clin. Periodontol. 31:996–1002 [DOI] [PubMed] [Google Scholar]
- 164. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 107:14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fouad AF. 2003. Diabetes mellitus as a modulating factor of endodontic infections. J. Dent. Educ. 67:459–467 [PubMed] [Google Scholar]
- 166. Fouad AF, Burleson J. 2003. The effect of diabetes mellitus on endodontic treatment outcome: data from an electronic patient record. J. Am. Dent. Assoc. 134:43–51 [DOI] [PubMed] [Google Scholar]
- 167. Armada-Dias L, Breda J, Provenzano JC, Breitenbach M, Rôças IN, Gahyva SM, Siqueira JF., Jr 2006. Development of periradicular lesions in normal and diabetic rats. J. Appl. Oral Sci. 14:371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Segura-Egea JJ, Jimenez-Pinzon A, Rios-Santos JV, Velasco-Ortega E, Cisneros-Cabello R, Poyato-Ferrera M. 2005. High prevalence of apical periodontitis amongst type 2 diabetic patients. Int. Endod. J. 38:564–569 [DOI] [PubMed] [Google Scholar]
- 169. Marotta PS, Fontes TV, Armada L, Lima KC, Rôças IN, Siqueira JF., Jr 2012. Type 2 diabetes mellitus and the prevalence of apical periodontitis and endodontic treatment in an adult Brazilian population. J. Endod. 38:297–300 [DOI] [PubMed] [Google Scholar]
- 170. de Sá AR, Moreira PR, Xavier GM, Sampaio I, Kalapothakis E, Dutra WO, Gomez RS. 2007. Association of CD14, IL1B, IL6, IL10 and TNFA functional gene polymorphisms with symptomatic dental abscesses. Int. Endod. J. 40:563–572 [DOI] [PubMed] [Google Scholar]
- 171. Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. 1998. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 102:1369–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Slots J, Sabeti M, Simon JH. 2003. Herpesviruses in periapical pathosis: an etiopathogenic relationship? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 96:327–331 [DOI] [PubMed] [Google Scholar]
- 173. Sabeti M, Simon JH, Slots J. 2003. Cytomegalovirus and Epstein-Barr virus are associated with symptomatic periapical pathosis. Oral Microbiol. Immunol. 18:327–328 [DOI] [PubMed] [Google Scholar]
- 174. Sabeti M, Valles Y, Nowzari H, Simon JH, Kermani-Arab V, Slots J. 2003. Cytomegalovirus and Epstein-Barr virus DNA transcription in endodontic symptomatic lesions. Oral Microbiol. Immunol. 18:104–108 [DOI] [PubMed] [Google Scholar]
- 175. Chen V, Chen Y, Li H, Kent K, Baumgartner JC, Machida CA. 2009. Herpesviruses in abscesses and cellulitis of endodontic origin. J. Endod. 35:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ferreira DC, Paiva SS, Carmo FL, Rôças IN, Rosado AS, Santos KR, Siqueira JF., Jr 2011. Identification of herpesviruses types 1 to 8 and human papillomavirus in acute apical abscesses. J. Endod. 37:10–16 [DOI] [PubMed] [Google Scholar]
- 177. Finlay BB, Falkow S. 1989. Common themes in microbial pathogenicity. Microbiol. Rev. 53:210–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Griffen AL, Lyons SR, Becker MR, Moeschberger ML, Leys EJ. 1999. Porphyromonas gingivalis strain variability and periodontitis. J. Clin. Microbiol. 37:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Baker PJ, Dixon M, Evans RT, Roopenian DC. 2000. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol. Immunol. 15:27–32 [DOI] [PubMed] [Google Scholar]
- 180. Özmeriç N, Preus HR, Olsen I. 2000. Genetic diversity of Porphyromonas gingivalis and its possible importance to pathogenicity. Acta Odontol. Scand. 58:183–187 [DOI] [PubMed] [Google Scholar]
- 181. Musser JM. 1996. Molecular population genetic analysis of emerged bacterial pathogens: selected insights. Emerg. Infect. Dis. 2:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sklavounos A, Legakis NJ, Ioannidou H, Patrikiou A. 1986. Anaerobic bacteria in dentoalveolar abscesses. Int. J. Oral Maxillofac. Surg. 15:288–291 [DOI] [PubMed] [Google Scholar]
- 183. Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG. 2008. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 4:e1000184 doi:10.1371/journal.ppat.1000184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Duan K, Sibley CD, Davidson CJ, Surette MG. 2009. Chemical interactions between organisms in microbial communities. Contrib. Microbiol. 16:1–17 [DOI] [PubMed] [Google Scholar]
- 185. Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50:1477–1491 [DOI] [PubMed] [Google Scholar]
- 186. Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. Rev. 25:193–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Rôças IN, Siqueira JF, Jr, Debelian GJ. 2011. Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J. Endod. 37:1206–1212 [DOI] [PubMed] [Google Scholar]
- 188. Sassone LM, Fidel RA, Faveri M, Guerra R, Figueiredo L, Fidel SR, Feres M. 2008. A microbiological profile of symptomatic teeth with primary endodontic infections. J. Endod. 34:541–545 [DOI] [PubMed] [Google Scholar]
- 189. Finlay BB, Falkow S. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bassler BL. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582–587 [DOI] [PubMed] [Google Scholar]
- 191. Withers H, Swift S, Williams P. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186–193 [DOI] [PubMed] [Google Scholar]
- 192. de Kievit TR, Iglewski BH. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Forng R-Y, Champagne C, Simpson W, Genco CA. 2000. Environmental cues and gene expression in Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Dis. 6:351–365 [DOI] [PubMed] [Google Scholar]
- 194. Kesavalu L, Holt SC, Ebersole JL. 2003. In vitro environmental regulation of Porphyromonas gingivalis growth and virulence. Oral Microbiol. Immunol. 18:226–233 [DOI] [PubMed] [Google Scholar]
- 195. Kesavalu L, Holt SC, Ebersole JL. 1999. Environmental modulation of oral treponeme virulence in a murine model. Infect. Immun. 67:2783–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Frias J, Olle E, Alsina M. 2001. Periodontal pathogens produce quorum sensing signal molecules. Infect. Immun. 69:3431–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zhang Y, Wang T, Chen W, Yilmaz O, Park Y, Jung IY, Hackett M, Lamont RJ. 2005. Differential protein expression by Porphyromonas gingivalis in response to secreted epithelial cell components. Proteomics 5:198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yuan L, Hillman JD, Progulske-Fox A. 2005. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect. Immun. 73:4146–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lewis MA, Carmichael F, MacFarlane TW, Milligan SG. 1993. A randomised trial of co-amoxiclav (Augmentin) versus penicillin V in the treatment of acute dentoalveolar abscess. Br. Dent. J. 175:169–174 [DOI] [PubMed] [Google Scholar]
- 200. Kuriyama T, Absi EG, Williams DW, Lewis MA. 2005. An outcome audit of the treatment of acute dentoalveolar infection: impact of penicillin resistance. Br. Dent. J. 25:759–763 [DOI] [PubMed] [Google Scholar]
- 201. Henry M, Reader A, Beck M. 2001. Effect of penicillin on postoperative endodontic pain and swelling in symptomatic necrotic teeth. J. Endod. 27:117–123 [DOI] [PubMed] [Google Scholar]
- 202. Nagle D, Reader A, Beck M, Weaver J. 2000. Effect of systemic penicillin on pain in untreated irreversible pulpitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90:636–640 [DOI] [PubMed] [Google Scholar]
- 203. Pickenpaugh L, Reader A, Beck M, Meyers WJ, Peterson LJ. 2001. Effect of prophylactic amoxicillin on endodontic flare-up in asymptomatic, necrotic teeth. J. Endod. 27:53–56 [DOI] [PubMed] [Google Scholar]
- 204. Walton RE, Chiappinelli J. 1993. Prophylactic penicillin: effect on posttreatment symptoms following root canal treatment of asymptomatic periapical pathosis. J. Endod. 19:466–470 [DOI] [PubMed] [Google Scholar]
- 205. Fouad AF, Rivera EM, Walton RE. 1996. Penicillin as a supplement in resolving the localized acute apical abscess. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 81:590–595 [DOI] [PubMed] [Google Scholar]
- 206. Baumgartner JC, Xia T. 2003. Antibiotic susceptibility of bacteria associated with endodontic abscesses. J. Endod. 29:44–47 [DOI] [PubMed] [Google Scholar]
- 207. Jacinto RC, Gomes BP, Ferraz CC, Zaia AA, Filho FJ. 2003. Microbiological analysis of infected root canals from symptomatic and asymptomatic teeth with periapical periodontitis and the antimicrobial susceptibility of some isolated anaerobic bacteria. Oral Microbiol. Immunol. 18:285–292 [DOI] [PubMed] [Google Scholar]
- 208. Kuriyama T, Williams DW, Yanagisawa M, Iwahara K, Shimizu C, Nakagawa K, Yamamoto E, Karasawa T. 2007. Antimicrobial susceptibility of 800 anaerobic isolates from patients with dentoalveolar infection to 13 oral antibiotics. Oral Microbiol. Immunol. 22:285–288 [DOI] [PubMed] [Google Scholar]
- 209. Flynn TR. 2011. What are the antibiotics of choice for odontogenic infections, and how long should the treatment course last? Oral Maxillofac. Surg. Clin. North Am. 23:519–536, v-vi [DOI] [PubMed] [Google Scholar]
- 210. Blandino G, Milazzo I, Fazio D, Puglisi S, Pisano M, Speciale A, Pappalardo S. 2007. Antimicrobial susceptibility and beta-lactamase production of anaerobic and aerobic bacteria isolated from pus specimens from orofacial infections. J. Chemother. 19:495–499 [DOI] [PubMed] [Google Scholar]
- 211. Paterson SA, Curzon ME. 1993. The effect of amoxycillin versus penicillin V in the treatment of acutely abscessed primary teeth. Br. Dent. J. 174:443–449 [DOI] [PubMed] [Google Scholar]
- 212. Lakhssassi N, Elhajoui N, Lodter JP, Pineill JL, Sixou M. 2005. Antimicrobial susceptibility variation of 50 anaerobic periopathogens in aggressive periodontitis: an interindividual variability study. Oral Microbiol. Immunol. 20:244–252 [DOI] [PubMed] [Google Scholar]
- 213. Gilmore WC, Jacobus NV, Gorbach SL, Doku HC, Tally FP. 1988. A prospective double-blind evaluation of penicillin versus clindamycin in the treatment of odontogenic infections. J. Oral Maxillofac. Surg. 46:1065–1070 [DOI] [PubMed] [Google Scholar]
- 214. von Konow L, Kondell PA, Nord CE, Heimdahl A. 1992. Clindamycin versus phenoxymethylpenicillin in the treatment of acute orofacial infections. Eur. J. Clin. Microbiol. Infect. Dis. 11:1129–1135 [DOI] [PubMed] [Google Scholar]
- 215. Sobottka I, Cachovan G, Sturenburg E, Ahlers MO, Laufs R, Platzer U, Mack D. 2002. In vitro activity of moxifloxacin against bacteria isolated from odontogenic abscesses. Antimicrob. Agents Chemother. 46:4019–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Cachovan G, Boger RH, Giersdorf I, Hallier O, Streichert T, Haddad M, Platzer U, Schon G, Wegscheider K, Sobottka I. 2011. Comparative efficacy and safety of moxifloxacin and clindamycin in the treatment of odontogenic abscesses and inflammatory infiltrates: a phase II, double-blind, randomized trial. Antimicrob. Agents Chemother. 55:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Handal T, Olsen I, Walker CB, Caugant DA. 2004. Beta-lactamase production and antimicrobial susceptibility of subgingival bacteria from refractory periodontitis. Oral Microbiol. Immunol. 19:303–308 [DOI] [PubMed] [Google Scholar]
- 218. Fosse T, Madinier I, Hitzig C, Charbit Y. 1999. Prevalence of beta-lactamase-producing strains among 149 anaerobic gram-negative rods isolated from periodontal pockets. Oral Microbiol. Immunol. 14:352–357 [DOI] [PubMed] [Google Scholar]
- 219. van Winkelhoff AJ, Winkel EG, Barendregt D, Dellemijn-Kippuw N, Stijne A, van der Velden U. 1997. Beta-lactamase producing bacteria in adult periodontitis. J. Clin. Periodontol. 24:538–543 [DOI] [PubMed] [Google Scholar]
- 220. Bernal LA, Guillot E, Paquet C, Mouton C. 1998. Beta-lactamase-producing strains in the species Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immunol. 13:36–40 [DOI] [PubMed] [Google Scholar]
- 221. Kuriyama T, Karasawa T, Nakagawa K, Yamamoto E, Nakamura S. 2001. Incidence of beta-lactamase production and antimicrobial susceptibility of anaerobic gram-negative rods isolated from pus specimens of orofacial odontogenic infections. Oral Microbiol. Immunol. 16:10–15 [DOI] [PubMed] [Google Scholar]
- 222. Herrera D, van Winkelhoff AJ, Dellemijn-Kippuw N, Winkel EG, Sanz M. 2000. Beta-lactamase producing bacteria in the subgingival microflora of adult patients with periodontitis. A comparison between Spain and The Netherlands. J. Clin. Periodontol. 27:520–525 [DOI] [PubMed] [Google Scholar]
- 223. Veloo AC, Seme K, Raangs E, Rurenga P, Singadji Z, Wekema-Mulder G, van Winkelhoff AJ. 2012. Antibiotic susceptibility profiles of oral pathogens. Int. J. Antimicrob. Agents 40:450–454 [DOI] [PubMed] [Google Scholar]
- 224. Brook I. 2004. Beta-lactamase-producing bacteria in mixed infections. Clin. Microbiol. Infect. 10:777–784 [DOI] [PubMed] [Google Scholar]
- 225. Kornman KS, Duff G. W. 2012. Personalized medicine: will dentistry ride the wave or watch from the beach? J. Dent. Res. 91:8S–11S [DOI] [PubMed] [Google Scholar]
- 226. Chan IS, Ginsburg GS. 2011. Personalized medicine: progress and promise. Annu. Rev. Genomics Hum. Genet. 12:217–244 [DOI] [PubMed] [Google Scholar]
- 227. Dunne WM, Jr, Westblade LF, Ford B. 2012. Next-generation and whole-genome sequencing in the diagnostic clinical microbiology laboratory. Eur. J. Clin. Microbiol. Infect. Dis. 31:1719–1726 [DOI] [PubMed] [Google Scholar]
- 228. Rôças IN, Siqueira JF., Jr 2012. Antibiotic resistance genes in anaerobic bacteria isolated from primary dental root canal infections. Anaerobe 18:576–580 [DOI] [PubMed] [Google Scholar]
- 229. Rolain JM, Mallet MN, Fournier PE, Raoult D. 2004. Real-time PCR for universal antibiotic susceptibility testing. J. Antimicrob. Chemother. 54:538–541 [DOI] [PubMed] [Google Scholar]
- 230. Siqueira JF, Jr, Rôças IN. 2010. Microbiology and treatment of endodontic infections, p 559–600 InHargreaves KM, Cohen S. (ed), Cohen's pathways of the pulp, 10th ed Mosby/Elsevier, St. Louis, MO [Google Scholar]
- 231. Griffee MB, Patterson SS, Miller CH, Kafrawy AH, Newton CW. 1980. The relationship of Bacteroides melaninogenicus to symptoms associated with pulpal necrosis. Oral Surg. Oral Med. Oral Pathol. 50:457–461 [DOI] [PubMed] [Google Scholar]
- 232. Yoshida M, Fukushima H, Yamamoto K, Ogawa K, Toda T, Sagawa H. 1987. Correlation between clinical symptoms and microorganisms isolated from root canals of teeth with periapical pathosis. J. Endod. 13:24–28 [DOI] [PubMed] [Google Scholar]
- 233. Hashioka K, Yamasaki M, Nakane A, Horiba N, Nakamura H. 1992. The relationship between clinical symptoms and anaerobic bacteria from infected root canals. J. Endod. 18:558–561 [DOI] [PubMed] [Google Scholar]
- 234. Gomes BP, Drucker DB, Lilley JD. 1994. Associations of specific bacteria with some endodontic signs and symptoms. Int. Endod. J. 27:291–298 [DOI] [PubMed] [Google Scholar]
- 235. Gomes BP, Lilley JD, Drucker DB. 1996. Clinical significance of dental root canal microflora. J. Dent. 24:47–55 [DOI] [PubMed] [Google Scholar]
- 236. Siqueira JF, Jr, Rôças IN, Souto R, de Uzeda M, Colombo AP. 2002. Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J. Endod. 28:168–172 [DOI] [PubMed] [Google Scholar]
- 237. Gomes BP, Pinheiro ET, Gade-Neto CR, Sousa EL, Ferraz CC, Zaia AA, Teixeira FB, Souza-Filho FJ. 2004. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 19:71–76 [DOI] [PubMed] [Google Scholar]
- 238. Foschi F, Cavrini F, Montebugnoli L, Stashenko P, Sambri V, Prati C. 2005. Detection of bacteria in endodontic samples by polymerase chain reaction assays and association with defined clinical signs in Italian patients. Oral Microbiol. Immunol. 20:289–295 [DOI] [PubMed] [Google Scholar]