Abstract
SUMMARY
Flexible endoscopy is a widely used diagnostic and therapeutic procedure. Contaminated endoscopes are the medical devices frequently associated with outbreaks of health care-associated infections. Accurate reprocessing of flexible endoscopes involves cleaning and high-level disinfection followed by rinsing and drying before storage. Most contemporary flexible endoscopes cannot be heat sterilized and are designed with multiple channels, which are difficult to clean and disinfect. The ability of bacteria to form biofilms on the inner channel surfaces can contribute to failure of the decontamination process. Implementation of microbiological surveillance of endoscope reprocessing is appropriate to detect early colonization and biofilm formation in the endoscope and to prevent contamination and infection in patients after endoscopic procedures. This review presents an overview of the infections and cross-contaminations related to flexible gastrointestinal endoscopy and bronchoscopy and illustrates the impact of biofilm on endoscope reprocessing and postendoscopic infection.
INTRODUCTION
The consequences of the use of contaminated endoscopes are a recurrent topic in the endoscopy literature. Flexible endoscopes may become heavily contaminated with blood, secretions, and microorganisms during use. These instruments are difficult to clean and disinfect and easy to damage because of their complex design, with narrow lumens and multiple internal channels (Fig. 1) (1). If the instruments are not properly cleaned, the disinfection and drying procedures can fail and increase the possibility of transmission of infection from one patient to another (2). In addition, the ability of bacteria to form biofilms in the endoscope channels, especially when these become damaged, can contribute to failure of the decontamination process (2, 3).
Fig 1.
Schematic drawing of a cross section of a flexible endoscope showing the complex design and multiple internal channels (inner diameter, 2.8 to 3.8 mm).
Accurate reprocessing of flexible endoscopes is a multistep procedure involving cleaning followed by high-level disinfection (HLD) with further rinsing and drying before storage. Endoscope reprocessing can be performed with the use of automated endoscope reprocessors (AERs) and manual methods. Since almost all outbreaks are related to breaches in reprocessing techniques, it is crucial that endoscope cleaning, disinfection, and drying are performed according to a strict protocol. However, process control of endoscope reprocessing does not guarantee prevention of settlement of biofilm during endoscopy (2).
Current endoscope reprocessing and infection control guidelines have been reported by various organizations (4–7), and under controlled conditions, these measures are adequate. The most common factors associated with microbial transmission involve inadequate cleaning, disinfection, and drying procedures; the use of contaminated AERs; and flaws in instrument design or the use of damaged endoscopes.
Endoscopy-related infections can be divided into two types: endogenous and exogenous. Endoscopic procedures most often result in endogenous infections (i.e., infections resulting from the patient's own microbial flora), and Escherichia coli, Klebsiella spp., Enterobacter spp., and enterococci are the species most frequently isolated (8). Examples of endogenous infections include pneumonia resulting from aspiration of oral secretions in a sedated patient during flexible bronchoscopy and bacteremia in patients with biliary obstruction during endoscopic retrograde cholangiopancreaticography (ERCP). Endogenous infections are associated with endoscopy but cannot be prevented by well-controlled disinfection procedures (9). The exogenous microorganisms most frequently associated with transmission are Pseudomonas aeruginosa and Salmonella spp. during flexible gastrointestinal (GI) endoscopy and P. aeruginosa and mycobacteria during bronchoscopy (10). These microorganisms can be transmitted from previous patients or contaminated reprocessing equipment by contaminated endoscopes or its accessory equipment. Exogenous infection should be prevented by strict endoscope disinfection procedures (9).
The purpose of this review is to present an overview of the infections and cross-contaminations related to flexible GI endoscopy and bronchoscopy and to illustrate the impact of biofilm on endoscope reprocessing and postendoscopic infection.
PRACTICAL ASPECTS OF FLEXIBLE ENDOSCOPE REPROCESSING
Relevance of Cleaning and Disinfection
According to the Spaulding classification system, medical devices are divided into three categories based on the risk of infection (11). Critical medical instruments (e.g., surgical instruments and prosthetic heart valves) that enter the vascular system and normally sterile tissues and that carry a high degree of infection risk if contaminated during use should be sterilized. Noncritical medical devices, such as stethoscopes, coming into contact with intact skin require low-level disinfection or simple cleaning with detergent and water. Most flexible endoscopes belong to semicritical devices which come into contact with mucous membranes during use and have a moderate degree of infection risk if contaminated at the time of use. They should receive at least HLD. HLD is a process that eliminates or kills all vegetative bacteria, mycobacteria, fungi, and viruses, except for small numbers of bacterial spores (12, 13). Sterilization results in the complete destruction of all forms of microbiological life, including bacterial spores.
Flexible endoscopes for therapeutic procedures (bronchoscopy and ERCP) and reusable accessories, such as biopsy forceps, are used in sterile body cavities and should be classified as critical devices (12, 13). They should be sterilized after each procedure. Due to their material composition, most flexible endoscopes cannot be steam sterilized (9). They tolerate ethylene oxide and hydrogen peroxide plasma sterilization, which are expensive and are not preferred by most institutions (14). No data are available demonstrating that sterilization results in a lower frequency of postendoscopic infection than does HLD. Ethylene oxide and hydrogen peroxide plasma sterilization have rapid and reliable efficacy compared to HLD (15). However, both sterilizers destroy chemical, biological, and mechanical properties of instruments, including flexible endoscopes. Gas sterilization with ethylene oxide may fail in the presence of organic debris after inadequate cleaning (16, 17) and when biofilm has settled in internal endoscope channels (2, 18).
Natural bioburden levels detected on flexible GI endoscopes range from 105 CFU/ml to 1010 CFU/ml after clinical use (19, 20). Cleaning must precede HLD or sterilization to remove organic debris (e.g., blood, feces, and respiratory secretions) from the external surface, lumens, and channels of flexible endoscopes (4, 21). Inadequate cleaning of flexible endoscopes has been frequently associated with microbial transmission during endoscopic procedures. Appropriate cleaning reduces the number of microorganisms and organic debris by 4 logs, or 99.99% (20).
The manual cleaning procedure for flexible endoscopes includes brushing of the external surface and removable parts (e.g., suction valves) and immersion in a detergent solution followed by irrigation of internal channels with a detergent. The endoscope and accessories should be inspected for damage, and a leak test should be performed before disinfection (4).
AERs are strongly recommended for reprocessing of flexible endoscopes to document all steps and to minimize contamination and contact with chemicals and contaminated instruments (5). However, contaminated and defective AERs can result in inadequate reprocessing and contamination of endoscopes and have been associated with outbreaks of endoscopy-related infections (22–26). Presence of biofilms in the AERs has been detected during these outbreaks (23, 25, 26).
Disinfecting agents used for HLD must have bactericidal, mycobactericidal, fungicidal, virucidal, and sporicidal activity (12, 13). According to their activity against bacteria, fungi, spores, and viruses, disinfectants can be classified into the following three groups: high-level (glutaraldehyde, peracetic acid, and ethylene oxide), intermediate-level (ethanol, formaldehyde, and phenolic solutions), and low-level (povidone-iodine, cetrimide, and benzalkonium chloride) disinfectants (11, 12, 27). Intermediate-level disinfectants do not have sporicidal activity. Disinfectants with low potency do not destroy Mycobacterium tuberculosis, atypical mycobacteria, and bacterial spores. Concentration and exposure time of a disinfecting agent are crucial; inappropriate dilution and insufficient exposure can result in a failure of effective reprocessing (28–30). Inappropriate disinfectants with low and intermediate potency are not recommended for HLD and have been replaced by glutaraldehyde, hydrogen peroxide, ortho-phthalaldehyde, peracetic acid, and superoxidized and electrolyzed acid water (31, 32). Advantages and disadvantages of commonly used high-level disinfectants are summarized in Table 1.
Table 1.
Advantages and disadvantages of commonly used high-level disinfectants
| High–level disinfectant | Advantage(s) (reference[s]) | Disadvantage(s) (reference[s]) |
|---|---|---|
| Glutaraldehyde | Excellent biocidal properties (5, 33–35) | Slow action against mycobacteria (35, 38) |
| Many studies published | Irritant to the respiratory tract, eyes, and skin; development of allergic reactions, contact dermatitis, asthma, acute colitis (36, 37) | |
| Does not damage endoscopes and processing equipment; noncorrosive to metal (5, 33) | Development of biocide resistance (39–42) | |
| Relatively inexpensive (5) | Coagulation and fixation of proteins (5) | |
| ortho-Phthalaldehyde | High biocidal activity (inclusive of mycobacteria) (5, 44) | Slow action against bacterial spores (5) |
| Does not damage endoscopes and processing equipment | Staining of the skin, clothing, instruments (46) | |
| Irritation of the respiratory tract and eyes; development of “anaphylaxis-like” reactions after repeated use (5, 36, 45) | ||
| Expensive | ||
| Peracetic acid | Excellent and fast biocidal activity at low concentrations (5, 7) | Irritant to the respiratory tract and eyes (5, 7, 36) |
| Can be used at low temperatures (5, 7) | Corrosive action depending on the pH value and concn (7) | |
| No development of resistance reported | Limited efficacy in biofilm removal and in killing bacteria within the biofilm (47–49) | |
| Electrolyzed acid and superoxidized water | Excellent and fast biocidal activity (15) | Reduced efficacy in the presence of organic soil after inappropriate cleaning (50) |
| Nontoxic to biological tissues; nonirritant to the respiratory tract, eyes, and skin (15) | ||
| Relatively inexpensive |
Glutaraldehyde (2 to 4%) is a disinfecting agent effective against bacteria, viruses, fungi, and spores that is relatively inexpensive, is noncorrosive to metal, and does not damage endoscopes and processing equipment (5, 33). Two percent aqueous solutions of glutaraldehyde killed vegetative bacteria in <2 min, fungi and viruses in <10 min, and spores of Bacillus spp. and Clostridium spp. in 3 h (33–35). However, glutaraldehyde has irritant properties for the respiratory tract, eyes, and skin and can cause allergic reactions, contact dermatitis, and asthma (36). For these reasons, the use of this high-level disinfectant should be done with containment to minimize aerosolization of glutaraldehyde. Acute colitis occurring after lower GI endoscopy was probably caused by glutaraldehyde residues in the endoscope after disinfection (37). Other disadvantages of glutaraldehyde are coagulation and fixation of proteins and failure to eliminate atypical mycobacteria within standard contact times (5, 35, 38).
Microorganisms possessing resistance to glutaraldehyde include atypical mycobacteria (Mycobacterium chelonae and Mycobacterium avium complex) (39, 40) and Cryptosporidium parvum (41). P. aeruginosa resistance to glutaraldehyde was responsible for three separate clinical episodes of ERCP-associated cholangitis (42). The mechanism of the high biocide resistance of mycobacteria is probably associated with the decreased penetration of a disinfectant through the hydrophobic lipid-rich cell wall (35). Two percent alkaline glutaraldehyde completely inactivated M. tuberculosis in bronchoscopes after 10 min of incubation (43). M. avium, Mycobacterium gordonae, and Mycobacterium intracellulare were more resistant to inactivation by 2% alkaline glutaraldehyde and survived the treatment for more than 10 min (38). Since mycobacteria are more resistant to glutaraldehyde than other bacteria, the manufacturers' instructions for flexible endoscopes and this high-level disinfectant should followed to determine the correct conditions.
ortho-Phthalaldehyde (0.55%) is a high-level disinfectant with a higher mycobactericidal efficacy than glutaraldehyde (5, 44). Disadvantages of this disinfectant include slow action against bacterial spores, irritation of the respiratory tract and eyes of the patients and staff, and the possibility of causing “anaphylaxis-like” reactions after repeated use (5, 36, 45). ortho-Phthalaldehyde stains skin, instruments, clothing, and surfaces and is more expensive than glutaraldehyde (46).
Peracetic acid is an oxidizing agent usually used for HLD of flexible endoscopes in AERs. It rapidly deactivates a large variety of pathogenic microorganisms, viruses, and spores at low concentrations (<0.3%) (5, 7). No development of microorganism resistance to peracetic acid has been reported. This disinfectant can be used at low temperatures and causes less irritation than glutaraldehyde but has corrosive action depending on the pH value and concentration (7). Several disinfectants that contain peracetic acid and hydrogen peroxide are available. Although the biocidal effect of peracetic acid on sessile microorganisms is well known, the effect of this disinfecting agent on microbial biofilms has not been completely studied. A recent study demonstrated insufficient efficacy of 1% peracetic acid disinfectant for 10 min against P. aeruginosa, Stenotrophomonas maltophilia, and Candida sp. biofilms (47). According to the literature, peracetic acid has the ability to fixate biofilms, and therefore, it can show limited efficacy in biofilm removal from the abiotic surfaces and in killing bacteria within the biofilm (48, 49). Vigorous cleaning with brushes or agitated liquids is important to remove as much biofilm as possible (4).
Electrolyzed acid and superoxidized water are relatively new disinfectants used for endoscope reprocessing (15). These disinfectants are produced by the electrolysis of sodium chloride solutions. They have excellent biocidal activity and are inexpensive, nontoxic to biological tissues, and nonirritating to the respiratory tract, eyes, and skin (15). However, antimicrobial efficacy can be reduced in the presence of organic soil after inappropriate cleaning (50).
After the disinfection phase, the remaining disinfectant must be removed from the exterior and from the internal channels by rinsing the endoscope with bacterium-free water. According to the Guideline Committee of the European Society of Gastrointestinal Endoscopy, sterile water is preferable for the final rinse to prevent recontamination (5). Many outbreaks of endoscopy-related infections and cross-contaminations due to P. aeruginosa, Serratia marcescens, M. chelonae, Mycobacterium xenopi, and Methylobacterium mesophilicum have been related to rinsing flexible endoscopes after disinfection with nonsterile tap water (30, 51–57). The accepted procedures to produce bacterium-free water in disinfectors for endoscopes include filtration, UV radiation, or heating followed by cooling (5). However, major U.S. guidelines recommend the use of sterile, filtered, or tap water to rinse the endoscope channels after HLD, followed by flushing of the endoscope channels with 70% to 90% ethyl alcohol or isopropyl alcohol (6, 7, 21). The rinse water must be discarded after each cycle. Water bottles used for irrigation during the procedure should be high-level disinfected or sterilized at least daily. Sterile water should be used to fill the water bottle.
Relevance of Drying and Storage
Accurate endoscope drying is crucial, whereas a humid environment facilitates microbial growth during storage. The final drying steps greatly reduce the risk of remaining pathogens as well as the possibility of recontamination of the endoscope by waterborne microorganisms such as Pseudomonas spp. and Acinetobacter spp. (58). Flexible endoscopes should be dried with filtered compressed air manually or in AERs between endoscopic procedures (a short drying cycle) and at the end of the day (an intensive final drying) (5, 59). Several guidelines recommend forced air drying, preceded by flushing of the internal channels with 70% to 90% ethanol or isopropanol at the end of a clinic day (6, 7, 21). Allen et al. (22) showed a high efficiency in preventing P. aeruginosa contaminations by flushing 70% ethanol through endoscope channels followed by drying with compressed air. Due to the fixative properties of ethanol, its use is not recommended in some countries.
Storage is an important factor in the maintenance of bacterium-free endoscopes. It is recommended that a dust-free drying cabinet be used for endoscope storage, in which the endoscopes are hung vertically (5, 60, 61). Noy et al. (62) noted that storing the endoscopes vertically in air after cleaning until the next use of the endoscope resulted in a drop of the contamination rate from 35 to 0%. According to the literature, endoscopes stay bacterium free after prolonged storage if an adequate drying procedure is applied (60, 61). When stored in the drying cabinet with a laminar airflow, no growth of bacteria and Candida spp. was found in endoscope channels 5 days after reprocessing (61). Low levels of microbial contamination on endoscopes stored in the drying and storage cabinet were detected, compared with the stable or increased microbial numbers on endoscopes stored outside the cabinet (60).
Many outbreaks of health care-associated infection after endoscopy and cross-contaminations due to P. aeruginosa, S. marcescens, M. tuberculosis, and M. chelonae have been associated with drying of flexible endoscopes without ethanol flushing or lack of a drying procedure (22–24, 30, 52, 57, 63–76).
EXOGENOUS INFECTIONS ASSOCIATED WITH FLEXIBLE ENDOSCOPY
Despite the large number of endoscopic procedures that are performed annually, documented data suggest that postendoscopic iatrogenic infections are rare. In GI endoscopy, the estimated rate of health care-associated infection is approximately 1 out of 1.8 million procedures (77). However, the true rate of transmission during endoscopy may go unrecognized because of technically inadequate surveillance, no surveillance at all, low frequency, or the absence of clinical symptoms (2, 9). Two hundred eighty-one patients infected after GI endoscopy were found in studies of endoscopy-related infections between 1966 and 1992 in the United States (8). Gorse and Messner (14) reported 6% iatrogenic infections after GI endoscopy in 116 hospitals. During the period of 1974 to 2004, 30 outbreaks of endoscopy-related infections and cross-contaminations involving 251 patients infected after GI endoscopic procedures were reported in the United States (78). Inadequate decontamination procedures and equipment malfunction were two leading causes of postendoscopic infection and contamination. More than 91% of the infections identified could be prevented if quality control systems were improved.
The important risk factors of infections in GI endoscopy are the number of microorganisms present inside the endoscope or the growth of a biofilm, invasive endoscopic procedures resulting in tissue damage, compromised immune status of the patient (human immunodeficiency virus [HIV] infection, neoplastic diseases, transplant patients, and immunosuppressive treatment), and the presence of infective foci (abscess and cholangitis) during an endoscopic procedure (79–81).
An overview of the exogenous endoscopy-related infections and cross-contaminations after flexible GI endoscopy and bronchoscopy is presented in Tables 2 to 5.
Table 2.
Infections associated with flexible upper gastrointestinal endoscopy
| Reference | Microorganism(s) | No. of contaminated patients after endoscopy | No. of infected patients | Infection type(s) | Detection of endoscope contamination | Cause(s) of contaminationa |
|---|---|---|---|---|---|---|
| 80 | P. aeruginosa | 3 | 2 | Sepsis | Yes | Inappropriate cleaning and disinfection (benzalkonium chloride) |
| 62 | P. aeruginosa | 4 | 3 | Pneumonia, lung abscess | Yes | Inappropriate cleaning and disinfection (cetrimide) |
| 65 | P. aeruginosa | 4 | 4 | Sepsis | Yes | Contaminated water bottle and water supply; inappropriate cleaning and disinfection |
| 23 | P. aeruginosa | 99 | No data | Bacteremia/sepsis, cholangitis, pneumonia | Yes | Contaminated AER (a flaw in design, presence of biofilm); drying with no ethanol flushing |
| 130 | H. pylori | 1 | 1 | Gastritis | Not tested | Biopsy forceps not sterilized |
| 128 | H. pylori | 2 | 2 | Gastritis | Yes | Inappropriate disinfection between patients (ethanol); biopsy forceps not sterilized |
| 131 | H. pylori | 1 | 1 | Gastritis | Yes | Inappropriate cleaning and disinfection between patients |
| 84 | Salmonella Typhi | 1 | 1 | Bacteremia | Not tested | Inappropriate cleaning and disinfection |
| 82 | Salmonella Typhimurium | 7 | 7 | Gastroenteritis, peritoneal abscess | Yes | Inappropriate cleaning and disinfection |
| 28 | Salmonella Typhimurium | 1 | 1 | Urinary tract infection | Yes | Lack of manual cleaning; insufficient disinfectant exposure |
| 88 | Salmonella Agona | 5 | 5 | Gastroenteritis, bacteremia/sepsis | Yes | Inappropriate cleaning and disinfection (cetrimide) |
| 86 | Salmonella Kedougou | 15 | 12 | Gastroenteritis | No | Inappropriate cleaning and disinfection (cetrimide) |
| 162 | HBV | 1 | 1 | HBV infection | Not tested | Inappropriate cleaning and disinfection (cetrimide); no disinfection between patients |
| 163 | HBV | 1 | 1 | HBV infection | Not tested | Endoscope reprocessing not described |
| 161 | HBV | 1 | 1 | HBV infection | Not tested | Lack of disinfection procedure |
| 183 | HCV | 9 | 9 | HCV infection | Not tested | Contaminated syringe or anesthetic vial; inappropriate cleaning and disinfection |
| 150 | Strongyloides stercoralis | 4 | 4 | Esophagitis | Not tested | Not found |
| 152 | Trichosporon spp. | 9 | 0 | No | Yes | Inappropriate cleaning and disinfection (cetrimide) |
| 151 | Trichosporon asahii | 1 | 1 | Esophagitis | Yes | Biopsy forceps not sterilized |
AER, automated endoscope reprocessor.
Table 5.
Infections associated with flexible bronchoscopy
| Reference | Microorganism(s) | No. of contaminated patients after endoscopy | No. of infected patients | Infection(s) | Detection of endoscope contamination | Cause(s) of contaminationa |
|---|---|---|---|---|---|---|
| 73 | P. aeruginosa | 11 | 1 | Pneumonia | Yes | Inappropriate cleaning and disinfection (povidone-iodine); drying with no ethanol flushing |
| 70 | P. aeruginosa | 8 | 0 | No | Yes | Lack of manual cleaning; drying with no ethanol flushing |
| 102 | P. aeruginosa | 35 | No data | No data | Yes | Contaminated AER |
| 104 | P. aeruginosa | 18 | 3 | Pneumonia | No data | Inadequate cleaning and disinfection (incorrect connectors) |
| 26 | P. aeruginosa | 8 | 8 | Sepsis, pneumonia | Yes | Contaminated AER (biofilm on internal plumbing); no protocol for manual cleaning; lack of drying procedure |
| 103 | P. aeruginosa | 18 | 3 | Pneumonia | Yes | Contaminated AER; incorrect AER connectors (obstruction of disinfectant flow) |
| 107 | P. aeruginosa, S. marcescens | 20 (P. aeruginosa), 6 (S. marcescens) | 1 | Pneumonia | Yes | Loose biopsy port cap of the contaminated bronchoscopes |
| 30 | P. aeruginosa, S. marcescens | 41 | 0 | No | Yes | Insufficient disinfectant exposure; rinsing with tap water; drying with no ethanol flushing |
| 93 | P. aeruginosa | 32 | 32 | Pneumonia, bacteremia/sepsis, bronchitis, sinusitis | Yes | Loose biopsy port cap of the contaminated bronchoscopes |
| 105 | P. aeruginosa | 16 | 4 | Pneumonia | Yes | Defective biopsy forceps and damaged endoscope channel |
| 64 | P. aeruginosa | 10 | 7 | Pneumonia, bronchitis | Not tested | Inappropriate disinfection (povidone-iodine); lack of drying procedure |
| 74 | P. aeruginosa | 7 | 7 | Pneumonia, bronchitis | Yes | Contaminated AER (detergent tank); inadequate manual cleaning; drying with no ethanol flushing |
| 106 | P. aeruginosa | 12 | 12 | Pneumonia, sepsis | Yes | Multiple manufacturing defects of the contaminated bronchoscope |
| 27 | M. tuberculosis | 1 | 0 | No | No data | Inappropriate disinfection (iodophor solution); lack of manual cleaning; drying procedure not described |
| 109 | M. tuberculosis | 2 | 1 | Lung tuberculosis | Yes | Inappropriate disinfection (iodophor solution) |
| 111 | M. tuberculosis | 3 | 1 | Lung tuberculosis | Yes | Contaminated suction valves (failure to disinfect with glutaraldehyde) |
| 63 | M. tuberculosis | 3 | 1 | Multidrug-resistant lung tuberculosis | Yes | Inadequate cleaning and disinfection (no immersion in disinfectant); drying with no ethanol flushing |
| 66 | M. tuberculosis | 1 | 0 | No | No data | Inappropriate cleaning and disinfection; lack of drying procedure |
| 108 | M. tuberculosis | 1 | 1 | Lung tuberculosis | No data | Inappropriate cleaning and disinfection; drying procedure not described |
| 125 | M. tuberculosis | 8 | 0 | No | Yes | Contaminated automated aspiration adaptor (no manual cleaning and disinfection) |
| 104 | M. tuberculosis | 4 | 0 | No | No | Inadequate cleaning and disinfection (biopsy port cap was not replaced before loading into the AER) |
| 110 | M. tuberculosis | 9 | 3 | Lung tuberculosis | No | Hole in the sheath of the bronchoscope (no leak testing performed) |
| 120 | M. tuberculosis | 2 | 0 | No | No | Inadequate cleaning and disinfection between uses in patients (incompatibility of the bronchoscope and AER) |
| 67 | M. chelonae | 7 | 0 | No | No | Contaminated AER; drying with no ethanol flushing |
| 124 | M. chelonae | 72 | 2 | No data | Yes | Contaminated suction channel (punctured channel) |
| 71 | M. chelonae | 7 | 0 | No | Yes | Rinsing with contaminated tap water; drying with no ethanol flushing |
| 126 | M. chelonae | 4 | 0 | No | Yes | Contaminated suction channel valve; inadequate endoscope reprocessing |
| 52 | M. chelonae | 14 | 0 | No | No data | Contaminated AER; inappropriate disinfection; rinsing with tap water; lack of drying procedure |
| 24 | M. chelonae | 14 | 0 | No | No data | Contaminated AER; inappropriate cleaning and disinfection; drying with no ethanol flushing |
| 68 | M. chelonae, M. gordonae | 14 | 0 | No | No data | Contaminated AER (rinse water tank); drying with no ethanol flushing |
| 122 | M. chelonae | 12 | 0 | No | Yes | Contaminated AER; inadequate endoscope reprocessing |
| 123 | M. chelonae | 13 | 0 | No | No data | Contaminated AER; inadequate endoscope reprocessing |
| 76 | M. chelonae | 15 | 0 | No | Yes | Contaminated AER; inappropriate disinfection; drying with no ethanol flushing |
| 127 | M. chelonae | 18 | 0 | No | Yes | Contaminated suction channels; inappropriate disinfection and drying |
| 25 | M. chelonae, Methylobacterium mesophilicum | 20 | 0 | No | Yes | Contaminated AER (presence of biofilm) |
| 111 | M. avium complex | 2 | 0 | No | Yes | Contaminated suction valve (failure to disinfect with glutaraldehyde) |
| 104 | M. avium complex | 7 | 0 | No | No | Inadequate cleaning and disinfection (incorrect connectors) |
| 56 | M. xenopi | 2 | 0 | No | No data | Rinsing with contaminated tap water |
| 51 | M. xenopi | 20 | 3 | Pulmonary infection, abscess | Yes | Rinsing with contaminated tap water; inadequate disinfection and drying between uses in patients |
| 121 | Mycobacterium spp. (patients), M. chelonae and M. fortuitum (AER) | 15 | 0 | No | No data | Contaminated AER; endoscope reprocessing not described |
| 142 | S. marcescens | 3 | 3 | Pneumonia | Yes | Inappropriate cleaning and disinfection (ethanol) |
| 55 | S. marcescens | 4 | 0 | No | Yes | Inappropriate disinfection (ethanol); rinsing with tap water |
| 57 | S. marcescens | 6 | 5 | Bacteremia/sepsis, pneumonia, wound infection | Yes | Inappropriate cleaning and disinfection; rinsing with tap water; lack of drying procedure |
| 72 | S. marcescens | 53 (no data about endoscopy) | Yes | Bacteremia/sepsis, pneumonia, wound infection | Yes | Drying with no ethanol flushing |
| 153 | R. rubra | 30 | 0 | No | No | Contaminated cleaning brushes |
| 69 | R. rubra | 11 | 0 | No | Yes | Contaminated suction channel; drying with no ethanol flushing; disinfection procedure not described |
| 156 | Bacillus sp. | 10 | 0 | No | Yes | Contaminated suction valves (no disassembling prior to cleaning); inappropriate cleaning and disinfection (ethanol) |
| 54 | M. mesophilicum | 7 | 0 | No | No | Rinsing with contaminated tap water; endoscope reprocessing not described |
| 154 | Blastomyces dermatitidis | 2 | 0 | No | Not tested | Inadequate manual cleaning; drying procedure not described |
| 155 | Legionella pneumophila | 3 | 0 | No | Not tested | Rinsing with contaminated tap water (inadequate maintenance of the filters) |
| 143 | Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii, Proteus vulgaris | 117 | 0 | No | Yes | Loose biopsy port cap of the contaminated bronchoscope |
AER, automated endoscope reprocessor.
Bacteria
Salmonella spp.
In the past, Salmonella spp. were the most common microorganisms associated with infections transmitted by GI endoscopy (28, 82–88). Many Salmonella outbreaks were related to an inappropriate use of disinfectants with intermediate and low potency instead of high-level disinfecting agents (Tables 2 and 3). There have been no reports of Salmonella infection since the current guidelines for HLD have been followed.
Table 3.
Infections associated with flexible sigmoidoscopy/colonoscopy
| Reference | Microorganism | No. of contaminated patients after endoscopy | No. of infected patients | Infection(s) | Detection of endoscope contamination | Cause(s) of contamination |
|---|---|---|---|---|---|---|
| 87 | Salmonella Lomita | 2 | 0 | No | Not tested | Inappropriate cleaning and disinfection (phenolic solution) |
| 28 | Salmonella Goerlitz | 1 | 1 | Gastroenteritis | Yes | Lack of manual cleaning; inappropriate disinfection (phenolic solution) |
| 85 | Salmonella Newport | 8 | 2 | Gastroenteritis | Yes | Biopsy forceps not sterilized; inappropriate disinfection (iodophor solution) |
| 181 | HCV | 2 | 2 | HCV infection | Not tested | Inadequate manual cleaning; insufficient disinfectant exposure; biopsy forceps not sterilized |
| 182 | HCV | 1 | 1 | HCV infection | Not tested | Contaminated syringe or anesthetic vial; inappropriate disinfection |
The following Salmonella serotypes were isolated from contaminated endoscopes or accessories: Salmonella enterica serotypes Typhi (84), Typhimurium (28, 82), Agona (88), Goerlitz (28), Newport (85), Oslo (83), Kedougou (86), and Lomita (87). A minority of patients involved were asymptomatic carriers and had stool or urine cultures positive for Salmonella spp. Salmonella infections developed within 1 to 9 days after a GI endoscopic procedure and included acute gastroenteritis (28, 82, 83, 85, 86), peritoneal abscess (82), urinary tract infection (28), and bacteremia/sepsis (83, 84, 88).
Pseudomonas aeruginosa.
P. aeruginosa, a Gram-negative opportunistic pathogen, is the most commonly reported microorganism responsible for transmission of infection during GI endoscopy and bronchoscopy. It is known for its preference for a moist environment (hospital water supply and wet endoscope channels after reprocessing) (10). Pseudomonas is able to form biofilms, and these biofilms are extremely difficult to remove from plumbing, AERs, and endoscope channels (2, 89). Serotype 10 of P. aeruginosa predominates in the published reports of Pseudomonas transmission (22, 23, 53, 73, 90, 91). It is possible that slime production by P. aeruginosa serotype 10 in a biofilm can form a barrier to antibiotics and disinfectants and lead to antibacterial resistance, but no explanation was offered as to why specifically this serotype was isolated.
Among healthy adults, P. aeruginosa can colonize many body sites, as evidenced by isolation from throat, sputum, and stool (92). Hospitalized patients, as well as patients with certain chronic lung diseases, have higher colonization rates. During health care-related outbreaks, Pseudomonas transmission can result in colonization of involved patients in the GI and respiratory tract with an absence of clinical symptoms and negative blood cultures, which was determined by molecular typing (93). Severe health care-associated postendoscopic infections due to P. aeruginosa include sepsis, liver abscess, and ascending cholangitis after flexible GI endoscopy (particularly after ERCP) and bloodstream infection and pneumonia after bronchoscopy. Post-ERCP P. aeruginosa infectious complications occur most frequently in patients with biliary obstruction undergoing endoscopic biliary stenting (94).
Many outbreaks of P. aeruginosa infection after GI endoscopy and bronchoscopy have been associated with inadequate cleaning and the use of inappropriate intermediate-level and low-level disinfectants (29, 62, 64, 73, 80, 90, 95–98), contaminated endoscope water bottles and the water supply to the endoscope (29, 65, 90, 99, 100), and drying of endoscope channels with no flushing with 70% ethanol after disinfection (22, 30, 70, 73–75) or lack of a drying procedure (26, 64) (Tables 2 to 5). Two recent outbreaks of multidrug-resistant P. aeruginosa post-ERCP infections have been related to the remaining contamination of the endoscope despite accurate reprocessing followed by negative surveillance endoscope cultures (2, 101). In an outbreak of post-ERCP sepsis described by Kovaleva et al. (2), the implicated endoscope was repetitively found to be positive by culturing for multidrug-resistant P. aeruginosa after HLD and contained biofilm in undamaged inner channels. The P. aeruginosa isolates from patients and the implicated endoscope underwent molecular typing and showed matching patterns.
Several postendoscopic P. aeruginosa outbreaks have been related to contaminated or defective AERs (22, 23, 26, 74, 75, 102, 103), the use of incorrect connectors between the endoscope and AER (103, 104), and defective endoscopes and accessories (93, 105–107) (Tables 2 to 5). The presence of biofilm deposits on the internal plumbing and detergent tank of AERs resulted in two outbreaks (23, 26).
In most outbreaks, the Pseudomonas strains obtained from patients were identical to those recovered from endoscopes, as determined by comparison of antimicrobial sensitivity patterns of the isolates, serotyping, and phage typing or by more recently developed molecular techniques (90, 97).
Mycobacteria.
M. tuberculosis and nontuberculous mycobacteria are microorganisms frequently associated with health care-associated transmission during endoscopic procedures, particularly during bronchoscopy. While transmission of nontuberculous mycobacteria is usually associated with contaminated AERs and rinsing water, contamination with M. tuberculosis generally comes from an infected patient during an endoscopic procedure (8). Most outbreaks due to nontuberculous mycobacteria have involved rapidly growing M. chelonae, intermediately growing M. gordonae, and slowly growing M. xenopi and M. avium complex strains.
Most publications about mycobacterial outbreaks described cross-contamination, a situation where the patient becomes nosocomially colonized with a strain from the endoscope in the absence of infection, but reports of bronchoscopy-related pulmonary tuberculosis were documented (63, 108–111). The majority of the patients was immunocompromised and had a history of lung cancer, HIV, or hematological malignancies. Most M. tuberculosis isolates were susceptible to antituberculosis drugs, but health care-associated transmission of multidrug-resistant tuberculosis has been reported (63). Nontuberculous mycobacteria, such as M. chelonae, M. avium, M. xenopi, M. gordonae, and M. fortuitum, can form the risk for development of colonization or infection in severely immunocompromised patients (112, 113).
The effectiveness of disinfectants against mycobacteria depends on the composition and concentration of the active agent, contact time, and presence of organic material (27, 113, 114). Activities of different disinfectants against M. tuberculosis and nontuberculous mycobacteria were tested in several studies. van Klingeren and Pullen (115) showed inadequate tuberculocidal activity of quaternary ammonium, good activity of phenolic disinfectants, and rapid killing of M. tuberculosis with 70% ethanol and 60% isopropanol. High-level effectiveness of 2% glutaraldehyde against M. tuberculosis and M. chelonae was demonstrated directly after cleaning and after 10- and 20-min exposures (43, 116, 117). Peracetic acid (0.26%) was effective against M. tuberculosis and M. avium complex strains within 10 to 20 min, with a 5-log reduction in viable bacteria (118).
Mycobacteria are known to possess resistance to many disinfecting agents, including aldehydes (35, 119). The mechanism of the high-level biocide resistance of mycobacteria is not completely understood; most likely, it is associated with decreased penetration of a disinfectant through the hydrophobic lipid-rich cell wall (35). Nontuberculous mycobacteria tend to be more resistant to antiseptics and disinfectants than M. tuberculosis (113). Slow-growing M. avium complex and intermediately growing M. gordonae strains survived treatment with 2% glutaraldehyde for more than 10 min (38). The M. chelonae strain resistant to glutaraldehyde and peracetic acid was isolated from a contaminated AER (40). Nomura et al. (39) reported a high percentage of glutaraldehyde-tolerant M. chelonae strains which were either resistant or intermediately resistant to 2 or 3 classes of antibiotics.
Most outbreaks of M. tuberculosis and nontuberculous mycobacterium transmission have been associated with inadequate cleaning, disinfection, and drying procedures (24, 27, 51, 52, 63, 66, 68, 71, 76, 104, 108, 109, 120), contaminated AERs and contaminated tap water used to rinse bronchoscopes after disinfection (24, 25, 51, 52, 56, 68, 71, 76, 121–123), and defective or contaminated endoscopes and accessories (104, 110, 111, 120, 124–127) (Tables 4 and 5). The presence of M. chelonae and M. mesophilicum biofilms was found in a contaminated AER during one outbreak (25).
Table 4.
Infections associated with endoscopic retrograde cholangiopancreaticographya
| Reference | Microorganism(s) | No. of contaminated patients after endoscopy | No. of infected patients | Infection(s) | Detection of endoscope contamination | Cause(s) of contamination |
|---|---|---|---|---|---|---|
| 95 | P. aeruginosa | 1 | 1 | Cholangitis, sepsis | Yes | Inappropriate cleaning and disinfection (ethanol) |
| 96 | P. aeruginosa | 14 | 0 | No | Yes | Inappropriate cleaning and disinfection (povidone-iodine/ethanol) |
| 97 | P. aeruginosa | 7 | 7 | Cholangitis | Yes | Inappropriate cleaning and disinfection (ethanol) |
| 100 | P. aeruginosa | 1 | 1 | Sepsis | Yes | Contaminated water bottles |
| 53 | P. aeruginosa | 4 | 3 | Sepsis | Yes | Inappropriate disinfection; rinsing with nonsterile tap water |
| 91 | P. aeruginosa | 5 | 5 | Cholangitis, sepsis, urinary tract infection | Yes | Inadequate cleaning and disinfection between uses in patients (tap water) |
| 22 | P. aeruginosa | 10 | 5 | Cholecystitis, liver abscess | Yes | Contaminated AER; inappropriate cleaning and disinfection; drying with no ethanol flushing |
| 328 | P. aeruginosa | 1 | 1 | Liver abscess | No | Not found; endoscope reprocessing not described |
| 98 | P. aeruginosa | 2 | 2 | Sepsis | Yes | Inappropriate cleaning and disinfection (cetrimide) |
| 90 | P. aeruginosa | 7 | 7 | Bacteremia/sepsis, cholangitis, pancreatitis | Yes | Contaminated water bottle; inadequate manual cleaning and disinfection between patients (isopropanol) |
| 99 | P. aeruginosa | 5 | 5 | Sepsis | Yes | Contaminated water bottle (not disinfected) |
| 23 | P. aeruginosa | 16 | No data | Bacteremia/sepsis, cholangitis, pneumonia | Yes | Contaminated AER (a flaw in design, presence of biofilm); drying with no ethanol flushing |
| 75 | P. aeruginosa | 25 | 25 | Bacteremia/sepsis | Yes | Failure to disinfect elevator channel in AER; drying with no ethanol flushing |
| 101 | P. aeruginosa | 5 | 3 | Cholangitis, sepsis | No | Not found; endoscope reprocessing not described |
| 29 | P. aeruginosa | 3 | 3 | Sepsis | Yes | Contaminated water bottle; inadequate manual cleaning; insufficient disinfectant exposure |
| 2 | P. aeruginosa | 3 | 3 | Sepsis | Yes | Presence of biofilm in intact endoscope channels |
| 83 | Salmonella Oslo | 3 | 2 | Gastroenteritis, sepsis | Not tested | Inappropriate cleaning and disinfection (povidone-iodine/ethanol) |
| 141 | Serratia marcescens | 1 | 0 | No | Yes | Inappropriate cleaning and disinfection (povidone-iodine) |
| 52 | M. chelonae | 14 | 0 | No | No data | Contaminated AER; inappropriate disinfection; rinsing with tap water; lack of drying procedure |
| 147 | Methylobacterium mesophilicum | 1 | 1 | Bacteremia | Yes | Contaminated endoscope channels |
| 144 | ESBL-producing K. pneumoniae | 16 | 12 | Bacteremia/sepsis, cholangitis | Yes | Contaminated endoscope channels; insufficient drying procedure |
| 145 | KPC-producing K. pneumoniae | 7 | 2 | Bacteremia | Yes | Contaminated endoscope channels; insufficient drying procedure |
| 184 | HCV | 1 | 1 | HCV infection | Not tested | Inadequate disinfection (low concn, insufficient exposure); failure to perfuse elevator channel |
AER, automated endoscope reprocessor; ESBL, extended-spectrum β-lactamase; KPC, Klebsiella pneumoniae carbapenemase.
Helicobacter pylori.
Although Helicobacter pylori is a common pathogen in patients with chronic gastritis, peptic ulcer, and gastric cancer, transmission of H. pylori by GI endoscopy is rare. Langenberg et al. (128) documented a 1.1% risk of endoscopic transmission of H. pylori in patients. Tytgat (129) estimated the frequency of transmission to be approximately 4 per 1,000 endoscopic procedures when the infection rate in the population was about 60%. However, the true incidence of H. pylori transmission may be underestimated because of the high prevalence of Helicobacter infection in the examined patient population and the asymptomatic or nonspecific clinical presentation of H. pylori infection (129).
H. pylori transmission was recognized with the introduction of new molecular techniques and was first demonstrated by using restriction enzyme DNA analysis (128, 130). Three H. pylori outbreaks after upper GI endoscopy were related to inadequate reprocessing of endoscopes and not-sterilized biopsy forceps (128, 130, 131). Four patients involved in these outbreaks developed postendoscopic gastritis.
Although H. pylori is readily killed by most disinfectants, including glutaraldehyde, povidone-iodine, and benzalkonium chloride, within 15 to 30 s (132), 70% ethanol failed to disinfect endoscopes between uses in patients (128). Disinfection with 2% glutaraldehyde for 5 and 10 min was effective in eliminating H. pylori DNA from flexible endoscopes (133, 134).
Clostridium difficile.
C. difficile, an obligatory anaerobic, spore-forming, Gram-positive rod, is responsible for a number of different intestinal diseases and is transmitted from patient to patient through the oral ingestion of its vegetative cells or endospores (135). Only one report of possible C. difficile transmission with development of fulminant pseudomembranous colitis after colonoscopy has been published (136). Thus, the risk of development of C. difficile-associated diarrhea after GI endoscopy is very low (137).
Commonly used high-level disinfectants have been studied to assess whether the vegetative cells and endospores of C. difficile are destroyed during different exposure times. Two percent glutaraldehyde and peracetic acid are capable of destroying large numbers of C. difficile endospores using exposure times of 5 to 20 min (138–140).
Other microorganisms.
Many health care-associated S. marcescens outbreaks have been related to inadequate disinfection and drying procedures (30, 55, 57, 72, 141, 142) and rinsing of endoscope channels with nonsterile tap water after disinfection (30, 55, 57) (Tables 4 and 5). A postbronchoscopic outbreak involved 117 patients who were colonized with different Enterobacteriaceae and was associated with two specific bronchoscopes contaminated with Klebsiella pneumoniae and Proteus vulgaris (143).
Duodenoscope-related nosocomial infections due to extended-spectrum-β-lactamase (ESBL)-producing and K. pneumoniae carbapenemase (KPC-2)-producing K. pneumoniae have been detected in two French hospital outbreaks (144, 145). K. pneumoniae producing ESBL type CTX-M-15 was isolated from patients with post-ERCP sepsis and cholangitis (144). One duodenoscope was indicated as the source of patient-to-patient transmission. Environmental cultures from the AERs and surfaces of the endoscopy rooms and routine surveillance cultures from endoscopes were negative. In the second outbreak, transmission of KPC-2-producing K. pneumoniae was associated with the use of a contaminated duodenoscope that had previously been used to examine an index patient transferred from a Greek hospital (145). An incomplete drying procedure was detected during endoscope reprocessing.
Methylobacterium is a slow-growing, pink-pigmented, Gram-negative rod and is a common contaminant in water (146). Cross-contaminations of Methylobacterium in patients by contaminated bronchoscopes have been related to contaminated tap water in the bronchoscopy unit and to biofilm-containing AERs (25, 54). It was considered a colonizer because no patient manifested true infection with this bacterium. Only one case of Methylobacterium bacteremia in a patient after ERCP and removal of a biliary tract prosthesis has been reported (147). Methylobacterium has a strong biofilm-producing ability and is highly resistant to dehydration, elevated temperatures, and ionizing radiation, which can explain the frequent occurrence and colonization of Methylobacterium in the hospital environment (148, 149).
A wide variety of other pathogens can be transmitted during endoscopic procedures. Health care-associated transmission of Strongyloides stercoralis (150), Trichosporon spp. (151, 152), and the yeast Rhodotorula rubra (69, 153) have been reported. Cross-contaminations of Blastomyces dermatitidis (154), Legionella pneumophila (155), and Bacillus spp. (156) after flexible bronchoscopy were related to inadequate cleaning and disinfection and a contaminated suction valve of the instrument.
The sensitivity of many unusual pathogens to disinfecting agents is mainly unknown. La Scola et al. (157) reported that HLD with 2% glutaraldehyde or peracetic acid disinfectants for 20 min may be ineffective to prevent transmission of Tropheryma whipplei by GI endoscopes. According to Muscarella (158), 2% glutaraldehyde and gas sterilization with ethylene oxide are able to destroy the vegetative cells and endospores of Bacillus anthracis. Transmissible cysts of intestinal protozoa such as Giardia intestinalis and Cryptosporidium are highly resistant to many disinfectants, including chlorine (159) and 2% glutaraldehyde (160).
Viruses
Hepatitis B virus.
Hepatitis B virus (HBV) is a highly infectious DNA virus that is easily transmitted through contact with blood or body fluids of an infected person. Despite the high infectivity of hepatitis B, only one case of endoscopic HBV transmission confirmed by molecular analysis has been documented after gastroscopy (161). Two other reports described HBV transmission in two patients after GI endoscopy with an instrument used in HBV-positive patients (162, 163). Both patients became HBsAg positive 9 months after endoscopy. Subtyping of the virus was not performed. The implicated endoscopes were inadequately disinfected between procedures.
Several clinical studies monitored patients after GI endoscopic procedures performed with an endoscope used during a preceding procedure in HBV-positive patients (164–171). No evidence of subsequent HBV infection related to the previous endoscopy was found, confirming that HBV transmission is not associated with GI endoscopy when appropriate disinfection procedures are performed.
Two studies have examined the sensitivity of HBV to disinfectants, including 2% glutaraldehyde at 20°C for 10 min and 80% ethanol at 11°C for 2 min, and heating at 98°C for 2 min (172, 173). All treatments were shown to be effective, indicating that the resistance level of HBV is not extreme.
Hepatitis C virus.
Hepatitis C virus (HCV) is a small, enveloped RNA virus that can be transmitted through contact with infected blood or body fluids during diagnostic and therapeutic invasive procedures (174). The overall risk for HCV transmission through GI endoscopy is controversial. Several studies found that GI endoscopic procedures were associated with HCV infection (175–178). Other clinical studies monitored HCV-negative patients who underwent GI endoscopy with the same endoscopes as those used on HCV-positive patients (179, 180). It was concluded that the risk for HCV transmission by endoscopy is low when adequate endoscope reprocessing is used.
Several cases of patient-to-patient HCV transmission have been related to inadequate cleaning and disinfection of GI endoscopes and accessories (181–184) and to the use of contaminated anesthetic vials or syringes (182, 183). In two cases, genotyping and nucleotide sequencing of the viral isolates showed the same HCV strain (181, 182).
Ciesek et al. (185) examined the sensitivity of HCV to hand antiseptics, high-level disinfectants, and high temperatures. Glutaraldehyde (0.5%) and 0.05% peracetic acid were able to completely inactivate HCV within 1 min of incubation. A >100-fold reduction of infectivity was observed after 5 min of exposure of the virus to 75°C.
HIV.
Recognition of the viral etiology of AIDS in 1983 led to great concern about possible health care-associated transmission of the virus. HIV might be inoculated during trauma into GI mucosa, for example, during insertion of a contaminated endoscope.
No cases of HIV transmission attributed to endoscopy have been reported so far. The virus is sensitive to many disinfectants, including 70% ethanol and 2% glutaraldehyde (186). Sampling of 20 gastroscopes immediately after use in patients with AIDS and before disinfection found contamination with commensal bacteria, P. aeruginosa, Candida albicans, HBV, and, in 35% of cases, HIV (187). Bronchoscopes were less heavily contaminated: HIV, HBV, commensal bacteria, and Pneumocystis jirovecii were detected by culturing, immunofluorescence tests, and PCR after bronchoscopy of patients with pulmonary manifestations of AIDS (187). Disinfection with 2% glutaraldehyde for 2 min completely eliminated HIV from the endoscopes artificially contaminated with high levels of virus (188).
Enteroviruses.
Enteroviruses are nonenveloped viruses that are more resistant to chemical disinfectants than enveloped viruses. No cases of endoscopic transmission of enteroviruses have been reported. Narang and Codd (189) found that 2% glutaraldehyde reduced poliovirus titers by at least 6 logs within a 30-min test period. Hanson et al. (190) studied elimination of enterovirus by 2% glutaraldehyde from endoscopes artificially contaminated with high virus levels. Samples were virus free after 2 min of disinfection. Virus dried on surfaces was inactivated in 1 min by 2% glutaraldehyde, with a reduction of >6 logs. Thus, disinfection was effective against a heavy contamination of endoscopes with enterovirus.
Creutzfeldt-Jakob Disease (Prion Disease)
Creutzfeldt-Jakob disease (CJD) and other transmissible spongiform encephalopathies are lethal degenerative neurological disorders with symptoms including dementia, ataxia, myoclonus, and pyramidal and extrapyramidal damage (191). These degenerative encephalopathies are transmitted by infectious agents called prions (protein particles without nucleic acid) and are characterized by accumulation and different distribution of the specific prion protein in the human body. CJD occurs in new-variant and classical (sporadic and iatrogenic) forms. Iatrogenic CJD followed administration of cadaveric human pituitary hormones (192, 193), dural graft transplants (194), corneal transplants (195), and the use of contaminated neurosurgical instruments (196).
In classical CJD, prion protein is concentrated in the central nervous system and is found less often in other organs (191). Intestinal tissue, blood, and saliva have a low risk for transmission of classical CJD. In new-variant CJD, large amounts of the prion protein are accumulated in lymphoid tissue, including the GI tract (197). Therefore, new-variant CJD transmission via a GI endoscopic procedure remains theoretically possible (198), but no reports of such transmission have been noted in the literature (199).
Prions are highly resistant to routine methods of decontamination and sterilization and can remain infectious for years (200). Dry heat, glutaraldehyde, and ethylene oxide were concluded to be ineffective disinfection and sterilization methods for medical devices (201, 202). Recommended chemical methods include a decontamination step with concentrated sodium hydroxide, sodium hypochlorite, or formic acid and prolonged steam sterilization (200, 203, 204). Most contemporary flexible endoscopes cannot be heat sterilized and disinfected with high concentrations of disinfectants without severe damage (1, 9). Therefore, flexible endoscopes should be discarded after endoscopy in patients with CJD (205).
ENDOGENOUS INFECTION ASSOCIATED WITH FLEXIBLE ENDOSCOPY
Flexible Gastrointestinal Endoscopy
Diagnostic and therapeutic upper gastrointestinal endoscopy.
Bacteremia occurs when the mucosal membrane is damaged (for example, during tooth cleaning). Microscopic tissue trauma occurring during endoscope insertion can result in transient bacteremia. In this case, microorganisms isolated from blood cultures belong to the oropharyngeal commensal microflora and are generally of low pathogenicity (206).
The reported incidence of bacteremia after diagnostic upper GI endoscopy, with or without biopsies, was less than 8% (207–215). The isolated microorganisms included mainly Staphylococcus epidermidis and Streptococcus spp. No infectious complications were detected in patients with bacteremia in a follow-up study 6 months to 2 years after endoscopy.
Therapeutic upper GI endoscopy, including esophageal sclerotherapy, variceal ligation, and esophageal dilatation, is associated with significantly more tissue trauma than diagnostic endoscopy (216). These endoscopic procedures are frequently performed in the setting of acute bleeding or a benign or malignant stricture of the esophagus and have a higher rate of bacteremia (30%) than diagnostic endoscopic procedures (12.5%) (214). The incidence of transient bacteremia ranges from 0% to 53% after esophageal sclerotherapy (65, 217–225), from 1% to 25% after endoscopic variceal ligation (211, 217, 219, 226), and from 2% to 54% after esophageal dilatation (227–230).
Other reported infectious complications after upper GI endoscopy include endocarditis of native and prosthetic valves (231–239), meningitis and/or cerebral abscess (240–245), and bacterial peritonitis (222, 246, 247). The majority of isolated organisms were Streptococcus viridans and Staphylococcus spp.
Colonoscopy and sigmoidoscopy.
Microorganisms found in the colon include anaerobic bacteria (Bacteroides fragilis and Clostridium spp.), Enterobacteriaceae (E. coli, Klebsiella spp., Enterobacter spp., and Proteus spp.), and enterococci (248). The reported incidence of bacteremia after colonoscopy, with or without biopsies and polypectomies, ranges from 0% to 25% (249–257). In immunocompetent patients, bacteremia during or after lower GI endoscopic procedures is usually transient and asymptomatic. Duration of colonoscopy with or without biopsy and/or polypectomy does not appear to increase the rate of bacteremia (253, 254). The incidence of transient bacteremia after flexible sigmoidoscopy ranges from 0% to 1% (258–260).
Other infectious complications after colonoscopy and sigmoidoscopy include acute appendicitis (261–267), bacterial peritonitis (268, 269), endocarditis (270–273), and septicemia (274–277). Two cases of Listeria monocytogenes meningitis and septicemia (278, 279) and one case of Fournier's gangrene of the perineum have been described (280). The most commonly isolated microorganisms are enterococci, Enterobacteriaceae, and Bacteroides spp.
Percutaneous endoscopic gastrostomy.
The most common complication of percutaneous endoscopic gastrostomy is peristomal wound infection, with the rate varying between 3% and 32% (281–286). The high risk of wound infection can be explained by contamination of the wound with oropharyngeal microflora during placement of a tube. More severe complications include necrotizing fasciitis (287–289), abdominal abscess, peritonitis, and septicemia (281, 290–295). The most common infecting organisms are Staphylococcus aureus, Gram-negative bacteria (Klebsiella spp., Enterobacter spp., and P. aeruginosa), enterococci, and C. albicans (291, 295).
Endoscopic retrograde cholangiopancreaticography.
ERCP is an endoscopic procedure associated with an incidence of severe infectious complications of between 2% and 4%, including sepsis, ascending cholangitis, liver abscess, acute cholecystitis, and infected pancreatic pseudocyst (296–299). A complication of ERCP can be defined as any event following the 30-day period after endoscopy that changed the health status of a patient negatively (300). Post-ERCP complications can be divided into mild (up to 3 days in the hospital), moderate (4 to 10 days of hospitalization), and severe (more than 10 days of hospitalization, surgical intervention, and/or death) complications. The important risk factors for post-ERCP infections are exogenous (because of contamination of endoscopes and accessory equipment) and endogenous patient-related factors, including obstruction of the bile or pancreatic duct, tissue damage during the procedure, and compromised immune status (2, 18, 75, 81, 99).
The rate of occurrence of bacteremia ranges from 0% to 15% after ERCP of unobstructed pancreatic or bile ducts (96, 301–304) and from 0% to 27% in patients with biliary obstruction by stones or a tumor (302–307). Not all bacteremic episodes result in a clinically relevant complication. ERCP-related sepsis is one of the most serious complications of this procedure, with a reported mortality rate as high as 29.4% despite the use of antibiotic therapy (307). The incidence of post-ERCP sepsis varies from 0.25% to 5.4% in different patient populations (296, 305, 308–311) and is significantly higher in patients with malignant biliary obstruction than in those with benign obstruction (21% versus 3%) (312).
Ascending cholangitis results mainly from inadequate drainage of an infected and obstructive biliary duct system (96). The rate of cholangitis after ERCP varies from 0.08% to 5% (296–299, 313–322). Post-ERCP cholangitis is defined by a typical clinical picture (temperature of >38°C, upper abdominal colicky pain, and cholestasis/jaundice) without evidence of other concomitant infections and with or without positive bile cultures obtained during biliary drainage (299). The most frequent organisms responsible for cholangitis/sepsis are enteric bacteria, including Enterobacteriaceae (E. coli, Klebsiella spp., and Enterobacter spp.), enterococci, and a variety of species of alpha-hemolytic streptococci (302, 306, 323).
Post-ERCP cholangitis is also a frequent complication of a biliary endoprosthesis insertion and occluded stents (323). Thus, stent removal and replacement frequently occur as a consequence of blockage caused by biofilm growth in the stent lumen (324). Enterococci, the most common organisms identified from patients with stents, are frequently isolated from infected bile in association with other microorganisms (323). Leung et al. (325) demonstrated a synergism between E. coli and enterococcal infection: colonization of a stent with E. coli facilitated attachment and biofilm formation by enterococci.
Other post-ERCP infectious complications include acute cholecystitis, with incidences ranging from 0.1% to 5.9% (296–299, 314, 321, 326, 327); liver abscess (296, 298, 328, 329); and infection of pancreatic pseudocysts (297, 314).
Antibiotic prophylaxis in flexible gastrointestinal endoscopy.
The purpose of antibiotic prophylaxis during GI endoscopy is to reduce the risk of iatrogenic infectious complications. Prophylactic antibiotic administration is not recommended for all GI endoscopic procedures (330). Therapeutic ERCP, percutaneous endoscopic gastrostomy, esophageal sclerotherapy, variceal ligation, and esophageal dilatation are endoscopic procedures associated with the highest rates of bacteremia and other infectious complications (216). Regimens of antibiotic prophylaxis for GI endoscopic procedures are summarized in Table 6.
Table 6.
Antibiotic prophylaxis recommended for gastrointestinal endoscopya
| Type of gastrointestinal endoscopic procedure and patient condition | Goal of prophylaxis | Prophylaxis |
|---|---|---|
| Percutaneous endoscopic gastrostomy | Prevention of peristomal infection | Cefazolin 1-g i.v. single dose 30 min before procedure, cefuroxime 1.5-g i.v. single dose, or amoxicillin-clavulanate 1.2-g i.v. single dose |
| Esophageal sclerotherapy, variceal ligation | Prevention of procedure-related bacteremia and peritonitis | Piperacillin-tazobactam 4.5-g i.v. single dose (some give 3 times daily), cefotaxime 2-g i.v. single dose, or ceftriaxone 2-g i.v. single dose |
| Cirrhosis with acute variceal bleeding | ||
| Esophageal dilatation | ||
| Benign/malignant stricture of the esophagus | ||
| Endoscopic retrograde cholangiopancreaticography | ||
| Biliary obstruction (e.g., primary sclerosing cholangitis and/or hilar cholangiocarcinoma) when complete biliary drainage is unlikely to be achieved | Prevention of cholangitis | Ciprofloxacin 750-mg single dose orally 1.5–2 h before procedure, piperacillin-tazobactam 4.5-g i.v. single dose 1 h before procedure, or i.v. single dose of gentamicin at 1.5 mg/kg of body weight at time of sedation |
| Communicating pancreatic cysts/pseudocysts | Prevention of cyst infection | See above (“Biliary obstruction”) |
| Biliary complications after liver transplantation | Prevention of cholangitis | Ciprofloxacin 750-mg single dose orally 1.5–2 h before procedure, gentamicin 1.5-mg/kg i.v. single dose at the time of sedation plus amoxicillin 1-g i.v. single dose, or vancomycin at 20 mg/kg i.v. over at least 1 h |
| Any gastrointestinal endoscopic procedure | ||
| Patients with cardiovascular risk factors | Prevention of infective endocarditis | Not recommended |
| Patients with synthetic vascular graft and other cardiovascular devices | Prevention of graft and device infection | Not recommended |
| Patients with prosthetic joints | Prevention of septic arthritis | Not recommended |
Percutaneous endoscopic gastrostomy is associated with a high risk of development of wound infection at the gastrostomy site (331). Prophylactic administration of antibiotics reduced the relative and absolute risk of wound infection by 73% and 17.5%, respectively, and is recommended for all patients undergoing percutaneous endoscopic gastrostomy (285, 331). The American Society for Gastrointestinal Endoscopy (ASGE) and the Endoscopy Committee of the British Society of Gastroenterology recommend antibiotic prophylaxis with an antibiotic that provides coverage of cutaneous microorganisms, such as a narrow- or expanded-spectrum cephalosporin or amoxicillin-clavulanate, for patients undergoing percutaneous endoscopic gastrostomy, 30 min before the procedure (332, 333).
Therapeutic upper GI endoscopic procedures associated with a high rate of postprocedure bacteremia and peritonitis include esophageal sclerotherapy, variceal ligation, and esophageal dilatation. Prophylactic administration of antibiotics is recommended for all patients with cirrhosis and acute variceal bleeding before esophageal sclerotherapy and variceal ligation and for patients with benign or malignant esophageal strictures before esophageal dilatation (333). A recent meta-analysis of 12 trials indicated a significant beneficial effect of antibiotic prophylaxis compared with placebo and no prophylaxis in decreasing the incidence of bacterial infections, bacteremia, pneumonia, spontaneous peritonitis, and mortality from bacterial infections in cirrhotic patients with GI bleeding (334). These benefits were observed independently of the antibiotic used. Administration of a broad-spectrum cephalosporin or piperacillin-tazobactam in a single dose or three doses during 1 day is recommended by the ASGE and the British Society of Gastroenterology (332, 333).
Recent studies examining antibiotic prophylaxis prior to ERCP concluded that it may reduce the incidence of bacteremia but did not reduce the incidence of clinical sepsis or cholangitis, and therefore, routine use of antibiotic prophylaxis cannot be recommended (300, 335, 336). Antibiotic prophylaxis is recommended before an ERCP in patients with biliary obstruction (e.g., primary sclerosing cholangitis and/or hilar cholangiocarcinoma) when complete biliary drainage is unlikely to be achieved and in patients with communicating pancreatic cysts or pseudocysts for prevention of cyst infection (332, 333). An adequate biliary drainage will prevent postprocedure cholangitis or sepsis without prophylactic administration of antibiotics. Continuation of antibiotics after ERCP in patients with biliary complications after liver transplantation may be beneficial, even when drainage is achieved (332, 333). Prophylactic antibiotics should cover biliary flora such as Enterobacteriaceae, Bacteroides spp., P. aeruginosa, and enterococci. An optimum benefit of antibiotics is obtained if therapeutic levels are present in the tissues at the time of the endoscopic procedure. Antibiotic prophylaxis should be started orally or intravenously at least 1 to 2 h before the procedure. Oral ciprofloxacin, intravenous piperacillin-tazobactam, or gentamicin is recommended (332, 333). Increasing microbial resistance (ciprofloxacin), poor penetration into bile (gentamicin), and limited activity against enterococci (gentamicin and ciprofloxacin) are disadvantages of these antibiotics. According to the British Society of Gastroenterology, for patients undergoing ERCP with biliary complications after liver transplantation, these antibiotics should be combined with amoxicillin or vancomycin to target Enterococcus spp. (333). However, local incidence of amoxicillin-resistant, vancomycin-sensitive enterococci and known colonization with vancomycin-resistant enterococci need to be considered. In hospitals with an increasing frequency of vancomycin-resistant enterococci, teicoplanin is recommended in preference to vancomycin for two reasons: first, it is simpler and quicker to administer, and second, more sustained blood levels occur following a single dose (337).
Antibiotic administration for prevention of infective endocarditis (in patients with cardiovascular risk factors) and graft and device infections (in patients with synthetic vascular graft and other cardiovascular devices) before GI endoscopy is no longer recommended by the ASGE and the American Heart Association (332, 338). This recommendation is based on an absence of clinically significant evidence of infective endocarditis and device infection being associated with GI procedures and on an absence of a conclusive link between GI endoscopy and development of any other type of infection (332). Antibiotic prophylaxis of septic arthritis during GI endoscopy is not recommended for patients with orthopedic prostheses. However, prophylactic antibiotic administration can be considered prior to any therapeutic GI endoscopic procedure in patients with a seriously impaired immune status (e.g., in cases of neutropenia and hematological malignancy) (333).
Flexible Bronchoscopy
Oropharyngeal microorganisms from the upper respiratory tract can be carried down into the lower respiratory tract during insertion of a bronchoscope into the lung through the mouth and can penetrate into the bloodstream. These microorganisms include viridans group streptococci, staphylococci, Moraxella spp., Neisseria spp., and anaerobic bacteria (103).
Bacteremia following bronchoscopy, with or without biopsy, is transient and occurs in <5% of patients (339–343). The microorganisms most frequently isolated from positive blood cultures are staphylococci and beta-hemolytic and viridans group streptococci, which are part of the normal upper airway flora. Fever after bronchoscopic procedures has been reported in many studies with a wide range of frequencies, from 0% to 27% (341, 343–349). Reported fevers were transient, developed during the first day after bronchoscopy, and were not associated with septic complications. One possible mechanism of development of fever after bronchoscopy is the release of proinflammatory cytokines from alveolar macrophages (350).
Pneumonia following bronchoscopic procedures has been reported, with a frequency of 0.6% to 6% (345, 346). Postbronchoscopy pneumonia was defined as the development of fever and a new or progressive infiltrate on a chest radiograph with peripheral leukocytosis and elevated levels of the C-reactive protein after the procedure. Several cases of fatal pneumonia, long abscess, and sepsis following bronchoscopy with isolation of Streptococcus pneumoniae, E. coli, Haemophilus influenzae, and Cryptococcus sp. from positive blood cultures and bronchial specimens have been described (351–355).
Antibiotic prophylaxis is not recommended during flexible bronchoscopy with or without biopsy because of the low incidence of bacteremia and endocarditis (356, 357). Only one case report of infective endocarditis following bronchoscopy in an HIV-positive patient with mitral valve prolapse has been published (358). The American Heart Association does not recommend antibiotic prophylaxis for routine flexible bronchoscopy (357). However, administration of 2.0 g amoxicillin orally 1 h prior to bronchoscopy is recommended for rigid bronchoscopy, particularly for high-risk patients, including patients with prosthetic cardiac valves, previous bacterial endocarditis, and complex cyanotic congenital heart disease (e.g., tetralogy of Fallot) (357). The British Thoracic Society Bronchoscopy Guidelines Committee recommends prophylactic administration of antibiotics before bronchoscopy for patients who are asplenic, have a heart valve prosthesis, or have a history of endocarditis (356).
IMPACT OF BIOFILM ON ENDOSCOPE REPROCESSING
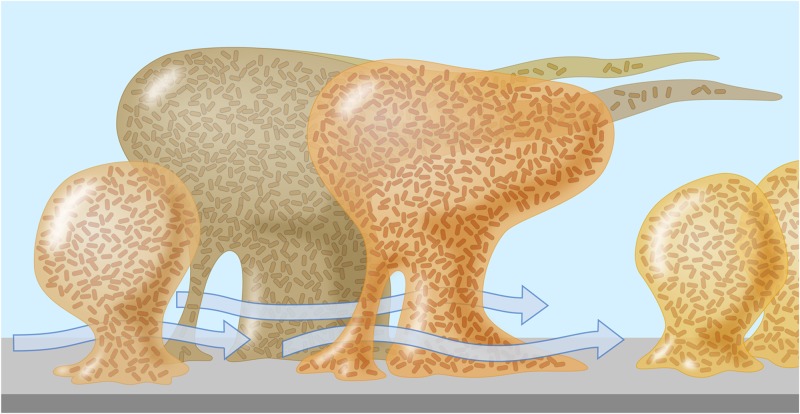
Microorganisms in nature do not generally grow in nutrient-rich suspensions (the so-called planktonic state) as in the laboratory but prefer to grow in surface-associated communities called biofilms. A biofilm is an assemblage of microbial cells that is irreversibly attached to a surface and enclosed in a matrix of exopolymeric substances (359). A typical biofilm will contain around 85% polymeric substances and only 15% bacterial mass, and cells are located in matrix-enclosed “towers” and “mushrooms” (Fig. 2) (360, 361). Biofilms may form on different surfaces, including living or dead tissues, medical devices, water supply systems, or endoscope channels (89, 359). Biofilm formation by microorganisms on inert surfaces has been extensively studied, and there is a direct relationship between the ability of the organism to form a biofilm and its pathogenicity (362). Many bacteria, including P. aeruginosa and atypical mycobacteria, are capable of existing in a planktonic state and can produce biofilms.
Fig 2.
A mature biofilm in a flowing environment comprises a complex mushroom-shaped architecture, long streamers, and water channels which permit the bulk fluid to penetrate deep within the biofilm, carrying oxygen and nutrients. (Courtesy of MSU Center for Biofilm Engineering, P. Dirckx; reprinted with permission.)
Bacteria growing within biofilms have a number of characteristics that distinguish them from planktonic populations. The ability to form biofilms allows microorganisms to survive under conditions of drying and chemical and antibiotic exposure (363, 364). Microorganisms in biofilms are protected from the host immune system and may be 1,000 times more resistant to antibiotics than planktonic cells (365). The increased resistance to antimicrobial agents can be explained by poor penetration of an antibiotic into a biofilm, low growth rate, and formation of resistant phenotypes of microorganisms within biofilms (363, 364).
Under adverse conditions, biofilms are capable of releasing their bacterial population into a planktonic state. This ability can be explained by intercellular communication within a biofilm (366). Signaling systems include quorum sensing (the release of chemical signals in response to increasing population density), biosignal blockers, pheromones, and butyrylhomoserine lactone. These signaling systems are important for regulation of a number of physiological processes, including antibiotic synthesis, plasmid transfer, and expression of virulence factors (367).
During endoscopy, the environment provides optimal conditions for contamination and subsequent growth of biofilms. Modern flexible endoscopes contain multiple channels and ports which can easily collect organic material. Even if valid endoscope reprocessing protocols are applied, microbial accumulation can lead to development of a biofilm inside narrow endoscope channels over time (89). Biofilm formation on the inner surface of endoscope channels, especially when these become scratched or damaged, can result in failure of the decontamination process. It can create a vicious circle of growth, disinfection, partial killing or inhibition, and regrowth, resulting in outbreaks of endoscopy-related infections in patients who underwent endoscopy with a biofilm-containing endoscope (2, 3).
The presence of biofilms on the inner surface of endoscope channels has been reported in the literature (2, 89). An outbreak of post-ERCP sepsis with multiresistant P. aeruginosa was related to biofilm development inside endoscope channels (2). Three outbreaks of postendoscopic infection and cross-contamination were attributed to contaminated AERs with the presence of biofilm deposits on the internal plumbing and detergent tank (23, 25, 26). Cross-contaminations of M. chelonae and M. mesophilicum resulted in colonization of patients after endoscopy with no infectious complications (25). Reported infections in two other outbreaks included post-ERCP P. aeruginosa bacteremia/sepsis and cholangitis (23) and sepsis and pneumonia after bronchoscopy (26).
Biofilms can be removed from artificial surfaces by physical and chemical methods (368). Physical methods such as ultrasound and manual cleaning are generally effective but difficult to control in practice. Chemical methods can be unsuccessful because of the resistance of biofilms to antibiotics, disinfectants, and biocides (363). The cleaning process is most critical for biofilm removal because multiple internal channels of flexible endoscopes cannot be inspected for cleanliness. The cleaning product must show good penetration and solubilization of organic debris and biofilms. In a study reported by Vickery et al. (368), enzymatic cleaners failed to reduce viable bacterial numbers more than 2 logs in E. coli biofilms in polyvinyl chloride tubing. Another study reported the failure of a commonly used enzymatic cleaner to completely remove test soil from endoscope channels (369). Nonenzymatic detergents showed a better inhibition of biofilm formation than enzymatic detergents (368, 370). The most efficient methods for biofilm removal were autoclaving and treatment with a concentrated bleach solution (49). High-temperature treatments (80°C to 90°C) were not effective for biofilm removal.
The use of antibiofilm-oxidizing agents with an antimicrobial coating inside washer disinfectors could reduce biofilm buildup inside endoscopes and AERs and decrease the risk of transmitting infections (15, 49). Sterilization can be helpful to destroy microorganisms within biofilms. However, ethylene oxide sterilization may fail in the presence of organic debris after an inadequate cleaning procedure before reprocessing of flexible endoscopes (16, 17).
MICROBIOLOGICAL SURVEILLANCE OF ENDOSCOPE REPROCESSING
Routine microbiological testing for endoscopes and AERs remains a controversial issue in many guidelines. Microbiological surveillance of endoscope reprocessing has been recommended by several medical specialist organizations (371–373). It is appropriate to trace contaminations of endoscopes and to prevent contaminations and infections in patients after endoscopic procedures (2, 3). The use of environmental endoscope culturing is a rapid and simple method to monitor the effectiveness of standard reprocessing procedures (374). Other organizations recommend microbiological surveillance of flexible endoscopes only in response to epidemiologic investigations when instruments may be microbial sources of health care-associated disease transmission or in the case of testing the effectiveness of new or modified cleaning or disinfection procedures (375). Microbiological surveillance systems have several important limitations. If there is a clinical demand for the reuse of an endoscope, surveillance culture results will not be obtained until after the endoscope is used on the next patient, because culture results take a minimum of 24 to 48 h to be produced (371). Efficient sampling of flexible endoscopes and their accessories may be hindered by a complex design, materials, construction, and fragility or by the absence of a standardized sampling protocol or an appropriate technique for sampling of the surfaces and channels of the flexible endoscope (375).
Some techniques are recommended for microbiological sampling of flexible endoscopes (375). A swab-rinse technique should be used for sampling the exterior surfaces and the distal opening of the suction-biopsy channel port. For adequate sampling of the inferior surface of endoscope channels, a “flush/brush/flush” technique should be performed with rinsing through the channel with a sterile fluid and using a sterile cleaning brush to obtain samples from the biopsy port. A simple flushthrough technique may be considered when brushing of the channel lumens is impossible, but it is less efficient (375).
According to the literature, endoscopes can be sampled in an anterograde and retrograde manner. For anterograde sampling, the last-rinse water from the endoscope is collected inside the washer-disinfector at the distal end of the endoscope (3, 9). For retrograde sampling, the suction-biopsy channel and the air-water channel of the endoscope are each flushed with 20 ml sterile demineralized water manually outside the AER from the distal to the proximal end. According to the literature, anterograde sampling is not sensitive enough (3, 9). Endoscope culturing with a retrograde technique is effective for monitoring endoscope reprocessing, and testing is easy to perform (3). Introduction of new techniques that are easier to perform on a daily basis and with faster results may improve the microbiological surveillance of endoscope reprocessing. New methods may be based on direct ATP measurement or quantitative PCR. These methods must be further optimized because of their low specificity (376, 377).
The literature provides no standards for the frequency of testing intervals of surveillance cultures. The Gastroenterological Society of Australia guideline recommends microbiological monitoring of duodenoscopes, bronchoscopes, and AERs every 4 weeks and all other GI endoscopes every 4 months (372). According to the European Society of Gastrointestinal Endoscopy guideline, routine microbiological testing of endoscopes is advised to be performed at intervals of no longer than 3 months (371). The use of routine environmental microbiological testing of therapeutic endoscopes once a month and diagnostic endoscopes once every 3 months has been reported (2, 3).
A contaminated endoscope should be inspected for potential defects. Unfortunately, it is not possible to adequately check all internal endoscope channels. Any small damage can be the source of bacterial contamination within the scope, which is difficult or impossible to detect by routine inspection and testing (2, 3). Some investigators have begun to explore sterile-sheathed endoscopes with new technology to reduce the risk of infectious complications (9, 378). The EndoSheath, for instance, is an endoscope system in which all parts of the endoscope that come into contact with the patient are fully disposable.
Literature related to the costs associated with endoscope reprocessing and economic consequences of endoscope contamination is very limited. Economic evaluation of exogenous endoscopy-related outbreaks should include an analysis of outcomes (number of prevented iatrogenic infections) and an estimation of the health care, non-health care, and indirect costs (78). A recently reported study analyzed the cost of 4-weekly surveillance microbiological tests of bronchoscopes, duodenoscopes, and AERs during a 5-year period (379). The overall annual cost of microbiological testing and cost in time for nursing staff to collect the samples was estimated to be $13,339. Another study calculated the annual costs of surveillance of endoscope reprocessing (cost of microbiological testing and cost in time for staff) to be $33,959 (18). It was also estimated that the cost of the post-ERCP outbreak of P. aeruginosa sepsis affecting six patients, i.e., direct health care costs for diagnosis, treatment, and hospitalization of six affected patients, was approximately $206,273 (18).
CONCLUSION
Contaminated endoscopes have been linked to many outbreaks of device-related nosocomial infections. The true incidence of endoscopy-related infections is unknown because of inadequate surveillance or no surveillance at all. Endoscopy-related infections can cause serious harm and can give rise to concerns over these procedures by physicians and patients. Flexible endoscopes can be cleaned and disinfected but not sterilized after use. This implies the risk of settlement of biofilm-producing species.
Process control of the cleaning and disinfection procedure does not guarantee prevention of biofilm formation during endoscopy. Implementation of microbiological surveillance of endoscope reprocessing is appropriate to detect early colonization and biofilm formation in the endoscope and to prevent contamination and infection in patients after endoscopic procedures. However, the returns of prevention of endoscopy-related infections should be reasonably in balance with costs of technical and laboratory procedures resulting from surveillance and the costs of reprocessing or servicing of the contaminated endoscope.
Biographies

Julia Kovaleva, M.D., is a clinical microbiologist in training at the Department of Medical Microbiology and Infection Control at the University Medical Center Groningen (UMCG), Groningen, The Netherlands. She is currently finishing her Ph.D. study focused on the microbiological surveillance of endoscope reprocessing and the impact of biofilm on endoscope reprocessing and development of postendoscopic infection.

Frans T. M. Peters, M.D., Ph.D., is a gastroenterologist at the Department of Gastroenterology and Hepatology at the University Medical Center Groningen (UMCG), Groningen, The Netherlands. He held the position of Medical Coordinator of the Endoscopy Center for 6 years. In this capacity, he was involved in the endoscope disinfection program. As a gastroenterologist, he is engaged in advanced endoscopy, especially endoscopic retrograde cholangiopancreaticography and endoscopic mucosal resection.

Henny C. van der Mei, Ph.D., is a full professor in microbiology at the Department of Biomedical Engineering at the University Medical Center Groningen (UMCG), Groningen, The Netherlands. In addition, she is the director of the W. J. Kolff Institute for Biomedical Engineering and Materials Science at the UMCG. Her research is focused on biomaterial-associated infections (BAI), because BAI remain the number one cause of failure of biomaterial implants or devices despite the development of various strategies to control BAI. Mostly, she looks at the physicochemical mechanisms of microbial adhesion to biomaterials and modifications of the biomaterial surface and how this influences microbial adhesion. She has been working in this area for almost 30 years.

John E. Degener, M.D., Ph.D., was trained at the Erasmus Medical Center in Rotterdam, The Netherlands. From 1979, he practiced as a clinical microbiologist at the Erasmus Medical Center and in the Public Health Laboratory in the province of Friesland. In 1996, he accepted a full professorship in clinical microbiology at the University Medical Center Groningen (UMCG), Groningen, The Netherlands. Until 2011, he chaired the Department of Medical Microbiology and Infection Control at the UMCG, where he is still responsible for the postdoctoral training of young medical microbiologists. His interests are antimicrobial resistance, antimicrobial therapy, clinical anaerobe bacteriology, and infection control, in which fields he has published many papers.
REFERENCES
- 1. Nelson DB, Barkun AN, Block KP, Burdick JS, Ginsberg GG, Greenwald DA, Kelsey PB, Nakao NL, Slivka A, Smith P, Vakil N. 2001. Technology status evaluation report. Transmission of infection by gastrointestinal endoscopy: May 2001. Gastrointest. Endosc. 54:824–828 [DOI] [PubMed] [Google Scholar]
- 2. Kovaleva J, Meessen NE, Peters FT, Been MH, Arends JP, Borgers RP, Degener JE. 2009. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy 41:913–916 [DOI] [PubMed] [Google Scholar]
- 3. Buss AJ, Been MH, Borgers RP, Stokroos I, Melchers WJ, Peters FT, Limburg AJ, Degener JE. 2008. Endoscope disinfection and its pitfalls—requirement for retrograde surveillance cultures. Endoscopy 40:327–332 [DOI] [PubMed] [Google Scholar]
- 4. Alvarado CJ, Reichelderfer M. 2000. APIC guidelines for infection prevention and control in flexible endoscopy. Association for Professionals in Infection Control. Am. J. Infect. Control 28:138–155 [PubMed] [Google Scholar]
- 5. Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Cimbro M, Kampf B, Rogers M, Schmidt V, ESGE Guidelines Committee, European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates 2008. ESGE-ESGENA guideline: cleaning and disinfection in gastrointestinal endoscopy. Endoscopy 40:939–957 [DOI] [PubMed] [Google Scholar]
- 6. Nelson DB, Jarvis WR, Rutala WA, Foxx-Orenstein AE, Isenberg G, Dash GR, Alvarado CJ, Ball M, Griffin-Sobel J, Petersen C, Ball KA, Henderson J, Stricof RL, Society for Heathcare Epidemiology of America 2003. Multi-society guideline for reprocessing flexible gastrointestinal endoscopes. Infect. Control Hosp. Epidemiol. 24:532–537 [DOI] [PubMed] [Google Scholar]
- 7. Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee 2008. Guidedline for disinfection and sterilization in healthcare facilities. CDC, Atlanta, GA: http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf [Google Scholar]
- 8. Spach DH, Silverstein FE, Stamm WE. 1993. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann. Intern. Med. 118:117–128 [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan A. 2003. Epidemiology and prevention of infections related to endoscopy. Curr. Infect. Dis. Rep. 5:467–472 [DOI] [PubMed] [Google Scholar]
- 10. Nelson DB, Muscarella LF. 2006. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J. Gastroenterol. 12:3953–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spaulding EH, Gröschel DH. 1974. Hospital disinfectants and antiseptics, p 852–857 In Lennette EH, Spaulding EH, Truant JP. (ed), Manual of clinical microbiology, 2nd ed American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Garner JS, Favero MS. 1986. CDC guidelines for the prevention and control of nosocomial infections. Guideline for handwashing and hospital environmental control, 1985. Supersedes guideline for hospital environmental control published in 1981. Am. J. Infect. Control 14:110–129 [DOI] [PubMed] [Google Scholar]
- 13. Simmons BP. 1983. CDC guidelines for the prevention and control of nosocomial infection. Guideline for hospital invironmental control. Am. J. Infect. Control 11:97–120 [DOI] [PubMed] [Google Scholar]
- 14. Gorse GJ, Messner RL. 1991. Infection control practices in gastrointestinal endoscopy in the United States: a national survey. Infect. Control Hosp. Epidemiol. 12:289–296 [DOI] [PubMed] [Google Scholar]
- 15. Rutala WA, Weber DJ. 2001. New disinfection and sterilization methods. Emerg. Infect. Dis. 7:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alfa MJ, DeGagne P, Olson N, Puchalski T. 1996. Comparison of ion plasma, vaporised hydrogen peroxide, and 100% ethylene oxide sterilisers to the 12/88 ethylene oxide gas steriliser. Infect. Control Hosp. Epidemiol. 17:92–100 [DOI] [PubMed] [Google Scholar]
- 17. Chaufour X, Deva AK, Vickery K, Zou J, Kumaradeva P, White GH, Cossart YE. 1999. Evaluation of disinfection and sterilization of reusable angioscopes with the duck hepatitis B model. J. Vasc. Surg. 30:277–282 [DOI] [PubMed] [Google Scholar]
- 18. Kovaleva J, Buss AJ. 2011. Usefulness of bacteriological monitoring of endoscope reprocessing, p 141–162 In Pascu O, Tantau M. (ed), Therapeutic gastrointestinal endoscopy, 1st ed InTech, Rijeka, Croatia [Google Scholar]
- 19. Chu NS, Favero M. 2000. The microbial flora of the gastrointestinal tract and the cleaning of flexible endoscopes. Gastrointest. Endosc. Clin. N. Am. 10:233–244 [PubMed] [Google Scholar]
- 20. Chu NS, McAlister D, Antonoplos PA. 1998. Natural bioburden levels detected on flexible gastrointestinal endoscopes after clinical use and manual cleaning. Gastrointest. Endosc. 48:137–142 [DOI] [PubMed] [Google Scholar]
- 21. Petersen BT, Chennat J, Cohen J, Cotton PB, Greenwald DA, Kowalski TE, Krinsky ML, Park WG, Pike IM, Romagnuolo J, ASGE Quality Assurance in Endoscopy Committee, Rutala WA, Society for Healthcare Epidemiology of America 2011. Multisociety guideline on reprocessing flexible GI endoscopes: 2011. Infect. Control Hosp. Epidemiol. 32:527–537 [DOI] [PubMed] [Google Scholar]
- 22. Allen JI, Allen MO, Olson MM, Gerding DN, Shanholtzer CJ, Meier PB, Vennes JA, Silvis SE. 1987. Pseudomonas infection of the biliary system resulting from use of a contaminated endoscope. Gastroenterology 92:759–763 [DOI] [PubMed] [Google Scholar]
- 23. Alvarado CJ, Stolz SM, Maki DG. 1991. Nosocomial infections from contaminated endoscopes: a flawed automated endoscope washer. An investigation using molecular epidemiology. Am. J. Med. 91(3B:S272–S280 [DOI] [PubMed] [Google Scholar]
- 24. Fraser VJ, Jones M, Murray PR, Medoff G, Zhang Y, Wallace RJ., Jr 1992. Contamination of flexible fiberoptic bronchoscopes with Mycobacterium chelonae linked to an automated bronchoscope disinfection machine. Am. Rev. Respir. Dis. 145:853–855 [DOI] [PubMed] [Google Scholar]
- 25. Kressel AB, Kidd F. 2001. Pseudo-outbreak of Mycobacterium chelonae and Methylobacterium mesophilicum caused by contamination of an automated endoscopy washer. Infect. Control Hosp. Epidemiol. 22:414–418 [DOI] [PubMed] [Google Scholar]
- 26. Schelenz S, French G. 2000. An outbreak of multidrug-resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J. Hosp. Infect. 46:23–30 [DOI] [PubMed] [Google Scholar]
- 27. Leers WD. 1980. Disinfecting endoscopes: how not to transmit Mycobacterium tuberculosis by bronchoscopy. CMAJ 123:275–280, 283 [PMC free article] [PubMed] [Google Scholar]
- 28. Hawkey PM, Davies AJ, Viant AC, Lush CJ, Mortensen NJ. 1981. Contamination of endoscopes by Salmonella species. J. Hosp. Infect. 2:373–376 [DOI] [PubMed] [Google Scholar]
- 29. Ranjan P, Das K, Ayyagiri A, Saraswat VA, Choudhuri G. 2005. A report of post-ERCP Pseudomonas aeruginosa infection outbreak. Indian J. Gastroenterol. 24:131–132 [PubMed] [Google Scholar]
- 30. Silva CV, Magalhães VD, Pereira CR, Kawagoe JY, Ikura C, Ganc AJ. 2003. Pseudo-outbreak of Pseudomonas aeruginosa and Serratia marcescens related to bronchoscopes. Infect. Control Hosp. Epidemiol. 24:195–197 [DOI] [PubMed] [Google Scholar]
- 31. Food and Drug Administration 2009. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices. Food and Drug Administration, Washington, DC: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofSingle-UseDevices/ucm133514.htm [Google Scholar]
- 32. Nelson DB. 2003. Infectious disease complications of GI endoscopy: part II, exogenous infections. Gastrointest. Endosc. 57:695–711 [DOI] [PubMed] [Google Scholar]
- 33. Russell AD. 1994. Glutaraldehyde: current status and uses. Infect. Control Hosp. Epidemiol. 15:724–733 [DOI] [PubMed] [Google Scholar]
- 34. Babb JR, Bradley CR, Ayliffe GA. 1980. Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment. J. Hosp. Infect. 1:63–75 [DOI] [PubMed] [Google Scholar]
- 35. McDonnell G, Russell AD. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rideout K, Teschke K, Dimich-Ward H, Kennedy SM. 2005. Considering risks to healthcare workers from glutaraldehyde alternatives in high-level disinfection. J. Hosp. Infect. 59:4–11 [DOI] [PubMed] [Google Scholar]
- 37. Rozen P, Somjen GJ, Baratz M, Kimel R, Arber N, Gilat T. 1994. Endoscope-induced colitis: description, probable cause by glutaraldehyde, and prevention. Gastrointest. Endosc. 40:547–553 [DOI] [PubMed] [Google Scholar]
- 38. Collins FM. 1986. Bactericidal activity of alkaline glutaraldehyde solution against a number of atypical mycobacterial species. J. Appl. Bacteriol. 61:247–251 [DOI] [PubMed] [Google Scholar]
- 39. Nomura K, Ogawa M, Miyamoto H, Muratani T, Taniguchi H. 2004. Antibiotic suspectibility of glutaraldehyde-tolerant Mycobacterium chelonae from bronchoscope washing machines. Am. J. Infect. Control 32:185–188 [DOI] [PubMed] [Google Scholar]
- 40. van Klingeren B, Pullen W. 1993. Glutaraldehyde resistant mycobacteria from endoscope washers. J. Hosp. Infect. 25:147–149 [DOI] [PubMed] [Google Scholar]
- 41. Barbee SL, Weber DJ, Sobsey MD, Rutala WA. 1999. Inactivation of Cryptosporidium parvum oocyst infectivity by disinfection and sterilization processes. Gastrointest. Endosc. 49:605–611 [DOI] [PubMed] [Google Scholar]
- 42. Kovacs BJ, Aprecio RM, Kettering JD, Chen YK. 1998. Efficacy of various disinfectants in killing a resistant strain of Pseudomonas aeruginosa by comparing zones of inhibition: implications for endoscopic equipment reprocessing. Am. J. Gastroenterol. 93:2057–2059 [DOI] [PubMed] [Google Scholar]
- 43. Hanson PJ, Chadwick MV, Gaya H, Collins JV. 1992. A study of glutaraldehyde disinfection of fiberoptic bronchoscopes experimentally contaminated with Mycobacterium tuberculosis. J. Hosp. Infect. 22:137–142 [DOI] [PubMed] [Google Scholar]
- 44. Gregory AW, Schaalje GB, Smart JD, Robison RA. 1999. The mycobactericidal efficacy of ortho-phthalaldehyde and the comparative resistances of Mycobacterium bovis, Mycobacterium terrae, and Mycobacterium chelonae. Infect. Control Hosp. Epidemiol. 20:324–330 [DOI] [PubMed] [Google Scholar]
- 45. Sokol WN. 2004. Nine episodes of anaphylaxis following cystoscopy caused by Cidex OPA (ortho-phthalaldehyde) high-level disinfectant in 4 patients after cystoscopy. J. Allergy Clin. Immunol. 114:392–397 [DOI] [PubMed] [Google Scholar]
- 46. Rutala WA, Weber DJ. 1999. Disinfection of endoscopes: review of new chemical sterilants used for high-level disinfection. Infect. Control Hosp. Epidemiol. 20:69–76 [DOI] [PubMed] [Google Scholar]
- 47. Kovaleva J, Degener JE, van der Mei HC. 2010. Mimicking disinfection and drying of biofilms in contaminated endoscopes. J. Hosp. Infect. 76:345–350 [DOI] [PubMed] [Google Scholar]
- 48. Loukili NH, Granbastien B, Faure K, Guery B, Beaucaire G. 2006. Effect of different stabilized preparations of peracetic acid on biofilm. J. Hosp. Infect. 63:70–72 [DOI] [PubMed] [Google Scholar]
- 49. Marion-Ferey K, Pasmore M, Stoodley P, Wilson S, Husson GP, Costerton JW. 2003. Biofilm removal from silicone tubing: an assessment of the efficacy of dialysis machine decontamination procedures using an in vitro model. J. Hosp. Infect. 53:64–71 [DOI] [PubMed] [Google Scholar]
- 50. Selkon JB, Babb JR, Morris R. 1999. Evaluation of the antimicrobial activity of a new super-oxidized water, Sterilox, for the disinfection of endoscopes. J. Hosp. Infect. 41:59–70 [DOI] [PubMed] [Google Scholar]
- 51. Bennett SN, Peterson DE, Johnson DR, Hall WN, Robinson-Dunn B, Dietrich S. 1994. Bronchoscopy-associated Mycobacterium xenopi pseudoinfections. Am. J. Respir. Crit. Care Med. 150:245–250 [DOI] [PubMed] [Google Scholar]
- 52. Centers for Disease Control 1991. Nosocomial infection and pseudoinfection from contaminated endoscopes and bronchoscopes: Wisconsin and Missouri. MMWR Morb. Mortal. Wkly. Rep. 40:675–678 [PubMed] [Google Scholar]
- 53. Cryan EM, Falkiner FR, Mulvihill TE, Keane CT, Keeling PW. 1984. Pseudomonas aeruginosa cross-infection following endoscopic retrograde cholangiopancreatography. J. Hosp. Infect. 5:371–376 [DOI] [PubMed] [Google Scholar]
- 54. Flournoy DJ, Petrone RL, Voth DW. 1992. A pseudo-outbreak of Methylobacterium mesophilica isolated from patients undergoing bronchoscopy. Eur. J. Clin. Microbiol. Infect. Dis. 11:240–243 [DOI] [PubMed] [Google Scholar]
- 55. Siegman-Igra Y, Inbar G, Campus A. 1985. An ‘outbreak’ of pulmonary pseudoinfection by Serratia marcescens. J. Hosp. Infect. 6:218–220 [DOI] [PubMed] [Google Scholar]
- 56. Sniadack DH, Ostroff SM, Karlix MA, Smithwick RW, Schwartz B, Sprauer MA, Silcox VA, Good RC. 1993. A nosocomial pseudo-outbreak of Mycobacterium xenopi due to a contaminated potable water supply: lessons in prevention. Infect. Control Hosp. Epidemiol. 11:636–641 [DOI] [PubMed] [Google Scholar]
- 57. Vandenbroucke-Grauls CM, Baars AC, Visser MR, Hulstaert PF, Verhoef J. 1993. An outbreak of Serratia marcescens traced to a contaminated bronchoscope. J. Hosp. Infect. 23:263–270 [DOI] [PubMed] [Google Scholar]
- 58. Alfa MJ, Sitter DL. 1991. In-hospital evaluation of contamination of duodenoscopes: a quantitative assessment of the effect of drying. J. Hosp. Infect. 19:89–98 [DOI] [PubMed] [Google Scholar]
- 59. Muscarella LF. 2006. Inconsistencies in endoscope-reprocessing and infection-control guidelines: the importance of endoscope drying. Am. J. Gastroenterol. 101:2147–2154 [DOI] [PubMed] [Google Scholar]
- 60. Pineau L, Villard E, Duc DL, Marchetti B. 2008. Endoscope drying/storage cabinet: interest and efficacy. J. Hosp. Infect. 68:59–65 [DOI] [PubMed] [Google Scholar]
- 61. Rejchrt S, Cermák P, Pavlatová L, Micková E, Bures J. 2004. Bacteriologic testing of endoscopes after high-level disinfection. Gastrointest. Endosc. 60:76–78 [DOI] [PubMed] [Google Scholar]
- 62. Noy MF, Harrison L, Holmes GK, Cockel R. 1980. The significance of bacterial contamination of fiberoptic endoscopes. J. Hosp. Infect. 1:53–61 [DOI] [PubMed] [Google Scholar]
- 63. Agerton T, Valway S, Gore B, Pozsik C, Plikaytis B, Woodley C, Onorato I. 1997. Transmission of a highly drug-resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosocomial transmission via a contaminated bronchoscope. JAMA 278:1073–1077 [PubMed] [Google Scholar]
- 64. Bou R, Aguilar A, Perpiñán J, Ramos P, Peris M, Lorente L, Zúñiga A. 2006. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. J. Hosp. Infect. 64:129–135 [DOI] [PubMed] [Google Scholar]
- 65. Brayko CM, Kozarek RA, Sanowski RA, Testa AW. 1985. Bacteremia during esophageal variceal sclerotherapy: its cause and prevention. Gastrointest. Endosc. 31:10–12 [DOI] [PubMed] [Google Scholar]
- 66. Breathnach AS, Taylor IK, Mitchison HC, Shrestha TL. 1997. Pseudo-infection with non-viable Mycobacterium tuberculosis following bronchoscopy. J. Infect. 35:321–322 [DOI] [PubMed] [Google Scholar]
- 67. Elston RA, Hay AJ. 1991. Acid-fast bacillus contamination of a bronchoscope washing machine. J. Hosp. Infect. 19:72–73 [DOI] [PubMed] [Google Scholar]
- 68. Gubler JG, Salfinger M, von Graevenitz A. 1992. Pseudoepidemic of nontuberculous mycobacteria due to a contaminated bronchoscope cleaning machine. Report of an outbreak and review of the literature. Chest 101:1245–1249 [DOI] [PubMed] [Google Scholar]
- 69. Hagan ME, Klotz SA, Bartholomew W, Potter L, Nelson M. 1995. A pseudoepidemic of Phodotorula rubra: a marker for microbial contamination of the bronchoscope. Infect. Control Hosp. Epidemiol. 16:727–728 [DOI] [PubMed] [Google Scholar]
- 70. Kolmos HJ, Lerche A, Kristoffersen K, Rosdahl VT. 1994. Pseudo-outbreak of Pseudomonas aeruginosa in HIV-infected patients undergoing fiberoptic bronchoscopy. Scand. J. Infect. Dis. 26:653–657 [DOI] [PubMed] [Google Scholar]
- 71. Nye K, Chadha DK, Hodgkin P, Bradley C, Hancox J, Wise R. 1990. Mycobacterium chelonei isolation from broncho-alveolar lavage fluid and its practical implications. J. Hosp. Infect. 16:257–261 [DOI] [PubMed] [Google Scholar]
- 72. Peltroche-Llacsahuanga H, Lütticken R, Haase G. 1999. Temporally overlapping nosocomial outbreaks of Serratia marcescens infections: an unexpected result revealed by pulsed-field gel electrophoresis. Infect. Control Hosp. Epidemiol. 20:387–388 [DOI] [PubMed] [Google Scholar]
- 73. Sammartino MT, Israel RH, Magnussen CR. 1982. Pseudomonas aeruginosa contamination of fiberoptic bronchoscopes. J. Hosp. Infect. 3:65–71 [DOI] [PubMed] [Google Scholar]
- 74. Shimono N, Takuma T, Tsuchimochi N, Shiose A, Murata M, Kanamoto Y, Uchida Y, Morita S, Matsumoto H, Hayashi J. 2008. An outbreak of Pseudomonas aeruginosa infections following thoracic surgeries occurring via the contamination of bronchoscopes and an automatic endoscope reprocessor. J. Infect. Chemother. 14:418–423 [DOI] [PubMed] [Google Scholar]
- 75. Struelens MJ, Rost F, Deplano A, Maas A, Schwam V, Serruys E, Cremer M. 1993. Pseudomonas aeruginosa and Enterobacteriaceae bacteremia after biliary endoscopy: an outbreak investigation using DNA macrorestriction analysis. Am. J. Med. 95:489–498 [DOI] [PubMed] [Google Scholar]
- 76. Takigawa K, Fujita J, Negayama K, Terada S, Yamaji S, Kawanishi K, Takahara J. 1995. Eradication of contaminating Mycobacterium chelonae from bronchofibrescopes and an automated bronchoscope disinfection machine. Respir. Med. 89:423–427 [DOI] [PubMed] [Google Scholar]
- 77. Kimmey MB, Burnett DA, Carr-Locke DL, DiMarino AJ, Jensen DM, Katon R, MacFadyen BV, Scobey MW, Stein TN, Steinberg SM. 1993. Transmission of infection by gastrointestinal endoscopy. Gastrointest. Endosc. 39:885–888 [Google Scholar]
- 78. Seoane-Vazquez E, Rodriguez-Monguio R, Visaria J, Carlson A. 2006. Exogenous endoscopy-related infections, pseudo-infections, and toxic reactions: clinical and economic burden. Curr. Med. Res. Opin. 22:2007–2021 [DOI] [PubMed] [Google Scholar]
- 79. Cowen AE. 1992. Infection and endoscopy: who infects whom? Scand. J. Gastroenterol. Suppl. 192:91–96 [DOI] [PubMed] [Google Scholar]
- 80. Greene WH, Moody M, Hartley R, Effman E, Aisner J, Young VM, Wiernik RH. 1974. Esophagoscopy as a source of Pseudomonas aeruginosa sepsis in patients with acute leukemia: the need for sterilization of endoscopes. Gastroenterology 67:912–919 [PubMed] [Google Scholar]
- 81. Kaw M, Przepiorka D, Sekas G. 1993. Infectious complications of endoscopic procedures in bone marrow transplant recipients. Dig. Dis. Sci. 38:71–74 [DOI] [PubMed] [Google Scholar]
- 82. Beecham HJ, III, Cohen ML, Parkin WE. 1979. Salmonella typhimurium: transmission by fiberoptic upper gastrointestinal endoscopy. JAMA 241:1013–1015 [DOI] [PubMed] [Google Scholar]
- 83. Chmel H, Armstrong D. 1976. Salmonella oslo: a focal outbreak in a hospital. Am. J. Med. 60:203–208 [DOI] [PubMed] [Google Scholar]
- 84. Dean AG. 1977. Transmission of Salmonella typhi by fiberoptic endoscopy. Lancet ii:134. [DOI] [PubMed] [Google Scholar]
- 85. Dwyer DM, Klein EG, Istre GR, Robinson MG, Neumann DA, McCoy GA. 1987. Salmonella newport infections transmitted by fiberoptic colonoscopy. Gastrointest. Endosc. 33:84–87 [DOI] [PubMed] [Google Scholar]
- 86. O'Connor BH, Bennett JR, Alexander JG, Sutton DR, Leighton I, Mawer SL, Dunlop JM. 1982. Salmonellosis infection transmitted by fibreoptic endoscopes. Lancet ii:864–866 [DOI] [PubMed] [Google Scholar]
- 87. Pearson HE, Grant WJ. 1970. Presumed transmission of Salmonella by sygmoidoscope. Calif. Med. 112:23–25 [PMC free article] [PubMed] [Google Scholar]
- 88. Schliessler KH, Rozendaal B, Taal C, Meawissen SG. 1980. Outbreak of Salmonella agona infection after upper intestinal fibreoptic endoscopy. Lancet ii:1246. [DOI] [PubMed] [Google Scholar]
- 89. Pajkos A, Vickery K, Cossart Y. 2004. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J. Hosp. Infect. 58:224–229 [DOI] [PubMed] [Google Scholar]
- 90. Classen DC, Jacobson JA, Burke JP, Jacobson JT, Evans RS. 1988. Serious Pseudomonas infections associated with endoscopic retrograde cholangiopancreatography. Am. J. Med. 84:590–596 [DOI] [PubMed] [Google Scholar]
- 91. Earnshaw JJ, Clark AW, Thom BT. 1985. Outbreak of Pseudomonas aeruginosa following endoscopic retrograde cholangiopancreatography. J. Hosp. Infect. 6:95–97 [DOI] [PubMed] [Google Scholar]
- 92. Grogan JB. 1966. Pseudomonas aeruginosa carriage in patients. J. Trauma 6:639–643 [DOI] [PubMed] [Google Scholar]
- 93. Srinivasan A, Wolfenden LL, Song X, Mackie K, Hartsell TL, Jones HD, Diette GB, Orens JB, Yung RC, Ross TL, Merz W, Scheel PJ, Haponik EF, Perl TM. 2003. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N. Engl. J. Med. 348:221–227 [DOI] [PubMed] [Google Scholar]
- 94. Motte S, Deviere J, Dumonceau JM, Serruys E, Thys JP, Cremer M. 1991. Risk factors for septicemia following endoscopic biliary stenting. Gastroenterology 101:1374–1381 [DOI] [PubMed] [Google Scholar]
- 95. Elson CO, Hattori K, Blackstone MO. 1975. Polymicrobial sepsis following endoscopic retrograde cholangiopancreatography. Gastroenterology 69:507–510 [PubMed] [Google Scholar]
- 96. Low DE, Mieflikier AB, Kennedy JK, Stiver HG. 1980. Infectious complications of endoscopic retrograde cholangiopancreatography. A prospective assessment. Arch. Intern. Med. 140:1076–1077 [PubMed] [Google Scholar]
- 97. Schousboe M, Carter A, Sheppard PS. 1980. Endoscopic retrograde cholangiopancreatography: related nosocomial infections. N. Z. Med. J. 92:275–277 [PubMed] [Google Scholar]
- 98. Siegman-Igra Y, Isakov A, Inbar G, Cahaner J. 1987. Pseudomonas aeruginosa septicemia following endoscopic retrograde cholangiopancreatography with a contaminated endoscope. Scand. J. Infect. Dis. 19:527–530 [DOI] [PubMed] [Google Scholar]
- 99. Bass DH, Oliver S, Bornman PC. 1990. Pseudomonas septicaemia after endoscopic retrograde cholangiopancreatography—an unresolved problem. S. Afr. Med. J. 77:509–511 [PubMed] [Google Scholar]
- 100. Doherty DE, Falko JM, Lefkovitz N, Rogers J, Fromkes J. 1982. Pseudomonas aeruginosa sepsis following retrograde cholangiopancreatography. Dig. Dis. Sci. 27:169–170 [DOI] [PubMed] [Google Scholar]
- 101. Fraser TG, Reiner S, Malczynski M, Yarnold PR, Warren J, Noskin GA. 2004. Multidrug-resistant Pseudomonas aeruginosa cholangitis after endoscopic retrograde cholangiopancreatography: failure of routine endoscope cultures to prevent an outbreak. Infect. Control Hosp. Epidemiol. 25:856–859 [DOI] [PubMed] [Google Scholar]
- 102. Blanc DS, Parret T, Janin B, Raselli P, Francioli P. 1997. Nosocomial infections from contaminated bronchoscopes: two-years follow up using molecular markers. Infect. Control Hosp. Epidemiol. 18:134–136 [DOI] [PubMed] [Google Scholar]
- 103. Sorin M, Segal-Maurer S, Mariano N, Urban C, Combest A, Rahal JJ. 2001. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect. Control Hosp. Epidemiol. 22:409–413 [DOI] [PubMed] [Google Scholar]
- 104. Centers for Disease Control and Prevention 1999. Bronchoscopy-related infections and pseudoinfections: New York, 1996 and 1998. MMWR Morb. Mortal. Wkly. Rep. 48:557–560 [PubMed] [Google Scholar]
- 105. Corne P, Godreuil S, Jean-Pierre H, Jonquet O, Campos J, Jumas-Bilak E, Parer S, Marchandin H. 2005. Unusual implication of biopsy forceps in outbreaks of Pseudomonas aeruginosa infections and pseudo-infections related to bronchoscopy. J. Hosp. Infect. 61:20–26 [DOI] [PubMed] [Google Scholar]
- 106. DiazGranados CA, Jones MY, Kongphet-Tran T, White N, Shapiro M, Wang YF, Ray SM, Blumberg HM. 2009. Outbreak of Pseudomonas aeruginosa infection associated with contamination of a flexible bronchoscope. Infect. Control Hosp. Epidemiol. 30:550–555 [DOI] [PubMed] [Google Scholar]
- 107. Kirschke DL, Jones TF, Craig AS, Chu PS, Mayernick GG, Patel JA, Schaffner W. 2003. Pseudomonas aeruginosa and Serratia marcescens contamination associated with a manufacturing defect in bronchoscopes. N. Engl. J. Med. 348:214–220 [DOI] [PubMed] [Google Scholar]
- 108. Michele TM, Cronin WA, Graham NM, Dwyer DM, Pope DS, Harrington S, Chaisson RE, Bishai WR. 1997. Transmission of Mycobacterium tuberculosis by a fiberoptic bronchoscope: identification by DNA fingerprinting. JAMA 278:1093–1095 [PubMed] [Google Scholar]
- 109. Nelson KE, Larson PA, Schraufnagel DE, Jackson J. 1983. Transmission of tuberculosis by flexible fiberbronchoscopes. Am. Rev. Respir. Dis. 127:97–100 [DOI] [PubMed] [Google Scholar]
- 110. Ramsey AH, Oemig TV, Davis JP, Massey JP, Török TJ. 2002. An outbreak of bronchoscopy-related Mycobacterium tuberculosis infections due to lack of bronchoscope leak testing. Chest 121:976–981 [DOI] [PubMed] [Google Scholar]
- 111. Wheeler PW, Lancaster D, Kaiser AB. 1989. Bronchopulmonary cross-colonization and infection related to mycobacterial contamination of suction valves of bronchoscopes. J. Infect. Dis. 159:954–958 [DOI] [PubMed] [Google Scholar]
- 112. Hanson PJ. 1988. Mycobacteria and AIDS. Br. J. Hosp. Med. 40:149. [PubMed] [Google Scholar]
- 113. Phillips MS, von Reyn CF. 2001. Nosocomial infections due to nontuberculous mycobacteria. Clin. Infect. Dis. 33:1363–1374 [DOI] [PubMed] [Google Scholar]
- 114. Sattar SA, Best M, Springthorpe VS, Sanani G. 1995. Mycobactericidal testing of disinfectants: an update. J. Hosp. Infect. 30(Suppl):372–382 [DOI] [PubMed] [Google Scholar]
- 115. van Klingeren B, Pullen W. 1987. Comparative testing of disinfectants against Mycobacterium tuberculosis and Mycobacterium terrae in a quantitative suspension test. J. Hosp. Infect. 10:292–298 [DOI] [PubMed] [Google Scholar]
- 116. Davis D, Bonekat HW, Andrews D, Shigeoka JW. 1984. Disinfection of the flexible fibreoptic bronchoscope against Mycobacterium tuberculosis and M. gordonae. Thorax 39:785–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Urayama S, Kozarek RA, Sumida S, Raltz S, Merriam L, Pethigal P. 1996. Mycobacteria and glutaraldehyde: is high-level disinfection of endoscopes possible? Gastrointest. Endosc. 43:451–456 [DOI] [PubMed] [Google Scholar]
- 118. Hernández A, Martró E, Puzo C, Matas L, Burgués C, Vázquez N, Castella J, Ausina V. 2003. In-use evaluation of Perasafe compared with Cidex in fibreoptic bronchoscope disinfection. J. Hosp. Infect. 54:46–51 [DOI] [PubMed] [Google Scholar]
- 119. Wallace RJ, Jr, Brown BA, Griffith DE. 1998. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu. Rev. Microbiol. 52:453–490 [DOI] [PubMed] [Google Scholar]
- 120. Larson JL, Lambert L, Stricof RL, Driscoll J, McGarry MA, Ridzon R. 2003. Potential nosocomial exposure to Mycobacterium tuberculosis from a bronchoscope. Infect. Control Hosp. Epidemiol. 24:825–830 [DOI] [PubMed] [Google Scholar]
- 121. Brown NM, Hellyar EA, Harvey JE, Reeves DS. 1993. Mycobacterial contamination of fibreoptic bronchoscopes. Thorax 48:1283–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Campagnaro RL, Teichtahl H, Dwyer B. 1994. A pseudoepidemic of Mycobacterium chelonae: contamination of a bronchoscope and autocleaner. Aust. N. Z. J. Med. 24:693–695 [DOI] [PubMed] [Google Scholar]
- 123. Shimoide H, Anzai E, Murata Y, Kusajima K, Ichihara H, Takano T, Hirayama N, Sato N, Kobayashi Y. 1995. Contamination of flexible fiberoptic bronchoscopes with Mycobacterium chelonae linked to an automated endoscope disinfection machine—on the relationship between the presence of the organism in the intestinal tract and contamination of disinfection machine, and a case of gallbladder and bile duct infection with M. chelonae. Kekkaku 70:571–577 [PubMed] [Google Scholar]
- 124. Pappas SA, Schaaff DM, DiCostanzo MB, King FW, Jr, Sharp JT. 1983. Contamination of flexible fiberoptic bronchoscopes. Am. Rev. Respir. Dis. 127:391–392 [DOI] [PubMed] [Google Scholar]
- 125. Prigogine TH, Glupczynski Y, Van Molle P, Schmerber J. 1988. Mycobacterial cross-contamination of bronchsocopy specimens. J. Hosp. Infect. 11:93–95 [DOI] [PubMed] [Google Scholar]
- 126. Uttley AH, Honeywell KM, Fitch LE, Yates MD, Collins CH, Simpson RA. 1990. Cross-contamination of bronchial washings. BMJ 301:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang HC, Liaw YS, Yang PC, Kuo SH, Luh KT. 1995. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibreoptic bronchoscope suction channel. Eur. Respir. Infect. 8:1259–1262 [DOI] [PubMed] [Google Scholar]
- 128. Langenberg W, Rauws EA, Oudbier JH, Tytgat GN. 1990. Patient-to-patient transmission of Campylobacter pylori infection by fiberoptic gastroduodenoscopy and biopsy. J. Infect. Dis. 161:507–511 [DOI] [PubMed] [Google Scholar]
- 129. Tytgat GN. 1995. Endoscopic transmission of Helicobacter pylori. Aliment. Pharmacol. Ther. 9(Suppl 2):105–110 [PubMed] [Google Scholar]
- 130. Graham DY, Alpert LC, Smith JL, Yoshimura HH. 1988. Iatrogenic Campylobacter pylori infection is a cause of epidemic achlorhydria. Am. J. Gastroenterol. 83:974–980 [PubMed] [Google Scholar]
- 131. Miyaji H, Kohli Y, Azuma T, Ito S, Hirai M, Ito Y, Kato T, Kuriyama M. 1995. Endoscopic cross-infection with Helicobacter pylori. Lancet 345:464. [PubMed] [Google Scholar]
- 132. Akamatsu T, Tabata K, Hironga M, Kawakami H, Uyeda M. 1996. Transmission of Helicobacter pylori infection via flexible fiberoptic endoscopy. Am. J. Infect. Control 24:396–401 [DOI] [PubMed] [Google Scholar]
- 133. Fantry GT, Zheng QX, James SP. 1995. Conventional cleaning and disinfection techniques eliminate the risk of endoscopic transmission of Helicobacter pylori. Am. J. Gastroenterol. 90:227–232 [PubMed] [Google Scholar]
- 134. Roosendaal R, Kuipers EJ, van den Brule AJ, Peña AS, Uyterlinde AM, Walboomers JM, Meuwissen SG, de Graaff J. 1994. Importance of the fiberoptic endoscope cleaning procedure for detection of Helicobacter pylori in gastric biopsy specimens by PCR. J. Clin. Microbiol. 32:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Poutanen SM, Simor AE. 2004. Clostridium difficile-associated diarrhea in adults. CMAJ 171:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Patterson DJ, Johnson EH, Schmulen AC. 1984. Fulminant pseudomembranous colitis occurring after colonoscopy. Gastrointest. Endosc. 30:249–253 [DOI] [PubMed] [Google Scholar]
- 137. Selinger CP, Greer S, Sutton CJ. 2010. Is gastrointestinal endoscopy a risk factor for Clostridium difficile associated diarrhea? Am. J. Infect. Control 38:581–582 [DOI] [PubMed] [Google Scholar]
- 138. Hughes CE, Gebhard RL, Peterson LR, Gerding DN. 1986. Efficacy of routine fiberoptic endoscope cleaning and disinfection for killing Clostridium difficile. Gastrointest. Endosc. 32:7–9 [DOI] [PubMed] [Google Scholar]
- 139. Rutala WA, Gergen MF, Weber DJ. 1993. Inactivation of Clostridium difficile spores by disinfectants. Infect. Control Hosp. Epidemiol. 14:36–39 [DOI] [PubMed] [Google Scholar]
- 140. Wullt M, Odenholt I, Walder M. 2003. Activity of three disinfectants and acidified nitrite against Clostridium difficile spores. Infect. Control Hosp. Epidemiol. 24:765–768 [DOI] [PubMed] [Google Scholar]
- 141. Godiwala T, Andry M, Agrawal N, Ertan A. 1988. Consecutive Serratia marcescens infections following endoscopic retrograde cholangiopancreatography. Gastrointest. Endosc. 34:345–347 [DOI] [PubMed] [Google Scholar]
- 142. Webb SF, Vall-Spinosa A. 1975. Outbreak of Serratia marcescens associated with the flexible fiberbronchoscope. Chest 68:703–708 [Google Scholar]
- 143. Cêtre J-C, Nicolle MC, Salord H, Pérol M, Tigaud S, David G, Bourjault M, Vanhems P. 2005. Outbreaks of contaminated broncho-alveolar lavage related to intrinsically defective bronchoscopes. J. Hosp. Infect. 61:39–45 [DOI] [PubMed] [Google Scholar]
- 144. Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, Bommelaer G, Traoré O. 2010. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy 42:895–899 [DOI] [PubMed] [Google Scholar]
- 145. Carbonne A, Thiolet JM, Fournier S, Fortineau N, Kassis-Chikhani N, Boytchev I, Aggoune M, Seguier JC, Senechal H, Tavolacci MP, Coignard B, Astagneau P, Jarlier V. 2010. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill. 15(48):pii=19734. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19734 [DOI] [PubMed] [Google Scholar]
- 146. Furuhata K, Kato Y, Goto K, Hara M, Yoshida S, Fukuyama M. 2006. Isolation and identification of Methylobacterium species from the tap water in hospitals in Japan and their antibiotic susceptibility. Microbiol. Immunol. 50:11–17 [DOI] [PubMed] [Google Scholar]
- 147. Imbert G, Seccia Y, La Scola B. 2005. Methylobacterium sp. bacteraemia due to a contaminated endoscope. J. Hosp. Infect. 61:268–270 [DOI] [PubMed] [Google Scholar]
- 148. Simões LC, Simões M, Vieira MJ. 2010. Adhesion and biofilm formation on polystyrene by drinking water-isolated bacteria. Antonie Van Leeuwenhoek 98:317–319 [DOI] [PubMed] [Google Scholar]
- 149. Van Aken B, Peres CM, Doty SL, Yoon JM, Schnoor JL. 2004. Methylobacterium populi sp. nov., a novel aerobic, pink-pigmented, facultatively methylotrophic, methane-utilizing bacterium isolated from poplar trees (Populus deltoides × nigra DN34). Int. J. Syst. Evol. Microbiol. 54:1191–1196 [DOI] [PubMed] [Google Scholar]
- 150. Mandelstam P, Sugawa C, Silvis SE, Nebel OT, Rogers BH. 1976. Complications associated with esophagogastroduodenoscopy and with esophageal dilation. Gastrointest. Endosc. 23:16–19 [DOI] [PubMed] [Google Scholar]
- 151. Lo Passo C, Pernice I, Celeste A, Perdichizzi G, Todaro-Luck F. 2001. Transmission of Trichosporon asahii esophagitis by a contaminated endoscope. Mycoses 44:13–21 [DOI] [PubMed] [Google Scholar]
- 152. Singh S, Singh N, Kochhar R, Mehta SK, Talwar P. 1989. Contamination of an endoscope due to Trichosporon beigelii. J. Hosp. Infect. 14:49–53 [DOI] [PubMed] [Google Scholar]
- 153. Hoffmann KK, Weber DJ, Rutala WA. 1989. Pseudoepidemic of Phodotorula rubra in patients indergoing fiberoptic bronchoscopy. Infect. Control Hosp. Epidemiol. 10:511–514 [DOI] [PubMed] [Google Scholar]
- 154. Nicolle LE, McLeod J, Romance L, Parker S, Paraskevas M. 1992. Pseudo-outbreak of blastomycosis associated with contaminated bronchoscopes. Infect. Control Hosp. Epidemiol. 13:324. [DOI] [PubMed] [Google Scholar]
- 155. Mitchell DH, Hicks LJ, Chiew R, Montanaro JC, Chen SC. 1997. Pseudoepidemic of Legionella pneumophila serogroup 6 associated with contaminated bronchoscopes. J. Hosp. Infect. 37:19–23 [DOI] [PubMed] [Google Scholar]
- 156. Goldstein B, Abrutyn E. 1985. Pseudo-outbreak of Bacillus spores related to fibreoptic bronchoscopy. J. Hosp. Infect. 6:194–200 [PubMed] [Google Scholar]
- 157. La Scola B, Rolain JM, Maurin M, Raoult D. 2003. Can Whipple's disease be transmitted by gastroscopes? Infect. Control Hosp. Epidemiol. 24:191–194 [DOI] [PubMed] [Google Scholar]
- 158. Muscarella LF. 2002. Anthrax: is there a risk of cross-infection during endoscopy? Gastroenterol. Nurs. 25:46–48 [DOI] [PubMed] [Google Scholar]
- 159. Jarroll EL, Bingham AK, Meyer EA. 1981. Effect of chlorine on Giardia lamblia cysts. Appl. Envinron. Microbiol. 41:483–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Campbell I, Tzipori AS, Hutchison G, Angus KW. 1982. Effect of disinfectants on survival of Cryptosporidium oocysts. Vet. Rec. 111:414–415 [DOI] [PubMed] [Google Scholar]
- 161. Birnie GG, Quigley EM, Clements GB, Follet EA, Watkinson G. 1983. Endoscopic transmission of hepatitis B virus. Gut 24:171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Morris IM, Cattle DS, Smits BJ. 1975. Letter: endoscopy and transmission of hepatitis B. Lancet ii:1152. [DOI] [PubMed] [Google Scholar]
- 163. Seefeld U, Bansky G, Jager M, Schmid M. 1981. Prevention of hepatitis B virus transmission by the gastrointestinal febrescope. Successful disinfection with an aldehyde liquid. Endoscopy 13:238–239 [DOI] [PubMed] [Google Scholar]
- 164. Ayoola EA. 1981. The risk of type B hepatitis infection in flexible endoscopy. Gastrointest. Endosc. 27:60–62 [DOI] [PubMed] [Google Scholar]
- 165. Chiaramonte M, Farini R, Truscia D, Zampieri L, Di Mario F, Pornaro E, Vecchiati U, Naccarato R. 1983. Risk of hepatitis B virus infection following upper gastrointestinal endoscopy: a prospective study in an endemic area. Hepatogastroenterology 30:189–191 [PubMed] [Google Scholar]
- 166. Hoofnagle JH, Blake J, Buskell-Bales Z, Seeff LB. 1980. Lack of transmission of type B hepatitis by fibreoptic upper endoscopy. J. Clin. Gastroenterol. 2:65–69 [DOI] [PubMed] [Google Scholar]
- 167. Koretz RL, Camacho R. 1979. Failure of endoscopic transmission of hepatitis B. Dig. Dis. Sci. 24:21–24 [DOI] [PubMed] [Google Scholar]
- 168. Lok AS, Lai C-L, Hui W-M, Ng MM, Wu P-C, Lam S-K, Leung EK. 1987. Absence of transmission of hepatitis B by fibreoptic upper gastrointestinal endoscopy. J. Gastroenterol. Hepatol. 2:175–180 [Google Scholar]
- 169. McClelland DB, Burrell CJ, Tonkin RW, Heading RC. 1978. Hepatitis B: absence of transmission by gastrointestinal endoscopy. Br. Med. J. i:23–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Moncada RE, Denes AE, Berquist KR, Fields HA, Murphy BL, Maynard JE. 1978. Inadvertent exposure of endoscopy patients to viral hepatitis B. Gastrointest. Endosc. 24:231–232 [DOI] [PubMed] [Google Scholar]
- 171. Villa E, Pasquinelli C, Rigo G, Ferrari A, Perini M, Ferretti I, Gandolfo M, Rubbiani L, Antonioli A, Barchi T, Manenti F. 1984. Gastrointestinal endoscopy and HBV infection: no evidence for a causal relationship. A prospective controlled study. Gastrointest. Endosc. 30:15–17 [DOI] [PubMed] [Google Scholar]
- 172. Bond WW, Favero MS, Petersen NJ, Ebert JW. 1983. Inactivation of hepatitis B virus by intermediate to high level disinfectant chemicals. J. Clin. Microbiol. 18:535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Kobayashi H, Tsuzuki M, Koshimizu K, Toyama H, Yoshihara N, Shikata T, Abe K, Mizuno K, Otomo N, Oda T. 1984. Susceptibility of hepatitis B virus to disinfectants or heat. J. Clin. Microbiol. 20:214–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, Cheung L, DiBisceglie A, Hoofnagle J, Shih JW, Kaslow R, Ness P, Alter HJ. 1996. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N. Engl. J. Med. 334:1691–1696 [DOI] [PubMed] [Google Scholar]
- 175. Andrieu J, Barny S, Colardelle P, Maisonneuve P, Giraud V, Robin E, Bréart G, Coste T. 1995. Prevalence and risk factors of hepatitis C virus infection in a hospitalized population in a gastroenterology unit. Role of endoscopic biopsies. Gastroenterol. Clin. Biol. 19:340–345 [PubMed] [Google Scholar]
- 176. Couroucé AM, Pillonel J, Saura C. 1998. Infections récentes par le virus de l'hépatite C chez les donneurs de sang et facteurs de risque. Bull. Epidemiol. Hebd. 4:13–14 [Google Scholar]
- 177. Crenn P, Gigou M, Passeron J, Dussaix E, Bismuth H, Feray C. 1988. Patient to patient transmission of hepatitis C virus during gastroscopy on neuroleptanalgesia. Gastroenterology 114(Suppl 1):A1229 [Google Scholar]
- 178. Mele A, Spada E, Sagliocca L, Ragni P, Tosti ME, Gallo G, Moiraghi A, Balocchini E, Sangalli M, Lopalco PL, Stroffoli T. 2001. Risk of parenterally transmitted hepatitis following exposure to surgery or other invasive procedures: results from the hepatitis surveillance system in Italy. J. Hepatol. 35:284–289 [DOI] [PubMed] [Google Scholar]
- 179. Ciancio A, Manzini P, Castagno F, D'Antico S, Reynaudo P, Coucourde L, Ciccone G, Del Piano M, Ballarè M, Peyre S, Rizzi R, Barletti C, Bruno M, Caronna S, Carucci P, de Bernardi Venon W, De Angelis C, Morgando A, Musso A, Repici A, Rizzetto M, Saracco G. 2005. Digestive endoscopy is not a major risk factor for transmitting hepatitis C virus. Ann. Intern. Med. 142:903–909 [DOI] [PubMed] [Google Scholar]
- 180. Mikhail NN, Lewis DL, Omar N, Taha H, El-Badawy A, Abdel-Mawgoud N, Abdel-Hamid M, Strickland GT. 2007. Prospective study of cross-infection from upper GI endoscopy in a hepatitis C-prevalent population. Gastrointest. Endosc. 65:584–588 [DOI] [PubMed] [Google Scholar]
- 181. Bronowicki JP, Venard V, Botté C, Monhoven N, Gastin I, Choné L, Hudziak H, Rihn B, Delanoë C, LeFaou A, Bigard MA, Gaucher P. 1997. Patient-to-patient transmission of hepatitis C virus during colonoscopy. N. Engl. J. Med. 337:237–240 [DOI] [PubMed] [Google Scholar]
- 182. Le Pogam S, Gondeau A, Bacq Y. 1999. Nosocomial transmission of hepatitis C virus. Ann. Intern. Med. 131:794. [DOI] [PubMed] [Google Scholar]
- 183. Muscarella LF, New York State Health Officials 2001. Recommendations for preventing hepatitis C virus infection: analysis of a Brooklyn endoscopy clinic's outbreak. Infect. Control Hosp. Epidemiol. 22:669. [DOI] [PubMed] [Google Scholar]
- 184. Tennenbaum R, Colardelle P, Chochon M, Maisonneuve P, Jean F, Andrieu J. 1993. Hepatitis C after retrograde cholangiography. Gastroenterol. Clin. Biol. 17:763–775 [PubMed] [Google Scholar]
- 185. Ciesek S, Friesland M, Steinmann J, Becker B, Wedemeyer H, Manns MP, Steinmann J, Pietschmann T, Steinmann E. 2010. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its suspectibility to chemical biocides. J. Infect. Dis. 201:1859–1866 [DOI] [PubMed] [Google Scholar]
- 186. Hanson PJ, Gor D, Jeffries DJ, Collins JV. 1989. Chemical inactivation of HIV on surfaces. BMJ 298:862–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Hanson PJ, Gor D, Clarke JR, Chadwick MV, Nicholson G, Shah N, Gazzard B, Jeffries DJ, Gaya H, Collins JV. 1989. Contamination of endoscopes used in AIDS patients. Lancet ii:86–88 [DOI] [PubMed] [Google Scholar]
- 188. Hanson PJ, Gor D, Jeffries DJ, Collins JV. 1990. Elimination of high titer HIV from fibreoptic endoscopes. Gut 31:657–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Narang HJ, Codd AA. 1983. Action of commonly used disinfectants against enteroviruses. J. Hosp. Infect. 4:209–212 [DOI] [PubMed] [Google Scholar]
- 190. Hanson PJ, Bennett J, Jeffries DJ, Collins JV. 1994. Enteroviruses, endoscopy and infection control: an applied study. J. Hosp. Infect. 27:61–67 [DOI] [PubMed] [Google Scholar]
- 191. Haywood AM. 1997. Transmissible spongiform encephalopathies. N. Engl. J. Med. 337:1821–1828 [DOI] [PubMed] [Google Scholar]
- 192. Brown P, Preece MA, Will RG. 1992. “Friendly fire” in medicine: hormones, homografts, and Creutzfeldt-Jakob disease. Lancet 340:24–27 [DOI] [PubMed] [Google Scholar]
- 193. Lewis AM, Yu M, DeArmond SJ, Dillon WP, Miller BL, Geschwind MD. 2006. Human growth hormone-related iatrogenic Creutzfeldt-Jakob disease with abnormal imaging. Arch. Neurol. 63:288–290 [DOI] [PubMed] [Google Scholar]
- 194. Preusser M, Ströbel T, Gelpi E, Eiler M, Broessner G, Schmutzhard E, Budka H. 2006. Alzheimer-type neuropathology in a 28 year old patient with iatrogenic Creutzfeldt-Jakob disease after dural grafting. J. Neurol. Neurosurg. Psychiatry 77:413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Allan B, Tuft S. 1997. Transmission of Creutzfeldt-Jakob disease in corneal grafts. BMJ 315:1553–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Johnson RT, Gibbs CJ., Jr 1998. Creutzfeldt-Jakob disease and related transmissible spongiform encephalopathies. N. Engl. J. Med. 339:1994–2004 [DOI] [PubMed] [Google Scholar]
- 197. Bruce ME, McConnell I, Will RG, Ironside JW. 2001. Detection of variant Creutzfeldt-Jakob disease infectivity in extraneural tissues. Lancet 358:208–209 [DOI] [PubMed] [Google Scholar]
- 198. Bramble MG, Ironside JW. 2002. Creutzfeldt-Jakob disease: implications for gastroenterology. Gut 50:888–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Axon AT, Beilenhoff U, Bramble MG, Ghosh S, Kruse A, McDonnell GE, Neumann C, Rey JF, Spencer K, Guidelines Committee, European Society of Gastrointestinal Endoscopy 2001. Variant Creutzfeldt-Jakob disease (vCJD) and gastrointestinal endoscopy. Endoscopy 33:1070–1080 [DOI] [PubMed] [Google Scholar]
- 200. Rutala WA, Weber DJ, Society for Healthcare Epidemiology of America 2010. Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect. Control Hosp. Epidemiol. 31:107–117 [DOI] [PubMed] [Google Scholar]
- 201. Ernst DR, Race RE. 1993. Comparative analysis of scrapie agent inactivation methods. J. Virol. Methods 41:193–202 [DOI] [PubMed] [Google Scholar]
- 202. Steelman VM. 1994. Creutzfeld-Jacob disease: recommendations for infection control. Am. J. Infect. Control 22:312–318 [DOI] [PubMed] [Google Scholar]
- 203. Steelman VM. 1999. Prion diseases—an evidence-based protocol for infection control. AORN J. 69:956–967 [DOI] [PubMed] [Google Scholar]
- 204. Weber DJ, Rutala WA. 2002. Managing the risk of nosocomial transmission of prion diseases. Curr. Opin. Infect. Dis. 15:421–425 [DOI] [PubMed] [Google Scholar]
- 205. Antloga K, Meszaros J, Malchesky PS, McDonnell GE. 2000. Prion disease and medical devices. ASAIO J. 46:S69–S72 doi:10.1097/00002480-200011000-00040 [DOI] [PubMed] [Google Scholar]
- 206. O'Connor HJ, Hamilton I, Lincoln C, Maxwell S, Axon AT. 1983. Bacteraemia with upper gastrointestinal endoscopy—a reappraisal. Endoscopy 15:21–23 [DOI] [PubMed] [Google Scholar]
- 207. Baltch AL, Buhac I, Agrawal A, O'Connor P, Bram M, Malatino E. 1977. Bacteremia after upper gastrointestinal endoscopy. Arch. Intern. Med. 137:594–597 [PubMed] [Google Scholar]
- 208. Kirk A, Graham-Brown R, Perinpanayagam RM, Smith RG, Bernardo DE. 1979. Bacteraemia and upper gastrointestinal fibre-endoscopy. J. R. Soc. Med. 72:409–411 [PMC free article] [PubMed] [Google Scholar]
- 209. Liebermann TR. 1976. Bacteremia and fiberoptic endoscopy. Gastrointest. Endosc. 23:36–37 [DOI] [PubMed] [Google Scholar]
- 210. Linnemann C, Weisman E, Wenger J. 1971. Blood cultures following endoscopy of the esophagus and stomach. South. Med. J. 64:1055–1062 [DOI] [PubMed] [Google Scholar]
- 211. Mellow MH, Lewis RJ. 1976. Endoscopy-related bacteremia. Incidence of positive blood cultures after endoscopy of upper gastrointestinal tract. Arch. Intern. Med. 136:667–669 [DOI] [PubMed] [Google Scholar]
- 212. Norfleet RG, Mitchell PD, Mulholland DD, Philo J. 1981. Does bacteremia follow upper gastrointestinal endoscopy? Am. J. Gastroenterol. 76:420–422 [PubMed] [Google Scholar]
- 213. Shull HJ, Jr, Greene BM, Allen SD, Dunn GD, Schenker S. 1975. Bacteremia with upper gastrointestinal endoscopy. Ann. Intern. Med. 83:212–214 [DOI] [PubMed] [Google Scholar]
- 214. Sontheimer J, Salm R, Freidrich G, von Wahlert J, Pelz K. 1991. Bacteremia following operative endoscopy of the upper gastrointestinal tract. Endoscopy 23:67–72 [DOI] [PubMed] [Google Scholar]
- 215. Stray N, Midtvedt T, Valnes K, Rosseland A, Pytte R, Höivik B. 1978. Endoscopy-related bacteremia. Scand. J. Gastroenterol. 13:345–347 [DOI] [PubMed] [Google Scholar]
- 216. Nelson DB. 2003. Infectious disease complications of GI endoscopy: part I, endogenous infections. Gastrointest. Endosc. 57:546–556 [DOI] [PubMed] [Google Scholar]
- 217. Berner JS, Gaing AA, Sharma R, Almenoff PL, Muhlfelder T, Korsten MA. 1994. Sequelae after esophageal variceal ligation and sclerotherapy: a prospective randomized study. Am. J. Gastroenterol. 89:852–858 [PubMed] [Google Scholar]
- 218. Camara DS, Gruber M, Barde CJ, Montes M, Caruana JA, Jr, Chung RS. 1983. Transient bacteremia following endoscopic injection sclerotherapy of esophageal varices. Arch. Intern. Med. 143:1350–1352 [PubMed] [Google Scholar]
- 219. da Silveira Rohr MR, Siqueira ES, Brant CQ, Morais M, Libera ED, Jr, Castro RR, Ferrari AP. 1997. Prospective study of bacteremia rate after elastic band ligation and sclerotherapy of esophageal varices in patients with hepatosplenic schistosomiasis. Gastrointest. Endosc. 46:321–323 [DOI] [PubMed] [Google Scholar]
- 220. Gerhartz HH, Sauerbruch T, Weinzierl M, Ruckdeschel G. 1984. Nosocomial septicemia in patients undergoing sclerotherapy for variceal hemorrhage. Endoscopy 16:129–130 [DOI] [PubMed] [Google Scholar]
- 221. Ho H, Zuckerman MJ, Wassem C. 1991. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology 101:1642–1648 [DOI] [PubMed] [Google Scholar]
- 222. Lo GH, Lai KH, Shen MT, Chang CF. 1994. A comparison of the incidence of transient bacteremia and infectious sequelae after sclerotherapy and rubber band ligation of bleeding esophageal varices. Gastrointest. Endosc. 40:675–679 [PubMed] [Google Scholar]
- 223. Lorgat F, Madden MV, Kew G, Roditi D, Krige JE, Bornman PC, Jonker MA, Terblanche J. 1990. Bacteremia after injection of esophageal varices. Surg. Endosc. 4:18–19 [DOI] [PubMed] [Google Scholar]
- 224. Low DE, Shoenut JP, Kennedy JK, Harding GK, Den Boer B, Micflikier AB. 1986. Infectious complications of endoscopic injection sclerotherapy. Arch. Intern. Med. 146:569–571 [PubMed] [Google Scholar]
- 225. Sauerbruch T, Holl J, Ruckdeschel G, Förstl J, Weinzierl M. 1985. Bacteraemia associated with endoscopic sclerotherapy of oesophageal varices. Endoscopy 17:170–172 [DOI] [PubMed] [Google Scholar]
- 226. Tseng CC, Green RM, Burke SK, Connors PJ, Carr-Locke DL. 1992. Bacteremia after endoscopic band ligation of esophageal varices. Gastrointest. Endosc. 38:336–337 [DOI] [PubMed] [Google Scholar]
- 227. Nelson DB, Sanderson SJ, Azar MM. 1998. Bacteremia with esophageal dilation. Gastrointest. Endosc. 48:563–567 [DOI] [PubMed] [Google Scholar]
- 228. Stephenson PM, Dorrington L, Harris OD, Rao A. 1977. Bacteraemia following oesophageal dilatation and oesophagogastroscopy. Aust. N. Z. J. Med. 7:32–35 [DOI] [PubMed] [Google Scholar]
- 229. Welsh JD, Griffiths WJ, McKee J, Wilkinson D, Flournoy DJ, Mohr JA. 1983. Bacteremia associated with esophageal dilatation. J. Clin. Gastroenterol. 5:109–112 [DOI] [PubMed] [Google Scholar]
- 230. Zuccaro G, Jr, Richter JE, Rice TW, Achkar E, Easley K, Lewis J, Gordon SM. 1998. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest. Endosc. 48:568–573 [DOI] [PubMed] [Google Scholar]
- 231. Baskin G. 1989. Prosthetic endocarditis after endoscopic variceal sclerotherapy: a failure of antibiotic prophylaxis. Am. J. Gastroenterol. 84:311–312 [PubMed] [Google Scholar]
- 232. Breuer GS, Yinnon AM, Halevy J. 1998. Infective endocarditis associated with upper endoscopy: case report and review. J. Infect. 36:342–344 [DOI] [PubMed] [Google Scholar]
- 233. Logan RF, Hastings JG. 1988. Bacterial endocarditis: a complication of gastroscopy. BMJ (Clin. Res. Ed.) 296:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Niv Y, Bat L, Motro M. 1985. Bacterial endocarditis after Hurst bougienage in a patient with a benign esophageal stricture and mitral valve prolapse. Gastrointest. Endosc. 31:265–267 [DOI] [PubMed] [Google Scholar]
- 235. Pritchard TM, Foust RT, Cantey JR, Leman RB. 1991. Prosthetic valve endocarditis due to Cardiobacterium hominis occurring after gastrointestinal endoscopy. Am. J. Med. 90:516–518 [PubMed] [Google Scholar]
- 236. Rumfeld W, Wallace G, Scott BB. 1980. Bacterial endocarditis after endoscopy. Lancet ii:1083. [DOI] [PubMed] [Google Scholar]
- 237. Shorvon PJ, Eykyn SJ, Cotton PB. 1983. Gastrointestinal instrumentation, bacteremia and endocarditis. Gut 24:1078–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Wong A, Rosenstein AH, Rutherford RE, James SP. 1997. Bacterial endocarditis following endoscopic variceal sclerotherapy. J. Clin. Gastroenterol. 24:90–91 [DOI] [PubMed] [Google Scholar]
- 239. Yin TP, Dellipiani AW. 1983. Bacterial endocarditis after Hurst bougienage in a patient with a benign oesophageal stricture. Endoscopy 15:27–28 [DOI] [PubMed] [Google Scholar]
- 240. Cohen FL, Koerner RS, Taub SJ. 1985. Solitary brain abscess following endoscopic injection sclerosis of esophageal varices. Gastrointest. Endosc. 31:331–333 [DOI] [PubMed] [Google Scholar]
- 241. Djupesland P, Solgaard T, Mair IW. 1991. Cerebral abscess complicating dilatation of a corrosive esophageal stricture. Eur. Arch. Otorhinolaryngol. 248:308–310 [DOI] [PubMed] [Google Scholar]
- 242. Kumar P, Mehta SK, Devi BI, Goenka MK, Khandelwal N, Kochhar S, Kak VK. 1991. Pyogenic meningitis and cerebral abscesses after endoscopic injection sclerotherapy. Am. J. Gastroenterol. 86:1672–1674 [PubMed] [Google Scholar]
- 243. Nagamine N, Kaneko Y, Kumakura Y, Ogawa Y, Ido K, Kimura K. 1999. Occurrence of pyogenic meningitis during the course of endoscopic variceal ligation therapy. Gastrointest. Endosc. 49:110–113 [DOI] [PubMed] [Google Scholar]
- 244. Toyoda K, Saku Y, Sadoshima S, Fujishima M. 1994. Purulent meningitis after endoscopic injection sclerotherapy for esophageal varices. Intern. Med. 33:706–709 [DOI] [PubMed] [Google Scholar]
- 245. Wang WM, Chen CY, Jan CM, Chen LT, Wu DC. 1990. Central nervous system infection after endoscopic injection sclerotherapy. Am. J. Gastroenterol. 85:865–867 [PubMed] [Google Scholar]
- 246. Lin OS, Wu SS, Chen YY, Soon MS. 2000. Bacterial peritonitis after elective endoscopic variceal ligation: a prospective study. Am. J. Gastroenterol. 95:214–217 [DOI] [PubMed] [Google Scholar]
- 247. Schembre D, Bjorkman DJ. 1991. Post-sclerotherapy bacterial peritonitis. Am. J. Gastroenterol. 86:481–486 [PubMed] [Google Scholar]
- 248. Nelson DB. 2003. Infection control during gastrointestinal endoscopy. J. Lab. Clin. Med. 141:159–167 [DOI] [PubMed] [Google Scholar]
- 249. Coughlin GP, Butler RN, Alp MH, Grant AK. 1977. Colonoscopy and bacteremia. Gut 18:678–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Dickman MD, Farrell R, Higgs RH, Wright LE, Humphries TJ, Wojcik JD, Chappelka R. 1976. Colonoscopy associated bacteremia. Surg. Gynecol. Obstet. 142:173–176 [PubMed] [Google Scholar]
- 251. Hartong WA, Barnes WG, Calkins WG. 1977. The absence of bacteremia during colonoscopy. Am. J. Gastroenterol. 67:240–244 [PubMed] [Google Scholar]
- 252. Kelley CJ, Ingoldby CJ, Blenkharn JI, Wood CB. 1985. Colonoscopy related endotoxemia. Surg. Gynecol. Obstet. 161:332–334 [PubMed] [Google Scholar]
- 253. Kiss A, Ferenci P, Graninger W, Pamperl H, Pötzi R, Meryn S. 1983. Endotoxaemia following colonoscopy. Endoscopy 15:24–26 [DOI] [PubMed] [Google Scholar]
- 254. Kumar S, Abcarian H, Prasad ML, Lakshmanan S. 1982. Bacteremia associated with lower gastrointestinal endoscopy: fact or fiction? I. Colonoscopy. Dis. Colon Rectum 25:131–134 [DOI] [PubMed] [Google Scholar]
- 255. London MT, Chapman BA, Faoagali JL, Cook HB. 1986. Colonoscopy and bacteraemia: an experience in 50 patients. N. Z. Med. J. 99:269–271 [PubMed] [Google Scholar]
- 256. Low DE, Shoenut JP, Kennedy JK, Sharma GP, Harding GK, Den Boer B, Micflikier AB. 1987. Prospective assessment of risk of bacteremia with colonoscopy and polypectomy. Dig. Dis. Sci. 32:1239–1243 [DOI] [PubMed] [Google Scholar]
- 257. Norfleet RG, Mulholland BS, Mitchell PD, Philo J, Walters EW. 1976. Does bacteremia follow colonoscopy? Gastroenterology 70:20–21 [PubMed] [Google Scholar]
- 258. Goldman GD, Miller SA, Furman DS, Brock D, Ryan JL, McCallum RW. 1985. Does bacteremia occur during flexible sigmoidoscopy? Am. J. Gastroenterol. 80:621–623 [PubMed] [Google Scholar]
- 259. Kumar S, Abcarian H, Prasad ML, Lakshmanan S. 1983. Bacteremia associated with lower gastrointestinal endoscopy: fact or fiction? II. Proctosigmoidoscopy. Dis. Colon Rectum 26:22–24 [DOI] [PubMed] [Google Scholar]
- 260. Llach J, Elizalde JI, Bordas JM, Gines A, Almela M, Sans M, Mondelo F, Pique JM. 1999. Prospective assessment of the risk of bacteremia in cirrhotic patients undergoing lower gastrointestinal endoscopy. Gastrointest. Endosc. 49:214–217 [DOI] [PubMed] [Google Scholar]
- 261. Brandt E, Naess A. 1989. Acute appendicitis following endoscopic polypectomy. Endoscopy 21:44 doi:10.1055/s-2007-1012894 [DOI] [PubMed] [Google Scholar]
- 262. Hirata K, Noguchi J, Yoshikawa I, Tabaru A, Nagata N, Murata I, Itoh H. 1996. Acute appendicitis immediately after colonoscopy. Am. J. Gastroenterol. 91:2239–2240 [PubMed] [Google Scholar]
- 263. Houghton A, Aston N. 1988. Appendicitis complicating colonoscopy. Gastrointest. Endosc. 34:489. [DOI] [PubMed] [Google Scholar]
- 264. Lipton S, Estrin J. 1999. Postcolonoscopy appendicitis: a case report. J. Clin. Gastroenterol. 28:255–256 [DOI] [PubMed] [Google Scholar]
- 265. Moorman ML, Miller JP, Khanduja KS, Price PD. 2010. Postcolonoscopy appendicitis. Am. Surg. 76:892–895 [PubMed] [Google Scholar]
- 266. Takagi Y, Abe T. 2000. Appendicitis following endoscopic polypectomy. Endoscopy 32:S49. [PubMed] [Google Scholar]
- 267. Vender R, Larson J, Garcia J, Topazian M, Ephraim P. 1995. Appendicitis as a complication of colonoscopy. Gastrointest. Endosc. 41:514–516 [DOI] [PubMed] [Google Scholar]
- 268. Petersen JH, Weesner RE, Gianella RA. 1987. Escherichia coli peritonitis after left-sided colonoscopy in a patient on continuous ambulatory peritoneal dialysis. Am. J. Gastroenterol. 82:171–172 [PubMed] [Google Scholar]
- 269. Shrake PD, Troiano F, Rex DK. 1989. Peritonitis following colonoscopy in a cirrhotic with ascites. Am. J. Gastroenterol. 84:453–454 [PubMed] [Google Scholar]
- 270. Murray JL. 1984. Enterococcal endocarditis after sigmoidoscopy. West. J. Med. 141:689–690 [PMC free article] [PubMed] [Google Scholar]
- 271. Norfleet RG. 1991. Infectious endocarditis after fiberoptic sigmoidoscopy. J. Clin. Gastroenterol. 13:448–451 [DOI] [PubMed] [Google Scholar]
- 272. Rigilano J, Mahapatra R, Barnhill H, Gutierrez J. 1984. Entercoccal endocarditis following sigmoidoscopy and mitral valve prolapse. Arch. Intern. Med. 144:850–851 [PubMed] [Google Scholar]
- 273. Rodriguez W, Levine JS. 1984. Enterococcal endocarditis following flexible sigmoidoscopy. West. J. Med. 140:951–953 [PMC free article] [PubMed] [Google Scholar]
- 274. Lee M, Munoz J. 1994. Septicemia occurring after colonoscopic polypectomy in a splenectomized patient taking corticosteroids. Am. J. Gastroenterol. 89:2245–2246 [PubMed] [Google Scholar]
- 275. Macrae FA, Tan KG, Williams CB. 1983. Toward safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut 24:376–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Patel RT, Deen KI, Fielding JW. 1994. Portal pyaemia following flexible sigmoidoscopy. Br. J. Surg. 81:1337. [DOI] [PubMed] [Google Scholar]
- 277. Thornton JR, Losowsky MS. 1991. Septicaemia after colonoscopy in patients with cirrhosis. Gut 32:450–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Sheehan GJ, Galbraith JC. 1993. Colonoscopy-associated listeriosis: report of a case. Clin. Infect. Dis. 17:1061–1062 [DOI] [PubMed] [Google Scholar]
- 279. Shemesh O, Bornstein IB, Weissberg N, Braverman D, Rudensky B. 1989. Listeria septicemia after colonoscopy in an ulcerative colitis patient receiving ACTH. Am. J. Gastroenterol. 84:1127–1128 [PubMed] [Google Scholar]
- 280. Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. 2002. Procedural success and complications of large-scale screening colonoscopy. Gastrointest. Endosc. 55:307–314 [DOI] [PubMed] [Google Scholar]
- 281. Akkersdijk WL, van Bergeijk JD, van Egmond T, Mulder CJ, van Berge Henegouwen GP, van der Werken C, van Erpecum KJ. 1995. Percutaneous endoscopic gastrostomy (PEG): comparison of push and pull methods and evaluation of antibiotic prophylaxis. Endoscopy 27:313–316 [DOI] [PubMed] [Google Scholar]
- 282. Gossner L, Keymling J, Hahn EG, Ell C. 1999. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy (PEG): a prospective randomized clinical trial. Endoscopy 31:119–124 [DOI] [PubMed] [Google Scholar]
- 283. Jain NK, Larson DE, Schroeder KW, Burton DD, Cannon KP, Thompson RL, DiMagno EP. 1987. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy. Ann. Intern. Med. 107:824–828 [DOI] [PubMed] [Google Scholar]
- 284. Jonas SK, Neimark S, Panwalker AP. 1985. Effect of antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am. J. Gastroenterol. 80:438–441 [PubMed] [Google Scholar]
- 285. Panigrahi H, Shreeve DR, Tan WC, Prudham R, Kaufman R. 2002. Role of antibiotic prophylaxis for wound infection in percutaneous endoscopic gastrostomy (PEG): result of a prospective double-blind randomized trial. J. Hosp. Infect. 50:312–315 [DOI] [PubMed] [Google Scholar]
- 286. Sturgis TM, Yancy W, Cole JC, Proctor DD, Minhas BS, Marcuard SP. 1996. Antibiotic prophylaxis in percutaneous endoscopic gastrostomy. Am. J. Gastroenterol. 91:2301–2304 [PubMed] [Google Scholar]
- 287. Cave DR, Robinson WR, Brotschi EA. 1986. Necrotizing fasciitis following percutaneous endoscopic gastrostomy. Gastrointest. Endosc. 32:294–296 [DOI] [PubMed] [Google Scholar]
- 288. Greif JM, Ragland JJ, Ochsner MG, Riding R. 1986. Fatal necrotizing fasciitis complicating percutaneous endoscopic gastrostomy. Gastrointest. Endosc. 32:292–294 [DOI] [PubMed] [Google Scholar]
- 289. Korula J, Rice HE. 1987. Necrotizing fasciitis and percutaneous endoscopic gastrostomy. Gastrointest. Endosc. 33:335–336 [DOI] [PubMed] [Google Scholar]
- 290. Abuksis G, Mor M, Segal N, Shemesh I, Plout S, Sulkes J, Fraser GM, Niv Y. 2000. Percutaneous endoscopic gastrostomy: high mortality rates in hospitalized patients. Am. J. Gastroenterol. 95:128–132 [DOI] [PubMed] [Google Scholar]
- 291. Ditesheim JA, Richards WO, Sharp K. 1989. Fatal and disastrous complications following percutaneous endoscopic gastrostomy. Am. Surg. 55:92–96 [PubMed] [Google Scholar]
- 292. Finucane P, Aslan SM, Duncan D. 1991. Percutaneous endoscopic gastrostomy in elderly patients. Postgrad. Med. J. 67:371–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Larson DE, Burton DD, Schroeder KW, DiMagno EP. 1987. Percutaneous endoscopic gastrostomy: indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology 93:48–52 [PubMed] [Google Scholar]
- 294. Miller RE, Castlemain B, Lacqua FJ, Kotler DP. 1989. Percutaneous endoscopic gastrostomy. Results in 316 patients and review of the literature. Surg. Endosc. 3:186–190 [DOI] [PubMed] [Google Scholar]
- 295. Pien EC, Hume KE, Pien FD. 1996. Gastrostomy tube infections in a community hospital. Am. J. Infect. Control 24:353–358 [DOI] [PubMed] [Google Scholar]
- 296. Christensen M, Matzen P, Schulze S, Rosenberg J. 2004. Complications of ERCP: a prospective study. Gastrointest. Endosc. 60:721–731 [DOI] [PubMed] [Google Scholar]
- 297. Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. 1996. Complications of endoscopic biliary sphincterotomy. N. Engl. J. Med. 335:909–918 [DOI] [PubMed] [Google Scholar]
- 298. Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. 1998. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest. Endosc. 48:1–10 [DOI] [PubMed] [Google Scholar]
- 299. Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A, Prada A, Passoni GR, Testoni PA. 2001. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am. J. Gastroenterol. 96:417–423 [DOI] [PubMed] [Google Scholar]
- 300. Cotton PB, Connor P, Rawls E, Romagnuolo J. 2008. Infection after ERCP and antibiotic prophylaxis: a sequential quality-improvement approach over 11 years. Gastrointest. Endosc. 67:471–475 [DOI] [PubMed] [Google Scholar]
- 301. Dutta S, Cox M, Williams RB, Eisenstat TE, Standiford HC. 1983. Prospective evaluation of the risk of bacteremia and the role of antibiotics in ERCP. J. Clin. Gastroenterol. 5:325–329 [DOI] [PubMed] [Google Scholar]
- 302. Kullman E, Borch K, Lindström E, Anséhn S, Ihse I, Anderberg B. 1992. Bacteremia following diagnostic and therapeutic ERCP. Gastrointest. Endosc. 38:444–449 [DOI] [PubMed] [Google Scholar]
- 303. Lam SK, Tsui JK, Chan PK, Wong KP, Ong GB. 1977. How often does bacteraemia occur following endoscopic retrograde cholangiopancreatography (ERCP)? Endoscopy 9:231–234 [DOI] [PubMed] [Google Scholar]
- 304. Siegel JH, Berger SA, Sable RA, Ho R, Rosenthal WS. 1979. Low incidence of bacteremia following endoscopic retrograde cholangiopancreatography (ERCP). Am. J. Gastroenterol. 71:465–468 [PubMed] [Google Scholar]
- 305. Anderson DJ, Shimpi RA, McDonald JR, Branch MS, Kanafani ZA, Harger J, Ely TM, Sexton DJ, Kaye KS. 2008. Infectious complications following endoscopic retrograde cholangiopancreatography: an automated surveillance system for detecting postprocedure bacteraemia. Am. J. Infect. Control 36:592–594 [DOI] [PubMed] [Google Scholar]
- 306. Leung JW, Ling TK, Chan RC, Cheung SW, Lai CW, Sung JJ, Chung SC, Cheng AF. 1994. Antibiotics, biliary sepsis, and bile duct stones. Gastrointest. Endosc. 40:716–721 [PubMed] [Google Scholar]
- 307. Low DE, Shoenut JP, Kennedy JK, Harding GK, Den Boer B, Micflikier AB. 1987. Risk of bacteremia with endoscopic sphincterotomy. Can. J. Surg. 30:421–423 [PubMed] [Google Scholar]
- 308. Palabiyikoglu I, Tekeli E, Aysev D, Sirlak M, Kaymakci S, Ozturk S. 2005. ERCP related sepsis. Am. J. Infect. Dis. 2:86–88 [Google Scholar]
- 309. Mollison LC, Desmond PV, Stockman KA, Andrew JH, Watson K, Shaw G, Breen K. 1994. A prospective study of septic complications of endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 9:55–59 [DOI] [PubMed] [Google Scholar]
- 310. Siegman-Igra Y, Spinradt S, Rattan J. 1988. Septic complications following endoscopic retrograde cholangiopancreatography: the experience in Tel Aviv Medical Center. J. Hosp. Infect. 12:7–12 [DOI] [PubMed] [Google Scholar]
- 311. Zimmon DS, Falkenstein DB, Riccobono C, Aaron B. 1975. Complications of endoscopic retrograde cholangiopancreatography: analysis of 300 consecutive cases. Gastroenterology 69:303–309 [PubMed] [Google Scholar]
- 312. Devière J, Motte S, Dumonceau JM, Serruys E, Thys JP, Cremer M. 1990. Septicemia after endoscopic retrograde cholangiopancreatography. Endoscopy 22:72–75 [DOI] [PubMed] [Google Scholar]
- 313. Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. 2007. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am. J. Gastroenterol. 102:1781–1788 [DOI] [PubMed] [Google Scholar]
- 314. Benchimol D, Bernard JL, Mouroux J, Dumas R, Elkaim D, Chazal M, Bourgeon A, Richelme H. 1992. Infectious complications of endoscopic retrograde cholangiopancreatography managed in a surgical unit. Int. Surg. 77:270–273 [PubMed] [Google Scholar]
- 315. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. 1991. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest. Endosc. 37:383–393 [DOI] [PubMed] [Google Scholar]
- 316. Mustard R, Jr, Mackenzie R, Jamieson C, Haber GB. 1984. Surgical complications of endoscopic sphincterotomy. Can. J. Surg. 27:215–217 [PubMed] [Google Scholar]
- 317. Safrany L. 1978. Endoscopic treatment of biliary-tract diseases. Lancet ii:983–985 [DOI] [PubMed] [Google Scholar]
- 318. Salminen P, Laine S, Gullichsen R. 2008. Severe and fatal complications after ERCP: analysis of 2555 procedures in a single experienced center. Surg. Endosc. 22:1965–1970 [DOI] [PubMed] [Google Scholar]
- 319. Vaira D, Ainley C, Williams S, Cairns S, Salmon P, Russell C, D'Anna L, Dowsett J, Baillie J, Croker J, Cotton P, Hatfield A. 1989. Endoscopic sphincterotomy in 1000 consecutive patients. Lancet ii:431–434 [DOI] [PubMed] [Google Scholar]
- 320. Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP, Jr, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, Van Dam J, Hughes M, Carr-Locke DL. 2002. Risk factors for complications after performance of ERCP. Gastrointest. Endosc. 56:652–656 [DOI] [PubMed] [Google Scholar]
- 321. Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS, Zhao Q, Shi RH, Tian ZB, Li YQ, Li W, Zhi FC. 2009. Risk factors for ERCP-related complications: a prospective multicenter study. Am. J. Gastroenterol. 104:31–40 [DOI] [PubMed] [Google Scholar]
- 322. Williams EJ, Taylor S, Fairclough P, Hamlyn A, Logan RF, Martin D, Riley SA, Veitch P, Wilkinson ML, Williamson PR, Lombard M. 2007. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy 39:793–801 [DOI] [PubMed] [Google Scholar]
- 323. Rerknimitr R, Fogel EL, Kalayci C, Esber E, Lehman GA, Sherman S. 2002. Microbiology of bile in patients with cholangitis or cholestasis with and without plastic biliary endoprosthesis. Gastrointest. Endosc. 56:885–889 [DOI] [PubMed] [Google Scholar]
- 324. Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. 2007. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin. Med. Res. 5:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Leung JW, Liu YL, Desta T, Libby E, Inciardi JF, Lam K. 1998. Is there a synergistic effect between mixed bacterial infection in biofilm formation on biliary stents? Gastrointest. Endosc. 48:250–257 [DOI] [PubMed] [Google Scholar]
- 326. Liguory C, Loriga P. 1978. Endoscopic sphincterotomy: analysis of 155 cases. Am. J. Surg. 136:609–613 [DOI] [PubMed] [Google Scholar]
- 327. Saito M, Tsuyuguchi T, Yamaguchi T, Ishihara T, Saisho H. 2000. Long-term outcome of endoscopic papillotomy for choledocholithiasis with cholecystolithiasis. Gastrointest. Endosc. 51:540–545 [DOI] [PubMed] [Google Scholar]
- 328. Davion T, Braillon A, Delamarre J, Delcenserie R, Joly JP, Capron JP. 1987. Pseudomonas aeruginosa liver abscesses following endoscopic retrograde cholangiography: report of a case without biliary tract disease. Dig. Dis. Sci. 32:1044–1046 [DOI] [PubMed] [Google Scholar]
- 329. Katsinelos P, Dimiropoulos S, Katsiba D, Arvaniti M, Tsolkas P, Galanis I, Papaziogas B, Limenopoulos V, Baltajiannis S, Vasilladis I. 2002. Pseudomonas aeruginosa liver abscesses after diagnostic endoscopic retrograde cholangiography in two patients with sphincter of Oddi dysfunction type 2. Surg. Endosc. 16:1638 doi:10.1007/s00464-002-4210-9 [DOI] [PubMed] [Google Scholar]
- 330. Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J, Faigel DO, Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy 2003. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 58:475–782 [DOI] [PubMed] [Google Scholar]
- 331. Sharma VK, Howden CW. 2000. Meta-analysis of randomized controlled trials of antibiotic prophylaxis before percutaneous endoscopic gastrostomy. Am. J. Gastroenterol. 95:3133–3136 [DOI] [PubMed] [Google Scholar]
- 332. ASGE Standards of Practice Committee, Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, Jagannath SB, Lichtenstein D, Fanelli RD, Lee K, van Guilder T, Stewart LE. 2008. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 67:791–798 [DOI] [PubMed] [Google Scholar]
- 333. Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS, Endoscopy Committee of the British Society of Gastroenterology 2009. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 58:869–880 [DOI] [PubMed] [Google Scholar]
- 334. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. 2010. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst. Rev. 2010:CD002907 doi:10.1002/14651858.CD002907.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Bai Y, Gao F, Gao J, Zou D-W, Li Z-S. 2009. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography induced cholangitis. A meta-analysis. Pancreas 38:126–130 [DOI] [PubMed] [Google Scholar]
- 336. Harris A, Chan C, Torres-Viera C, Hammett R, Carr-Locke D. 1999. Meta-analysis of antibiotic prophylaxis in endoscopic retrograde cholangiopancreatography. Endoscopy 31:718–724 [DOI] [PubMed] [Google Scholar]
- 337. Harding I, Sorgel F. 2000. Comparative pharmacokinetics of teicoplanin and vancomycin. J. Chemother. 12(Suppl 5):15–20 [DOI] [PubMed] [Google Scholar]
- 338. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association. A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 116:1736–1754 [DOI] [PubMed] [Google Scholar]
- 339. Haynes J, Greenstone MA. 1987. Fibreoptic bronchoscopy and the use of antibiotic prophylaxis. BMJ (Clin. Res. Ed.) 294:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Pedro-Botet ML, Ruiz J, Sabria M, Roig J, Abad J, Carrasco I, Manterolas JM. 1991. Bacteremia after fibrobronchoscopy. Prospective study. Enferm. Infecc. Microbiol. Clin. 9:159–161 [PubMed] [Google Scholar]
- 341. Sharif-Kashani B, Shahabi P, Behzadnia N, Mohammad-Taheri Z, Mansouri D, Masjedi MR, Zargari L, Salimi Negad L. 2010. Incidence of fever and bacteriemia following flexible fiberoptic bronchoscopy: a prospective study. Acta Med. Iran. 48:385–388 [PubMed] [Google Scholar]
- 342. Vasanthakumar V, Bhan GL, Perera BS, Taft P. 1990. A study to assess the efficacy of chemoprophylaxis in the prevention of endoscopy-related bacteraemia in patients aged 60 and over. Q. J. Med. 75:647–653 [PubMed] [Google Scholar]
- 343. Witte MC, Opal SM, Gilbert JG, Pluss JL, Thomas DA, Olsen JD, Perry ME. 1986. Incidence of fever and bacteremia following transbronchial needle aspiration. Chest 89:85–87 [DOI] [PubMed] [Google Scholar]
- 344. de Blic J, Marchac V, Scheinmann P. 2002. Complications of flexible bronchoscopy in children: prospective study of 1,328 procedures. Eur. Respir. J. 20:1271–1276 [DOI] [PubMed] [Google Scholar]
- 345. Park JS, Lee CH, Yim JJ, Yang SC, Yoo CG, Chung HS, Kim YW, Han SK, Shim YS, Kim DK. 2011. Impact of antibiotic prophylaxis on postbronchoscopy fever: a randomised controlled study. Int. J. Tuberc. Lung Dis. 15:528–535 [DOI] [PubMed] [Google Scholar]
- 346. Pereira W, Kovnat DM, Khan MA, Iacovino JR, Spivack ML, Snider GL. 1975. Fever and pneumonia after flexible fiberoptic bronchoscopy. Am. Rev. Respir. Dis. 112:59–64 [DOI] [PubMed] [Google Scholar]
- 347. Pereira W, Jr, Kovnat DM, Snider G. 1978. A prospective cooperative study of complications following flexible fiberoptic bronchoscopy. Chest 73:813–816 [DOI] [PubMed] [Google Scholar]
- 348. Rennard SI, Aalbers R, Bleecker E, Klech H, Rosenwasser L, Olivieri D, Sibille Y. 1998. Bronchoalveolar lavage: performance, sampling procedure, processing and assessment. Eur. Respir. J. 26:13S–15S [PubMed] [Google Scholar]
- 349. Sinha S, Guleria R, Pande JN, Pandey RM. 2004. Bronchoscopy in adults at a tertiaty centre: indications and complications. J. Indian Med. Assoc. 102:152–153 [PubMed] [Google Scholar]
- 350. Krause A, Hohberg B, Heine F, John M, Burmester GR, Witt C. 1997. Cytokines derived from alveolar macrophages induce fever after bronchoscopy and bronchoalveolar lavage. Am. J. Respir. Crit. Care Med. 155:1793–1797 [DOI] [PubMed] [Google Scholar]
- 351. Aelony Y, Finegold SM. 1979. Serious infectious complications after flexible fibreoptic bronchoscopy. West. J. Med. 131:327–333 [PMC free article] [PubMed] [Google Scholar]
- 352. Alexander WJ, Baker GL, Hunker FD. 1979. Bacteremia and meningitis following fiberoptic bronchoscopy. Arch. Intern. Med. 139:580–583 [PubMed] [Google Scholar]
- 353. Beyt BE, Jr, King DK, Glew RH. 1977. Fatal pneumonitis and septicemia after fiberoptic bronchoscopy. Chest 72:105–107 [DOI] [PubMed] [Google Scholar]
- 354. de Fijter JW, van der Hoeven JG, Eggelmeijer F, Meinders AE. 1993. Sepsis syndrome and death after bronchoalveolar lavage. Chest 104:1296–1297 [DOI] [PubMed] [Google Scholar]
- 355. Gillis S, Dann EJ, Berkman N, Koganox Y, Kramer MR. 1993. Fatal Haemophilus influenzae septicemia following bronchoscopy in a splenectomized patient. Chest 104:1607–1609 [DOI] [PubMed] [Google Scholar]
- 356. British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of British Thoracic Society 2001. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 56(Suppl 1):i1–i21 doi:10.1136/thorax.56.suppl_1.i1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, Gewitz MH, Shulman ST, Nouri S, Newburger JW, Hutto C, Pallasch TJ, Gage TW, Levison ME, Peter G, Zuccaro G., Jr 1997. Prevention of bacterial endocarditis. Recommendation by the American Heart Association. JAMA 277:1794–1801 [PubMed] [Google Scholar]
- 358. Jurado RL, Klein S. 1998. Infective endocarditis associated with fibreoptic bronchoscopy in a patient with mitral valve prolapse. Clin. Infect. Dis. 26:768–769 [DOI] [PubMed] [Google Scholar]
- 359. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Chicurel M. 2000. Bacterial biofilms and infections. Slimebusters. Nature 408:284–286 [DOI] [PubMed] [Google Scholar]
- 361. O'Toole G, Kaplan HB, Kolter R. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49–79 [DOI] [PubMed] [Google Scholar]
- 362. Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. 2002. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 40:1244–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 364. Patel R. 2005. Biofilms and antimicrobial resistance. Clin. Orthop. Relat. Res. 437:41–47 [DOI] [PubMed] [Google Scholar]
- 365. Trautner BW, Darouiche RO. 2004. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 164:842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298 [DOI] [PubMed] [Google Scholar]
- 367. Van Delden C, Iglewski BH. 1998. Cell-to-cell signalling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Vickery K, Pajkos A, Cossart Y. 2004. Removal of biofilm from endoscopes: evaluation of detergent efficiency. Am. J. Infect. Control 32:170–176 [DOI] [PubMed] [Google Scholar]
- 369. Hervé R, Keevil CW. 2013. Current limitations about the cleaning of luminal endoscopes. J. Hosp. Infect. 83:22–29 [DOI] [PubMed] [Google Scholar]
- 370. Fang Y, Shen Z, Li L, Cao Y, Gu LY, Gu Q, Zhong XQ, Yu CH, Li YM. 2010. A study of the efficacy of bacterial biofilm cleanout for gastrointestinal endoscopes. World J. Gastroenterol. 16:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Schmidt V, ESGE Guidelines Committee 2007. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy 39:175–181 [DOI] [PubMed] [Google Scholar]
- 372. Gastroenterological Society of Australia, Gastroenterological Nurses College of Australia 2010. Infection control in endoscopy, 3rd ed Gastroenterological Society of Australia, Sydney, Australia: http://www.gesa.org.au/files/editor_upload/File/Professional/Endoscopy_infection_control%20%28low%29.pdf [Google Scholar]
- 373. Systchenko R, Marchetti B, Canard JN, Palazzo L, Ponchon T, Rey JF, Sautereau D, French Society of Digestive Endoscopy 2000. Guidelines of the French Society of Digestive Endoscopy: recommendations for setting up cleaning and disinfection procedures in gastrointestinal endoscopy. Endoscopy 32:807–818 [DOI] [PubMed] [Google Scholar]
- 374. Moses FM, Lee J. 2003. Surveillance cultures to monitor quality of gastrointestinal endoscope reprocessing. Am. J. Gastroenterol. 98:77–81 [DOI] [PubMed] [Google Scholar]
- 375. Bond WW, Sehulster L. 2004. Microbiological assay of environmental and medical-device surfaces, p 13.10.1–13.10.12 In Isenberg HD. (ed), Clinical microbiology procedures handbook, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 376. Obee PC, Griffith CJ, Cooper RA, Cooke RP, Bennion NE, Lewis M. 2005. Real-time monitoring in managing the decontamination of flexible gastrointestinal endoscopes. Am. J. Infect. Control 33:202–206 [DOI] [PubMed] [Google Scholar]
- 377. Sciortino CV, Jr, Xia EL, Mozee A. 2004. Assessment of a novel approach to evaluate the outcome of endoscope reprocessing. Infect. Control Hosp. Epidemiol. 25:284–290 [DOI] [PubMed] [Google Scholar]
- 378. Colt HG, Beamis JJ, Harrell JH, Mathur PM. 2000. Novel flexible bronchoscope and single-use disposable-sheath endoscope system. A preliminary technology evaluation. Chest 118:183–187 [DOI] [PubMed] [Google Scholar]
- 379. Gillespie EE, Kotsanas D, Stuart RL. 2008. Microbiological monitoring of endoscopes: 5-year review. J. Gastroenterol. Hepatol. 23:1069–1074 [DOI] [PubMed] [Google Scholar]