Abstract
SUMMARY
For the optimization of dosing regimens of anti-infective agents, it is imperative to have a good understanding of pharmacokinetics (PK) and pharmacodynamics (PD). Whenever possible, drug efficacy needs to be related to unbound concentrations at the site of action. For anti-infective drugs, the infection site is typically located outside plasma, and a drug must diffuse through capillary membranes to reach its target. Disease- and drug-related factors can contribute to differential tissue distribution. As a result, the assumption that the plasma concentration of drugs represents a suitable surrogate of tissue concentrations may lead to erroneous conclusions. Quantifying drug exposure in tissues represents an opportunity to relate the pharmacologically active concentrations to an observed pharmacodynamic parameter, such as the MIC. Selection of an appropriate specimen to sample and the advantages and limitations of the available sampling techniques require careful consideration. Ultimately, the goal will be to assess the appropriateness of a drug and dosing regimen for a specific pathogen and infection.
INTRODUCTION
I do not care about protein binding because I always measure free, unbound concentrations.
—Nick H. Holford, Paul Ehrlich meeting, Nuremberg, Germany, 2004
Most drugs bind to plasma and tissue proteins, resulting in a decrease in free, pharmacologically active concentrations. As described by the law of mass action, the binding is reversible, and equilibrium between the protein-bound and unbound drug is quickly established. The extent of binding is dependent on the structure and physicochemical properties of the drug molecule, which dictate affinity for the protein, as well as the drug and protein concentrations, experimental conditions, and the species in which the binding is studied (1, 2). The fraction unbound in plasma (fU) is computed as the ratio of free, unbound (CU) and total (CT) drug concentrations, as shown in equation 1 (3). Assuming that a drug has a single binding site, fU can also be expressed as a function of the equilibrium dissociation constant (KD), the maximum binding capacity (Bmax), and CU. The relationship becomes more complex if more than one binding site is involved, but a detailed mathematical description of this scenario is outside the scope of this paper.
| (1) |
At commonly prescribed doses, most drugs display linear binding, whereby the fraction unbound remains unchanged as drug concentrations increase. Under these conditions, CU is much lower than K, and the function may be simplified according to equation 2 (3):
| (2) |
When unbound concentrations exceed the number of available binding sites (CU > KD), this simple relationship does not hold, and protein binding becomes concentration dependent (i.e., saturable binding). Ceftriaxone, cefazolin, cefonicid, and ertapenem are examples of antimicrobials for which nonlinear protein binding has been reported in the literature (4–7).
Binding to protein can occur in both intra- and extravascular spaces and is an important determinant of a drug's pharmacokinetics (PK), as it will impact distribution and elimination processes. Tissue binding increases the fraction of drug outside plasma and away from systemic drug elimination organs. Similarly, plasma protein binding limits the unbound drug concentration available for drug metabolism, filtration, and/or excretion by the kidneys. For the liver, the scenario is more complex, and plasma protein binding may or may not affect drug clearance depending on the affinity of the drug for the metabolizing enzyme (i.e., intrinsic clearance) (8). Depending on the PK properties of the drug, drug elimination may be impacted. When applying the well-stirred model of hepatic elimination, low-extraction drugs will be dependent on fU and the intrinsic clearance, whereas the hepatic blood flow is the limiting factor for high-extraction drugs.
For anti-infective drugs, the impact of protein binding is of prime interest because the free, unbound drug concentrations at the site of action/infection are responsible for the drug's effect. Frequently, however, the site of infection is not the bloodstream, and a drug's ability to cross capillary membranes to reach the site of action is critical to its efficacy. For drugs where the interstitial fluid is the site of infection, and where disease-related barriers or efflux mechanisms do not impair drug distribution, plasma concentrations often represent a reasonable surrogate for tissue concentrations due to the establishment of a rapid equilibrium between plasma and tissues (9). For drugs where these conditions do not hold, selection of an appropriate measure of drug exposure requires careful consideration.
Table 1 summarizes the extent of plasma protein binding for drugs across various anti-infective drug classes (10). The impact of protein binding on drug efficacy will depend on the extent of the binding, PK properties, and intrinsic activity of the drug (11–15). In vitro, the impact of protein binding on antimicrobial activity is often investigated through determination of the MIC, time-kill curves, and cell culture assays (13, 16). Numerous in vitro studies have been published, which evaluated the impact of protein binding on antimicrobials (17–28), antivirals (29, 30), and antifungals (31, 32) by using protein supplements and/or serum to mimic in vivo conditions. In the majority of studies, free drug concentrations are not measured directly in the experimental setting, and the extent of protein binding is accounted for by the using binding values reported in the literature. This approach can be misleading if the protein binding reported in the literature differs from the actual protein binding in the experimental setting (16, 33).
Table 1.
Percentage of protein bound drug in plasma for anti-infective drug classesa
| Drug | % PB (mean ± SD or range) |
|---|---|
| Antimicrobials | |
| Amoxicillin | 18 |
| Amikacin | 4 ± 8 |
| Azithromycin | 7–50b |
| Cefazolin | 89 ± 2 |
| Cefdinir | 89 |
| Cefixime | 67 ± 1 |
| Ceftazidime | 21 ± 6 |
| Cefuroxime | 33 ± 6 |
| Cephalexin | 14 ± 3 |
| Cefepime | 16–19 |
| Ceftriaxone | 83–96b |
| Ciprofloxacin | 40 |
| Clarithromycin | 42–50 |
| Clindamycin | 93.6 ± 0.2 |
| Dapsone | 73 ± 1 |
| Daptomycin | 92 |
| Doxycycline | 88 ± 5 |
| Ertapenem | 84–96b |
| Erythromycin | 84 ± 3 |
| Fosfomycin | Negligible |
| Gentamicin | <10 |
| Imipenem-cilastatin | I, <20; C, ∼35 |
| Levofloxacin | 24–38 |
| Linezolid | 31 |
| Minocycline | 76 |
| Moxifloxacin | 39.4 ± 2.4 |
| Nitrofurantoin | 62 ± 4 |
| Rifampin | 60–90 |
| Sulfamethoxazole | 53 ± 5 |
| Telithromycin | 70 |
| Tigecycline | 71–89 |
| Trimethoprim | 37 ± 5 |
| Vancomycin | 30 ± 11 |
| Antivirals | |
| Acyclovir | 15 ± 4 |
| Atazanavir | 86 |
| Cidofovir | <6 |
| Darunavir | 95 |
| Didanosine | <5 |
| Efavirenz | 99.5–99.75 |
| Ganciclovir | 1–2 |
| Emtricitabine | <4 |
| Foscarnet | 14–17 |
| Lopinavir | 98–99 |
| Maraviroc | 76 |
| Raltegravir | 83 |
| Ribavirin | 0 |
| Ritonavir | 98–99 |
| Tenofovir | <1 |
| Valacyclovir | 13.5–17.9 |
| Zidovudine | <25 |
| Antifungals | |
| Amphotericin B | >90 |
| Caspofungin | 96.5 |
| Fluconazole | 11 ± 1 |
| Itraconazole | 99.8 |
| Micafungin | 99 |
| Posaconazole | 98 |
| Voriconazole | 58 |
| Atypical | |
| Chloroquine | S, 66.6 ± 3.3; R, 42.7 ± 2.1 |
| Ethambutol | 6–30 |
| Albendazole | 70 |
| Hydroxychloroquine | 45 ± 3 |
| Isoniazid | Negligible |
| Mefloquine | 98.2 |
| Metronidazole | 11 ± 3 |
| Pyrazinamide | 10 |
Protein binding values reported are taken from the same reference (10). % PB, percentage of protein-bound drug in plasma for anti-infective drug classes; I, imipenem; C, cilastatin; S, S isomer; R, R isomer.
Concentration-dependent binding.
In vivo, animal infection models serve as one mechanism to evaluate the impact of protein binding on tissue concentrations and antimicrobial efficacy (34–37), although in most cases, unbound drug concentrations are not measured directly, but rather, total drug concentrations are corrected according to the extent of protein binding measured in vitro.
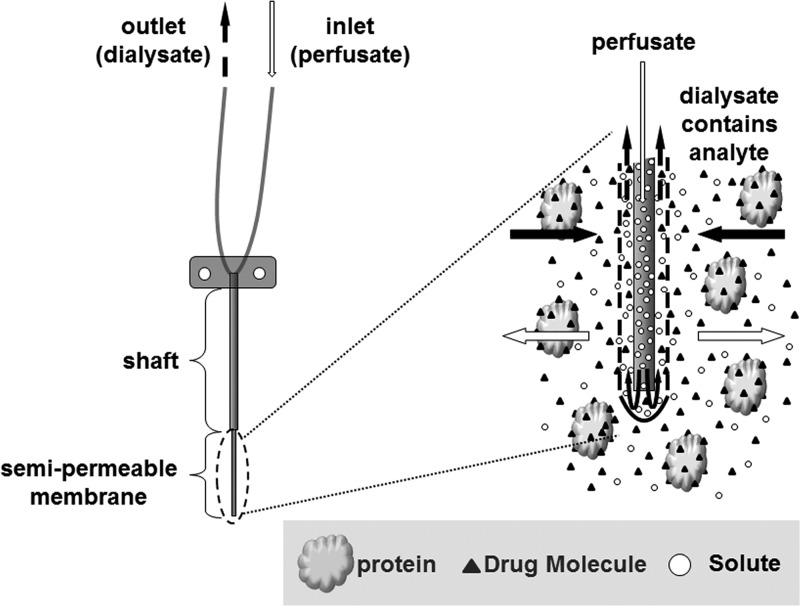
For anti-infective drugs where free plasma concentrations do not represent a reliable surrogate of drug exposure at the site of infection, direct measurements of free tissue concentrations offer a more meaningful approach. There are various techniques that are routinely applied to measure unbound tissue concentrations in humans. These techniques include microdialysis, tissue biopsy, imaging techniques, and saliva or blister fluid sampling (38–47). Each of these techniques has advantages and limitations. Microdialysis is an innovative technique that is being increasingly used to determine free, unbound concentrations in the interstitial space fluid (ISF) of various tissues (Fig. 1). It is a semi-invasive sampling technique whereby a probe containing a semipermeable membrane is implanted into a tissue and infused with a solution mimicking tissue conditions at a constant rate. Movement of drug molecules via passive diffusion, and determination of the probe recovery value, allow for a direct measurement of unbound drug concentrations in tissues over time without the need for a sample purification method. In contrast to measuring whole tissue concentrations in homogenized samples, where measured concentrations represent a mixture of intra- and extracellular contents, microdialysis quantifies unbound drug concentrations in the extracellular fluid, the site of action for many antimicrobials (42).
Fig 1.
Pictorial representation of a microdialysis probe inserted into a medium containing an analyte of interest and a drug binding protein.
Once free drug concentrations at or at least close to the site of infection are measured, they can be correlated to the pharmacodynamic (PD) effect to help guide the selection of an optimal dose. The goal of this review paper is to outline the benefit of measuring and relating unbound drug concentrations to anti-infective drug efficacy.
METHODS AVAILABLE TO MEASURE PROTEIN BINDING AND TISSUE DISTRIBUTION
Protein Binding Determination
Frequently, free drug concentrations in plasma are estimated by correcting total plasma concentrations for protein binding. Available techniques for measurement of protein binding include equilibrium dialysis, ultrafiltration, ultracentrifugation, microdialysis, chromatographic methods, capillary electrophoresis, fluorescence spectroscopy, and ultrafast immunoextraction. These methods have been compared extensively in published review articles, and only an overview is provided here (16, 48, 49). With the exception of microdialysis, which may be applied in vivo to measure unbound drug concentrations, these methods are used in vitro for protein binding determination. Measurement of the extent of protein binding may then be used to correct total concentrations for protein binding and to compute unbound drug concentrations in vivo.
The experimental setup varies between these methods. Equilibrium dialysis and ultrafiltration methods use a semipermeable membrane to separate the bound and unbound drug. In the case of ultrafiltration, separation is driven by centrifugal forces, whereas passive diffusion from plasma to a physiological buffer facilitates separation in the equilibrium dialysis setup. Equilibrium dialysis is considered to be the “gold standard” due to the reliability of the results and the robustness of the procedure. Likewise, ultrafiltration is considered to be simple and practical, as it does not require the use of a physiological buffer, the time to equilibration need not be determined a priori, and sample processing time is relatively short (16). A limitation of both methods is the potential of drug adsorption to the semipermeable membrane. In addition, for equilibrium dialysis, due to a greater osmotic pressure in the plasma (or serum) compartment, movement of physiological buffer into the plasma can dilute the drug concentration (16). Assuming that drug adsorption and volume shifts do not occur, the unbound drug fraction may be calculated by relating the unbound drug concentrations (CU) in the buffer (equilibrium dialysis) or ultrafiltrate (ultrafiltration) to the drug concentration in plasma (or serum) at the start of the experiment (CT), according to equation 1.
For ultracentrifugation, bound and unbound drug concentrations are separated by centrifugal forces only. As a result, membrane binding and fluid shifts need not be considered. Following centrifugation, a sample partitions into three layers: a top layer containing very-low-density lipoproteins and chylomicrons; a middle aqueous layer, which is protein free; and a bottom layer, which contains larger plasma proteins and lipoproteins (50). fU can be calculated by comparing the CT obtained prior to centrifugation and the CU obtained from the aqueous, middle layer. A major disadvantage of the method is that it is relatively low throughput, limiting how many samples may be processed at once (50).
Microdialysis uses a semipermeable membrane at the tip of a flexible probe to measure CU. The probes differ in size, geometry, and molecular weight cutoff, allowing for customization based on individual research needs. The microdialysis probe is perfused with a fluid (i.e., the “perfusate”) that resembles the composition of body fluids in the tissue of interest at a constant flow rate. Drug present in the sample matrix diffuses across the semipermeable membrane and into the perfusate. The collected sample (i.e., the “dialysate”) is then analyzed, and the drug concentration is then determined. At typical flow rates (1 to 10 μl/min), total equilibrium is not established between the tissue and perfusate. However, the constant flow of solute and unbound analyte across the semipermeable membrane results in the establishment of a pseudoequilibrium, which can be used to determine a calibration factor, i.e., the recovery. The flow of drug is assumed to be equal in both directions, resulting in identical recovery values. This assumption should always be tested in vitro, for example, by loss-of-drug (retrodialysis) and gain-of-drug (extraction efficiency) calibration experiments, before performing a clinical study. There is a plethora of calibration techniques available to experimentally determine the recovery value. These techniques include retrodialysis, extraction efficiency, no-net-flux, and internal standard calibration methods (51, 52). In a retrodialysis setup, a known drug concentration is added to the perfusate, and the relative recovery is calculated by relating the concentrations in the perfusate (Cperfusate) and dialysate (Cdialysate) (equation 3). The retrodialysis method is frequently applied in vivo for recovery determination.
| (3) |
For the extraction efficiency method, the ability of drug present in the matrix to cross the membrane and appear in the dialysate is evaluated by comparing the known concentration in the surrounding fluid (Cfluid) with Cdialysate (equation 4).
| (4) |
In theory, the relative recoveries determined by both methods should be identical and can be used to compute the true unbound drug concentrations in the ISF of the tissue by correcting Cdialysate by the experimentally determined recovery value (equation 5).
| (5) |
Improvements in technology have led to recent advances in the methods available to study drug-protein interactions. For example, high-performance affinity chromatography (HPAC), a technique coupling high-performance liquid chromatography (HPLC) with affinity columns containing proteins of interest, has been applied to measure the extent of protein binding in blood and characterize the binding process through estimation of equilibrium constants (53–57). Moreover, immunoaffinity chromatography, a chromatographic method that uses drug binding antibodies, has been applied to measure the free drug fraction of warfarin in a sample containing human serum albumin (58). One major advantage of these techniques is the ability to analyze a large number of samples in a short period of time. Fluorescence and UV absorption spectroscopy methods can also be used to characterize binding characteristics with plasma proteins (59). These techniques are useful to systematically study the binding mechanism and the extent of drug binding.
Target Site Exposure
If complete equilibration between unbound plasma and tissue concentrations is not achieved, evaluating a drug's tissue PK may offer considerable insight into its distribution properties. For anti-infective drugs, disease-specific factors may contribute to poor distribution to the site of infection. Direct measurements of tissue drug exposure may aid in accounting for differences in differential tissue penetration and facilitate rational dose selection for clinical drug development. Linking these measured target site concentrations to a PD effect provides a more meaningful approach for determining safe and effective dosing regimens.
Of special note, once a drug reaches the extracellular fluid, there may be an additional barrier to reaching the site of action, the cell membrane. This last step is critical for intracellular infections, where a pathogen may replicate and hide from a host's immune system. As with plasma and extracellular fluid concentrations, drug exposures inside and outside the cell may be different. Once within the cell, a drug must be able to reach its target, whose location will depend on the specific pathogen involved. For example, some species reside primarily in the cytosol (e.g., Listeria, Shigella, and Rickettsia), whereas others remain in phagosomes that may or may not fuse with endosomes (e.g., Mycobacterium) or lysosomes (60). Using antibiotics as an example, the extent of intracellular accumulation varies considerably between drug classes, and factors such as physicochemical properties, binding to cellular structures (e.g., phospholipids), and the presence of antibiotic efflux pumps play a key role in determining the extent of accumulation within the cell (60–62). Although within a drug class there are differences in the extent of intra- versus extracellular distribution, macrolides and fluoroquinolones accumulate extensively within cells, whereas β-lactams distribute only into the extracellular body water (60, 63).
The ability of an antibiotic to accumulate intracellularly is studied in vitro by using specific target cells (e.g., macrophages, polymorphonuclear neutrophils, and lung parenchyma cells) and/or in vivo, for example, by using a murine peritonitis infection model (64–67). In vivo studies provide an opportunity to evaluate drug distribution to the target site and the immune system's response to an infection. In human subjects, collection of plasma and microdialysis samples, coupled with isolation of white blood cells and subsequent determination of intracellular drug concentrations, provides an opportunity to assess tissue distribution in various compartments (68).
Tissue homogenates are frequently used to determine drug concentrations within a specific organ. However, during the homogenization process, intra- and extracellular components are mixed. Determined concentrations therefore represent average values for this particular tissue, which are not reflective of specific target site concentrations, such as concentrations within the ISF, the cell, and organelles, etc. (42). In fact, this approach underestimates concentrations of drugs that equilibrate exclusively with the interstitial fluid (e.g., β-lactams and aminoglycosides) and overestimates the concentrations of those that accumulate within cells (e.g., fluoroquinolones and macrolides) (9, 40, 69). Alternative methods for assessment of drug tissue distribution include microdialysis, tissue biopsy, skin blister fluid sampling, saliva sampling, and imaging techniques such as positron emission tomography (PET) and magnetic resonance spectroscopy (MRS). Major differences between these methods include the matrix sampled (blood, plasma, ISF, and saliva, etc.), invasive nature, collection period (continuous versus single time point), and direct measurement of unbound drug concentrations (40). However, a routine measurement of free target site concentrations may not always be feasible under certain conditions due to the accessibility of the tissue (e.g., the brain) or the health status of the patient (e.g., in critically ill patients).
A plethora of clinical microdialysis studies have been performed to study the extent to which antimicrobials reach the extracellular fluid of specific tissues (Table 2) (7, 46, 70–102). Although most have been performed with healthy volunteers, this technique has also been applied to measure unbound drug concentrations of the ISF of critically ill patients. Frequently, these studies are of small sample size and focus on soft tissue penetration.
Table 2.
Summary of clinical microdialysis studies performed with antimicrobials
| Drug(s) | Subject group | Tissue(s) | No. of subjects | Collection period (h) | Dosage(s)a | Reference |
|---|---|---|---|---|---|---|
| β-Lactams | ||||||
| Cefaclor | Healthy volunteers | Muscle | 12 | 5.5 | Single dose, various doses* | 70 |
| Cefazolin | Aortic valve replacement | Muscle, subcutaneous | 7 | 12 | 2 doses, 4 and 2 g | 71 |
| Cefpirome | Healthy volunteers | Muscle, subcutaneous | 12 | 8 | Single dose, 2 g | 72 |
| Cefpirome | Sepsis and healthy controls | Subcutaneous | 20 | 4 | Single dose, 2 g | 73 |
| Cefpirome, cefodizime | Healthy volunteers | Muscle, subcutaneous | 6 | 5 | Single dose, 2 g | 74 |
| Cefpodoxime, cefixime | Healthy volunteers | Muscle | 6 | 8 | Single doses, 400 mg each | 75 |
| Cefuroxime | Morbidly obese, abdominal surgery | Muscle, subcutaneous | 6 | 6 | Single dose, 1.5 g | 76 |
| Cefuroxime | Cardiac surgery | Muscle | 9 | 6 | Multiple dosing, various doses** | 77 |
| Ceftobiprole | Healthy volunteers | Muscle, subcutaneous | 12 | 0–12, 16, 24 | Single dose, 0.5 g | 78 |
| Doripenem | Healthy volunteers | Muscle, subcutaneous | 6 | 8 | Single dose, 0.5 g | 79 |
| Ertapenem | Healthy volunteers | Muscle, subcutaneous | 6 | 12 | Single dose, 1 g | 7 |
| Imipenem | Critically ill | Muscle, subcutaneous | 11 | 8 | Single and multiple dosing, 500 mg 3 or 4 times daily | 80 |
| Meropenem | Acute brain injury | Brain | 2 | 7 | Multiple doses, 1 g 3 times daily | 81 |
| Meropenem | Septic shock | Peritoneal fluid | 6 | 7 | 2 1-g doses | 82 |
| Meropenem | Pneumonia | Lung | 7 | 8 | Single dose, 1 g | 83 |
| Piperacillin | Aortic valve replacement | Muscle, subcutaneous | 6 | 4 | Single dose, 4 g | 84 |
| Piperacillin | Pneumonia | Lung | 5 | 8 | Single dose, 4 g | 85 |
| Aminoglycosides | ||||||
| Gentamicin | Healthy volunteers | Subcutaneous | 7 | 6 | Single dose, 240 mg | 86 |
| Macrolides-ketolides | ||||||
| Clarithromycin | Healthy volunteers | Muscle, subcutaneous | 6 | 8 | 250 mg (single dose) and 500 mg twice daily | 87 |
| Telithromcyin | Healthy volunteers | Muscle, subcutaneous | 10 | 8 | Single dose, 800 mg orally | 88 |
| Fluoroquinolones | ||||||
| Ciprofloxacin | Healthy volunteers | Muscle, subcutaneous | 8 | 8 | Single dose, 200 mg | 89 |
| Ciprofloxacin | Healthy volunteers | Muscle, subcutaneous | 8 | 12 | Single doses, 400 mg (i.v.) or 500 mg (oral) | 90 |
| Ciprofloxacin | Obese and lean subjects | Muscle, subcutaneous | 24 | 6 | Single dose, 2.85 mg/kg of body wt | 91 |
| Ciprofloxacin | Diabetics | Foot lesion, subcutaneous | 6 | 5 | Single dose, 200 mg | 92 |
| Gemifloxacin | Healthy volunteers | Muscle, subcutaneous | 12 | 10 | Single dose, 320 mg | 93 |
| Levofloxacin | Cardiac surgery | Lung | 10 | 8 | Single dose, 500 mg | 94 |
| Levofloxacin | Cardiac surgery | Lung | 6 | 8 | Single dose, 500 mg | 95 |
| Moxifloxacin | Healthy volunteers | Muscle, subcutaneous | 13 | 12 | Single dose, 400 mg | 46 |
| Oxazolidinones | ||||||
| Linezolid | Sepsis or septic shock | Muscle, subcutaneous | 12 | 8 | Single and multiple doses, 600 mg every 12 h | 96 |
| Linezolid | Healthy volunteers | Muscle, subcutaneous | 10 | 8 | Single and multiple doses, 600 mg twice daily | 97 |
| Linezolid | Healthy volunteers | Muscle, subcutaneous | 9 | 8 | Multiple doses, 600 mg twice daily | 98 |
| Tedizolid | Healthy volunteers | Muscle, subcutaneous | 12 | 12 | Single dose, 600 mg | 99 |
| Lipopeptides | ||||||
| Daptomycin | Diabetic and healthy controls | Subcutaneous | 12 | 24 | Single, 4 mg/kg | 100 |
| Miscellaneous | ||||||
| Fosfomycin | Healthy volunteers | Muscle, subcutaneous | 6 | 8 | Single, 4 or 8 g | 101 |
| Metronidazole | Gynecological | Muscle | 6 | 10 | Single, 500 mg | 102 |
i.v., intravenous. *, modified release, 500 and 750 mg; immediate release, 500 mg. **, cefuroxime, 3 g i.v. with anesthesia induction, then 1.5 g i.v. after cardiopulmonary bypass with protamine sulfate, and 1.5 g i.v. 8 h after surgery.
Similar to microdialysis, the skin blister technique attempts to evaluate tissue distribution through measurement of interstitial drug concentrations. First reported 40 years ago (103), the basic principle of the technique involves the separation of the dermis and epidermis through applied suction on the skin surface (104). The resulting fluid-filled blisters serve as a surrogate of interstitial fluid. In addition to drug sampling, the technique has also been used to quantify concentrations of endogenous, inflammatory mediators (105–107). Limitations of this technique include the discomfort resulting from skin blister formation, limited sampling times, difficulties related to standardization, and the presence of inflammatory proteins and mediators in the blister fluid (40, 104, 105). One study sought to compare the skin blister and microdialysis techniques to evaluate the subcutaneous penetration of fluconazole following single-dose, oral administration. Microdialysis concentrations measured in the subcutaneous tissue were similar to unbound concentrations in plasma, although a lag time of 0.5 h was observed (108). On the other hand, fluconazole concentrations measured in blister fluid were significantly low and delayed compared to plasma concentrations. In this study, microdialysis appeared to more appropriately capture the time course of drug concentrations in subcutaneous tissue. In separate studies, less stark differences were observed between these methods when evaluating dermal PK for famciclovir and acetylsalicylic acid (104, 109). To date, there is no widely accepted gold-standard technique, and study-specific factors must be considered in the design of a clinical study and the interpretation of its results.
Saliva drug sampling represents another potential measure of extravascular drug penetration. Drug-related factors impacting salivary drug concentrations include molecular size, lipophilicity, pKa, and protein binding, whereas salivary flow and clearance mechanisms, salivary pH, and pathophysiological factors will also dictate drug distribution in saliva (47, 110). Two studies comparing the microdialysis, skin blister, and salivary drug sampling techniques using theophylline and paracetamol showed that salivary concentrations were poorly predictive of unbound plasma concentrations (111, 112). Moreover, with theophylline, microdialysis proved to be the more reliable technique, with unbound drug concentrations in plasma and tissue being highly correlated after accounting for protein binding. In the case of paracetamol, a drug with negligible protein binding, drug levels determined by both microdialysis and the skin blister technique closely mirrored serum drug levels. However, there are also examples in the literature that suggest method-specific differences in determined free drug concentrations. For example, it was shown that following oral administration, ciprofloxacin penetrates preferentially into inflamed lesions, such as cantharis-induced skin blisters (area under the concentration-time curve for blister fluid [AUCblister]/AUCplasma = 1.44 ± 0.16), a fact that would have been missed based on measurements from different sampling sites, such as saliva (AUCsaliva/AUCplasma = 0.33 ± 0.01) or capillary blood (AUCcapillary blood/AUCplasma = 0.88 ± 0.07) (90).
Imaging techniques, namely, MRS and PET, provide an opportunity to visualize patterns of drug distribution in key organs. MRS, a technique based on nuclear magnetic resonance (NMR), uses radiofrequency pulses and magnetic fields to capture resonance signals emitted by specific nuclei (e.g., 1H and 13C) on molecules of interest (40, 45). MRS can provide continuous monitoring of both the parent drug and metabolites (40, 113–116). PET is a noninvasive imaging technique that uses a radionuclide attached to the molecule of interest to elucidate distribution patterns and drug target binding characteristics and expression patterns (43, 44). Although PET can also provide continuous monitoring, the length of time depends on the half-life of the radioisotope. Also, because PET records nuclear decay events altogether, it cannot exclusively quantify unbound drug concentrations (40). However, one study combined microdialysis and PET imaging to study intracellular ciprofloxacin concentrations (117). The authors of that study were able to relate unbound extracellular concentrations measured by using microdialysis to total concentrations quantified by using PET to assess the extent of intracellular uptake and retention.
APPROACHES FOR ESTABLISHING PK/PD RELATIONSHIPS
In Vitro Studies
In vitro studies of anti-infective drugs are often designed to mimic conditions observed in vivo. Of the various factors that must be taken into consideration (e.g., pH, growth media, electrolytes, and fatty acids), the appropriate resemblance of free, active drug concentrations in these in vitro settings is of utmost importance.
To do so, two general approaches have been established. First, protein supplements, such as human or bovine serum albumin or human serum, may be added to the growth media to mimic physiological binding conditions in in vitro settings. Although there is no general consensus on the amount of protein to be added, 4 g/dl is typically regarded as the target concentration, as it resembles normal physiological conditions (16, 17, 20, 21, 26, 27, 118–122). Selection of the serum concentration is even more difficult, as bacterial growth is often inhibited once the serum content exceeds 70% of the growth medium (16). Moreover, variability in the extent of drug binding between protein supplements, binding characteristics dissimilar from those in vivo, differences in experimental conditions, and application of literature protein binding values have led to various conclusions related to the significance of protein binding for antimicrobials (16, 25, 33). A second approach is to account for protein binding through simulation of unbound concentrations in vivo, circumventing the need to add a protein supplement (26, 123, 124).
An in vitro study compared the antimicrobial activity of cefditoren against penicillin-resistant Streptococcus pneumoniae in the presence of Mueller-Hinton broth plus 5% lysed horse blood (MH), MH plus 90% human serum, MH plus human serum albumin (4 g/dl), and a kill curve assuming a final drug concentration corresponding to 88% protein binding (26). Three separate strains with increasing MIC values (0.12, 0.25, and 0.5 mg/liter) were tested (the MIC corresponded to the lowest concentration inhibiting visible bacterial growth after 18 to 24 h). For the strain with the lowest MIC, no significant differences were observed between the four scenarios. In contrast, for the other two strains, significant reductions in bacterial counts at 24 h were observed with human serum compared to the study arms containing broth alone, human serum albumin, or a simulated unbound concentration. This may be explained partly by the presence of other serum constituents (e.g., gammaglobulins) which can enhance bactericidal activity. Moreover, as noted by the authors of that study, the fact that only in the presence of human serum was cefditoren's bactericidal activity unaffected may suggest that accounting for protein binding effects through the use of reported protein binding values can result in poor conclusions based on the observed data.
A similar study performed by using the antifungals voriconazole and anidulafungin sought to compare antifungal activities against Aspergillus fumigatus and Aspergillus flavus in the presence of RPMI broth alone, human serum, human serum albumin, and expected unbound drug concentrations based on theoretical protein binding (voriconazole, 58%; anidulafungin, 99%) (32). By using the XTT {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide} assay to assess metabolic activity, anidulafungin activity was reduced in the presence of human serum and human serum albumin, while the pattern of voriconazole's activity remained unchanged following 48 h of incubation. A few additional points are worth noting. First, as in the above-described example, the impact of protein binding was dependent on the culture medium. Anidulafungin exhibited greater activity in the presence of human serum than in the presence of human serum albumin. This difference was still apparent despite the fact that heat-inactivated serum was used, suggesting that perhaps some additional non-protein-mediated mechanism may help explain this phenomenon. Second, for both drugs, greater bactericidal activity was observed with human serum than with the use of a theoretical protein binding value. This may suggest that simply accounting for protein binding through a theoretical fraction that is unavailable to exert a drug effect may be too simplistic. Finally, the impact of protein binding may be species dependent in some cases. Anidulafungin's increased activity in the presence of serum was particularly evident for A. flavus. All these factors explain why it may be difficult to account for protein binding in in vitro experimental settings.
Although greater protein binding can reduce unbound drug concentrations at the site of action, this may not always translate into an impact on drug efficacy. For example, telavancin and daptomycin display bactericidal activity against Gram-positive organisms despite being highly protein bound, 93 and 92%, respectively (21, 23, 125). Various factors have been proposed to explain these observations. For example, weak binding to plasma proteins relative to the drug target may play a role (21, 27). In the case of telavancin, the molecule's ability to disrupt bacterial plasma membrane function, in addition to inhibiting peptidoglycan synthesis, was proposed to partly explain its efficacy despite significant binding to plasma proteins (126).
Regardless of the approach used to account for the impact of protein binding, free drug concentrations should be measured whenever feasible. For example, one study sought to simulate total and unbound serum concentrations by using a two-compartment, in vitro dynamic model for two compounds which differ in the extent of protein binding (cefpodoxime, 21%; cefditoren, 88%) (123). In the experimental setup, no protein supplements were added, and target drug concentrations were simulated by using estimates of the extent of drug binding reported in the literature. Target drug concentrations were compared with experimental total and unbound concentrations quantified by taking aliquots from the peripheral compartment at predefined time points and analyzing the samples by using a bioassay. The measured drug concentrations were then compared directly with the reduction in bacterial counts over time for two strains of Streptococcus pneumoniae.
In Vivo Studies
Animal models.
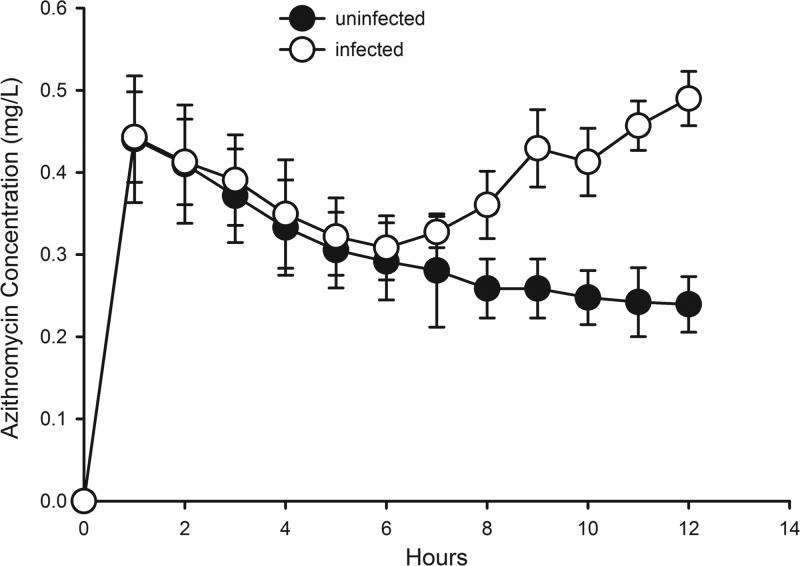
Animal infection models provide an opportunity to test the efficacy of anti-infective drugs in vivo across a range of scenarios, including multiple pathogens, various drugs and dosing regimens, and different types of infection. Lung and thigh infection models are often used to study cyclophosphamide-induced neutropenia (127). When evaluating the PK in these animal models, frequently, total plasma concentrations are determined and corrected by using protein binding values reported in the literature. A limited number of studies that evaluated unbound tissue concentrations in animal infection models are available. For example, two separate studies sought to compare unbound plasma and tissue concentrations of voriconazole and fluconazole in healthy and Candida species-infected rats (128, 129). In both cases, unbound plasma levels could serve as a good surrogate for unbound drug levels in the kidneys. In contrast, a separate study that compared azithromycin tissue concentrations in uninfected and infected tissue by using a rat thigh infection model (Staphylococcus aureus) noted greater drug exposure in the infected tissue (Fig. 2) (130). The azithromycin example clearly demonstrates the potential impact of disease on free, active concentrations at the site of action/infection. Extrapolation of findings from healthy animals directly to diseased conditions can consequently be misleading, and the underlying assumptions warrant further investigation.
Fig 2.
Azithromycin concentration versus time in uninfected and infected (S. aureus) tissue in a rat thigh infection model. (Reprinted from reference 130.)
Clinical studies.
Clinical studies evaluating drug distribution are frequently conducted with healthy subjects. Although there are several practical advantages to performing these studies with healthy subjects, disease-related changes can result in significant differences between healthy subjects and the intended patient population. It may consequently be misleading to translate findings from healthy subjects directly to patients without considering the underlying pathophysiological changes. For example, antibiotics, such as beta-lactams or aminoglycosides, that freely distribute in extracellular water can be “dragged” into the interstitial space of a critically ill patient with “leaky capillaries” by extravascular fluid movement, resulting in an increase of the distribution volume (131). As a result, plasma concentrations will decrease for a given dose, requiring an increased loading dose to compensate for the drug “lost” to the tissue. Once the patient's condition improves, the distribution volume will slowly return to its original value. The time course of changes in the PK of these drugs therefore mirrors that of the patient's pathophysiology (131), a phenomenon observed for vancomycin (132, 133), amikacin (134), and beta-lactams (135), to name a few. On the other hand, for drugs that distribute into extra- and intracellular spaces, such as quinolones (136), moderate changes in the volume of distribution as a result of disease are less important and are likely to require no adjustment of the loading dose.
In addition to fluid-shift-related phenomena, changes in the rate at which the primary eliminating organs (i.e., the liver and the kidneys) are perfused may alter the PK of antibiotics in patients (131). For example, altered cardiac output due to position changes during surgery and/or anesthesia or due to the administration of large fluid volumes may substantially alter drug clearance. It was shown for the kidneys that an augmented clearance was not appropriately reflected by measured serum creatinine concentrations, the clinical surrogate for the glomerular filtration rate. While serum creatinine concentrations were in some cases within the normal range, the renal clearance rate was significantly elevated, making more frequent dosing necessary (137). The situation becomes even more complex if patients require additional hemodialysis, as the anti-infective agent itself may also be removed during the process. In addition to these distribution- and clearance-related factors, pH changes, tissue and/or plasma protein binding alterations, as well as drug delivery via macrophages or neutrophils may impact target site concentrations (138, 139).
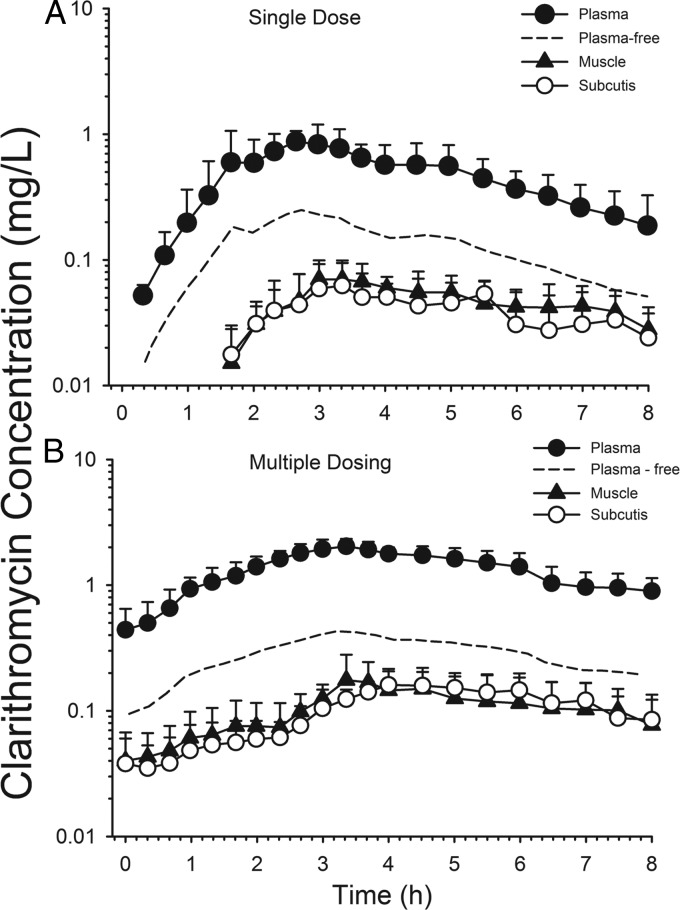
Microdialysis has been applied to study the differential tissue penetration of anti-infective drugs in humans, frequently in healthy volunteers but also in patients. For example, a clinical microdialysis study performed with six healthy volunteers evaluated the distribution of clarithromycin in muscle and subcutaneous tissue and compared it with unbound plasma concentrations (87). Following a single dose of clarithromycin (250 mg), the ratios of the area under the unbound drug concentration-time curve from 0 to 24 h (fAUC0–24) in subcutaneous tissue and skeletal muscle versus plasma were 0.29 ± 0.17 and 0.42 ± 0.18, respectively (Fig. 3A). At steady state, similar ratios were observed, although less stark differences between subcutaneous tissue and muscle were noted (subcutis, 0.39 ± 0.04; skeletal muscle, 0.41 ± 0.19) (Fig. 3B). As noted by the authors of that study, these findings are at odds with measurements of clarithromycin in biopsy specimen homogenates, which supported greater exposure in tissue than in plasma (140, 141). This example nicely illustrates the fact that free, unbound concentrations in plasma (although not directly measured in that study) may not always be an appropriate surrogate for unbound concentrations in the interstitial space fluid of various tissues. The use of free, unbound plasma concentrations for predicting outcome would have resulted in an overprediction of clarithromycin's free tissue concentrations, which can result in therapeutic failures and resistance development.
Fig 3.
Clarithromycin concentration versus time in plasma, subcutis, and skeletal muscle after a 250-mg single dose (A) and 500 mg twice daily for 3 to 5 days (B). (Reprinted from reference 87.)
Whenever possible, drug concentrations should be measured directly at the site of infection and in the patient population of interest. Doing so would prevent erroneous conclusions based on differences in penetration between tissues and due to disease-related changes. For example, one study which evaluated levofloxacin concentrations in peripheral soft tissues and the lung found 2-fold-lower concentrations in the latter (142). Soft tissue concentrations are consequently a poor predictor of levofloxacin's exposure in the lung, and the respective dosing recommendations may result in therapeutic failures.
The type of infection generally determines the site at which target concentrations should be sampled (9). For lung infections, epithelial lining fluid (ELF) and lung interstitial fluid have been studied, and the respective concentrations have been used to predict the probability of success or failure for a given therapy. For example, measurement of dapsone concentrations in ELF of patients infected with human immunodeficiency virus type 1 (HIV-1) showed that appropriate tissue penetration is achieved, and a twice-daily prophylactic treatment regimen resulted in sufficient drug exposure (143). Improvements in the methodology used to sample ELF drug concentrations have addressed known limitations of the technique (144), namely, the inaccuracies associated with quantifying the ELF volume and the inability to sample the same site multiple times (145). Microdialysis has also been applied as a means to quantify lung concentrations of antimicrobials in human subjects (83, 85, 94, 142, 146, 147). However, a limitation of the technique in this setting is that pulmonary infections may be located in different compartments (e.g., alveoli, bronchioles, and intracellularly) (148). Consequently, it is important to choose a sampling technique that allows the determination of concentrations at or at least as close as possible to the infection site. Failure to do so may result in treatment failures due to insufficient knowledge about target site exposure.
Even when unbound drug concentrations are measured at or near the site of infection, data need to be interpreted cautiously. One contributor of differential tissue penetration is a difference in pH between milieus, as may be caused by disease states. Depending on the physicochemical properties of a drug, a pH gradient can facilitate movement of neutral molecules across membranes, where ionization then restricts further diffusion, a phenomenon known as “ion trapping” (149, 150). Although higher unbound drug concentrations may be measured at the site of infection, the charged species may not be able to diffuse across the bacterial cell wall, and thus, no additional therapeutic benefit would be obtained (151). The potential role of ion trapping may be considered not only by relating drug concentrations in plasma and tissue but also by taking into account potential differences in pH between plasma and tissue, under the given set of circumstances. For example, moxifloxacin has been shown to accumulate in prostatic secretions in healthy subjects, with a prostatic secretion-to-plasma ratio of 1.57 (152). In this scenario, as minimal differences have been observed between plasma and prostatic secretion pH in healthy subjects (153), it is unlikely that ion trapping explains the observations, but rather, differences in lipophilicty, binding to cellular matrices, and/or rapid cellular uptake/release kinetics may play a role (154).
PK/PD RELATIONSHIPS
In isolation, information on the PK of a drug is of limited meaningfulness. Only the link between a drug's PK and the corresponding drug effect provides a meaningful rationale for the selection of a safe and effective dose. For antimicrobials, the drug effect is typically determined in vitro by using MIC or time-kill curve experiments. The determined PD/susceptibility breakpoints for a specific pathogen or group of pathogens are then correlated to the PK parameters of a certain anti-infective agent. To date, the majority of these correlations are MIC based due to the relative simplicity of its experimental determination, resulting in three main indices: fT>MIC, fAUC/MIC, and fCmax/MIC. In general, for β-lactams, a drug class associated with time-dependent killing, it is the time that drug concentrations stay above the MIC (T>MIC) that correlates best with drug efficacy. In the case of aminoglycosides and fluoroquinolones, where bacterial killing is concentration dependent, it is the maximum concentration of the drug in serum divided by the MIC (Cmax/MIC) and AUC/MIC ratio that are important (155, 156). Since only the unbound drug is pharmacologically active, these indices should be expressed in terms of unbound drug exposure (fT>MIC, fCmax/MIC, and fAUC/MIC) (157). These same three PK/PD indices may also be applied to antifungals (158). Similar principles have also been applied to antiviral agents, but the respective PK/PD correlations oftentimes use more stringent PD endpoints (e.g., 95% effective dose [ED95]or ED99) than antibacterials or antifungals to achieve a faster elimination of the virus from the body. In many cases, the relationship between host, virus, and antiviral agent is somewhat more complex, requiring more sophisticated modeling approaches.
Although MIC is the most widely used PD parameter, there are limitations to its use (159). It is a single-point estimate after 18 to 24 h of incubation, which does not provide information on the time course of the drug effect or the presence of a postantibiotic effect. MIC values determined in vitro may differ from the actual susceptibility breakpoints in vivo due to faster growth of bacteria in nutrition-rich growth media and the absence of immune factors. The static nature also allows for only a direct comparison of drug effects at a limited concentration range. It should also be noted that the concentrations of the starting inoculum routinely employed for MIC testing (∼5 × 105 to 5 × 106 CFU/ml) can be quite different from the bacterial burden observed in vivo, depending on the type of infection (e.g., >1010 in pneumonic lung). As a result, PK/PD indices based on standardized in vitro values may not accurately reflect the in vivo situation. Time-kill curve experiments and particularly hollow-fiber models have been proposed as more robust approaches to determine the concentration-effect relationship over time. Due to the time-consuming and labor-intensive nature of these experiments, they have so far been used primarily in drug development and research settings, yet technical advancement and automation may make this approach more attractive for routine application in the future.
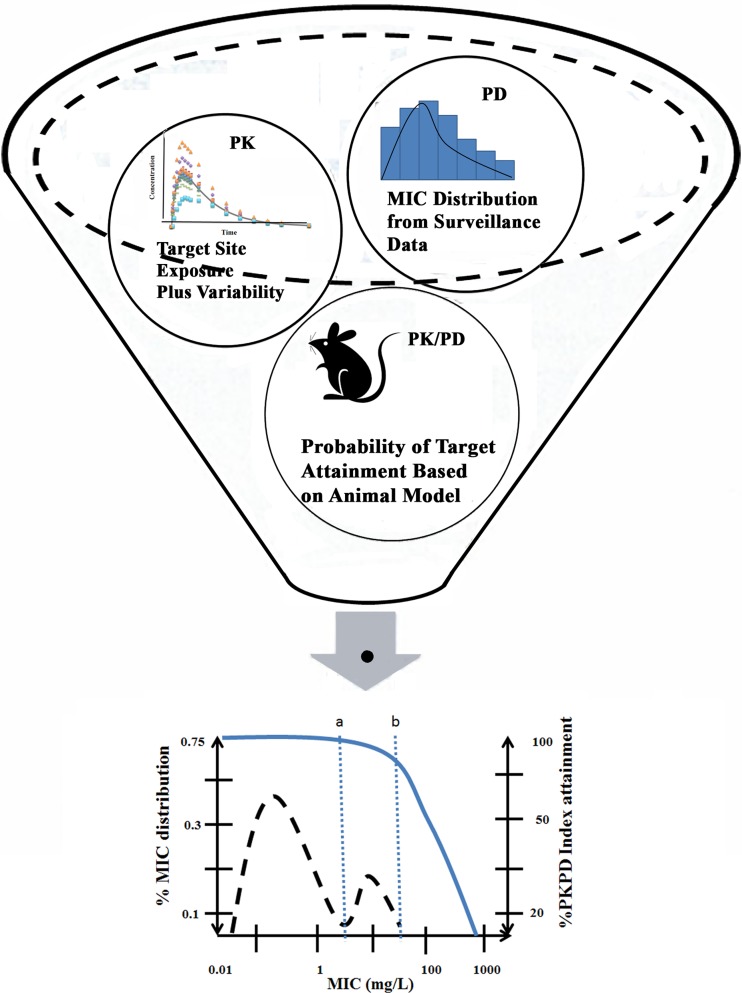
PD parameters, such as the concentration necessary to produce 50% of the maximum effect (EC50), determined from these in vitro experiments can then be linked to in vivo drug exposure to predict the concentration-effect relationship at the site of infection and account for between-subject variability in treatment response (Fig. 4) (160). Through a simulation-based approach, the developed models may then be applied to evaluate “what-if” scenarios, which may help to gauge the appropriateness of a particular drug or dosing regimen against a pathogen (161). For example, one study applied a population PK/PD model developed in healthy subjects and cystic fibrosis patients to guide dosage selection for piperacillin (162). The model predicted that continuous (9 g/day) or 4-h (3 g every 8 h) infusions resulted in a greater or similar target attainment (fT>MIC ≥ 50%) compared to a higher daily dose (3 g every 4 h) administered as a 30-min infusion.
Fig 4.
Application of PK/PD modeling and simulation to integrate in vitro and in vivo data and guide drug and dosage selection. Dosage recommendations are further supported by determination of inflection points (a and b) in the MIC distribution obtained from clinical surveillance data and estimates of target drug exposure. (Adapted from reference 160 [Fig. 12.5] with kind permission from Springer Science+Business Media B.V.)
CONCLUSIONS
Free, unbound drug concentrations are responsible for the PD effect of anti-infective agents. Although plasma protein binding is an important PK parameter, it is only a surrogate for the free, unbound concentrations at the site of action causing the drug effect. As a consequence, free drug concentrations should be experimentally determined at or at least close to the site of action whenever possible. For anti-infective agents, the site of infection is typically located outside plasma, and thus, a drug must diffuse to reach its target. There are many available techniques to quantify tissue concentrations. Selection of an impropriate sampling technique requires careful consideration of the infection site and the advantages and limitations of each approach.
ACKNOWLEDGMENTS
D.G. is funded by the NICHD under the Best Pharmaceuticals for Children Act through grant T32 GM086330.
Biographies

Daniel Gonzalez, Pharm.D., Ph.D., received his Pharm.D. from the University of Florida in 2008. He then obtained his Ph.D. in Pharmaceutical Sciences from the University of Florida in 2012 under the direction of Professor Hartmut Derendorf. Dr. Gonzalez is currently a postdoctoral clinical pharmacology fellow at The University of North Carolina at Chapel Hill and the Duke Clinical Research Institute working under the mentorship of Professors Kim L. R. Brouwer, Daniel Benjamin, Jr., and Paul Watkins. His research interests include the application of modeling and simulation techniques to characterize the pharmacokinetics and pharmacodynamics of drugs, guide dosage selection, and improve drug safety in the pediatric population. Dr. Gonzalez is also interested in the application of microdialysis as a means to assess tissue penetration of drugs.

Stephan Schmidt, Ph.D., received his B.S. in Pharmaceutical Sciences from the Friedrich-Alexander University in Erlangen, Germany, in 2004 and his license to practice as a pharmacist in Germany in 2005. He obtained his Ph.D. in Pharmaceutical Sciences from the University of Florida, Gainesville, FL, in 2008 under the supervision of Professor Hartmut Derendorf. Dr. Schmidt joined the Division of Pharmacology at the Leiden/Amsterdam Center for Drug Research in the Netherlands as a postdoctoral fellow in January 2009, where he was a member of the Dutch Top Institute Pharma mechanism-based PK/PD modeling platform under the supervision of Professor Meindert Danhof. In January 2012, he rejoined the University of Florida as an Assistant Professor at the Center for Pharmacometrics and Systems Pharmacology in Lake Nona (Orlando). His primary research interest is on the development and application of quantitative clinical analysis techniques as well as on the development of mechanism-based disease system models.

Hartmut Derendorf, Ph.D., is Distinguished Professor and Chairman of the Department of Pharmaceutics at the University of Florida College of Pharmacy in Gainesville. Professor Derendorf has published over 380 scientific publications and six textbooks in English and German. He is Associate Editor of the Journal of Clinical Pharmacology, British Journal of Pharmacology, and European Journal of Pharmaceutical Sciences and Editor of the International Journal of Clinical Pharmacology and Therapeutics, International Journal of Antiinfective Agents, and Die Pharmazie and serves on the Editorial Board of eight other journals. Professor Derendorf has served as President of the American College of Clinical Pharmacology (ACCP) and President of the International Society of Antiinfective Pharmacology. He was awarded the Distinguished Research Award and the Nathaniel T. Kwit Distinguished Service Award of the ACCP, the Research Achievement Award in Clinical Science of the American Association of Pharmaceutical Sciences, and the Volwiler Award of the American Association of Colleges of Pharmacy.
REFERENCES
- 1. Kratochwil NA, Huber W, Müller F, Kansy M, Gerber PR. 2002. Predicting plasma protein binding of drugs: a new approach. Biochem. Pharmacol. 64:1355–1374 [DOI] [PubMed] [Google Scholar]
- 2. Craig W, Welling P. 1977. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin. Pharmacokinet. 2:252–268 [DOI] [PubMed] [Google Scholar]
- 3. Toutain P, Bousquet-Melou A. 2002. Free drug fraction vs free drug concentration: a matter of frequent confusion. J. Vet. Pharmacol. Ther. 25:460–463 [DOI] [PubMed] [Google Scholar]
- 4. Perry T, Schentag JJ. 2001. Clinical use of ceftriaxone: a pharmacokinetic-pharmacodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin. Pharmacokinet. 40:685–694 [DOI] [PubMed] [Google Scholar]
- 5. Vella-Brincat JW, Begg EJ, Kirkpatrick CMJ, Zhang M, Chambers ST, Gallagher K. 2007. Protein binding of cefazolin is saturable in vivo both between and within patients. Br. J. Clin. Pharmacol. 63:753–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dudley M, Shyu W, Nightingale C, Quintiliani R. 1986. Effect of saturable serum protein binding on the pharmacokinetics of unbound cefonicid in humans. Antimicrob. Agents Chemother. 30:565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burkhardt O, Brunner M, Schmidt S, Grant M, Tang Y, Derendorf H. 2006. Penetration of ertapenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers measured by in vivo microdialysis. J. Antimicrob. Chemother. 58:632–636 [DOI] [PubMed] [Google Scholar]
- 8. Benet LZ, Hoener B. 2002. Changes in plasma protein binding have little clinical relevance. Clin. Pharmacol. Ther. 71:115–121 [DOI] [PubMed] [Google Scholar]
- 9. Theuretzbacher U. 2007. Tissue penetration of antibacterial agents: how should this be incorporated into pharmacodynamic analyses? Curr. Opin. Pharmacol. 7:498–504 [DOI] [PubMed] [Google Scholar]
- 10. Brunton LL, Chabner BA, Knollmann BC. (ed). 2011. Goodman & Gilman's the pharmacological basis of therapeutics, 12th ed McGraw-Hill Professional, New York, NY [Google Scholar]
- 11. Kunin CM, Craig WA, Kornguth M, Monson R. 1973. Influence of binding on the pharmacologic activity of antibiotics. Ann. N. Y. Acad. Sci. 226:214–224 [DOI] [PubMed] [Google Scholar]
- 12. Craig W, Ebert S. 1989. Protein binding and its significance in antibacterial therapy. Infect. Dis. Clin. North Am. 3:407–415 [PubMed] [Google Scholar]
- 13. Boffito M, Back DJ, Blaschke TF, Rowland M, Bertz RJ, Gerber JG, Miller V. 2003. Protein binding in antiretroviral therapies. AIDS Res. Hum. Retroviruses 19:825–835 [DOI] [PubMed] [Google Scholar]
- 14. Zeitlinger MA, Derendorf H, Mouton JW, Cars O, Craig WA, Andes D, Theuretzbacher U. 2011. Protein binding: do we ever learn? Antimicrob. Agents Chemother. 55:3067–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berezhkovskiy LM. 2010. On the influence of protein binding on pharmacological activity of drugs. J. Pharm. Sci. 99:2153–2165 [DOI] [PubMed] [Google Scholar]
- 16. Beer J, Wagner CC, Zeitlinger M. 2009. Protein binding of antimicrobials: methods for quantification and for investigation of its impact on bacterial killing. AAPS J. 11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cafini F, Aguilar L, González N, Giménez MJ, Torrico M, Alou L, Sevillano D, Vallejo P, Prieto J. 2007. In vitro effect of the presence of human albumin or human serum on the bactericidal activity of daptomycin against strains with the main resistance phenotypes in Gram-positives. J. Antimicrob. Chemother. 59:1185–1189 [DOI] [PubMed] [Google Scholar]
- 18. Boswell FJ. 2002. Effect of protein binding on the in vitro activity and pharmacodynamics of faropenem. J. Antimicrob. Chemother. 50:525–532 [DOI] [PubMed] [Google Scholar]
- 19. Lamp K, Rybak M. 1993. Teicoplanin and daptomycin bactericidal activities in the presence of albumin or serum under controlled conditions of pH and ionized calcium. Antimicrob. Agents Chemother. 37:605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee BL, Sachdeva M, Chambers HF. 1991. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob. Agents Chemother. 35:2505–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cha R, Rybak MJ. 2004. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 54:259–262 [DOI] [PubMed] [Google Scholar]
- 22. Ghobrial O, Derendorf H, Hillman JD. 2010. Human serum binding and its effect on the pharmacodynamics of the lantibiotic MU1140. Eur. J. Pharm. Sci. 41:658–664 [DOI] [PubMed] [Google Scholar]
- 23. Leuthner KD, Cheung CM, Rybak MJ. 2006. Comparative activity of the new lipoglycopeptide telavancin in the presence and absence of serum against 50 glycopeptide non-susceptible staphylococci and three vancomycin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:338–343 [DOI] [PubMed] [Google Scholar]
- 24. Perl T, Pfaller M, Houston A, Wenzel R. 1990. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrob. Agents Chemother. 34:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt S, Röck K, Sahre M, Burkhardt O, Brunner M, Lobmeyer MT, Derendorf H. 2008. Effect of protein binding on the pharmacological activity of highly bound antibiotics. Antimicrob. Agents Chemother. 52:3994–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sevillano D, Giménez MJ, Alou L, Aguilar L, Cafini F, Torrico M, González N, Echeverría O, Coronel P, Prieto J. 2007. Effects of human albumin and serum on the in vitro bactericidal activity of cefditoren against penicillin-resistant Streptococcus pneumoniae. J. Antimicrob. Chemother. 60:156–158 [DOI] [PubMed] [Google Scholar]
- 27. Tsuji BT, Leonard SN, Rhomberg PR, Jones RN, Rybak MJ. 2008. Evaluation of daptomycin, telavancin, teicoplanin, and vancomycin activity in the presence of albumin or serum. Diagn. Microbiol. Infect. Dis. 60:441–444 [DOI] [PubMed] [Google Scholar]
- 28. Zeitlinger M, Sauermann R, Fille M, Hausdorfer J, Leitner I, Müller M. 2008. Plasma protein binding of fluoroquinolones affects antimicrobial activity. J. Antimicrob. Chemother. 61:561–567 [DOI] [PubMed] [Google Scholar]
- 29. Bilello AJA, Bilello PA, Prichard M, Robins T, Drusano GL. 1995. Reduction of the in vitro activity of A77003, an inhibitor of human virus protease, by human serum alpha-1 acid glycoprotein immunodeficiency. J. Infect. Dis. 171:546–551 [DOI] [PubMed] [Google Scholar]
- 30. Molla A, Vasavanonda S, Kumar G, Sham HL, Johnson M, Grabowski B, Denissen JF, Kohlbrenner W, Plattner JJ, Leonard JM, Norbeck DW, Kempf DJ. 1998. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human immunodeficiency virus. Virology 250:255–262 [DOI] [PubMed] [Google Scholar]
- 31. Zhanel GG, Saunders DG, Hoban DJ, Karlowsky JA. 2001. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob. Agents Chemother. 45:2018–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cafini F, Sevillano D, Alou L, Gomez-Aguado F, Corcuera MT, Gonzalez N, Guinea J, Prieto J. 2012. Effect of protein binding on the activity of voriconazole alone or combined with anidulafungin against Aspergillus spp. using a time-kill methodology. Rev. Esp. Quimioter. 25:47–55 [PubMed] [Google Scholar]
- 33. Schmidt S, Gonzalez D, Derendorf H. 2010. Significance of protein binding in pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 99:1107–1122 [DOI] [PubMed] [Google Scholar]
- 34. Safdar N, Andes D, Craig W. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tawara S, Matsumoto S, Kamimura T, Goto S. 1992. Effect of protein binding in serum on therapeutic efficacy of cephem antibiotics. Antimicrob. Agents Chemother. 36:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodnutt G, Berry V, Mizen L. 1995. Effect of protein binding on penetration of beta-lactams into rabbit peripheral lymph. Antimicrob. Agents Chemother. 39:2678–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerding D, Van Etta L, Peterson L. 1982. Role of serum protein binding and multiple antibiotic doses in the extravascular distribution of ceftizoxime and cefotaxime. Antimicrob. Agents Chemother. 22:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joukhadar C, Derendorf H, Müller M. 2001. Microdialysis. A novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211–219 [DOI] [PubMed] [Google Scholar]
- 39. Brunner M, Derendorf H, Müller M. 2005. Microdialysis for in vivo pharmacokinetic/pharmacodynamic characterization of anti-infective drugs. Curr. Opin. Pharmacol. 5:495–499 [DOI] [PubMed] [Google Scholar]
- 40. Brunner M, Langer O. 2006. Microdialysis versus other techniques for the clinical assessment of in vivo tissue drug distribution. AAPS J. 8:E263–E271 doi:10.1208/aapsj080230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langer O, Müller M. 2004. Methods to assess tissue-specific distribution and metabolism of drugs. Curr. Drug Metab. 5:463–481 [DOI] [PubMed] [Google Scholar]
- 42. Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237 [DOI] [PubMed] [Google Scholar]
- 43. Matthews PM, Rabiner EA, Passchier J, Gunn RN. 2012. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 73:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matthews PM, Rabiner I, Gunn R. 2011. Non-invasive imaging in experimental medicine for drug development. Curr. Opin. Pharmacol. 11:501–507 [DOI] [PubMed] [Google Scholar]
- 45. Port RE, Wolf W. 2003. Noninvasive methods to study drug distribution. Invest. New Drugs 21:157–168 [DOI] [PubMed] [Google Scholar]
- 46. Müller M, Stass H, Brunner M, Möller JG, Lackner E, Eichler HG. 1999. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob. Agents Chemother. 43:2345–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jusko WJ, Milsap RL. 1993. Pharmacokinetic principles of drug distribution in saliva. Ann. N. Y. Acad. Sci. 694:36–47 [DOI] [PubMed] [Google Scholar]
- 48. Liu Z, Li F, Huang Y. 1999. Determination of unbound drug concentration and protein-drug binding fraction in plasma. Biomed. Chromatogr. 13:262–266 [DOI] [PubMed] [Google Scholar]
- 49. Zhang F, Xue J, Shao J, Jia L. 2012. Compilation of 222 drugs' plasma protein binding data and guidance for study designs. Drug Discov. Today 17:475–485 [DOI] [PubMed] [Google Scholar]
- 50. Howard ML, Hill JJ, Galluppi GR, McLean MA. 2010. Plasma protein binding in drug discovery and development. Comb. Chem. High Throughput Screen. 13:170–187 [DOI] [PubMed] [Google Scholar]
- 51. Plock N, Kloft C. 2005. Microdialysis—theoretical background and recent implementation in applied life-sciences. Eur. J. Pharm. Sci. 25:1–24 [DOI] [PubMed] [Google Scholar]
- 52. De Lange EC, De Boer A, Breimer GDD. 2000. Methodological issues in microdialysis sampling for pharmacokinetic studies. Adv. Drug Deliv. Rev. 45:125–148 [DOI] [PubMed] [Google Scholar]
- 53. Hage DS. 2002. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 768:3–30 [DOI] [PubMed] [Google Scholar]
- 54. Yoo MJ, Smith QR, Hage DS. 2009. Studies of imipramine binding to human serum albumin by high-performance affinity chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joseph K, Hage D. 2010. Characterization of the binding of sulfonylurea drugs to HSA by high-performance affinity chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878:1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tong Z, Hage DS. 2011. Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. J. Chromatogr. A 1218:6892–6897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hage DS, Jackson A, Sobansky M, Schiel JE, Yoo MJ, Joseph K. 2009. Characterization of drug-protein interactions in blood using high-performance affinity chromatography. J. Sep. Sci. 32:835–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clarke W, Chowdhuri AR, Hage DS. 2001. Analysis of free drug fractions by ultrafast immunoaffinity chromatography. Anal. Chem. 73:2157–2164 [DOI] [PubMed] [Google Scholar]
- 59. Xie M-X, Long M, Liu Y, Qin C, Wang Y-D. 2006. Characterization of the interaction between human serum albumin and morin. Biochim. Biophys. Acta 1760:1184–1191 [DOI] [PubMed] [Google Scholar]
- 60. Carryn S, Chanteux H, Seral C, Mingeot-Leclercq M-P, Van Bambeke F, Tulkens PM. 2003. Intracellular pharmacodynamics of antibiotics. Infect. Dis. Clin. North Am. 17:615–634 [DOI] [PubMed] [Google Scholar]
- 61. Seral C, Carryn S, Tulkens PM, Van Bambeke F. 2003. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167–1173 [DOI] [PubMed] [Google Scholar]
- 62. de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. 1974. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 23:2495–2531 [DOI] [PubMed] [Google Scholar]
- 63. Mandell GL, Coleman E. 2001. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 45:1794–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barcia-Macay M, Mouaden F, Mingeot-Leclercq M-P, Tulkens PM, Van Bambeke F. 2008. Cellular pharmacokinetics of telavancin, a novel lipoglycopeptide antibiotic, and analysis of lysosomal changes in cultured eukaryotic cells (J774 mouse macrophages and rat embryonic fibroblasts). J. Antimicrob. Chemother. 61:1288–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lemaire S, Glupczynski Y, Duval V, Joris B, Tulkens PM, Van Bambeke F. 2009. Activities of ceftobiprole and other cephalosporins against extracellular and intracellular (THP-1 macrophages and keratinocytes) forms of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2289–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sandberg A, Lemaire S, Van Bambeke F, Tulkens PM, Hughes D, Von Eiff C, Frimodt-Møller N. 2011. Intra- and extracellular activities of dicloxacillin and linezolid against a clinical Staphylococcus aureus strain with a small-colony-variant phenotype in an in vitro model of THP-1 macrophages and an in vivo mouse peritonitis model. Antimicrob. Agents Chemother. 55:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sandberg A, Jensen KS, Baudoux P, Van Bambeke F, Tulkens PM, Frimodt-Møller N. 2010. Intra- and extracellular activity of linezolid against Staphylococcus aureus in vivo and in vitro. J. Antimicrob. Chemother. 65:962–973 [DOI] [PubMed] [Google Scholar]
- 68. Krasniqi S, Matzneller P, Kinzig M, Sörgel F, Hüttner S, Lackner E, Müller M, Zeitlinger M. 2012. Blood, tissue, and intracellular concentrations of erythromycin and its metabolite anhydroerythromycin during and after therapy. Antimicrob. Agents Chemother. 56:1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Theuretzbacher U. 2005. Clinical implications of tissue concentrations. Abstr. 7th Eur. Congr. Chemother. Infect., Florence, Italy http://www.isap.org/2005/7th-ECC-Florence/slides/Theuretzbacher/Theuretzbacher-7th-ECC-ISAP-2005.pdf [Google Scholar]
- 70. De la Pena A, Brunner M, Eichler H, Rehak E, Gross J, Thyroff-Friesinger U, Müller M, Derendorf H. 2002. Comparative target site pharmacokinetics of immediate and modified-release formulations of cefaclor in humans. J. Clin. Pharmacol. 42:403–411 [DOI] [PubMed] [Google Scholar]
- 71. Hutschala D, Skhirtladze K, Kinstner C, Mayer-Helm B, Müller M, Wolner E, Tschernko EM. 2007. In vivo microdialysis to measure antibiotic penetration into soft tissue during cardiac surgery. Ann. Thorac. Surg. 84:1605–1610 [DOI] [PubMed] [Google Scholar]
- 72. Hollenstein U, Brunner M, Mayer BX, Delacher S, Erovic B, Eichler HG, Müller M. 2000. Target site concentrations after continuous infusion and bolus injection of cefpirome to healthy volunteers. Clin. Pharmacol. Ther. 67:229–236 [DOI] [PubMed] [Google Scholar]
- 73. Sauermann R, Delle-Karth G, Marsik C, Steiner I, Zeitlinger M, Mayer-Helm B-X, Georgopoulos A, Müller M, Joukhadar C. 2005. Pharmacokinetics and pharmacodynamics of cefpirome in subcutaneous adipose tissue of septic patients. Antimicrob. Agents Chemother. 49:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Müller M, Rohde B, Kovar A, Georgopoulos A, Eichler H-G, Derendorf H. 1997. Relationship between serum and free interstitial concentrations of cefodizime and cefpirome in muscle and subcutaneous adipose tissue of healthy volunteers measured by microdialysis. J. Clin. Pharmacol. 37:1108–1113 [DOI] [PubMed] [Google Scholar]
- 75. Liu P, Müller M, Grant M, Obermann B, Derendorf H. 2005. Tissue penetration of cefpodoxime and cefixime in healthy subjects. J. Clin. Pharmacol. 45:564–569 [DOI] [PubMed] [Google Scholar]
- 76. Barbour A, Schmidt S, Rout WR, Ben-David K, Burkhardt O, Derendorf H. 2009. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int. J. Antimicrob. Agents 34:231–235 [DOI] [PubMed] [Google Scholar]
- 77. Mand'ak J, Pojar M, Malakova J, Lonsky V, Palicka V, Zivny P. 2007. Tissue and plasma concentrations of cephuroxime during cardiac surgery in cardiopulmonary bypass—a microdialysis study. Perfusion 22:129–136 [DOI] [PubMed] [Google Scholar]
- 78. Barbour A, Schmidt S, Sabarinath SN, Grant M, Seubert C, Skee D, Murthy B, Derendorf H. 2009. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob. Agents Chemother. 53:2773–2776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Burian B, Zeitlinger M, Donath O, Reznicek G, Sauermann R. 2012. Penetration of doripenem into skeletal muscle and subcutaneous adipose tissue in healthy volunteers. Antimicrob. Agents Chemother. 56:532–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tegeder I, Schmidtko A, Bräutigam L, Kirschbaum A, Geisslinger G, Lötsch J. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325–333 [DOI] [PubMed] [Google Scholar]
- 81. Dahyot-Fizelier C, Timofeev I, Marchand S, Hutchinson P, Debaene B, Menon D, Mimoz O, Gupta A, Couet W. 2010. Brain microdialysis study of meropenem in two patients with acute brain injury. Antimicrob. Agents Chemother. 54:3502–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Karjagin J, Lefeuvre S, Oselin K, Kipper K, Marchand S, Tikkerberi A, Starkopf J, Couet W, Sawchuk RJ. 2008. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin. Pharmacol. Ther. 83:452–459 [DOI] [PubMed] [Google Scholar]
- 83. Tomaselli F, Maier A, Matzi V, Smolle-Jüttner FM, Dittrich P. 2004. Penetration of meropenem into pneumonic human lung tissue as measured by in vivo microdialysis. Antimicrob. Agents Chemother. 48:2228–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brunner M, Pernerstorfer T, Mayer BX, Eichler HG, Müller M. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754–1759 [DOI] [PubMed] [Google Scholar]
- 85. Tomaselli F, Dittrich P, Maier A, Woltsche M, Matzi V, Pinter J, Nuhsbaumer S, Pinter H, Smolle J, Smolle-Jüttner FM. 2003. Penetration of piperacillin and tazobactam into pneumonic human lung tissue measured by in vivo microdialysis. Br. J. Clin. Pharmacol. 55:620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lorentzen H, Kallehave F, Kolmos HJ, Knigge U, Bülow J, Gottrup F. 1996. Gentamicin concentrations in human subcutaneous tissue. Antimicrob. Agents Chemother. 40:1785–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Traunmüller F, Zeitlinger M, Zeleny P, Müller M, Joukhadar C. 2007. Pharmacokinetics of single- and multiple-dose oral clarithromycin in soft tissues determined by microdialysis. Antimicrob. Agents Chemother. 51:3185–3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gattringer R, Urbauer E, Traunmu F, Zeitlinger M, Dehghanyar P, Zeleny P, Graninger W, Mu M, Joukhadar C. 2004. Pharmacokinetics of telithromycin in plasma and soft tissues after single-dose administration to healthy volunteers. Antimicrob. Agents Chemother. 48:4650–4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brunner M, Hollenstein U, Delacher S, Jäger D, Schmid R, Lackner E, Georgopoulos A, Eichler HG, Müller M. 1999. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob. Agents Chemother. 43:1307–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Brunner M, Stabeta H, Möller J, Schrolnberger C, Erovic B, Hollenstein U, Zeitlinger M, Eichler H, Müller M. 2002. Target site concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob. Agents Chemother. 46:3724–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hollenstein UM, Brunner M, Schmid R, Müller M. 2001. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int. J. Obes. Relat. Metab. Disord. 25:354–358 [DOI] [PubMed] [Google Scholar]
- 92. Müller M, Brunner M, Hollenstein U, Joukhadar C, Schmid R, Minar E, Ehringer H, Eichler HG. 1999. Penetration of ciprofloxacin into the interstitial space of inflamed foot lesions in non-insulin-dependent diabetes mellitus patients. Antimicrob. Agents Chemother. 43:2056–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Islinger F, Bouw R, Stahl M, Lackner E, Zeleny P, Brunner M, Mu M, Eichler HG, Joukhadar C. 2004. Concentrations of gemifloxacin at the target site in healthy volunteers after a single oral dose. Antimicrob. Agents Chemother. 48:4246–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hutschala D, Kinstner C, Skhirtladze K, Mayer-Helm B-X, Zeitlinger M, Wisser W, Müller M, Tschernko E. 2008. The impact of perioperative atelectasis on antibiotic penetration into lung tissue: an in vivo microdialysis study. Intensive Care Med. 34:1827–1834 [DOI] [PubMed] [Google Scholar]
- 95. Hutschala D, Skhirtladze K, Zuckermann A, Wisser W, Jaksch P, Mayer-Helm B-X, Burgmann H, Wolner E, Müller M, Tschernko EM. 2005. In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob. Agents Chemother. 49:5107–5111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. 2006. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 50:2455–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dehghanyar P, Bu C, Zeitlinger M, Islinger F, Kovar F, Mu M, Kloft C, Joukhadar C. 2005. Penetration of linezolid into soft tissues of healthy volunteers after single and multiple doses. Antimicrob. Agents Chemother. 49:2367–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Islinger F, Dehghanyar P, Sauermann R, Bürger C, Kloft C, Müller M, Joukhadar C. 2006. The effect of food on plasma and tissue concentrations of linezolid after multiple doses. Int. J. Antimicrob. Agents 27:108–112 [DOI] [PubMed] [Google Scholar]
- 99. Sahre M, Sabarinath S, Grant M, Seubert C, Deanda C, Prokocimer P, Derendorf H. 2012. Skin and soft tissue concentrations of tedizolid (formerly torezolid), a novel oxazolidinone, following a single oral dose in healthy volunteers. Int. J. Antimicrob. Agents 40:51–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim A, Suecof LA, Sutherland CA, Gao L, Kuti JL, Nicolau DP. 2008. In vivo microdialysis study of the penetration of daptomycin into soft tissues in diabetic versus healthy volunteers. Antimicrob. Agents Chemother. 52:3941–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frossard M, Joukhadar C, Erovic BM, Dittrich P, Mrass PE, Van Houte M, Burgmann H, Georgopoulos A, Müller M. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Karjagin J, Pähkla R, Starkopf J. 2004. Perioperative penetration of metronidazole into muscle tissue: a microdialysis study. Eur. J. Clin. Pharmacol. 59:809–813 [DOI] [PubMed] [Google Scholar]
- 103. Kiistala U. 1968. Suction blister device for separation of viable epidermis from dermis. J. Invest. Dermatol. 50:129–138 [DOI] [PubMed] [Google Scholar]
- 104. Benfeldt E, Serup J, Menné T. 1999. Microdialysis vs. suction blister technique for in vivo sampling of pharmacokinetics in the human dermis. Acta Derm. Venereol. 79:338–342 [DOI] [PubMed] [Google Scholar]
- 105. Day RM, Harbord M, Forbes A, Segal AW. 2001. Cantharidin blisters: a technique for investigating leukocyte trafficking and cytokine production at sites of inflammation in humans. J. Immunol. Methods 257:213–220 [DOI] [PubMed] [Google Scholar]
- 106. Dinh PHD, Corraza F, Mestdagh K, Kassengera Z, Doyen V, Michel O. 2011. Validation of the cantharidin-induced skin blister as an in vivo model of inflammation. Br. J. Clin. Pharmacol. 72:912–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dearman RJ, Bhushan M, Cumberbatch M, Kimber I, Griffiths CEM. 2004. Measurement of cytokine expression and Langerhans cell migration in human skin following suction blister formation. Exp. Dermatol. 13:452–460 [DOI] [PubMed] [Google Scholar]
- 108. Sasongko L, Williams KM, Day RO, McLachlan AJ. 2003. Human subcutaneous tissue distribution of fluconazole: comparison of microdialysis and suction blister techniques. Br. J. Clin. Pharmacol. 56:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Borg N, Götharson E, Benfeldt E, Groth L, Ståhle L. 1999. Distribution to the skin of penciclovir after oral famciclovir administration in healthy volunteers: comparison of the suction blister technique and cutaneous microdialysis. Acta Derm. Venereol. 79:274–277 [DOI] [PubMed] [Google Scholar]
- 110. Haeckel R. 1993. Factors influencing the saliva/plasma ratio of drugs. Ann. N. Y. Acad. Sci. 694:128–142 [DOI] [PubMed] [Google Scholar]
- 111. Müller M, Brunner M, Schmid R, Putz EM, Schmiedberger A, Wallner I, Eichler HG. 1998. Comparison of three different experimental methods for the assessment of peripheral compartment pharmacokinetics in humans. Life Sci. 62:PL227–PL234 doi:10.1016/S0024-3205(98)00071-X [DOI] [PubMed] [Google Scholar]
- 112. Brunner M, Schmiedberger A, Schmid R, Jäger D, Piegler E, Eichler HG, Müller M. 1998. Direct assessment of peripheral pharmacokinetics in humans: comparison between cantharides blister fluid sampling, in vivo microdialysis and saliva sampling. Br. J. Clin. Pharmacol. 46:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Singh M, Waluch V. 2000. Physics and instrumentation for imaging in-vivo drug distribution. Adv. Drug Deliv. Rev. 41:7–20 [DOI] [PubMed] [Google Scholar]
- 114. Lyoo IK, Renshaw PF. 2002. Magnetic resonance spectroscopy: current and future applications in psychiatric research. Biol. Psychiatry 51:195–207 [DOI] [PubMed] [Google Scholar]
- 115. Bolo NR, Hodé Y, Nédélec JF, Lainé E, Wagner G, Macher JP. 2000. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology 23:428–438 [DOI] [PubMed] [Google Scholar]
- 116. Strauss WL, Dager SR. 2001. Magnetization transfer of fluoxetine in the human brain using fluorine magnetic resonance spectroscopy. Biol. Psychiatry 49:798–802 [DOI] [PubMed] [Google Scholar]
- 117. Langer O, Karch R, Müller U, Dobrozemsky G, Abrahim A, Zeitlinger M, Lackner E, Joukhadar C, Dudczak R, Kletter K, Müller M, Brunner M. 2005. Combined PET and microdialysis for in vivo assessment of intracellular drug pharmacokinetics in humans. J. Nucl. Med. 46:1835–1841 [PubMed] [Google Scholar]
- 118. Alou L, Giménez M-J, Cafini F, Aguilar L, Sevillano D, González N, Torrico M, Prieto J, García-Rey C, García-Escribano N. 2009. In vitro effect of physiological concentrations of human albumin on the antibacterial activity of tigecycline. J. Antimicrob. Chemother. 64:1230–1233 [DOI] [PubMed] [Google Scholar]
- 119. Gustafsson I. 2004. The influence of protein binding on the antibacterial activity of faropenem against Haemophilus influenzae. Clin. Microbiol. Infect. 10:934–937 [DOI] [PubMed] [Google Scholar]
- 120. Palmer SM, Kang SL, Cappelletty DM, Rybak MJ. 1995. Bactericidal killing activities of cefepime, ceftazidime, cefotaxime, and ceftriaxone against Staphylococcus aureus and beta-lactamase-producing strains of Enterobacter aerogenes and Klebsiella pneumoniae in an in vitro infection model. Antimicrob. Agents Chemother. 39:1764–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Odenholt I, Löwdin E, Cars O. 2007. Pharmacodynamic effects of telavancin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains in the presence of human albumin or serum and in an in vitro kinetic model. Antimicrob. Agents Chemother. 51:3311–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tsuji BT, Rybak MJ. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Echeverria O, Alou L, Sevillano D, Gonzalez N, Gomez-Lus M, Aguilar L, Prieto J. 2007. Pharmacodynamics of simulated total versus free-drug serum concentrations of a low versus high protein bound third-generation oral cephalosporin (cefpodoxime versus cefditoren) against Streptococcus pneumoniae. J. Chemother. 19:288–294 [DOI] [PubMed] [Google Scholar]
- 124. González N, Aguilar L, Sevillano D, Giménez M-J, Alou L, Cafini F, Torrico M, López A-M, Coronel P, Prieto J. 2011. Efficacy of simulated cefditoren versus amoxicillin-clavulanate free concentrations in countering intrastrain ftsI gene diffusion in Haemophilus influenzae. Antimicrob. Agents Chemother. 55:2788–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shaw J, Seroogy J, Kaniga K, Higgins D, Kitt M, Barriere S. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hegde S, Reyes N, Wiens T, Vanasse N, Skinner R, McCullough J, Kaniga K, Pace J, Thomas R, Shaw J-P, Obedencio G, Judice J. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Drusano GL. 2005. Infection site concentrations: their therapeutic importance and the macrolide and macrolide-like class of antibiotics. Pharmacotherapy 25:150S–158S doi:10.1592/phco.2005.25.12part2.150S [DOI] [PubMed] [Google Scholar]
- 128. De Araujo BV, Da Silva CF, Haas SE, Dalla Costa T. 2009. Free renal levels of voriconazole determined by microdialysis in healthy and Candida sp.-infected Wistar rats. Int. J. Antimicrob. Agents 33:154–159 [DOI] [PubMed] [Google Scholar]
- 129. Azeredo FJ, De Araújo BV, Haas SE, Torres B, Pigatto M, De Andrade C, Dalla Costa T. 2012. Comparison of fluconazole renal penetration levels in healthy and Candida albicans-infected Wistar rats. Antimicrob. Agents Chemother. 56:5852–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lucini V, Grosso S, Pannacci M, Scaglione F. 2006. Azithromycin kinetics in infected tissue evaluated by microdialysis, abstr A-335. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 131. Lipman J, Udy AA, Roberts JA. 2011. Do we understand the impact of altered physiology, consequent interventions and resultant clinical scenarios in the intensive care unit? The antibiotic story. Anaesth. Intensive Care 39:999–1000 [DOI] [PubMed] [Google Scholar]
- 132. Chung J, Oh JM, Cho EM, Jang HJ, Hong SB, Lim CM, Koh YS. 2011. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth. Intensive Care 39:1030–1037 [DOI] [PubMed] [Google Scholar]
- 133. Gous A, Dance M, Lipman J, Luyt DK, Mathivha R, Scribante J. 1995. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth. Intensive Care 23:678–682 [DOI] [PubMed] [Google Scholar]
- 134. Marik PE. 1993. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth. Intensive Care 21:172–173 [DOI] [PubMed] [Google Scholar]
- 135. Roberts JA, Kirkpatrick CMJ, Roberts MS, Robertson TA, Dalley AJ, Lipman J. 2009. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J. Antimicrob. Chemother. 64:142–150 [DOI] [PubMed] [Google Scholar]
- 136. Gous A, Lipman J, Scribante J, Tshukutsoane S, Hon H, Pinder M, Mathivha R, Verhoef L, Stass H. 2005. Fluid shifts have no influence on ciprofloxacin pharmacokinetics in intensive care patients with intra-abdominal sepsis. Int. J. Antimicrob. Agents 26:50–55 [DOI] [PubMed] [Google Scholar]
- 137. Udy A, Boots R, Senthuran S, Stuart J, Deans R, Lassig-Smith M, Lipman J. 2010. Augmented creatinine clearance in traumatic brain injury. Anesth. Analg. 111:1505–1510 [DOI] [PubMed] [Google Scholar]
- 138. Gonzalez D, Conrado DJ, Theuretzbacher U, Derendorf H. 2011. The effect of critical illness on drug distribution. Curr. Pharm. Biotechnol. 12:2030–2036 [DOI] [PubMed] [Google Scholar]
- 139. Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 37:840–851; quiz 859 [DOI] [PubMed] [Google Scholar]
- 140. Fish DN, Gotfried MH, Danziger LH, Rodvold KA. 1994. Penetration of clarithromycin into lung tissues from patients undergoing lung resection. Antimicrob. Agents Chemother. 38:876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Honeybourne D, Kees F, Andrews JM, Baldwin D, Wise R. 1994. The levels of clarithromycin and its 14-hydroxy metabolite in the lung. Eur. Respir. J. 7:1275–1280 [DOI] [PubMed] [Google Scholar]
- 142. Zeitlinger MA, Traunmüller F, Abrahim A, Müller MR, Erdogan Z, Müller M, Joukhadar C. 2007. A pilot study testing whether concentrations of levofloxacin in interstitial space fluid of soft tissues may serve as a surrogate for predicting its pharmacokinetics in lung. Int. J. Antimicrob. Agents 29:44–50 [DOI] [PubMed] [Google Scholar]
- 143. Cruciani M, Gatti G, Mengoli C, Cazzadori A, Lazzarini L, Miletich F, Graziani MS, Malena M, Bessetti D. 1997. Penetration of dapsone into pulmonary lining fluid of human immunodeficiency virus type 1-infected patients. Antimicrob. Agents Chemother. 41:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yamazaki K, Ogura S, Ishizaka A, Oh-hara T, Nishimura M. 2003. Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am. J. Respir. Crit. Care Med. 168:1304–1307 [DOI] [PubMed] [Google Scholar]
- 145. Fish D. 2003. Bronchoscopic sampling of drug concentrations: penetration to tissue is the issue. Am. J. Respir. Crit. Care Med. 168:1263–1265 [DOI] [PubMed] [Google Scholar]
- 146. Herkner H, Müller MR, Kreischitz N, Mayer BX, Frossard M, Joukhadar C, Klein N, Lackner E, Müller M. 2002. Closed-chest microdialysis to measure antibiotic penetration into human lung tissue. Am. J. Respir. Crit. Care Med. 165:273–276 [DOI] [PubMed] [Google Scholar]
- 147. Burkhardt O, Majcher-Peszynska J, Borner K, Mundkowski R, Drewelow B, Derendorf H, Welte T. 2005. Penetration of ertapenem into different pulmonary compartments of patients undergoing lung surgery. J. Clin. Pharmacol. 45:659–665 [DOI] [PubMed] [Google Scholar]
- 148. Zeitlinger M, Müller M, Joukhadar C. 2005. Lung microdialysis—a powerful tool for the determination of exogenous and endogenous compounds in the lower respiratory tract. AAPS J. 7:E600–E608 doi:10.1208/aapsj070362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lipsky BA, Byren I, Hoey CT. 2010. Treatment of bacterial prostatitis. Clin. Infect. Dis. 50:1641–1652 [DOI] [PubMed] [Google Scholar]
- 150. Barza M, Cuchural G. 1985. General principles of antibiotic tissue penetration. J. Antimicrob. Chemother. 15(Suppl A):59–75 [DOI] [PubMed] [Google Scholar]
- 151. Naber KG, Sörgel F. 2003. Antibiotic therapy—rationale and evidence for optimal drug concentrations in prostatic and seminal fluid and in prostatic tissue. Andrologia 35:331–335 [PubMed] [Google Scholar]
- 152. Wagenlehner FME, Kees F, Weidner W, Wagenlehner C, Naber KG. 2008. Concentrations of moxifloxacin in plasma and urine, and penetration into prostatic fluid and ejaculate, following single oral administration of 400 mg to healthy volunteers. Int. J. Antimicrob. Agents 31:21–26 [DOI] [PubMed] [Google Scholar]
- 153. Fair WR, Cordonnier JJ. 1978. The pH of prostatic fluid: a reappraisal and therapeutic implications. J. Urol. 120:695–698 [DOI] [PubMed] [Google Scholar]
- 154. Perletti G, Wagenlehner FME, Naber KG, Magri V. 2009. Enhanced distribution of fourth-generation fluoroquinolones in prostatic tissue. Int. J. Antimicrob. Agents 33:206–210 [DOI] [PubMed] [Google Scholar]
- 155. Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2:289–300 [DOI] [PubMed] [Google Scholar]
- 156. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 157. Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607 [DOI] [PubMed] [Google Scholar]
- 158. Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 159. Mueller M, De La Peña A, Derendorf H. 2004. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: kill curves versus MIC. Antimicrob. Agents Chemother. 48:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jumbe NL, Drusano GL. 2011. A model-based PK/PD antimicrobial chemotherapy drug development platform to simultaneously combat infectious diseases and drug resistance, p 251–280 In Kimko HHC, Peck CC. (ed), Clinical trial simulations: applications and trends. Springer, New York, NY [Google Scholar]
- 161. Bradley JS, Dudley MN, Drusano GL. 2003. Predicting efficacy of antiinfectives with pharmacodynamics and Monte Carlo simulation. Pediatr. Infect. Dis. J. 22:982–992 [DOI] [PubMed] [Google Scholar]
- 162. Bulitta JB, Duffull SB, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano GL, Sörgel F. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]