Abstract
SUMMARY
Plasmodium knowlesi is a malaria parasite that is found in nature in long-tailed and pig-tailed macaques. Naturally acquired human infections were thought to be extremely rare until a large focus of human infections was reported in 2004 in Sarawak, Malaysian Borneo. Human infections have since been described throughout Southeast Asia, and P. knowlesi is now recognized as the fifth species of Plasmodium causing malaria in humans. The molecular, entomological, and epidemiological data indicate that human infections with P. knowlesi are not newly emergent and that knowlesi malaria is primarily a zoonosis. Human infections were undiagnosed until molecular detection methods that could distinguish P. knowlesi from the morphologically similar human malaria parasite P. malariae became available. P. knowlesi infections cause a spectrum of disease and are potentially fatal, but if detected early enough, infections in humans are readily treatable. In this review on knowlesi malaria, we describe the early studies on P. knowlesi and focus on the epidemiology, diagnosis, clinical aspects, and treatment of knowlesi malaria. We also discuss the gaps in our knowledge and the challenges that lie ahead in studying the epidemiology and pathogenesis of knowlesi malaria and in the prevention and control of this zoonotic infection.
INTRODUCTION
Malaria is caused by protozoan parasites belonging to the genus Plasmodium. Over 150 species have been described to date, infecting mammals, birds, and reptiles (1). Despite having such a large number of hosts, in general, malaria parasites tend to be host specific. For example, humans are the natural hosts for 4 species, P. falciparum, P. vivax, P. malariae, and P. ovale, while long-tailed macaques (Macaca fascicularis) are hosts for 5, P. knowlesi, P. fieldi, P. coatneyi, P. cynomolgi, and P. inui (2). Zoonotic malaria was considered to be extremely rare until a large focus of P. knowlesi infections in the Kapit Division of Sarawak, Malaysian Borneo, was described in 2004 (3). Since then, human cases have been described in virtually all Southeast Asian countries, and P. knowlesi is now considered the fifth species of Plasmodium causing malaria in humans (4, 5). In this review on knowlesi malaria, we describe the early studies on P. knowlesi and focus on the epidemiology, diagnosis, clinical features, and treatment of knowlesi malaria. We also discuss the gaps in our knowledge and the challenges that lie ahead in studying the epidemiology and pathogenesis of knowlesi malaria and in the prevention and control of this zoonotic infection.
Life Cycle of Plasmodium in Humans
The life cycle of malaria parasites in humans and other primates begins when a female anopheline mosquito injects sporozoites into the host while taking a blood meal (1). These parasites are taken by the bloodstream to the liver, where they invade hepatocytes, undergo asexual multiplication, and develop into schizonts. The hepatic schizonts that rupture release thousands of merozoites that invade erythrocytes (RBCs) to continue their development. Within the erythrocyte, the merozoite develops into a ring or early trophozoite form, which in turn develops into a mature trophozoite that undergoes asexual multiplication to form a schizont containing numerous merozoites. The erythrocytic schizont ruptures, releasing merozoites that invade erythrocytes, thereby completing the erythrocytic cycle. Some of the merozoites also develop within the erythrocytes into male and female gametocytes, which are taken up during a blood meal by female anopheline mosquitoes, in which they continue their development.
There are no clinical signs and symptoms when the malaria parasites are developing in the liver. These are associated with the cycle of the parasites in the erythrocytes. The duration of the erythrocytic cycle depends on the species of Plasmodium: P. knowlesi has the shortest cycle, approximately 24 h, while for P. falciparum, P. vivax, and P. ovale, it is approximately 48 h, and for P. malariae, it is 72 h (1). Therefore, if untreated, the parasite counts or parasitemia will continue to increase approximately every 24, 48, or 72 h, depending on the species of Plasmodium. In synchronous infections of single clones, particularly for P. knowlesi, P. vivax, P. ovale, and P. malariae, there is a fever peak which occurs following the release of merozoites by rupturing schizonts, resulting in quotidian, tertian, or quartan fever patterns. However, the fever patterns may be daily and may not be at these regular intervals for all species of Plasmodium early in infection and also in cases where mixed species or more than one brood of parasites is present (6, 7).
Discovery and Early Studies
P. knowlesi was first isolated and studied in detail at the Kolkata School of Tropical Medicine in India in the early 1930s, after it was noticed by Campbell in a blood film from a long-tailed macaque that had been imported from Singapore (8–10). Campbell and Napier, who were working on leishmaniasis, inoculated P. knowlesi-infected blood into two long-tailed macaques and a rhesus macaque (Macaca mulatta) and reported that it produced a fulminating infection in the rhesus macaque and a mild infection in the long-tailed macaques. Knowing that malaria was the research focus of Knowles and Das Gupta, they handed the macaque to them for a series of experiments that confirmed that blood passage of P. knowlesi among its natural hosts, Macaca fascicularis, resulted in low parasitemia (8). In contrast, blood passage into rhesus macaques, which are indigenous to India and are not the natural hosts of P. knowlesi, resulted in extremely high parasitemia and fatal infections, unless the macaques were treated with antimalarials. Knowlesi and Das Gupta then successfully infected three human volunteers with knowlesi malaria following macaque blood passage in two volunteers and human blood passage in the third volunteer (8). Those researchers observed that all three human recipients of P. knowlesi-infected blood developed malaria and commented that although the fever pattern was daily, further work was required to confirm the periodicity of the fever. They also observed that the morphology of the parasites resembled that of P. malariae. The quotidian nature of the fever pattern, indicating that P. knowlesi had a 24-h erythrocytic cycle, was confirmed by Sinton and Mulligan (11, 12). They studied the morphology of the parasite in detail in nonhuman primate hosts and named this new malaria parasite in honor of Robert Knowles.
From the 1920s until the mid-1950s, prior to the discovery of penicillin, patients with tertiary neurosyphilis were successfully treated by inducing fever with P. vivax, a treatment that was pioneered by Wagner-Juaregg (13) and often referred to as malariotherapy (14). Since P. knowlesi had a shorter erythrocytic cycle than P. vivax, it was used for malariotherapy by physicians in a number of countries (15–18), particularly by Ciuca and coworkers in Romania (19–21). Those researchers stopped using the P. knowlesi strain when they noticed that it had become more pathogenic following 170 serial passages in patients with neurosyphilis (21).
It was known that humans could acquire knowlesi malaria by blood passage soon after P. knowlesi was isolated in 1931. However, the first case of a natural infection in a human was only reported 34 years later, when a U.S. Army surveyor acquired the infection while working in the forest in the state of Pahang in Peninsular Malaysia (22). This finding was the result of an extraordinary sequence of events. The surveyor had returned to the United States, where his doctor observed malaria parasites that resembled the ring forms of P. falciparum. His doctor sent him to the National Institutes of Health Clinical Center in Bethesda, MD, where they saw “band forms” of malaria parasites resembling P. malariae. They obtained a blood sample before treating him and sent the sample to Atlanta, GA, where chemotherapy trials on P. malariae were being conducted on volunteers at the U.S. Penitentiary in Atlanta. Inoculation of blood into the first volunteer and six other human volunteers produced quotidian or daily fever patterns, and subsequent inoculation of infected human blood into three rhesus macaques produced fatal infections, thereby confirming that the surveyor was naturally infected with P. knowlesi (23). This single case was followed by a presumptive human case of knowlesi malaria that was acquired in Johore, Peninsular Malaysia, in 1971, where detection was based on parasite morphology and serological methods, since no pretreatment blood was available for inoculation into rhesus macaques (24).
The accidental infection of humans by mosquito bites in the early 1960s in two different laboratories in the United States with P. cynomolgi (25, 26), another malaria parasite of long-tailed macaques (1), resulted in the initiation of studies to investigate whether malaria was a zoonosis, since zoonotic malaria would have hampered the Malaria Eradication Program launched by the WHO. These investigations by a team from the U.S. National Institutes of Health working in close collaboration with colleagues at the Institute for Medical Research in Kuala Lumpur, Peninsular Malaysia, were intensified with the report of the naturally acquired human infection with P. knowlesi (22). A total of 1,117 blood samples were collected from villagers living in the vicinity where the American army surveyor had been working in the forest in Pahang State, Peninsular Malaysia (27). These samples, comprising only 28 that were malaria positive by microscopy, were pooled and injected into rhesus macaques, but none of the macaques acquired malaria. However, P. knowlesi was identified in 2 of 4 long-tailed macaques from the study area. The researchers therefore concluded that natural infections of humans with P. knowlesi and other simian malaria parasites within the study population were extremely rare and that there probably existed two cycles of transmission of P. knowlesi: one among the macaques at the canopy level and another, less common, one from macaques to humans. Subsequently, malariologists downplayed the significance of the zoonotic potential of malaria.
The generally held view that zoonotic malaria was an extremely rare event changed following the discovery of a large focus of human infections with P. knowlesi in the Kapit Division of Sarawak, Malaysian Borneo (3). Singh and colleagues were prompted to study microscopy-confirmed cases of P. malariae since there appeared to be foci of these cases in Sarawak. One of these foci was in the Kapit Division, where microscopy-confirmed P. malariae cases in 1999 accounted for 40% of the 270 microscopy-confirmed cases for the state of Sarawak. Furthermore, in contrast to P. malariae infections, which normally result in asymptomatic infections with low parasitemia (<5,000 parasites per μl blood) and affects people of all age groups (1, 6), most of the cases in Kapit were reported in adults seeking treatment at hospitals, and parasitemia was higher than that expected for P. malariae malaria. Initial investigation of 4 such “P. malariae” samples from Kapit Hospital by nested PCR assays indicated that Plasmodium DNA was present but that the patients were not infected with P. malariae or with the other 3 species of Plasmodium tested. DNA sequencing of two nuclear genes, the small-subunit (SSU) rRNA genes and the circumsporozoite gene, followed by phylogenetic analyses indicated that 8 “P. malariae” patients were infected with P. knowlesi (3). PCR primers that were specific for P. knowlesi were developed, and a prospective study was undertaken with 208 blood samples collected from patients admitted to Kapit Hospital with malaria, 141 of whom had been diagnosed with P. malariae by microscopy (3). PCR assays revealed that knowlesi malaria accounted for 58% of 208 admissions for malaria at Kapit Hospital: 112 were single P. knowlesi infections, 8 were P. knowlesi coinfections with other Plasmodium species, and none were P. malariae.
EPIDEMIOLOGY
Distribution
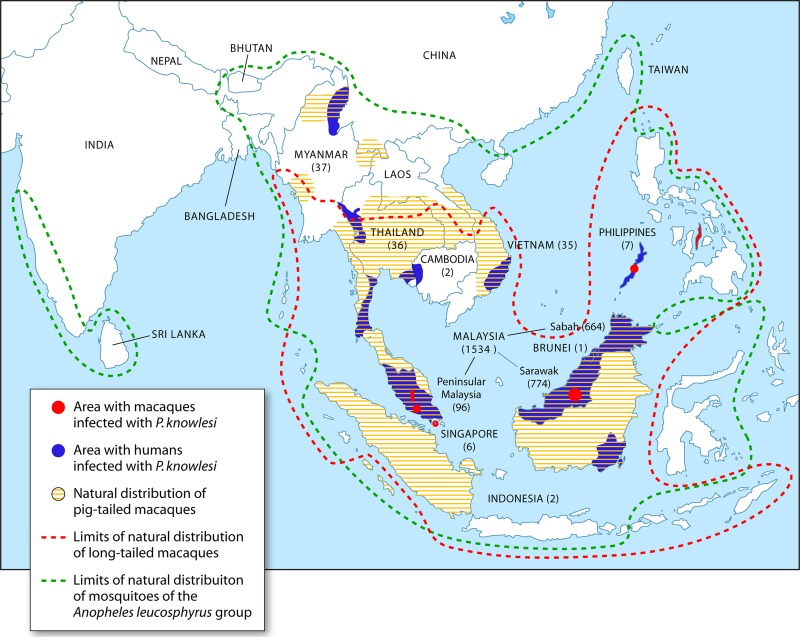
Following the description of the large focus of human knowlesi malaria cases in the Kapit Division of Malaysian Borneo in 2004 (3), there have been reports of infections acquired in Kapit and other locations in Malaysian Borneo (28–43) and in Peninsular Malaysia (28, 44–47). In some hospitals in Malaysian Borneo, knowlesi malaria accounts for the majority of malaria cases admitted to hospitals (29, 38, 43). Human cases are not restricted to Malaysia, with reports of infections acquired in Thailand (48–53), the Philippines (54–56), Myanmar (57, 58), Singapore (59–61), Vietnam (62, 63), Indonesia (64, 65), Brunei (66), and Cambodia (67) (Fig. 1). Transmission of knowlesi malaria to humans has therefore been reported in all the countries in Southeast Asia except Laos. Most of the human knowlesi malaria cases have been detected in Sarawak and Sabah, Malaysian Borneo, mainly because extensive studies utilizing molecular detection methods have been undertaken in these two states. With regard to Sarawak, since molecular methods for malaria detection were employed at the Malaria Research Centre, Universiti Malaysia Sarawak, over 881 local P. knowlesi cases and only 6 P. malariae cases have been identified from 2000 to 2011 (3, 5, 37, 38). All 6 were logging camp workers who had acquired their infections overseas, thereby indicating that there are no indigenous cases of P. malariae in Sarawak. The situation appears to be similar in Sabah, where the number of P. knowlesi infections detected by PCR at the Public Health Laboratory and at Universiti Malaysia Sabah greatly outnumbers those of P. malariae (30, 40, 41). The reports to date indicate that adults are more commonly infected than children. The actual incidence of knowlesi malaria in each of the countries in Southeast Asia, including Malaysia, is unknown, mainly because only a limited number of studies utilizing molecular detection methods have been undertaken, and it is not possible to accurately identify P. knowlesi by microscopy due to its morphological similarities with P. malariae and P. falciparum (68). Additional extensive studies utilizing molecular detection methods would probably uncover more human knowlesi malaria cases in Southeast Asia.
Fig 1.
Plasmodium knowlesi infections reported in humans and macaques and limits of natural distribution of mosquito vectors and of macaques. The numbers in parentheses represent numbers of P. knowlesi cases reported for each Southeast Asian country or region in Malaysia. (Adapted from reference 5 with permission from Elsevier.)
Reservoir Hosts
The natural hosts of P. knowlesi that were initially identified were long-tailed (M. fascicularis) and pig-tailed (Macaca nemestrina) macaques from Singapore (8) and Peninsular Malaysia (69, 70), and there has also been a report of a single P. knowlesi isolation from a leaf monkey (Presbytis melalophos) from Peninsular Malaysia (71). Subsequently, P. knowlesi infections were detected in macaques from Cebu (72) and Palawan Island (73), Philippines. More recent reports, using molecular detection methods and sequencing, have identified P. knowlesi infections in wild long-tailed macaques in Sarawak, Malaysian Borneo (74); Peninsular Malaysia (44); Singapore (61); and Southern Thailand (49) and in wild pig-tailed macaques in Southern Thailand (49) and Sarawak (74). These two macaque species are distributed throughout Southeast Asia (75, 76) (Fig. 1) and are the most common nonhuman primates in this region. The observation that peridomestic long-tailed macaques in Singapore (61) and macaques living close to a temple in Thailand did not harbor P. knowlesi or other simian malaria parasites is a reflection of the absence of a competent mosquito vector for parasite transmission. The highest prevalence of P. knowlesi has been observed in wild macaques of the Kapit Division in Sarawak, Malaysian Borneo, where 87% of 83 long tailed-macaques and 50% of 26 pig-tailed macaques were P. knowlesi positive. The very high prevalence of P. knowlesi and other malaria parasites in these macaques (94% were malaria positive) from 17 locations indicates that malaria transmission is intense among the wild-macaque population in the Kapit Division of Malaysian Borneo.
Vectors
The vectors of knowlesi malaria are forest-dwelling mosquitoes that belong to the Anopheles leucosphyrus group, and their distribution in Southeast Asia largely overlaps that of long-tailed and pig-tailed macaques (77, 78) (Fig. 1). The first vector found in nature to be infected with P. knowlesi was A. hackeri, a predominantly zoophilic mosquito, in Selangor State, Peninsular Malaysia, in 1961 (79). Experimental studies in the United States undertaken in the 1960s with the H strain of P. knowlesi (isolated from the American surveyor) showed that among the anophelines tested for the presence of sporozoites in the salivary glands and their capability of transmitting P. knowlesi to rhesus macaques, A. balabacensis was the most competent vector, followed by A. stephensi, A. maculatus, and A. freeborni (80, 81). Human-to-human, monkey-to-human, and human-to-monkey transmission of P. knowlesi was demonstrated by using A. balabacensis, with incubation periods in the vector of 12 to 13 days. In Kapit, Malaysian Borneo, where most of the human knowlesi malaria cases have been described, A. latens has been incriminated as the vector (82, 83). This mosquito species feeds mainly between 7 and 10 p.m. in the forest, is attracted to both long-tailed macaques and humans, and prefers to feed on macaques at higher elevations. A. cracens has recently been incriminated as a vector for knowlesi malaria in Pahang, Peninsular Malaysia, with peak biting times between 8 and 9 p.m. (44, 84). This species is highly zoophilic and was previously found to feed on macaques at the canopy level and on humans at the ground level. None of these vectors of P. knowlesi were found to be infected with the human malaria parasites P. falciparum, P. vivax, P. malariae, and P. ovale during recent entomological surveys in Sarawak (82, 83) and Peninsular Malaysia (44). Interestingly, at 4 collection sites in the forest and forest fringe areas near a village in south central Vietnam, A. dirus mosquitoes were found to harbor sporozoites of P. knowlesi alone and mixed with P. vivax or P. falciparum and also mixed with both P. falciparum and P. vivax (62, 85). Since A. dirus is the only known vector for human malaria in this area (62), this raises the possibility that human-to-human transmission of P. knowlesi could occur and may actually be occurring in this region in Vietnam.
Populations at Risk
For any vector-borne disease, transmission is highly dependent on the bionomics and distribution of the vectors. Since the vectors of P. knowlesi are restricted to members of the A. leucosphyrus group, which are found in the forest and forest fringe, populations that are particularly at risk of acquiring knowlesi malaria are people who live there or those that venture into the ecological habitat of macaques and the anopheline vectors of P. knowlesi for either work or leisure. For example, in Sarawak, Malaysian Borneo, where most cases of knowlesi malaria have been reported, the majority of knowlesi malaria patients are adults who are subsistence farmers, hunters, and logging camp workers (3, 5, 28). Those acquiring their infections in Vietnam appear to be very similar to those in Sarawak in that they are people in the forest fringe who enter the forests to collect bamboo and rattan and work on their farms on mountain slopes (62), and there have also been cases of children living in forest communities with knowlesi malaria (63). In Singapore, servicemen acquired knowlesi malaria while training in a forested area (61). Visitors to Southeast Asia have not been spared, with reports of adult travelers from Sweden (31), Finland (47), France (52), Spain (53), the Netherlands (42), Taiwan (56), the United States (55), and the United Kingdom (66) returning home with P. knowlesi infections following visits to Sarawak (31, 42), the Philippines (55, 56), Peninsular Malaysia (47), Brunei (66), and Thailand (52). Furthermore, a helicopter pilot returned to New Zealand with knowlesi malaria following a working stint in the interior of Sarawak, Malaysian Borneo (36), and an Australian acquired it while working in South Kalimantan Province, Indonesian Borneo (64).
Molecular Epidemiology and Evolutionary and Demographic History
In order to expand our understanding of the epidemiology of knowlesi malaria, P. knowlesi isolates derived from humans and macaques have been characterized and compared. The studies from the Kapit Division of Sarawak revealed that some of the P. knowlesi isolates derived from wild macaques shared identical mitochondrial DNA (mtDNA) and circumsporozoite protein gene (csp) sequences with those derived from humans (74). Furthermore, wild macaques had higher numbers of haplotypes of mtDNA and alleles of the csp gene per infection than humans, probably reflecting the high level of malaria transmission among wild macaques in Kapit. Limited molecular studies in Peninsular Malaysia (44) and Singapore (61) also demonstrated shared alleles of csp among humans and macaques, while in Thailand, alleles of the merozoite surface protein 1 gene (msp-1) were found to be similarly diverse in both hosts (49). In the Sarawak studies, the mtDNA haplotypes and the mtDNA lineage were not associated exclusively with either vertebrate host, and the cumulative molecular evidence together with the epidemiological and entomological data support the view that knowlesi malaria is primarily a zoonosis and that wild macaques are the reservoir hosts.
By comparing DNA sequence data of nuclear genes of P. knowlesi derived from both macaque and human hosts, it is not possible to ascertain whether knowlesi malaria is a newly emergent zoonosis. However, through the analysis of P. knowlesi mtDNA sequences, it is possible to extend our understanding of the evolutionary and demographic history of P. knowlesi. Through such analyses, the estimated time to the most recent common ancestor (TMRCA) of P. knowlesi was 98,000 to 478,000 years ago (74), which indicates that P. knowlesi is derived from an ancestral parasite population. It is as old as or older than the human malaria parasites P. falciparum and P. vivax, for which the TMRCAs have been estimated to be 50,000 to 330,000 years ago (86, 87) and 53,000 to 265,000 years ago (88, 89), respectively. The emergence of P. knowlesi from an ancestral parasite predates the arrival and settlement of humans in Southeast Asia approximately 70,000 years ago (90) but not that of the genus Macaca. Macaques migrated to Asia from Africa approximately 5.5 million years ago, and the M. fascicularis group emerged about 3.7 million to 4.0 million years ago (91–93). It is highly likely that P. knowlesi migrated with the natural macaque hosts to Borneo during the Pleistocene era, when sea levels were lower than they are now and the island of Borneo was connected to mainland Southeast Asia (94). Analysis of P. knowlesi mtDNA sequence data also showed that P. knowlesi parasites in Sarawak underwent rapid population growth between 30,000 and 40,000 years ago, concordant with a time of human population growth in Southeast Asia (95). Similar analyses of mtDNA of M. fascicularis and M. nemestrina have not been undertaken, so it is not possible to ascertain whether the population expansion of P. knowlesi was due to that of the human or the macaque hosts or even that of the mosquito vectors. Nevertheless, molecular evidence indicates that P. knowlesi is an ancient parasite and strongly suggests that it is not newly emergent in the human population. Precisely when humans first became infected with P. knowlesi is not known. In Sarawak, the first malaria survey using microscopy was undertaken in 1952, where out of 421 malaria cases detected during community surveys in 6 areas, 142 (33.7%) were P. malariae cases (96). In two areas, P. malariae accounted for 68.8% and 76.3% of malaria cases detected. Analysis by PCR of DNA extracted from archival “P. malariae” slides from 1996 in Sarawak (37) indicates that these were P. knowlesi infections, and recent studies showed that parasites identified by microscopy as P. malariae were P. knowlesi. Slides from the 1952 surveys are not available for analysis by PCR assays, but it is highly likely that these microscopy-confirmed P. malariae infections were actually P. knowlesi infections. In conclusion, the molecular data indicate that P. knowlesi is an ancient parasite and that it has most probably been infecting humans in Southeast Asia ever since it first emerged in macaques in this region.
LABORATORY DIAGNOSIS
Microscopy
The most widely used method for detection of malaria in rural settings is microscopy, since it is a relatively cheap, rapid, quantitative, and sensitive technique. Microscopists in Southeast Asia are largely trained to identify the three main species of Plasmodium that cause malaria in humans in the region, namely, P. falciparum, P. vivax, and P. malariae. Each of these species has morphological characteristics that should enable a well-trained microscopist to identify them fairly accurately (97). For example, for both P. falciparum and P. malariae, there is no enlargement of malaria-infected RBCs. However, due to sequestration of late trophozoites and schizonts of P. falciparum-infected erythrocytes in blood capillaries, only ring forms or early trophozoite and crescent-shaped gametocytes are observed in peripheral blood films prepared from patient samples (Fig. 2), unless parasitemia is very high (98). For P. malariae, all erythrocytic stages are seen in blood films, and some trophozoites stretch across the erythrocyte and appear as “band forms.” However, even well-trained microscopists not uncommonly have difficulty distinguishing early trophozoites of P. vivax from those of P. falciparum, particularly when parasitemia is low. Furthermore, although these species of Plasmodium should be correctly identified by microscopy, misidentification does occur, as exemplified by a study by Cox-Singh et al., where 43/440 (10%) patients with PCR-confirmed P. vivax infection in Sarawak were misdiagnosed as having P. malariae/P. knowlesi infection (28).
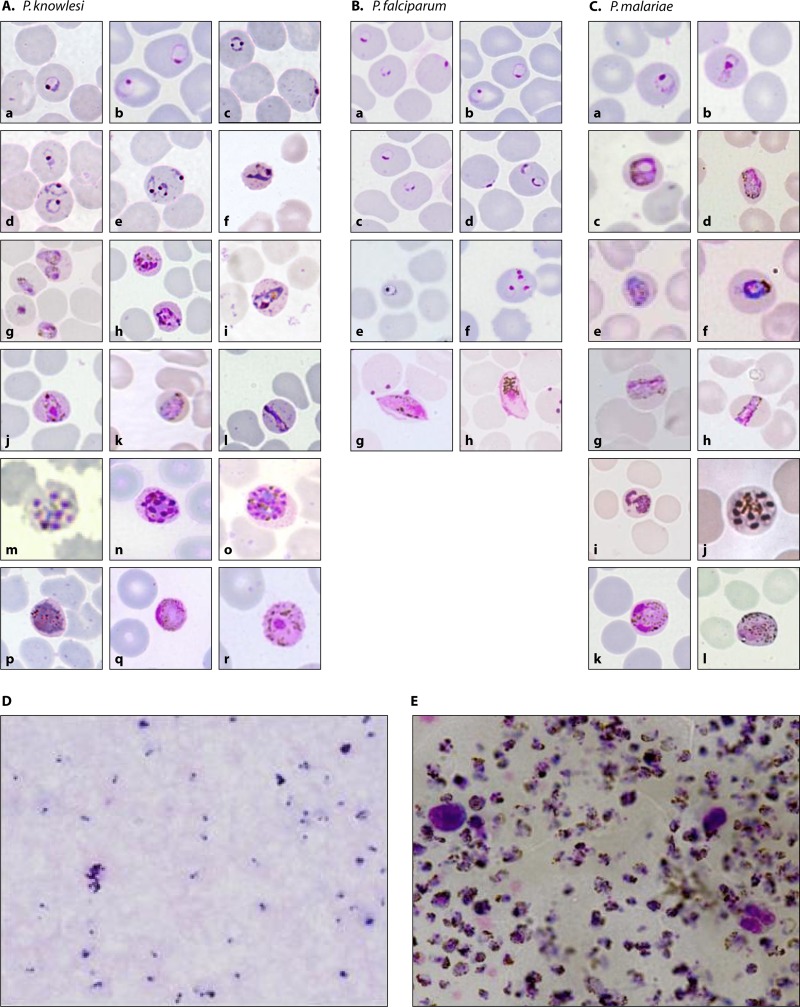
Fig 2.
Erythrocytic stages of P. knowlesi, P. malariae, and P. falciparum observed in Giemsa-stained peripheral blood films. (A to C) Thin blood films with early trophozoites of P. knowlesi (Aa to e), P. falciparum (Ba to f), and P. malariae (Ca); late trophozoites, including band forms, of P. knowlesi (Af to l) and P. malariae (Cb to i); schizonts of P. knowlesi (Am to o) and P. malariae (Cj); and gametocytes of P. knowlesi (Ap to r), P. falciparum (Bg and h), and P. malariae (Ck and l). (D and E) Thick blood films showing early trophozoites of P. knowlesi resembling those of P. falciparum (D) and heavy parasitemia from a fatal P. knowlesi infection (E). (Photographs in panels Aa to e, m, and o and Cc, j, and l have been reproduced from reference 5 with permission from Elsevier, and photographs in panels A and C have been reproduced from reference 68.)
It is not possible to accurately identify P. knowlesi by microscopy, since the morphological features of the early trophozoites of P. knowlesi are identical to those of P. falciparum, with double-chromatin dots, multiple infections per erythrocyte, and no enlargement of infected erythrocytes. The rest of the blood stages of P. knowlesi resemble those of P. malariae, including band-form trophozoites (Fig. 2). The morphological similarities between P. knowlesi and P. malariae were first noted by Knowles and Das Gupta when they induced knowlesi malaria by blood passage in three human subjects in 1932 (8). They wrote that in humans, the parasites show little or no amoeboid activity; the red corpuscles are not enlarged; with Leishman's or Giemsa's stain, no stippling is seen; and the general morphology rather recalls that of P. malariae of humans.
Careful examination of well-stained slides shows minor differences in morphology between P. knowlesi and P. malariae, such as a certain proportion of early trophozoites of P. knowlesi with double-chromatin dots and schizonts of P. knowlesi having up to 16 merozoites, compared to 6 to 12 for P. malariae (68). However, early trophozoites and mature schizonts would not be observed in all P. knowlesi infections, but more importantly, these minor differences between the two species would be missed in a busy routine diagnostic laboratory in a developing country, where only thick blood films are normally examined when parasitemia is low and where microscopists have limited time to screen a large number of samples. Although parasitemia in knowlesi malaria infections can be extremely high (Fig. 2), low parasitemia is relatively common in knowlesi malaria patients. In a prospective malaria study in Kapit, 30% of 107 knowlesi malaria patients presented with parasitemia of <500 parasites/μl blood (33). Most P. knowlesi infections have been identified as P. malariae infections in routine diagnostic laboratories, as exemplified by the observation that out of 349 samples identified as single P. knowlesi infections by PCR, and before the microscopists at 12 hospitals in Sarawak were informed about P. knowlesi, 317 (91%) were identified as P. malariae, 18 (5%) were identified as P. vivax, and 14 (4%) were identified as P. falciparum by microscopy (28). Accurate identification of P. knowlesi by microscopy is a diagnostic challenge, particularly when parasitemia is low. Perhaps the best observation and prediction were made by Garnham, who wrote in his book entitled Malaria Parasites and Other Haemosporidia that “a P. knowlesi infection in a human being could easily pass unrecognized as such in routine laboratories, where it would probably be diagnosed as P. malariae, or if rings only were present, as P. falciparum” (1). Due to the difficulties in distinguishing P. knowlesi from P. malariae by microscopy, and given that P. knowlesi infections can lead to death (28, 32, 34, 43), at a recent WHO consultation meeting on P. knowlesi, it was recommended that in areas where P. knowlesi has been described, microscopists should report all P. malariae-positive results as P. malariae/P. knowlesi (99). Similar reporting should also be made by microscopists in areas where the disease is not endemic for slides from travelers who have visited the forest or forest fringe areas in Southeast Asian countries where human P. knowlesi cases have been described.
Molecular Detection Methods
Molecular detection methods have been developed for the accurate identification of malaria parasites, and these methods have consistently proven to be more sensitive and specific than microscopy (100–104). The molecular detection assays that have been described for P. knowlesi, the gene targets for the PCR primers in each assay, and the number of P. knowlesi infections detected in field isolates at each laboratory are summarized in Table 1 (3, 28, 30–34, 37–41, 44–46, 51, 54, 58–60, 62, 63, 67, 102, 105–110). The first PCR assay developed for the detection of P. knowlesi was a nested PCR assay with primers Pmk8 and Pmkr9, based on the small-subunit rRNA genes (3). This gene was selected because a widely used nested PCR assay for P. falciparum, P. vivax, P. malariae, and P. ovale was being utilized for molecular epidemiological studies of malaria in Sarawak, Malaysian Borneo (101, 111). Genus-specific primers (primer rPLU6 with either primer rPLU1 or rPLU5) are used in the first round of PCR amplification, followed by species-specific primers in a separate second round of PCR amplification. The sensitivity of this method of detection is between 1 and 6 parasites/μl blood, when a DNA template is prepared with a simple boiling procedure with a chelating agent from blood spots collected onto filter paper (112). The initial knowlesi-specific primers that were developed in Sarawak, Pmk8 and Pmkr9, were found in certain laboratories to nonspecifically amplify a proportion of P. vivax isolates, resulting in P. vivax infections being identified as mixed P. vivax and P. knowlesi infections (63, 65, 113). In epidemiological studies undertaken in Sarawak, 512 single P. vivax infections and 28 P. vivax infections mixed with P. knowlesi infections have been detected (3, 28, 33). If these mixed infections were all due to nonspecific amplification of P. vivax, this would represent 5.2% false-positive P. knowlesi results for the P. vivax samples. In contrast, a laboratory in Thailand reported that 20% (6 of 30) of samples from P. vivax patients resulted in PCR amplification by P. knowlesi primers Pmk8 and Pmkr9 (113). With any PCR assay, optimization of amplification parameters is crucial, and further optimization of the assay for knowlesi malaria may have reduced the percentage of false-positive results observed. Nevertheless, new P. knowlesi-specific primers based on the SSU rRNA have now been developed by the laboratory in Thailand (primers PkF1140 and PkR1550) (113) and by the laboratory in Sarawak (primers Kn1f and Kn3r) (74). Recently, a single-step PCR assay for the detection of P. knowlesi was described, with a sensitivity of detection of 10 parasites/μl blood, using a DNA template prepared with a commercial DNA extraction kit, but this assay has yet to be validated for specificity and sensitivity with clinical and other samples from the field (108).
Table 1.
Molecular detection assays for P. knowlesia
| Type of assay | Gene target | Primers or probe(s) | Sensitivity | No. of human P. knowlesi infections identified (location of laboratory) | Reference(s) |
|---|---|---|---|---|---|
| Nested PCR | SSU rRNA (S type) | Pmk8 + Pmkr9 | 1–6 parasites/μl blood | 852 (Sarawak) | 3, 28, 31–33, 38, 54, 67 |
| 405 (Sabah) | 40, 41 | ||||
| 65 (Sabah) | 30 | ||||
| 77 (Peninsular Malaysia) | 44 | ||||
| 7 (Peninsular Malaysia) | 45 | ||||
| 2 (Peninsular Malaysia) | 46 | ||||
| 1 (Singapore) | 59 | ||||
| 1 (Singapore) | 60 | ||||
| 32 (Vietnam) | 62 | ||||
| 3 (Belgium) | 63 | ||||
| 1 (Thailand) | 51 | ||||
| 36 (China) | 58 | ||||
| 1 (Netherlands) | 39 | ||||
| PkF1060 + PkR1550 | 1–10 parasite genomes/sample | 1 (Sarawak) | 67 | ||
| 130 (Australia) | 43 | ||||
| Kn1f + Kn3r | 1–6 parasites/μl blood | 25 (Sarawak) | 74 | ||
| PK18SF + PK18SRc | NR | 35 (Thailand) | 48–50 | ||
| 1 (Cambodia) | 67 | ||||
| Dihydrofolate reductase-thymidylate synthase | PK-Lin-F + PK-Lin-R | 0 (Thailand) | 102 | ||
| LAMP | Apical membrane antigen 1 | 10 plasmid copies/sample | 13 (Peninsular Malaysia) | 106 | |
| β-Tubulin | F3, B3, FIP, BIP, FLP, BLP | 100 plasmid copies/sample | 0 (Obihiro, Japan) | 107 | |
| Single-step PCR | Unidentified genes | 1 parasite/μl blood | 0 (Atlanta, GA) | 108 | |
| Real-time PCR | SSU rRNA | PK1 + PK2 | 10 copies/μl | 0 (Rochester, NY) | 109 |
| NVPK-P | 5 copies/reaction | 1 (Netherlands) | 42 | ||
| PKe′F, PKg′R | 100 copies/μl | 2 (France) | 110 | ||
| Pk | 10 copies/μl | 40 (Sarawak) | 105 |
LAMP, loop-mediated isothermal amplification; NR, not reported.
A number of real-time PCR assays targeting the SSU rRNA genes for detection of P. knowlesi have also been described (Table 2). However, with the exception of the assay described by Divis et al. (105), none of the assays have been validated against a significant number of clinical samples. Real-time PCR assays have an advantage over nested PCR assays and single-step PCR assays in that they are more rapid at providing identification and can give quantitative data, but they are expensive to run and require a substantial initial financial investment. These assays are more likely to be available in diagnostic laboratories in developed countries and referral laboratories in developing countries. Due to their relatively high costs, real-time PCR assays and other molecular detection assays, such as loop-mediated isothermal amplification (LAMP) assays (Table 2), will not replace microscopy in routine diagnostic laboratories in rural areas in the tropics where malaria is prevalent.
Table 2.
Rapid diagnostic tests that have been used to detect P. knowlesi
| RDTa | Result(s) | Parasitemiab | Place where test was conducted | Reference |
|---|---|---|---|---|
| Tests on human samples | ||||
| BinaxNOW Malaria | Negative (P. falciparum), positive (pan-malaria) | 84,000 parasites/μl blood | Rotterdam, Netherlands | 39 |
| Negative (P. falciparum and pan-malaria) | 1,587 parasites/μl blood | |||
| Negative (P. falciparum and pan-malaria) | 138 parasites/μl blood | |||
| Negative (P. falciparum), positive (pan-malaria) | 0.8% | Toulouse, France | 52 | |
| Positive (P. falciparum and pan-malaria) | 7,700 parasites/μl blood (0.2%) | Singapore | 60 | |
| Negative (P. falciparum and pan-malaria) | 0.1% | Stockholm, Sweden | 31 | |
| Negative (P. falciparum and pan-malaria) | 250 parasites/μl blood | Madrid, Spain | 53 | |
| Negative (P. falciparum and pan-malaria) | 185 parasites/μl blood | Enoggera, Australia | 64 | |
| Negative (P. falciparum and pan-malaria) | 0.0005% | Amsterdam, Netherlands | 42 | |
| Negative (P. falciparum and pan-malaria) | NR | Auckland, New Zealand | 36 | |
| OptiMAL | Positive (P. falciparum and pan-malaria) | 84,000 parasites/μl blood | Rotterdam, Netherlands | 39 |
| Positive (P. falciparum and pan-malaria) | 1,587 parasites/μl blood | |||
| Negative (P. falciparum and pan-malaria) | 138 parasites/μl blood | |||
| Positive (P. falciparum), negative (pan-malaria) | 7,700 parasites/μl blood (0.2%) | Singapore | 60 | |
| Core Malaria Pan/Pv/Pf | Positive (P. vivax and pan-malaria) | 0.8% | Toulouse, France | 52 |
| Tests on macaque samples | ||||
| OptiMal-IT | Positive (P. falciparum and pan-malaria) | 28.3% | Japan | 115 |
| Positive (P. falciparum and pan-malaria) | 11.2% | |||
| Negative | 0.04% | |||
| Entebe Malaria Cassette | Positive (P. vivax), negative (P. falciparum) | 28.3% | Japan | 115 |
| Positive (P. vivax), negative (P. falciparum) | 11.2% | |||
| Negative (P. vivax and P. falciparum) | 0.04% |
BinaxNOW detects HRP-2 of P. falciparum and aldolase of Plasmodium; OptiMal detects LDH of P. falciparum and Plasmodium; Core Malaria Pan/Pv/Pf detects HRP-2 of P. falciparum, LDH of P. vivax, and LDH of Plasmodium; and Entebe Malaria Cassette detects HRP-2 of P. falciparum, LDH of P. vivax, and LDH of Plasmodium.
NR, not reported.
Rapid Diagnostic Tests
Immunochromatographic rapid diagnostic tests (RDTs) have been developed for detection of malaria and are useful for investigations of outbreaks in rural settings where electricity is unavailable and in laboratories in developed countries where laboratory technologists are unfamiliar with detecting malaria by microscopy (114). The RDTs contain antibodies that are specific for histidine-rich protein 2 (HRP-2) of P. falciparum or are specific for lactate dehydrogenase (LDH) of either P. falciparum or P. vivax. They commonly also include “pan-malarial antibodies” directed at aldolase or LDH of Plasmodium. All the current commercially available RDTs were developed before it was discovered that P. knowlesi is a significant cause of human malaria, and consequently, these RDTs were not evaluated against P. knowlesi.
Four different RDTs have been used on travelers with knowlesi malaria, and one has been used on macaques experimentally infected with P. knowlesi, producing mixed results (Table 2) (31, 39, 42, 52, 53, 64, 115). For the BinaxNOW RDT, with the exception of one report where there was a positive P. falciparum and pan-malaria result, all other reports indicated a positive pan-malaria result and a negative P. falciparum result. Furthermore, negative pan-malaria results were observed for 5 out of 9 samples examined, with parasitemia ranging from 185 to 1,587 parasites per μl blood and from 0.0005 to 0.1%, highlighting the low sensitivity of detection of knowlesi malaria with BinaxNOW. The OptiMal test and the OptiMal-IT tests, which use antibodies to LDH of P. falciparum that have been found to cross-react with P. knowlesi (116), indicated the presence of P. falciparum or P. falciparum mixed with either P. vivax, P. ovale, or P. malariae in 4 samples from humans and 3 from macaques. From this limited number of reports so far, it appears that the Plasmodium species identified in a sample from a knowlesi malaria patient depends on the RDT being utilized, with OptiMal identifying the infection as a single or mixed P. falciparum infection, BinaxNOW identifying it as a non-P. falciparum malaria infection, and the other two RDTs identifying it as a P. vivax infection. More importantly, P. knowlesi infection in patients with low parasitemia would not be detected by these RDTs. Since P. knowlesi has a short 24-h erythrocytic cycle and has the potential to be fatal (28), it is important that cases with low parasitemia are correctly diagnosed. For travelers from areas where the disease is not endemic, it is recommended that microscopists examine blood films for malaria parasites when RDTs are negative. However, there is a danger that for patients with a negative RDT where blood films are not examined, the attending physician would attribute the signs and symptoms to nonmalaria infections, and appropriate antimalarial treatment would not be provided. There is a need to evaluate the currently available commercial RDT kits against clinical isolates of P. knowlesi and also to develop new sensitive and rapid detection tests suitable for use in remote rural areas.
CLINICAL COURSE
Historical Data
Only recently have we begun to understand the complete clinical spectrum of naturally acquired P. knowlesi infections in humans. However, the first reports of the clinical impact can be found among the initially reported experimental infections of humans by Knowles and Das Gupta (8) and subsequent studies on therapeutic P. knowlesi infections in patients with neurosyphilis (16, 18–21). From these reports, the impact of infection by blood passage had a variable effect on patients. Nicol believed that the disease induced was mild and brief, prompting him to be skeptical over the application of P. knowlesi for use in malariotherapy (18). On the other hand, Ciuca and coworkers in Romania noted that the parasite became too virulent for use following 170 human-to-human blood passages, noting an increase in the need to terminate infections with antimalarials and parasitemia frequently reaching 500,000 parasites per μl of blood (21). Detailed descriptions by van Rooyen and Pile (15) indicated that patients could become unwell with shock, while Milam and Kusch (16) noted that the infections could suddenly change “from moderate severity to one of rather serious proportions.” Different P. knowlesi parasite strains and the effect of multiple subpassages between humans may have accounted for the variable clinical outcomes observed by these early workers. Two studies in the United States also provided evidence that infections in African Americans were milder than those in Caucasians and that some African Americans were refractory to infection by P. knowlesi through blood passage (16, 117).
Although it is difficult to extrapolate these findings to knowlesi malaria infections in previously healthy individuals, it is clear that a full spectrum of illness may be present, including severe disease. In the first reported naturally acquired case of knowlesi malaria in the U.S. Army surveyor, symptoms of anorexia, mild fatigue, and some nausea were followed by a sore throat, shaking chills, high fever, and profuse sweating (22), typically nonspecific infectious symptoms commonly seen in malaria. The P. knowlesi strain isolated from this patient, the H strain, was used in a series of experiments where malaria was induced by blood passage and mosquito bites in 20 volunteers at the U.S. Penitentiary in Atlanta, GA, in the 1960s (22, 23, 97). In contrast to what had been observed previously (16, 117), these experiments showed that African Americans were infected easily, and there were no differences observed in infections between African Americans and Caucasians (97). Among the 12 blood-induced infections, the clinical manifestations were reported as “moderate to severe with attacks terminating spontaneously after 2 weeks.” The clinical course for the 8 sporozoite-induced infections was observed to be not much different from that of blood-induced infections (97). However, after 8 to 11 days of parasitemia and fever, three of the sporozoite-induced cases required chloroquine to terminate the infections, since they were deemed to have severe infections (23). Although clinical details were not provided in this small series of experiments, they indicated that infection with a single strain of P. knowlesi could lead to a spectrum of disease.
Recent Studies
The first report of clinical details for a large number of naturally acquired knowlesi malaria cases appeared in 2004, when retrospective clinical data for 106 patients in Sarawak, Malaysian Borneo, were described (3). In the same year, a case of naturally acquired knowlesi malaria from southern Thailand was reported (48). Between 2004 and 2008, clinical details of knowlesi malaria were reported in a further eight cases, including cases in Sarawak, the Philippines, Singapore, and Peninsular Malaysia (28, 54, 56, 59). The most notable of these reports was that by Cox-Singh et al. (28), where four cases of fatal Plasmodium knowlesi from Sarawak were described. Our understanding now of clinical symptoms seen in naturally acquired human infections with P. knowlesi is derived from single case reports (31, 32, 36, 39, 46–48, 52, 53, 55–57, 59, 64); a small case series of 7 patients in the Klang Valley, Peninsular Malaysia (45); a retrospective study of 94 patients in Kapit, Sarawak (3); three prospective studies from Kapit, Sarikei, and Sabah, with 107, 110, and 130 patients, respectively (33, 43, 118); and three retrospective studies from Sabah (29, 34, 35). Although the literature covers patients from a wide geographical zone across Southeast Asia and of many ethnicities and various background levels of malaria immunity, most of the data are weighted to Malaysia.
Demographics
The majority of cases of knowlesi malaria have been reported for adults, with fewer reports and smaller case series for children. This is likely to reflect generally low transmission rates combined with environmental interactions that predispose adults to come into contact with infected vectors. Similarly, males are more represented in the literature, especially in case reports (31, 36, 39, 47, 52, 53), case series (35), and two recent prospective studies in Sarawak and Sabah (35, 118), while studies in the Kapit region of Sarawak have found a more equal distribution (3, 33). Reports from selected areas of Sabah indicated that 10% of patients were under 15 years of age, and males accounted for 74% of cases (41). The percentage of females was found to be higher for patients with severe disease in initial studies (33, 35), but Barber et al. reported that there were only 8 (21%) females out of 38 severe cases (43). There is a wide age range among patients, including older patients, where comorbidities may be present. These factors combined with low endemicity and limited malaria exposure may explain the observation of an association between age, sex, and severity of disease (33, 35), although a recent study found this association to be confounded by parasitemia in a multivariate analysis (43).
Symptoms
The symptoms of acute knowlesi infection are of a nonspecific infectious illness similar to those seen in falciparum and vivax malaria (33, 43). Fevers, chills, and rigors are the most dominant features reported, while headaches, myalgia/arthralgia, malaise, and poor appetite are also commonly present. Cough (48 and 56%), abdominal pain (31 and 52%), and diarrhea (18 and 29%) were additional symptoms noted in prospective studies of 107 and 130 patients, respectively, presenting with acute knowlesi malaria (33, 43). Gastrointestinal symptoms were also dominant features in four fatal cases described by Cox-Singh et al. (32), and parasitemia in knowlesi malaria has been associated with abdominal pain (43). In Vietnam, where coinfection with other malaria species was present, the clinical features appeared to be less dominant, with only 6 of 32 (19%) patients reporting fever (62). The median duration of illness prior to presentation to a health care facility for knowlesi malaria has been reported to be between 4 and 5 days (3, 33, 35, 43). In some cases, however, the duration of illness was several weeks (33, 64).
Clinical Examination Findings
The most common examination findings reported for 107 prospectively studied knowlesi malaria patients were tachypnea, fever, and tachycardia (33). Palpable liver and spleen were reported in 24 to 40% and 15 to 26% of cases, respectively, in two prospective studies of 107 and 130 patients (33, 43). Clinical signs of severe disease including low oxygen saturations, tachypnea, chest crackles (indicating acute respiratory distress or coexisting pneumonia), hypotension, and jaundice have been documented (28, 32–35, 43, 45). In one fatal case with a history of poorly controlled hypertension, focal neurology was present, but it is not clear whether this was a coexistent cerebrovascular event, since brain imaging was unavailable. A cerebral malaria-like syndrome has not been reported, but consciousness may be impaired secondary to the severity of illness in the context of multiorgan failure or hypoglycemia (32, 33).
Laboratory Findings
Thrombocytopenia.
Thrombocytopenia is the most frequently reported blood abnormality and appears to be almost universal in knowlesi malaria infections (28, 31–36, 39, 42, 43, 45, 47, 48, 52, 53, 55, 59, 64, 118). In the prospective study undertaken at Kapit Hospital, 98% of 107 patients presented with thrombocytopenia, and within 24 h of admission, the remaining 2% became thrombocytopenic (33). Despite the extremely high proportion of patients with thrombocytopenia, and a third of these patients being severely thrombocytopenic (<50,000 platelets per μl blood), bleeding complications were rarely seen. In limited comparative studies, the severity and frequency of thrombocytopenia were higher with knowlesi malaria than with falciparum and vivax malaria (33), while an inverse association between platelet counts and parasitemia has been observed (43), a feature also seen with both falciparum and vivax malaria infections (33, 119, 120). Willmann et al. recently described laboratory markers of severity in knowlesi malaria infections and found thrombocytopenia (≤45,000 platelets per μl blood) to be associated with complicated disease, with a sensitivity of 71% but a positive predictive value of 22% (118).
Throughout Southeast Asia, dengue fever is a differential cause of a febrile illness associated with thrombocytopenia (121). For the first case of a naturally acquired P. knowlesi infection in Singapore, the initial diagnosis was dengue fever, and a blood film was examined only on the third day following admission to the hospital (59). We observed that for 104 patients with acute thrombocytopenia during a 4-month period, 4 patients (10% of knowlesi malaria cases) at Kapit Hospital were given an initial working diagnosis of dengue fever (C. Daneshvar, unpublished data). For knowlesi malaria, where thrombocytopenia can occur with very low parasitemia, careful examination of well-prepared blood films is needed and should be repeated daily if results are initially negative. Delays in diagnosis of knowlesi malaria could be critical to the outcome, and thrombocytopenia seems an appropriate warning flag to intensify clinical suspicion of this rapidly dividing parasite in patients with a recent history of travel to the forest and forest fringes of Southeast Asia.
Anemia.
Unlike falciparum malaria (122), severe anemia is not a commonly reported feature at the time of presentation for adults with knowlesi malaria. Mild anemia may be observed, with a frequency of <5% in 107 patients prospectively studied (33). The mean red blood cell volume (MCV) was preserved, (median, 85.6 fl), with 8 (7.5%) patients having mild microcytosis (<80 fl) (Daneshvar, unpublished). During the course of admission, there was an initial drop in hemoglobin levels, compatible with data reported for falciparum infections (123), which recovered in all 87 of the patients studied by day 28 (33). In a recent study of 130 patients with acute knowlesi malaria, two patients met the criteria for severe anemia (hemoglobin concentration of <7 g/dl) on day 1 and day 8 of admission (43).
Other hematological findings.
For knowlesi malaria, the prothrombin and thromboplastin times are usually preserved (33). However, in a retrospective case series including severe disease, 7 patients had significant derangement, but no associated bleeding complications were noted (35). Other hematological abnormalities may be seen, including lymphopenia (lymphocyte counts of <800 per μl), which was observed in 6.5% of 107 patients in a prospective study at Kapit Hospital (33).
Renal function.
Renal function may be significantly deranged; of 107 patients with acute knowlesi malaria infections, 3 (2.8%) had established renal failure (creatinine level of >265 μmol/liter after fluid resuscitation for >24 h) (33). Two recent prospective studies of 130 and 110 cases reported frequencies of renal failure of 6.9% and 14.5%, respectively (43, 118). Careful fluid resuscitation and antimalarial treatment are usually sufficient, and renal impairment is reversible. A recent case series from the Klang Valley of Peninsular Malaysia reported that 2 out of 7 knowlesi malaria patients had acute renal failure (45). It is, however, an ominous sign associated with fatal cases (28, 34). Electrolyte abnormalities, including hyponatremia (Na concentration of <136 mmol/liter), are frequently seen (24% of 107 patients) and are self-correcting with treatment of malaria (33). Sodium concentrations have been reported to be negatively associated with parasitemia (43).
Liver function.
Liver function may be abnormal, and a mild elevation of levels of aminotransferase enzyme is frequently seen in cases of knowlesi malaria. Generally, synthetic functions (clotting factors) appear to be preserved; however, albumin concentrations are lower in patients with severe disease at the time of admission (33).
Hematological and biochemical parameters respond rapidly following treatment, with the exception of hemoglobin levels, serum albumin concentrations, and liver enzyme levels, which typically return to normal limits by day 28 (33).
Clinical Aspects in Children
There are few reports on knowlesi malaria in children. Barber et al. described retrospective findings of P. knowlesi infections in 16 children from the district of Kudat in Sabah, Malaysian Borneo (29). No children were found to have severe disease upon admission, and they all successfully responded to antimalarial treatment. Thrombocytopenia was observed for 94% of children with single knowlesi infections, and anemia (<11 g/dl) was present at admission in 56% children and developed in all children during hospital admission. Compared with 14 children with falciparum malaria, significant differences were found in hemoglobin concentrations (higher in knowlesi than in falciparum malaria infection) and platelet counts (lower in knowlesi than in falciparum malaria infection). Of 188 patients at Kapit Hospital reviewed consecutively, 8 out of 121 (6.6%) cases of single knowlesi infections occurred in children (Daneshvar, unpublished). The median age of the children was 11 years (range, 9 to 12 years), and the median parasite count was 940 parasites per μl blood (range, 440 to 26,270 parasites per μl blood). All children were thrombocytopenic, two were mildly anemic, two had increased alanine aminotransferase concentrations, and one had jaundice (bilirubin level of 38 μmol/liter). In one patient with a parasite count of 26,270 parasites per μl blood, a petechial rash over the shins and retinal hemorrhage were found upon examination. All eight children with knowlesi malaria responded to treatment.
Complicated Knowlesi Malaria
Severe disease.
The first reported cases of fatal P. knowlesi infections were of four patients aged between 39 and 69 years (28). They presented with a 3- to 7-day history of a fever associated with nonspecific features that included shortness of breath, abdominal pain, and vomiting. All four cases had high parasitemia (75,000, 112,000 and 764,720 parasites per μl, scored as “++++”), severe thrombocytopenia, renal failure, hypotension, jaundice, and deranged liver enzymes. In the first patient, malaria was not considered for 3 days due to the dominance of abdominal symptoms, leading clinicians to a diagnosis of presumed bacterial gastroenteritis, while the second patient had a perforated gastric ulcer with associated pneumoperitoneum. The third case died within 4 h of admission to the hospital, while the fourth case developed acute respiratory distress after 4 days of admission, required a prolonged period of care in the intensive care unit, and then died of complications from a tracheostomy hemorrhage.
Features of severe malaria defined by laboratory findings reflect extensive studies of P. falciparum infections (122). Such prognostic markers include a white cell peripheral leucocytosis count of >12,000 cells/μl, a serum creatinine concentration of >265 μmol/liter, a urea concentration of >21.5 mmol/liter, a hemoglobin concentration of <7.1 g/dl, and a blood glucose level of <2.2 mmol/liter (124–126). A 3-fold increase in aminotransferase enzyme levels and increased serum lactate and low bicarbonate concentrations are also associated with a poor outcome in falciparum malaria (122). The relevance of these thresholds and application to knowlesi malaria require further evaluation.
Although most cases of knowlesi malaria respond to treatment and resolve without complications, complicated and fatal cases are being increasingly reported (Table 3) (28, 32–35, 43, 45, 127). In a prospective study that excluded patients with significant comorbidities with apparent end-organ disease, the application of the World Health Organization criteria for severe falciparum malaria (122) indicated that 7 of 107 (6.5%) patients had signs and laboratory features of severe disease at the time of presentation, and a further 3 developed severe disease during admission (33). Two patients in this study died, with a case fatality rate of 1.8% (confidence intervals [CI], 0.6% to 6.6%). Recently, further studies have contributed to our understanding. A retrospective study of admissions to a tertiary referral hospital in Sabah, Malaysian Borneo, reported complications occurring in 22 of 56 (39%) cases (35). It should be noted that 17 of the 22 severe cases had been referred from district hospitals, and this number is therefore likely to be an overestimation of the true prevalence of complicated disease. A more recent comparative prospective study from the same site reported complications in 38 (29%) of 130 patients, where the referral criteria included having moderate parasitemia from referring district hospitals (43). That study also found that having knowlesi malaria was associated with an increased odds ratio of developing severe malaria over falciparum malaria (odds ratio, 2.96; 95% CI, 1.19 to 7.38). More extensive studies are needed to determine the case fatality rate, but these studies demonstrate the breadth of complications and severity of disease that may occur in knowlesi malaria infections.
Table 3.
Summary of published reports on severe and fatal knowlesi malaria cases
| Study type (reference) | Study location | No. of cases | Avg age of patients (yr) | No. (%) of patients with clinical signh |
Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperparasitemiaa | Hypotensionb | Acute kidney injuryc | Jaundiced | Hypoglycemiae | Lactic acidosisf | ARDS/pulmonary edemag | |||||
| Retrospective fatal case series (28) | Sarawak | 4 | 53 | 3 (75) | 3 (75) | 4 (100) | 4 (100) | 1 (100)j | 1 out of 1 | 3 (75) | All died |
| Prospective (33) | Sarawak | 10 | 63.5 | 3 (30) | 2 (20) | 3 (30) | 4 (100) | 1 (10) | 1 (10) | 6 (60) | 2 died (20%) |
| Fatal case report (32) | Sabah | 1 | 40 | 1 (100) | 1 (100) | 1 (100) | NA | NA | NA | 1 (100) | Died |
| Retrospective (45) | Peninsular Malaysia | 3 | 55 | 3 (100) | 2 (67) | 2 (67) | 2 (67) | 1 out of 1 | 1 out of 1 | 2 (67) | All survived |
| Retrospective case series (35) | Sabah | 22 | 56.5 | NA | 11 (50) | 12 (55) | 9 (41) | 3 | 6 | 13 (59) | 6 died (27%) |
| Prospective (127) | Sarawak | 2 | 32.5 | 1 (50) | 0 | 1 (50) | 1 | NA | NA | NA | All survived |
| Prospective (43) | Sabah | 38 | 55 | 18 (47) | 13 (34) | 9 (24) | 20 (53)i | 0 (0) | 4 (11) | 14 (37) | All survived |
| Prospective (118) | Sarawak | 17 | 49.6 | 8 (47) | 1 (5.9) | 16 (94.1) | 6 (35.3)i | NA | NA | 3 (17.6) | 4 died (23.5%) |
Parasite counts of >100,000 parasites/μl.
Systolic blood pressure of <80 mm Hg or started on ionotropes.
Creatinine level of >265 μmol/liter (3 mg/dl) or requiring dialysis.
Total bilirubin level of ≥43 μmol/liter (3 mg/dl).
Glucose level of <2.2 mmol/liter (40 mg/dl).
Lactate level of >6 mmol/liter.
Respiratory rate of >30 breaths per minute, presence of pulmonary infiltrates, or requiring ventilation.
NA, data not available.
Total bilirubin level of ≥43 μmol/liter plus parasitemia of >20,000 parasites/μl or creatinine level of >132 μmol/liter (1.49 mg/dl).
Data available for one patient.
Acute respiratory distress syndrome.
Patients may present with tachypnea, hypoxemia, and pulmonary infiltrates on chest radiograph, consistent with acute respiratory distress syndrome (ARDS). The two largest case series reported frequencies of 5.6% and 10.7% for 107 and 130 knowlesi malaria cases studied, respectively (33, 43). In severe cases of knowlesi malaria reported in the literature with sufficient detail, ARDS was present in 43 out of 83 (52%) cases, with a crude mortality rate of 37% (Fig. 3). Frequently associated complications were reported to be present (median = 3), and in four patients, ARDS developed following admission, a pattern similar to that seen with falciparum malaria (128–130). Logistic regressions in limited case series indicated positive and independent associations with parasitemia and neutrophilia and an inverse association with hemoglobin concentrations at admission (33, 43).
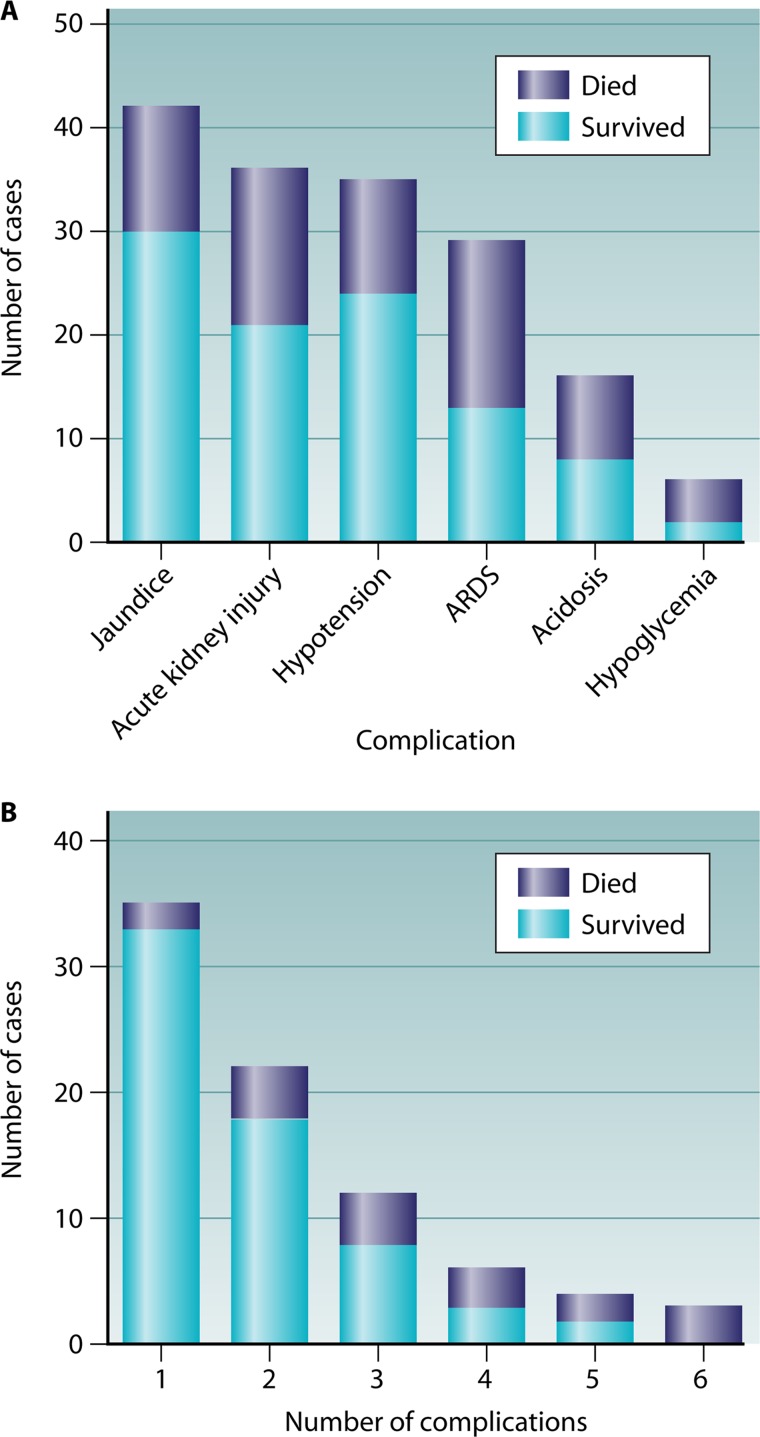
Fig 3.
Complications and outcomes (A) and the number of complications and outcomes (B) for 86 cases of severe knowlesi malaria. ARDS, hypoxia with a respiratory rate of >30, presence of pulmonary infiltrates, or requiring ventilation; acute kidney injury, creatinine levels of >265 μmol/liter (3 mg/dl) or requiring dialysis; hypotension, systolic blood pressure of <80 mm Hg or started on ionotropes; jaundice, total bilirubin level of ≥43 μmol/liter (3 mg/dl); acidosis, lactate level of >6 mmol/liter; hypoglycemia, glucose level of <2.2 mmol/liter (40 mg/dl). (Data obtained from references 28, 32–35, 43, 45, and 127.)
Acute renal failure.
Overall, acute renal failure was reported in 3 of 107 (3%) prospectively studied patients with acute knowlesi infections (33). Willmann et al. observed acute kidney injury in 16 (94.1%) of 17 cases with severe knowlesi malaria (118). In pooled data for complicated disease, acute renal failure was present in 36 out of 86 (42%) reported cases, with an associated mortality rate of 42% (Fig. 3). Acute tubular necrosis was observed postmortem in a fatal case of knowlesi malaria (32). Full renal support may be required, although supportive treatment may be sufficient with normalization of renal function (33, 35, 45). Renal failure in association with a “Blackwater fever” clinical phenotype has been reported (35). Parasitemia, neutrophilia, and age are independently associated with serum creatinine, although this complication may occur in apparently young healthy patients (33, 35, 43). Interleukin-1ra (IL-1ra) levels were found to positively correlate with the serum creatinine (131).
Other features.
Hypotension after fluid resuscitation requiring ionotropic support has been reported for knowlesi malaria (33, 35, 45). This occurred in 35 of 86 (41%) reported complicated cases and was associated with a mortality rate of 31%. Hypoglycemia is not a dominant feature of knowlesi malaria infections; however, when present, it is associated with other multiple complications (median, 5.5) and has a very high mortality rate (4 of 6 cases). Acidosis is also a feature of severe knowlesi malaria infections associated with a high mortality rate (Fig. 3). Cerebral malaria-like syndromes have not been reported for knowlesi malaria.
Parasitemia appears to be a strong predictor of complications in knowlesi malaria infection, with an area under the receiver operating characteristic (ROC) curve of 0.9 (95% confidence interval, 0.82 to 0.98; P < 0.001) (33). The specificity at a threshold of 100,000 parasites per μl was 100%, while the sensitivity of 30% indicates that this threshold is probably too high and highlights that severe cases can occur at relatively low parasitemia. Further studies have confirmed this, with thresholds of >20,000 or >35,000 parasites per μl having an 11- or 10-fold increase in the odds of having severe disease, respectively (43, 118).
Complications in knowlesi malaria may occur as single-organ dysfunction (35 of 86 [41%] cases) but more commonly occur with multiorgan involvement (Fig. 3). From published reports, three or more complications were present in 25 of 86 cases (30%). As one would expect, the more complications present, the higher the mortality rate (Fig. 3). Further work involving a larger number of cases is needed to ascertain whether such complications are present upon admission or develop over the course of admission as well as their role in predicting outcome in knowlesi malaria.
Pathogenesis.
The pathogenesis of severe knowlesi disease is not fully understood. A recent study reported postmortem findings for a patient in Sabah, Malaysian Borneo, who died within 2 h of admission, having presented in shock and found to have multiorgan failure (32). This case showed accumulation of infected erythrocytes, indicating possible sequestration of malaria parasites and hemorrhagic complications in vital organs, but a lack of chronic inflammatory infiltrate. This suggests that there are some histological similarities with falciparum malaria but that a distinct pathophysiology may occur in severe knowlesi malaria.
A study of pretreatment cytokine concentrations at admission showed that knowlesi malaria patients with complicated disease had higher levels of cytokines including tumor necrosis factor alpha (TNF-α), IL-6, IL-8, IL-1ra, and IL-10 than patients with uncomplicated disease (131). The anti-inflammatory cytokines IL-1ra and IL-10 were associated with parasitemia in knowlesi malaria. For patients with uncomplicated knowlesi malaria, the overall levels of proinflammatory, anti-inflammatory, and macrophage-derived cytokines were lower than those for patients with uncomplicated falciparum malaria. Patients with severe knowlesi malaria differed from those with severe falciparum malaria, with higher IL-6, TNF-α, and macrophage inflammatory protein 1β (MIP-1β) (CCL4) levels but not higher IL-10 levels. While further studies are clearly needed to understand the immunopathology of knowlesi malaria, this study indicates that the pathogenesis of severe disease may be different from that seen in falciparum malaria.
A small study of ex vivo cytoadherence demonstrated that late-trophozoite- and schizont-infected erythrocytes from patients with knowlesi malaria have the capacity to bind to the human endothelial cell receptors intracellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule (VCAM) but not to CD36 (127). The role of this in the pathogenesis of knowlesi malaria is not yet understood; however, the potential for these endothelial cell receptors to be expressed in knowlesi malaria patients gives rise to the possibility of sequestration of P. knowlesi-infected erythrocytes in blood capillaries of different organs. Whether total sequestration or sequestration of a subset of P. knowlesi-infected erythrocytes truly occurs during the natural life cycle or occurs only in a proportion of patients during an intense inflammatory response and whether this has a role in pathogenesis need to be determined. Further studies examining the dynamic changes in cytokine levels and gene expression and the role of host and parasite genetics will complement our understanding of pathogenesis while also revealing insights into other malaria species.
TREATMENT
Although patients with knowlesi malaria have been successfully treated with a wide range of antimalarial drugs, the optimal treatment for either uncomplicated or complicated disease is unknown. From the neurosyphilis era in the 1930s, quinacrine (Atebrin), pamaquine (Plasmochin), proguanil (Paludrine), and quinine were used to terminate fevers, often in combination (15, 16, 132). van Rooyen and Pile described “the almost dramatic destruction of parasites” following administration of quinine intramuscularly (15). One may assume that there is little antimalarial drug resistance, as knowlesi malaria is primarily a zoonosis, so P. knowlesi parasites have not been subjected to any significant antimalarial drug pressure. Indeed, in nonhuman primate studies, tetracycline, clindamycin, trimethoprim, erythromycin, and artemisinins have all been shown to have an antiparasitic effect in P. knowlesi infections (133–138). Despite this, quinacrine was not effective in controlling acute parasitemia in humans (15), and it was noted that resistance was easily induced with recurrent exposure to mefloquine, proguanil, and pyrimethamine in rhesus macaques (139, 140). Interestingly, quinacrine is closely related to mefloquine. In fact, a recent case report indicated that despite treatment with mefloquine, parasitemia continued to increase (46).
In the earliest reports of naturally acquired knowlesi malaria infection, chloroquine was provided as a clinical cure (3, 22, 48). In Malaysia, the combination of chloroquine and primaquine (primarily as a gametocidal drug) is recommended for the treatment of P. malariae and was effective for 82 patients at Kapit Hospital subsequently identified as having single knowlesi malaria infections (3). Also, in this retrospective study, 2 patients received quinine, and 10 patients received a combination of chloroquine, primaquine, and sulfadoxine-pyrimethamine (Fansidar). There were no reported deaths or treatment failures in any of these patients, and the overall median parasite clearance time was 2.4 days.
Case reports indicated that chloroquine alone and atovaquone with proguanil, mefloquine, artemisinins, quinine, and doxycycline can be successfully used to treat knowlesi malaria (Table 4). An observational prospective study supported successful treatment with chloroquine (141). In that study, 73 patients with uncomplicated knowlesi malaria who had not been exposed to antimalarial drugs in the preceding 14 days were treated with a total dose of 25 mg/kg of body weight of chloroquine, administered as 10 mg/kg, followed by 5 mg/kg at 6, 24, and 48 h. In line with the Malaysian Ministry of Health treatment guidelines for P. malariae infections, 2 doses of primaquine (15 mg) were administered at 24 and 48 h, mainly as a gametocidal drug. That study showed that patients subjectively felt better within 24 h and that the median fever clearance time was 26 h. Parasite clearance was rapid, with a time to 50% reduction in admission parasitemia (PCT50) and a PCT90 of 3.1 and 10.3 h, respectively, significantly faster than for vivax infections. For most patients, parasite clearance occurred within 48 h. No resistance, recrudescence, or reinfection was observed during 28 days of follow-up for 60 of these patients. Chloroquine appeared to have gametocidal activity, although the parasite clearance time (PCT) was less rapid than for asexual erythrocytic stages of P. knowlesi (PCT50, 10.4 h [95% CI, 9.0 to 12.2 h]; PCT90, 34.4 h [95% CI, 29.9 to 40.4 h]). In view of this, primaquine is unlikely to have a role in the treatment of knowlesi malaria, since there is not known to be a latent liver (hypnozoite) stage for P. knowlesi (1). Limited data on the PCTs of quinine and artemether-lumefantrine are also available (35). This retrospective study reported PCTs of 2.5 days and 1 day for quinine (n = 10) and artemether-lumefantrine (n = 8), respectively, for patients with uncomplicated infection.
Table 4.
Antimalarials for treatment of naturally acquired human P. knowlesi infections
| Treatment | No. of cases | No. of deaths | Parasite clearance time(s)a | Reference(s) |
|---|---|---|---|---|
| Chloroquine | 247 | 3 | PCT50, 3 hb; PCT, 1 day (n = 97); PCT, 2.5 days (n = 15); PCT, 2 days (n = 13) | 3, 29, 33–35, 39, 43, 45, 48, 52, 56, 59 |
| Artesunate followed by artemether-lumefantrinec | 58 | 0 | PCT, 2 days | 43 |
| Quinine | 48 | 9 | PCT, 2 days (n = 3); PCT, 2.5 days (n = 11); PCT, 4 days (n = 16) | 3, 28, 29, 33, 35, 45, 47 |
| Artemether-lumefantrine | 36 | 0 | PCT, 1 day (n = 8); PCT, 2 days (n = 28) | 35, 43 |
| Artemether-mefloquined | 10 | 0 | NR | 43 |
| Dihydroartemisinin-piperaquine | 7 | 0 | PCT, 1 day | 43 |
| Artesunate | 7 | 2 | PCT, 2 days | 34, 35 |
| Atovaquone-proguanil | 3 | 0 | PCT, 2 days (n = 1) | 42, 55, 64 |
| Mefloquine | 1 | 0 | NR | 31 |
| Atovaquone-proguanil-artemether-lumefantrine | 1 | 0 | NR | 36 |
| Chloroquine-doxycycline | 1 | 0 | NR | 45 |
| Chloroquine-sulfadoxine-pyrimethamine | 2 | 2 | NR | 28 |
| Chloroquine-sulfadoxine-pyrimethamine-primaquine-quinine | 1 | 1 | NR | 28 |
| Mefloquine-quinine-artemether-lumefantrine | 1 | 0 | Early treatment failure with mefloquine | 46 |
| Quinine-artemether-lumefantrine-doxycycline | 1 | 0 | NR | 45 |
| Sulfadoxine-pyrimethamine | 1 | 1 | NR | 34 |
| Quinine-chloroquine-doxycycline-primaquine | 1 | 1 | NR | 34 |
PCT50, time to 50% reduction in admission parasitemia; PCT, time to complete parasite clearance. NR, not reported.
For gametocidal activity, the PCT50 was 10.4 h (95% CI, 9.0 to 12.2 h), and the PCT90 was 34.4 h (95% CI, 29.9 to 40.4 h).
Patients received at least one dose of intravenous artesunate.
Three patients also received at least one dose of intravenous artesunate.
Given the 24-h replication cycle of P. knowlesi, early aggressive treatment is warranted for patients with relatively high parasitemia, to prevent sudden increases in parasitemia and the development of complications. In prospectively studied patients with uncomplicated infection, one-third of knowlesi malaria patients experienced an increase in parasitemia during the first 6 h of treatment with chloroquine, twice that seen for vivax malaria (141). In this setting, patients with knowlesi malaria were identified and immediately treated. Unfortunately, this is not always the case. Delayed diagnosis and confusion over the morphological appearances, leading to a diagnosis of P. malariae still being issued by diagnostic laboratories in rural hospitals, complicate the situation further. This was recently illustrated in a report reviewing retrospective data on 6 knowlesi malaria fatalities in Sabah, Malaysian Borneo. Despite convincing demographic evidence being available on the existence of knowlesi malaria and the paucity of P. malariae infections, 5 patients with high parasitemia and severe malaria were diagnosed as having P. malariae infection, and only two received immediate parenteral treatment (34). At the recent WHO consultation meeting on P. knowlesi, it was recommended that in the absence of assays for molecular confirmation of P. knowlesi infection, hospital laboratories in areas where P. knowlesi has been described should report all microscopy-diagnosed P. malariae cases as P. malariae/P. knowlesi so that clinicians can treat and manage patients accordingly (99).
There are currently no reported randomized control trials of treatment for severe and uncomplicated knowlesi malaria. William et al. reported retrospective data on 22 patients with severe disease in Sabah, Malaysian Borneo, where intravenous quinine and artesunate were used in 16 and 6 patients, respectively (35). Those treated with intravenous quinine had a PCT of 4 days, with a case fatality rate of 31%, while patients in the artesunate group had a parasite clearance time of 2 days, with a case fatality rate of 17%. Barber et al. reported the use of artemisinins (as combined oral therapies and intravenously as a single agent) in severe and nonsevere knowlesi malaria (n = 119) and reported a median fever clearance time of 1 day and a PCT of 2 days (43). In that study, there were no fatal cases, which those authors attributed to the early use of intravenous artesunate. These findings support the recommendations of the WHO informal consultation meeting on P. knowlesi, where a pragmatic approach with artemisinin combination therapies was advocated together with managing complicated disease according to guidelines for management of severe falciparum malaria (99).
FUTURE DIRECTIONS AND CHALLENGES
Our current knowledge on the clinical course, treatment, pathogenesis, and epidemiology of knowlesi malaria has been derived from a limited number of case reports, small retrospective and prospective studies, and relatively small field studies. The data acquired indicate that knowlesi malaria is primarily a zoonotic disease with long-tailed and pig-tailed macaques as the major reservoir hosts. Furthermore, the studies showed that although P. knowlesi infections cause a spectrum of disease and can be potentially fatal, knowlesi malaria can be successfully treated with a number of antimalarials. However, there is still much more that needs to be known about knowlesi malaria. There remains a paucity of data from large prospective clinical studies to enable physicians to appreciate the true spectrum of knowlesi malaria. As yet, the largest prospective study including all patients with acute knowlesi malaria infections attending a regional health care facility has studied only 107 patients (33), and a more recent one based at a referral hospital included only 130 knowlesi malaria patients (43). Larger prospective studies on well-defined cohorts with the full spectrum of disease will enable us to define criteria for what constitutes severe disease in knowlesi malaria. Further studies will be able to provide additional information on regional variations in clinical outcomes.
Treatment studies are urgently needed to inform the optimum management of patients with knowlesi malaria infections. At a practical level, with the widespread use of artemisinin combination therapies, such studies may not initially be attractive; however, defining baseline parasite clearance and stage specificity and extended in vivo resistance studies will serve as markers for detecting resistance in the future. It may be that the rapid clearance of parasites will need specific methods to be devised to accurately define some of these parameters, and a role for quantified molecular techniques will be explored. The pharmacodynamics of antimalarials, both orally and parentally, in patients with severe and uncomplicated disease need to be established.
The unusually frequent presence of complications of knowlesi malaria reported in the literature makes it essential to undertake studies of the optimal treatment for clearance of parasites and of how best to manage the associated syndromes (28, 34, 43). There have been no detailed studies exploring management strategies for acute respiratory distress syndrome, renal failure, and fluid status; the use of adjunctive therapies such as dialysis, blood transfusions, or ionotropes; and the role for antibiotics. Such studies will be possible only with multicentered approaches, collaboration, and endorsement by the Ministries of Health in the countries affected. Crucial to such studies will also be the ability to rapidly detect knowlesi malaria while excluding the presence of falciparum malaria. In theory, such studies may seem simple; however, those working in these environments are well aware of the logistical problems encountered.
Detailed prospective clinical studies can generate the information and material required to explore the pathogenesis of knowlesi malaria in depth and will undoubtedly give insights into the pathogenesis of other malaria species. In fact, in some respects, the presence of knowlesi malaria in a population in a region of low endemicity may help to simplify studies on pathogenesis, while the existence of a natural animal model could provide further insights. Although it is clear that research on severe knowlesi malaria needs to be undertaken, accurate diagnostic difficulties, the relatively low incidence of knowlesi malaria and the low prevalence of severe cases, low parasite burdens, and difficulty in accurately quantifying serial changes suggest that there are significant challenges ahead.
The data contributed by hospital-based studies may be only the tip of the iceberg of P. knowlesi infections, and there is a need for longitudinal studies of communities that live in the forest or forest fringe to ascertain whether there is a significant burden of infection in these exposed populations that either resolves without treatment or never becomes symptomatic. Furthermore, there is a need to conduct large-scale malaria surveys with molecular detection methods in areas other than Sarawak and Sabah in Southeast Asia, to determine the actual prevalence of knowlesi malaria. The utilization of molecular tools will also assist in determining whether human-to-human transmission is currently occurring. Central to these studies is the need to develop inexpensive and sensitive rapid diagnostic tests that are easy to use in rural settings. In addition, P. knowlesi-specific serological assays need to be developed, since they will be able to provide valuable information with regard to previous exposure to P. knowlesi in communities living in the forest fringe and forests. Such denominators will provide crucial information to guide us toward a better understanding of the knowlesi malaria landscape.
It is unknown whether the recent increase in the number of reported knowlesi malaria cases in Southeast Asia is a genuine increase or whether it is the result of heightened awareness and enhanced efforts for detection with recently developed sensitive and specific molecular detection methods coupled with a decline in the number of human malaria cases. Detailed genetic analyses of sequences of P. knowlesi isolates obtained from both human and macaque infections from different parts of Southeast Asia, similar to the study undertaken in Kapit, Sarawak, would assist in determining whether there is any regional variation in the emergence of knowlesi malaria.
The studies on the vectors of knowlesi malaria in nature that have been undertaken to date have all had very restricted geographic and temporal samplings. For example, in the study in Kapit, sampling was undertaken at a single longhouse, a farm, and only one location in the forest in the Kapit District, one of three districts within the Kapit Division, which has an area of 38,934 km2. In a large state such as Sarawak (124,000 km2), the vectors for knowlesi and other malarias may vary at different locations. Therefore, there is a need for more extensive entomological studies to be undertaken to identify the vectors of knowlesi malaria and to study their bionomics in different ecological habitats in Malaysia and other regions in Southeast Asia where human knowlesi malaria cases have been reported. A detailed understanding of the vector bionomics and transmission of knowlesi malaria is crucial to the successful implementation of malaria control programs.
Now that malaria elimination is back on the agenda (142), one of the main challenges is the prevention and control of zoonotic knowlesi malaria in rural areas where there is a large pool of parasites in the macaque reservoir hosts. A reduction in the number of these macaque reservoir hosts would be one option, but implementing such a measure would be impractical and difficult. Currently used methods for malaria control, which include provision of insecticide-treated bed nets and residual spraying of houses with insecticides, would not be efficient against vectors that are predominantly outdoor feeders, like the ones identified in the Kapit Division of Sarawak, Malaysian Borneo (82, 83). Prevention of humans from being bitten by vectors would be the main method of prevention and control of knowlesi malaria in these settings. This would involve the use of insect repellents, but these are prohibitively expensive for subsistence farmers, hunters, logging camp workers, and other rural people whose daily activities take them to the forest and forest fringe. The use of insecticide-impregnated hammocks has been successful for controlling forest malaria in Vietnam (143), but hammocks are not traditionally used in Malaysian Borneo, where most P. knowlesi cases occur, and so such a control measure would be difficult to implement successfully.
In conclusion, although much is known about the epidemiology and clinical manifestations of knowlesi malaria, these data are based on limited studies, and there remain many unanswered questions. What is the actual burden of knowlesi malaria in Southeast Asia? What are the criteria and the optimum management strategies for severe knowlesi malaria? Is the pathophysiology of knowlesi malaria different from that of falciparum malaria? Is human-to-human transmission by mosquitoes currently occurring? Will deforestation or ecological changes to the forests by logging lead to changes in vector behavior and composition and eventually cause P. knowlesi to switch hosts from macaques to humans? With renewed interest in knowlesi malaria and the availability of appropriate molecular tools, some of these questions may be answered in the near future, while for others, it will require patience, persistence, and continued commitment from clinicians, laboratory-based scientists, entomologists, and epidemiologists. In the meantime, clinicians and health care workers should be made aware of the potential for fatal outcomes in P. knowlesi infections, and there should be continued surveillance of knowlesi malaria.
ACKNOWLEDGMENTS
We acknowledge the support of our collaborators, the doctors, nurses, and malaria control program staff of the Sarawak Health Department, and of the many people who willingly donated their blood samples for us to be able to undertake our studies on knowlesi malaria. We thank Dayang Shuaisah Awang Mohamad and Khamisah Abdul Kadir for their assistance in preparing the figures.
We also acknowledge the financial support received from the Wellcome Trust; the Malaysian Ministry of Science, Technology and Innovation; and Universiti Malaysia Sarawak for our work on knowlesi malaria.
Biographies

Balbir Singh, Ph.D., is the Director of the Malaria Research Centre at Universiti Malaysia Sarawak, Kuching, Sarawak, Malaysia. He received his graduate degree in Biochemistry, his M.Sc. in Medical Entomology and Applied Parasitology, and his Ph.D. in Microbiology and Immunology from the University of Liverpool, United Kingdom. He started working on malaria in 1984 at the Liverpool School of Tropical Medicine, initially as a postdoctoral Research Assistant on cytoadherence in Plasmodium falciparum and later as a Beit Medical Fellow on molecular aspects of malaria. He returned to Malaysia in 1992, where he was a lecturer at the University of Science Malaysia for 7 years before he moved to Universiti Malaysia Sarawak. His research interests have centered on the molecular epidemiology, evolution, pathogenesis, and population genetics of malaria, with a particular interest in Plasmodium knowlesi.

Cyrus Daneshvar (M.R.C.P) is a United Kingdom training respiratory physician (Oxford University Hospitals). He earned his medical degree (M.B. Ch.B.) from the University of Birmingham, United Kingdom, in 1999. During his training, he spent 6 months working in South Africa and Mozambique, completed his M.R.C.P. exams while rotating through the medical specialties, and attended the Tropical Medicine in Practice course in Blantyre, Malawi. Following the completion of his diploma in Tropical Medicine and Hygiene at the London School of Tropical Medicine and Hygiene in 2005, he spent 3 years studying the clinical aspects of knowlesi malaria in Sarawak, working as a Clinical Research Fellow at the Malaria Research Centre, Universiti Malaysia Sarawak. He has an interest in infection and in particular malaria. He has been a technical adviser to the WHO.
REFERENCES
- 1. Garnham PCC. 1966. Malaria parasites and other haemosporidia. Blackwell Scientific Publications, Oxford, United Kingdom [Google Scholar]
- 2. Butcher GA, Cohen S, Garnham PC. 1970. Passive immunity in Plasmodium knowlesi malaria. Trans. R. Soc. Trop. Med. Hyg. 64:850–856 [DOI] [PubMed] [Google Scholar]
- 3. Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ. 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363:1017–1024 [DOI] [PubMed] [Google Scholar]
- 4. White NJ. 2008. Plasmodium knowlesi: the fifth human malaria parasite. Clin. Infect. Dis. 46:172–173 [DOI] [PubMed] [Google Scholar]
- 5. Cox-Singh J, Singh B. 2008. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 24:406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandosham AA. 1959. Malariology with special reference to Malaya. University of Malaya Press, Singapore [Google Scholar]
- 7. Hoffman SL, Campbell C, White CNJ. 2011. Malaria, p 646–675 In Guerrant RL, Walker DH, Weller PF. (ed), Tropical infectious diseases: principles, pathogens and practice, 3rd ed Elsevier Saunders, Oxford, United Kingdom [Google Scholar]
- 8. Knowles RM, Das Gupta B. 1932. A study of monkey malaria and its experimental transmission to man. Indian Med. Gaz. 67:301–320 [PMC free article] [PubMed] [Google Scholar]
- 9. Knowlesi R. 1935. Monkey malaria. Br. Med. J. ii:1020 [Google Scholar]
- 10. Napier LE, Campbell HGM. 1932. Observations on a Plasmodium infection which causes haemoglobinuria in certain species of monkey. Indian Med. Gaz. 67:246–249 [PMC free article] [PubMed] [Google Scholar]
- 11. Sinton JA, Mulligan HW. 1932. A critical review of the literature relating to the identification of the malaria parasites recorded from monkeys of the families Cercopithecidae and Colobidae. Rec. Malar. Surv. India III:24 [Google Scholar]
- 12. Sinton JA, Mulligan HW. 1933. A critical review of the literature relating to the identification of the malaria parasites recorded from monkeys of the families Cercopithecidae and Colobidae. Rec. Malar. Surv. India III:62 [Google Scholar]
- 13. Wagner-Jauregg J. 1918. Über die Einwirkung der Malaria auf die progressive Paralyse. Psychiatr. Neurol. Wochenschr. 20:132–134 [Google Scholar]
- 14. Shute PG. 1958. Thirty years of malaria-therapy. J. Trop. Med. Hyg. 61:57–61 [PubMed] [Google Scholar]
- 15. van Rooyen CE, Pile GR. 1935. Observations on infection by Plasmodium knowlesi (ape malaria) in the treatment of general paralysis of the insane. Br. Med. J. ii:662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milam DF, Kusch E. 1938. Observations of Plasmodium knowlesi malaria in general paresis. South. Med. J. 31:947–949 [Google Scholar]
- 17. Chopra RN, Das Gupta BM. 1936. A preliminary note on the treatment of neuro-syphilis with monkey malaria. Indian Med. Gaz. 1936:187–189 [PMC free article] [PubMed] [Google Scholar]
- 18. Nicol WD. 1935. Malaria in general paresis of the insane. Br. Med. J. ii:760 [Google Scholar]
- 19. Ciuca M, Ballif L, Chelarescu M, Lavrinenko M, Zotta E. 1937. Contributions a l'étude de l'action pathogene de Pl. knowlesi pour l'homme (considerations sur l'immunite naturelle et l'immunité acquise contre cette espece de parasite). Bull. Soc. Pathol. Exot. 30:305–315 [Google Scholar]
- 20. Ciuca M, Tomescu P, Badenski G, Badenski A, Terintianu PIM. 1937. Contribution à l'étude de la virulence du Pl. knowlesi chez l'homme. Caractères de la maladie et biologie du parasitie. Arch. Roum. Pathol. Exp. Microbiol. 10:5–28 [Google Scholar]
- 21. Ciuca M, Chelarescu M, Sofletes A, Constantinescu P, Teritaenu E, Cortez P, Balanovschi G, Ilies M. (ed). 1955. Contribution experimentale a l'etude de l'immunite dans le paludisme. Editions Acad. Rep. Pop. Roumaine. L'Academie, Bucharest, Romania [Google Scholar]
- 22. Chin W, Contacos PG, Coatney GR, Kimball HR. 1965. A naturally acquired quotidian-type malaria in man transferrable to monkeys. Science 149:865. [DOI] [PubMed] [Google Scholar]
- 23. Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. 1968. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am. J. Trop. Med. Hyg. 17:355–358 [DOI] [PubMed] [Google Scholar]
- 24. Fong YL, Cadigan FC, Coatney GR. 1971. A presumptive case of naturally occurring Plasmodium knowlesi malaria in man in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 65:839–840 [DOI] [PubMed] [Google Scholar]
- 25. Eyles DE, Coatney GR, Getz ME. 1960. Vivax-type malaria parasite of macaques transmissible to man. Science 131:1812–1813 [DOI] [PubMed] [Google Scholar]
- 26. Schmidt LH, Greenland R, Genther CS. 1961. The transmission of Plasmodium cynomolgi to man. Am. J. Trop. Med. Hyg. 10:679–688 [DOI] [PubMed] [Google Scholar]
- 27. Warren M, Cheong WH, Fredericks HK, Coatney GR. 1970. Cycles of jungle malaria in West Malaysia. Am. J. Trop. Med. Hyg. 19:383–393 [DOI] [PubMed] [Google Scholar]
- 28. Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Rahman HA, Conway DJ, Singh B. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barber BE, William T, Jikal M, Jilip J, Dhararaj P, Menon J, Yeo TW, Anstey NM. 2011. Plasmodium knowlesi malaria in children. Emerg. Infect. Dis. 17:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Joveen-Neoh WF, Chong KL, Wong CM, Lau TY. 2011. Incidence of malaria in the interior division of Sabah, Malaysian Borneo, based on nested PCR. J. Parasitol. Res. 2011:104284 doi:10.1155/2011/104284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bronner U, Divis PC, Färnert A, Singh B. 2009. Swedish traveller with Plasmodium knowlesi malaria after visiting Malaysian Borneo. Malar. J. 8:15 doi:10.1186/1475-2875-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, Wong KT, Adem P, Zaki SR, Singh B, Krishna S. 2010. Severe malaria—a case of fatal Plasmodium knowlesi infection with post-mortem findings: a case report. Malar. J. 9:10 doi:10.1186/1475-2875-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daneshvar C, Davis TM, Cox-Singh J, Rafa'ee MZ, Zakaria SK, Divis PC, Singh B. 2009. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin. Infect. Dis. 49:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. 2012. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar. J. 11:284 doi:10.1186/1475-2875-11-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, Khoo S, Frederick C, Jelip J, Anstey NM, Yeo TW. 2011. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg. Infect. Dis. 17:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoosen A, Shaw MT. 2011. Plasmodium knowlesi in a traveller returning to New Zealand. Travel Med. Infect. Dis. 9:144–148 [DOI] [PubMed] [Google Scholar]
- 37. Lee KS, Cox-Singh J, Brooke G, Matusop A, Singh B. 2009. Plasmodium knowlesi from archival blood films: further evidence that human infections are widely distributed and not newly emergent in Malaysian Borneo. Int. J. Parasitol. 39:1125–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh B, Daneshvar C. 2010. Plasmodium knowlesi malaria in Malaysia. Med. J. Malaysia 65:166–172 [PubMed] [Google Scholar]
- 39. van Hellemond JJ, Rutten M, Koelewijn R, Zeeman AM, Verweij JJ, Wismans PJ, Kocken CH, van Genderen PJ. 2009. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg. Infect. Dis. 15:1478–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anderios F, Mohamed Z, Ratnam S, Ibrahim MY, Awang TAM. 2008. Detection of malaria parasites in Sabah by nested polymerase chain reaction: a focus of naturally acquired Plasmodium knowlesi infections. Sains Malaysiana 37:137–141 [Google Scholar]
- 41. Naing DKS, Anderios F, Lin Z. 2011. Geographic and ethnic distribution of P. knowlesi infection in Sabah, Malaysia. Int. J. Collab. Res. Int. Med. Public Health 3:391–400 [Google Scholar]
- 42. Link L, Bart A, Verhaar N, van Gool T, Pronk M, Scharnhorst V. 2012. Molecular diagnosis of Plasmodium knowlesi in a Dutch traveler by real-time PCR. J. Clin. Microbiol. 50:2523–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, Anstey NM, Yeo TW. 19 October 2012. A prospective comparative study of knowlesi, falciparum and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and P. vivax but no mortality with early referral and artesunate therapy. Clin. Infect. Dis. doi:10.1093/cid/cis902 [DOI] [PubMed] [Google Scholar]
- 44. Vythilingam I, Noorazian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, Norparina I, Noorrain A, Lokmanhakim S. 2008. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit. Vectors 1:26 doi:10.1186/1756-3305-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee CE, Adeeba K, Freigang G. 2010. Human Plasmodium knowlesi infections in Klang Valley, Peninsula Malaysia: a case series. Med. J. Malaysia 65:63–65 [PubMed] [Google Scholar]
- 46. Lau YL, Tan LH, Chin LC, Fong MY, Noraishah MA, Rohela M. 2011. Plasmodium knowlesi reinfection in human. Emerg. Infect. Dis. 17:1314–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kantele A, Marti H, Felger I, Müller D, Jokiranta TS. 2008. Monkey malaria in a European traveler returning from Malaysia. Emerg. Infect. Dis. 14:1434–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jongwutiwes S, Putaporntip C, Iwasaki T, Sata T, Kanbara H. 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg. Infect. Dis. 10:2211–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jongwutiwes S, Buppan P, Kosuvin R, Seethamchai S, Pattanawong U, Sirichaisinthop J, Putaporntip C. 2011. Plasmodium knowlesi malaria in humans and macaques, Thailand. Emerg. Infect. Dis. 17:1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Putaporntip C, Hongsrimuang T, Seethamchai S, Kobasa T, Limkittikul K, Cui L, Jongwutiwes S. 2009. Differential prevalence of Plasmodium infections and cryptic Plasmodium knowlesi malaria in humans in Thailand. J. Infect. Dis. 199:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sermwittayawong N, Singh B, Nishibuchi M, Sawangjaroen N, Vuddhakul V. 2012. Human Plasmodium knowlesi infection in Ranong province, southwestern border of Thailand. Malar. J. 11:36 doi:10.1186/1475-2875-11-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berry A, Iriart X, Wilhelm N, Valentin A, Cassaing S, Witkowski B, Benoit-Vical F, Menard S, Olagnier D, Fillaux J, Sire S, Le Coustumier A, Magnaval JF. 2011. Imported Plasmodium knowlesi malaria in a French tourist returning from Thailand. Am. J. Trop. Med. Hyg. 84:535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tang T-HT, Salas A, Ali-Tammam M, Martínez MC, Lanza M, Arroyo E, Rubio JM. 2010. First case of detection of Plasmodium knowlesi in Spain by real time PCR in a traveller from Southeast Asia. Malar. J. 9:219 doi:10.1186/1475-2875-9-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Nolder D, Sutherland C, Lee KS, Singh B. 2008. Human infections with Plasmodium knowlesi, the Philippines. Emerg. Infect. Dis. 14:811–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ennis JG, Teal AE, Habura A, Madison-Antenucci S, Keithly JS, Arguin PM, Barnwell JW, Collins WE, Mali S, Slutsker L, Dasilva A, Hwang J. 2009. Simian malaria in a US traveler—New York, 2008. MMWR Morb. Mortal. Wkly. Rep. 58:229–23219282815 [Google Scholar]
- 56. Kuo M-C, Chiang T-Y, Chan C-W, Tsai W-S, Ji D-D. 2009. A case report of simian malaria Plasmodium knowlesi, in a Taiwanese traveler from Palawan Island. Taiwan Epidemiol. Bull. 25:178–191 [Google Scholar]
- 57. Zhu HM, Li J, Zheng H. 2006. Human natural infection of Plasmodium knowlesi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 24:70–71 [PubMed] [Google Scholar]
- 58. Jiang N, Chang Q, Sun X, Lu H, Yin J, Zhang Z, Wahlgren M, Chen Q. 2010. Co-infections with Plasmodium knowlesi and other malaria parasites, Myanmar. Emerg. Infect. Dis. 16:1476–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Pei SW, Tu TM, Loh JP, Leo YS. 2008. Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg. Infect. Dis. 14:814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ong CW, Lee SY, Koh WH, Ooi EE, Tambyah PA. 2009. Monkey malaria in humans: a diagnostic dilemma with conflicting laboratory data. Am. J. Trop. Med. Hyg. 80:927–928 [PubMed] [Google Scholar]
- 61. Jeslyn WP, Huat TC, Vernon L, Irene LM, Sung LK, Jarrod LP, Singh B, Ching NL. 2011. Molecular epidemiological investigation of Plasmodium knowlesi in humans and macaques in Singapore. Vector Borne Zoonotic Dis. 11:131–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Marchand RP, Culleton R, Maeno Y, Quang NT, Nakazawa S. 2011. Co-infections of Plasmodium knowlesi, P. falciparum, and P. vivax among humans and Anopheles dirus mosquitoes, southern Vietnam. Emerg. Infect. Dis. 17:1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Van den Eede P, Van HN, Van Overmeir C, Vythilingam I, Duc TN, Hung LX, Manh HN, Anné J, D'Alessandro U, Erhart A. 2009. Human Plasmodium knowlesi infections in young children in central Vietnam. Malar. J. 8:249 doi:10.1186/1475-2875-8-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Figtree M, Lee R, Bain L, Kennedy T, Mackertich S, Urban M, Cheng Q, Hudson BJ. 2010. Plasmodium knowlesi in human, Indonesian Borneo. Emerg. Infect. Dis. 16:672–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sulistyaningsih E, Fitri LE, Löscher T, Berens-Riha N. 2010. Diagnostic difficulties with Plasmodium knowlesi infection in humans. Emerg. Infect. Dis. 16:1033–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Health Protection Agency UK 2011. Imported malaria cases and deaths, United Kingdom: 1992–2011. Health Protection Agency UK, London, United Kingdom: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733773780 [Google Scholar]
- 67. Khim N, Siv S, Kim S, Mueller T, Fleischmann E, Singh B, Divis PC, Steenkeste N, Duval L, Bouchier C, Duong S, Ariey F, Menard D. 2011. Plasmodium knowlesi infection in humans, Cambodia, 2007-2010. Emerg. Infect. Dis. 17:1900–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee KS, Cox-Singh J, Singh B. 2009. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar. J. 8:73 doi:10.1186/1475-2875-8-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edeson JFB, Davey DG. 1953. Isolation of a virulent strain of Plasmodium knowlesi. Trans. R. Soc. Trop. Med. Hyg. 47:259–260 [Google Scholar]
- 70. Eyles DE, Laing ABG, Dobrovolny CG. 1962. The malaria parasites of the pig-tailed macaque, Macaca nemestrina nemestrina (Linnaeus), in Malaya. Indian J. Malariol. 16:285–298 [Google Scholar]
- 71. Eyles DE, Laing ABG, Warren M, Sandosham AA. 1962. Malaria parasites of Malaysan leaf monkeys of the genus Presbytis. Med. J. Malaya 17:85–86 [Google Scholar]
- 72. Lambrecht FL, Dunn FL, Eyles DE. 1961. Isolation of Plasmodium knowlesi from Philippine macaques. Nature 191:1117–1118 [DOI] [PubMed] [Google Scholar]
- 73. Tsukamoto M, Miyata A. 1978. Surveys on simian malaria parasites and their vector in Palawan Island, the Philippines. Trop. Med. 20:39–50 [Google Scholar]
- 74. Lee KS, Divis PC, Zakaria SK, Matusop A, Julin RA, Conway DJ, Cox-Singh J, Singh B. 2011. Plasmodium knowlesi: reservoir hosts and tracking the emergence in humans and macaques. PLoS Pathog. 7:e1002015 doi:10.1371/journal.ppat.1002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fooden J. 1982. Ecogeographic segregation of macaque species. Primates 23:574–579 [Google Scholar]
- 76. Fooden J. 2006. Comparative review of fascicularis-group species of macaques (Primates: Macaca). Fieldiana Zool. 107:1–43 [Google Scholar]
- 77. Sallum MA, Peyton EL, Wilkerson RC. 2005. Six new species of the Anopheles leucosphyrus group, reinterpretation of An. elegans and vector implications. Med. Vet. Entomol. 19:158–199 [DOI] [PubMed] [Google Scholar]
- 78. Peyton EL. 1990. A new classification for the Leucosphyrus group of Anopheles (Cellia). Mosq. Syst. 21:197–205 [Google Scholar]
- 79. Wharton RH, Eyles DE. 1961. Anopheles hackeri, a vector of Plasmodium knowlesi in Malaya. Science 134:279–280 [DOI] [PubMed] [Google Scholar]
- 80. Collins WE, Contacos PG, Guinn EG. 1967. Studies on the transmission of simian malarias. II. Transmission of the H strain of Plasmodium knowlesi by Anopheles balabacensis balabacensis. J. Parasitol. 53:841–844 [PubMed] [Google Scholar]
- 81. Collins WE, Contacos PG, Skinner JC, Guinn EG. 1971. Studies on the transmission of simian malaria. IV. Further studies on the transmission of Plasmodium knowlesi by Anopheles balabacensis balabacensis mosquitoes. J. Parasitol. 57:961–966 [PubMed] [Google Scholar]
- 82. Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B. 2008. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar. J. 7:52 doi:10.1186/1475-2875-7-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. 2006. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans. R. Soc. Trop. Med. Hyg. 100:1087–1088 [DOI] [PubMed] [Google Scholar]
- 84. Jiram AI, Vythilingam I, NoorAzian YM, Yusof YM, Azahari AH, Fong M-Y. 2012. Entomologic investigation of Plasmodium knowlesi vectors in Kuala Lipis, Pahang, Malaysia. Malar. J. 11:213 doi:10.1186/1475-2875-11-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nakazawa S, Marchand RP, Quang NT, Culleton R, Manh ND, Maeno Y. 2009. Anopheles dirus co-infection with human and monkey malaria parasites in Vietnam. Int. J. Parasitol. 39:1533–1537 [DOI] [PubMed] [Google Scholar]
- 86. Joy DA, Feng X, Mu J, Furuya T, Chotivanich K, Krettli AU, Ho M, Wang A, White NJ, Suh E, Beerli P, Su XZ. 2003. Early origin and recent expansion of Plasmodium falciparum. Science 300:318–321 [DOI] [PubMed] [Google Scholar]
- 87. Krief S, Escalante AA, Pacheco MA, Mugisha L, André C, Halbwax M, Fischer A, Krief JM, Kasenene JM, Crandfield M, Cornejo OE, Chavatte JM, Lin C, Letourneur F, Grüner AC, McCutchan TF, Rénia L, Snounou G. 2010. On the diversity of malaria parasites in African apes and the origin of Plasmodium falciparum from Bonobos. PLoS Pathog. 6:e1000765 doi:10.1371/journal.ppat.1000765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Escalante AA, Cornejo OE, Freeland DE, Poe AC, Durrego E, Collins WE, Lal AA. 2005. A monkey's tale: the origin of Plasmodium vivax as a human malaria parasite. Proc. Natl. Acad. Sci. U. S. A. 102:1980–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mu J, Joy DA, Duan J, Huang Y, Carlton J, Walker J, Barnwell J, Beerli P, Charleston MA, Pybus OG, Su XZ. 2005. Host switch leads to emergence of Plasmodium vivax malaria in humans. Mol. Biol. Evol. 22:1686–1693 [DOI] [PubMed] [Google Scholar]
- 90. Macaulay V, Hill C, Achilli A, Rengo C, Clarke D, Meehan W, Blackburn J, Semino O, Scozzari R, Cruciani F, Taha A, Shaari NK, Raja JM, Ismail P, Zainuddin Z, Goodwin W, Bulbeck D, Bandelt HJ, Oppenheimer S, Torroni A, Richards M. 2005. Single, rapid coastal settlement of Asia revealed by analysis of complete mitochondrial genomes. Science 308:1034–1036 [DOI] [PubMed] [Google Scholar]
- 91. Ziegler T, Abegg C, Meijaard E, Perwitasari-Farajallah D, Walter L, Hodges JK, Roos C. 2007. Molecular phylogeny and evolutionary history of Southeast Asian macaques forming the M. silenus group. Mol. Phylogenet. Evol. 42:807–816 [DOI] [PubMed] [Google Scholar]
- 92. Tosi AJ, Morales JC, Melnick DJ. 2003. Paternal, maternal, and biparental molecular markers provide unique windows onto the evolutionary history of macaque monkeys. Evolution 57:1419–1435 [DOI] [PubMed] [Google Scholar]
- 93. Delson E. 1980. Fossil macaques, phyletic relationships and a scenario of deployment, p 10–30 In Lindburg DG. (ed), The macaques: studies in ecology, behavior and evolution. Van Nostrand Rheinhold, Amsterdam, Netherlands [Google Scholar]
- 94. Voris HK. 2000. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. J. Biogeogr. 27:1153–1167 [Google Scholar]
- 95. Atkinson QD, Gray RD, Drummond AJ. 2008. mtDNA variation predicts population size in humans and reveals a major southern Asian chapter in human prehistory. Mol. Biol. Evol. 25:468–474 [DOI] [PubMed] [Google Scholar]
- 96. de Zulueta J. 1956. Malaria in Sarawak and Brunei. Bull. World Health Organ. 15:651–671 [PMC free article] [PubMed] [Google Scholar]
- 97. Coatney GR, Collins WE, Warren M, Contacos PG. 1971. The primate malarias. US Department of Health, Education, and Welfare, Bethesda, MD [Google Scholar]
- 98. Feild JW. 1949. Blood examination and prognosis in acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 43:33–48 [DOI] [PubMed] [Google Scholar]
- 99. World Health Organization 2011. Informal consultation on the public health importance of Plasmodium knowlesi meeting report. World Health Organization Regional Office for the Western Pacific Press, Manila, Philippines [Google Scholar]
- 100. Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. 1999. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am. J. Trop. Med. Hyg. 60:687–692 [DOI] [PubMed] [Google Scholar]
- 101. Snounou G, Singh B. 2002. Nested PCR analysis of Plasmodium parasites. Methods Mol. Med. 72:189–203 [DOI] [PubMed] [Google Scholar]
- 102. Tanomsing N, Imwong M, Theppabutr S, Pukrittayakamee S, Day NP, White NJ, Snounou G. 2010. Accurate and sensitive detection of Plasmodium species in humans by use of the dihydrofolate reductase-thymidylate synthase linker region. J. Clin. Microbiol. 48:3735–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Singh B, Cox-Singh J, Miller AO, Abdullah MS, Snounou G, Rahman HA. 1996. Detection of malaria in Malaysia by nested polymerase chain reaction amplification of dried blood spots on filter papers. Trans. R. Soc. Trop. Med. Hyg. 90:519–521 [DOI] [PubMed] [Google Scholar]
- 104. Roper C, Elhassan IM, Hviid L, Giha H, Richardson W, Babiker H, Satti GM, Theander TG, Arnot DE. 1996. Detection of very low level Plasmodium falciparum infections using the nested polymerase chain reaction and a reassessment of the epidemiology of unstable malaria in Sudan. Am. J. Trop. Med. Hyg. 54:325–331 [DOI] [PubMed] [Google Scholar]
- 105. Divis PC, Shokoples SE, Singh B, Yanow SK. 2010. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar. J. 9:344 doi:10.1186/1475-2875-9-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lau YL, Fong MY, Mahmud R, Chang PY, Palaeya V, Cheong FW, Chin LC, Anthony CN, Al-Mekhlafi AM, Chen Y. 2011. Specific, sensitive and rapid detection of human Plasmodium knowlesi infection by loop-mediated isothermal amplification (LAMP) in blood samples. Malar. J. 10:197 doi:10.1186/1475-2875-10-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, Igarashi I. 2010. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J. Clin. Microbiol. 48:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lucchi NW, Poorak M, Oberstaller J, DeBarry J, Srinivasamoorthy G, Goldman I, Xayavong M, da Silva AJ, Peterson DS, Barnwell JW, Kissinger J, Udhayakumar V. 2012. A new single-step PCR assay for the detection of the zoonotic malaria parasite Plasmodium knowlesi. PLoS One 7:e31848 doi:10.1371/journal.pone.0031848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Babady NE, Sloan LM, Rosenblatt JE, Pritt BS. 2009. Detection of Plasmodium knowlesi by real-time polymerase chain reaction. Am. J. Trop. Med. Hyg. 81:516–518 [PubMed] [Google Scholar]
- 110. Oddoux O, Debourgogne A, Kantele A, Kocken CH, Jokiranta TS, Vedy S, Puyhardy JM, Machouart M. 2011. Identification of the five human Plasmodium species including P. knowlesi by real-time polymerase chain reaction. Eur. J. Clin. Microbiol. Infect. Dis. 30:597–601 [DOI] [PubMed] [Google Scholar]
- 111. Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315–320 [DOI] [PubMed] [Google Scholar]
- 112. Cox-Singh J, Mahayet S, Abdullah MS, Singh B. 1997. Increased sensitivity of malaria detection by nested polymerase chain reaction using simple sampling and DNA extraction. Int. J. Parasitol. 27:1575–1577 [DOI] [PubMed] [Google Scholar]
- 113. Imwong M, Tanomsing N, Pukrittayakamee S, Day NP, White NJ, Snounou G. 2009. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J. Clin. Microbiol. 47:4173–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Murray CK, Bennett JW. 2009. Rapid diagnosis of malaria. Interdiscip. Perspect. Infect. Dis. 2009:415953 doi:10.1155/2009/415953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kawai S, Hirai M, Haruki K, Tanabe K, Chigusa Y. 2009. Cross-reactivity in rapid diagnostic tests between human malaria and zoonotic simian malaria parasite Plasmodium knowlesi infections. Parasitol. Int. 58:300–302 [DOI] [PubMed] [Google Scholar]
- 116. McCutchan TF, Piper RC, Makler MT. 2008. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg. Infect. Dis. 14:1750–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Milam DF, Coggeshall LT. 1938. Duration of Plasmodium knowlesi infections in man. Am. J. Trop. Med. 18:331–338 [Google Scholar]
- 118. Willmann M, Ahmed A, Siner A, Tien WI, Woon LC, Singh B, Krishna S, Cox-Singh J. 2012. Laboratory markers of disease severity in Plasmodium knowlesi infection: a case control study. Malar. J. 11:363 doi:10.1186/1475-2875-11-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, Meshnick SR, Gasser RA, Wongsrichanalai C. 2004. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am. J. Trop. Med. Hyg. 70:8–14 [PubMed] [Google Scholar]
- 120. Eriksson B, Hellgren U, Rombo L. 1989. Changes in erythrocyte sedimentation rate, C-reactive protein and hematological parameters in patients with acute malaria. Scand. J. Infect. Dis. 21:434–441 [PubMed] [Google Scholar]
- 121. Schexneider KI, Reedy EA. 2005. Thrombocytopenia in dengue fever. Curr. Hematol. Rep. 4:145–148 [PubMed] [Google Scholar]
- 122. World Health Organization Communicable Disease Cluster 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl 1):S1–S90 [PubMed] [Google Scholar]
- 123. Price RN, Simpson JA, Nosten F, Luxemburger C, Hkirjaroen L, ter Kuile F, Chongsuphajaisiddhi T, White NJ. 2001. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 65:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner RC. 1983. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N. Engl. J. Med. 309:61–66 [DOI] [PubMed] [Google Scholar]
- 125. White NJ, Warrell DA, Looareesuwan S, Chanthavanich P, Phillips RE, Pongpaew P. 1985. Pathophysiological and prognostic significance of cerebrospinal-fluid lactate in cerebral malaria. Lancet i:776–778 [DOI] [PubMed] [Google Scholar]
- 126. Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, Harinasuta T. 1982. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N. Engl. J. Med. 306:313–319 [DOI] [PubMed] [Google Scholar]
- 127. Fatih FA, Siner A, Ahmed A, Woon LC, Craig AG, Singh B, Krishna S, Cox-Singh J. 2012. Cytoadherence and virulence—the case of Plasmodium knowlesi malaria. Malar. J. 11:33 doi:10.1186/1475-2875-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Losert H, Schmid K, Wilfing A, Winkler S, Staudinger T, Kletzmayr J, Burgmann H. 2000. Experiences with severe P. falciparum malaria in the intensive care unit. Intensive Care Med. 26:195–201 [DOI] [PubMed] [Google Scholar]
- 129. Salord F, Allaouchiche B, Gaussorgues P, Boibieux A, Sirodot M, Gerard-Boncompain M, Biron F, Peyramond D, Robert D. 1991. Severe falciparum malaria (21 cases). Intensive Care Med. 17:449–454 [DOI] [PubMed] [Google Scholar]
- 130. Gachot B, Wolff M, Nissack G, Veber B, Vachon F. 1995. Acute lung injury complicating imported Plasmodium falciparum malaria. Chest 108:746–749 [DOI] [PubMed] [Google Scholar]
- 131. Cox-Singh J, Singh B, Daneshvar C, Planche T, Parker-Williams J, Krishna S. 2011. Anti-inflammatory cytokines predominate in acute human Plasmodium knowlesi infections. PLoS One 6:e20541 doi:10.1371/journal.pone.0020541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chopra R, Das Gupta B, Sen B. 1938. Experimental studies on ape malaria with reference to its use in malaria therapy for nervous conditions. Proc. Natl. Inst. Sci. India 4:165–169 [Google Scholar]
- 133. Dutta GP, Singh PP. 1979. Blood schizontocidal activity of some antibiotics against Plasmodium knowlesi infection in Assamese monkey. Indian J. Med. Res. 70(Suppl):91–94 [PubMed] [Google Scholar]
- 134. Warhurst DC, Robinson BL, Peters W. 1983. The blood schizontocidal action of erythromycin against Plasmodium knowlesi infections in Macaca mulatta. Ann. Trop. Med. Parasitol. 77:231–237 [DOI] [PubMed] [Google Scholar]
- 135. Bajpai R, Dutta GP, Vishwakarma RA. 1989. Blood schizontocidal activity of a new antimalarial drug, arteether (alpha/beta), against Plasmodium knowlesi in rhesus monkeys. Trans. R. Soc. Trop. Med. Hyg. 83:484. [DOI] [PubMed] [Google Scholar]
- 136. Powers KG, Aikawa M, Nugent KM. 1976. Plasmodium knowlesi: morphology and course of infection in rhesus monkeys treated with clindamycin and its N-demethyl-4′-pentyl analog. Exp. Parasitol. 40:13–24 [DOI] [PubMed] [Google Scholar]
- 137. Nair CP, Ray AP, Singh J. 1953. Studies on Nuri strain of P. knowlesi. II. Therapeutic effect of pyrimethamine, proguanil and quinine. Indian J. Malariol. 7:371–376 [PubMed] [Google Scholar]
- 138. Shi YL, Li GF, Zhao JB, Yang JD, Ding DB. 1999. Schizontocidal effects of oral artesunate on Plasmodium berghei in mice and P. knowlesi in monkeys. Zhongguo Yao Li Bao 20:755–758 [PubMed] [Google Scholar]
- 139. Singh J, Ray AP, Basu PC, Nair CP. 1952. Acquired resistance to proguanil in Plasmodium knowlesi. Trans. R. Soc. Trop. Med. Hyg. 46:639–649 [DOI] [PubMed] [Google Scholar]
- 140. Singh J, Nair CP, Ray AP. 1954. Studies on Nuri strain of P. knowlesi. V. Acquired resistance to pyrimethamine. Indian J. Malariol. 8:187–195 [PubMed] [Google Scholar]
- 141. Daneshvar C, Davis TM, Cox-Singh J, Rafa'ee MZ, Zakaria SK, Divis PC, Singh B. 2010. Clinical and parasitological response to oral chloroquine and primaquine in uncomplicated human Plasmodium knowlesi infections. Malar. J. 9:238 doi:10.1186/1475-2875-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tanner M, de Savigny D. 2008. Malaria eradication back on the table. Bull. World Health Organ. 86:82 doi:10.2471/BLT.07.050633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Thang ND, Erhart A, Speybroeck N, Xa NX, Thanh NN, Ky PV, Hung LX, Thuan LK, Coosemans M, D'Alessandro U. 2009. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS One 4:e7369 doi:10.1371/journal.pone.0007369 [DOI] [PMC free article] [PubMed] [Google Scholar]