Abstract
Johne's disease (JD) is prevalent worldwide and has a significant impact on the global agricultural economy. In the present study, we evaluated the protective efficacy of a leuD (Δleud) mutant and gained insight into differential immune responses after challenge with virulent M. avium subsp. paratuberculosis in a caprine colonization model. The immune response and protective efficacy were compared with those of the killed vaccine Mycopar. In vitro stimulation of peripheral blood mononuclear cells with johnin purified protein derivative showed that Mycopar and ΔleuD generated similar levels of gamma interferon (IFN-γ) but significantly higher levels than unvaccinated and challenged phosphate-buffered saline controls. However, only with ΔleuD was the IFN-γ response maintained. Flow cytometric analysis showed that the increase in IFN-γ correlated with proliferation and activation (increased expression of CD25) of CD4, CD8, and γδT cells, but this response was significantly higher in ΔleuD-vaccinated animals at some time points after challenge. Both Mycopar and ΔleuD vaccines upregulated Th1/proinflammatory and Th17 cytokines and downregulated Th2/anti-inflammatory and regulatory cytokines at similar levels at almost all time points. However, significantly higher levels of IFN-γ (at weeks 26 and 30), interleukin-2 (IL-2; week 18), IL-1b (weeks 14 and 22), IL-17 (weeks 18 and 22), and IL-23 (week 18) and a significantly lower level of IL-10 (weeks 14 and 18) and transforming growth factor β (week 18) were detected in the ΔleuD-vaccinated group. Most importantly, ΔleuD elicited an immune response that significantly limited colonization of tissues compared to Mycopar upon challenge with wild-type M. avium subsp. paratuberculosis. In conclusion, the ΔleuD mutant is a promising vaccine candidate for development of a live attenuated vaccine for JD in ruminants.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis is the causative agent of Johne's disease (JD) in cattle, sheep, goats, and other ruminant species worldwide (1, 2). The infection causes chronic untreatable granulomatous enteritis, with symptoms that include poor nutrient uptake, severe diarrhea, emaciation, and eventually death of the infected host (3). According to a recent report from the National Animal Health Monitoring System, the prevalence in U.S dairy herds is estimated to be 68% and costs the dairy industry approximately $250 million annually (4). In the United States, huge economic losses result from early culling or death, reduced reproductive and feed efficiency, and decreased milk production (4). M. avium subsp. paratuberculosis is also implicated in the pathogenesis of Crohn's disease, an inflammatory bowel disease in humans, as this bacterium has been isolated from both adults and children with the disease (5–7). M. avium subsp. paratuberculosis has also been implicated as a trigger for type 1 diabetes and ulcerative colitis (8–10). JD is controlled by vaccination of animals with whole killed M. avium subsp. paratuberculosis (e.g., Gudiar, CSL) (11, 12) in other countries. Mycopar, another killed vaccine, is available in the United States but is not currently used to control JD. These vaccines provide inadequate protection and induce a severe local inflammatory reaction at the site of injection. More importantly, they do not prevent infection or shedding of M. avium subsp. paratuberculosis in the feces (11, 13). Additionally, the immune responses generated by these vaccines interfere with tests to identify Mycobacterium tuberculosis- or Mycobacterium bovis-infected animals (14–16). These limitations highlight the need for the development of an improved vaccine for JD.
Attempts have been made to identify protective antigens and use them as subunit vaccines. Although vaccines based on these subunit antigens are effective, they are expensive and require strong adjuvants, which cause toxicity and local inflammatory reactions. Moreover, these vaccines fail to provide complete protection, and infected animals shed M. avium subsp. paratuberculosis in their feces. Because of the limitations associated with currently available subunit vaccines, there has been increased interest in alternative strategies, such as creation of genetically attenuated mutants for evaluation as vaccines for JD. Several attempts have been made to successfully produce mutant strains of M. avium subsp. paratuberculosis with vaccine potential by exploiting transposon mutagenesis and allelic exchange (17–19). The latter technology affords a method to select genes associated with virulence or function for gene disruption. Use of allelic exchange has thus far yielded one mutant with a disrupted relA gene (ΔrelA) that meets two important criteria for a live vaccine: it elicits an immune response that clears the infection with the mutant and limits colonization by wild-type M. avium subsp. paratuberculosis following challenge (20). Further studies are now needed to determine if disruption of other genes will yield similar results or an immune response that prevents establishment of infection. Further studies are also needed to determine how the immune response differs from the response elicited by wild-type M. avium subsp. paratuberculosis. The leuD gene encodes an isopropyl malate isomerase, an essential enzyme for leucine biosynthesis. leuD is involved in an oxidative stress response, a part of the PhoPR system in M. tuberculosis, and is a component of the codY regulon in Listeria monocytogenes (21–23). A mutant created by deletion of the leuD gene (GeneID 2717943) using allelic exchange was found to be protective in both mice and cattle against challenge with virulent M. tuberculosis or M. bovis (24, 25). In our previous study, we created a leuD mutant (ΔleuD) and demonstrated its protective potential against M. avium subsp. paratuberculosis challenge in a mouse model (26, 27). In the present study, we compared the immune response and protective efficacy of the mutant with the immune response to Mycopar in one of the natural hosts (goat challenge model).
MATERIALS AND METHODS
Animals.
A total of 18 castrated male or female Boer goats mixed with dairy goats, between 8 to 9 weeks old, were obtained from a local farm and used in this study. The goats were housed collectively in groups of 6 animals. Fecal samples taken from the goats before the immunization experiments were negative for M. avium subsp. paratuberculosis and other pathogens, both by culture and by PCR for the IS900 gene. The caudal fold tuberculin test (CFT) was performed pre- and postvaccination. Briefly, the goats were injected intradermally with 0.1 ml of tuberculin purified protein derivative (PPD; National Veterinary Services Laboratory, Ames, IA) 4 days before vaccination, 4 days before challenge, and 1 week before euthanasia. Skin fold thickness was measured at the injection site with a caliper before injection and 72 h after injection. Results are expressed as the increase (in millimeters) of skin thickness to determine a positive or negative reaction. All the goats were negative on the intradermal skin test (IDT) at all time points. All of the experimental work was conducted in compliance with the regulations, policies, and principles of the Animal Welfare Act, the Public Health Service Policy on Humane Care and Use of Laboratory Animals Used in Testing, Research, and Training, the National Research Council's Guide for the Care and Use of Laboratory Animals (54), and the New York State Department of Public Health.
Bacterial strains.
M. avium subsp. paratuberculosis 66115-98, a clinical isolate, was used to challenge the goats after immunization (28, 29). This strain is IS900 positive and mycobactin dependent. The bacterium was grown in 7H9 medium supplemented with 10% oleic acid–albumin–dextrose–catalase (Becton, Dickinson Co., Sparks, MD) and mycobactin J (Allied Monitor, Inc., Fayette, MO). After culturing for 8 weeks, the organisms were harvested by centrifugation at 4,000 × g for 10 min and washed twice with phosphate-buffered saline (PBS; 10 mM; pH 7.2). The organisms were diluted in PBS to the required concentration and used to challenge the goats. Mycopar (heat-killed M. avium subsp. paratuberculosis bacteria with oil adjuvant) was obtained from Boehringer Ingelheim Vetmedica, Inc., and injected with the dose recommended by the manufacturer.
Construction of the leuD mutant.
The ΔleuD mutant was constructed from strain K-10 by allelic exchange using methods described previously (20, 26). The primer pairs UF primer (5′-CTGAGATCTTCAAGACGATGGCGGTCAACGTCGAC-3′)/UR primer (5′-CTACTCGAGCTCATCCCTTCACGGTCGAATACGTC-3′) and DF primer (5′-GACTCTAGAAGCGACGTATCCCGATTGGAAACCG-3′)/DR primer (5′-GTCGGTACCAGGACGTGCTCTGCCTACTTGCGG-3′) were designed using the M. avium subsp. paratuberculosis K-10 genome sequence database to amplify a 939-bp upstream fragment and a 910-bp downstream fragment from K-10 genomic DNA. After digesting the upstream fragment of the leuD gene with BglII and XhoI and the downstream fragment with XbaI and KpnI, both fragments were cloned into pYUB854 on either side of the HygB resistance (Hygr) cassette to generate the allelic exchange substrate (AES). The pYUB854 plasmid containing the AES was digested with PacI and ligated with plasmid phAE87. The resulting plasmid was packaged with in vitro λ packaging extract (Gigapack III-XL; Stratagene) and incubated with Escherichia coli HB101 on a low-salt LB agar plate containing 100 μg/ml of HygB. The pooled phAE87-AES plasmid DNA was prepared from Hygr colonies and electroporated into Mycobacterium smegmatis mc2 155 to generate the phage particles. After incubation at the permissive temperature (30°C) for 3 days, several plaques were picked up for amplification on a 7H10 plate. The high-titer transducing mycobacteriophage was prepared by washing the amplified plaques with MP buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 10 mM MgSO4, 2 mM CaCl2). M. avium subsp. paratuberculosis K-10 was cultured in 40 ml of 7H9 broth medium containing Tween 80 in a T75 tissue culture flask until the optical density at 600 nm (OD600) was 0.6 to 0.8. The culture was removed to a 50-ml tube and allowed to stand for 10 min to allow large clumps of bacteria to settle out by gravity. Thirty-five milliliters of the top layer of the culture was then removed into a new 50-ml tube and centrifuged at 1,500 × g for 10 min. The bacterial pellet was resuspended in an equal volume of MP buffer and centrifuged again to remove residual Tween 80. The pellet was resuspended carefully in 1/10 of the original volume in MP buffer. Equal volumes of high-titer mycobacteriophage stock and bacterial cells were mixed in a 2-ml screw-cap tubes and incubated at 37°C for 4 to 6 h. The mixture was added to 2 ml of 7H9 broth medium containing Casitone (BD), and cultured at 37°C for an additional 48 h for recovery, and the cells were then harvested by centrifugation at 2,000 × g for 10 min. The pellet was resuspended with 1 ml 7H9 medium, and each 200 μl of the resuspended culture was then plated on 7H10 medium with 75 μg/ml HygB. After 6 weeks of incubation, 20 colonies were selected for analysis; the genomic DNA of colonies containing the Hyg resistance cassette was prepared for PCR and DNA sequencing in order to confirm allelic exchange.
Immunization.
The goats were divided into three groups of 6 animals, group A (ΔleuD), B (PBS), and C (Mycopar) (Table 1), and immunized, bled, or challenged according to the schedule presented in Table 2. The animals were immunized subcutaneously (s.c.) in the lower left side of the neck. Three weeks after the primary immunization, the goats were boosted with the same regimen. All the animals were euthanized 6 months postchallenge. One goat in the Mycopar group was euthanized due to sickness unrelated to this study.
Table 1.
Study treatment groups
| Vaccine | No. of goats | Goat ID no. | Dose | Route |
|---|---|---|---|---|
| ΔleuD mutant | 6 | 2152, 2154, 2156, 2158, 2161, 2163 | 5 × 108 CFU in 1 ml PBS | s.c. |
| PBS | 6 | 2194, 2198, 2199, 2200, 2201, 2202 | 1 ml PBS | s.c. |
| Mycopar | 6 | 2206, 2207, 2214, 2217, 2218, 2221 | 0.5 ml | s.c. |
Table 2.
Schedule for vaccination and challenge of goats
| Time post-primary vaccination (wks) | Procedures conducted |
|---|---|
| 0 | Vaccination, bleeding (10 ml, jugular vein), feces collection (10 g) |
| 3 | Booster vaccination, bleeding (10 ml, jugular vein), feces collection (10 g) |
| 6 | Challenge with 5 × 108 CFU orally (7 days), bleeding (10 ml), feces collection (10 g) |
| 10 | Bleeding (10 ml), feces collection (10 g) |
| 14 | Bleeding (10 ml), feces collection (10 g) |
| 18 | Bleeding (10 ml), feces collection (10 g) |
| 22 | Bleeding (10 ml), feces collection (10 g) |
| 26 | Bleeding (10 ml), feces collection (10 g) |
| 30 | Bleeding (10 ml), feces collection (10 g), necropsy |
Challenge.
Three weeks after the booster, all 18 goats were challenged orally with 5 × 108 CFU of M. avium subsp. paratuberculosis 66115-98 in 10 ml PBS for 7 consecutive days. Fecal cultures were prepared from each animal on days 2, 4, and 6 after each challenge and then once every month.
Antibody response.
Sera were harvested from blood collected at the indicated time points, and an indirect enzyme-linked immunosorbent assay (ELISA) was performed to detect antibodies, as previously described (30). Briefly, 96-well flat-bottom plates (Nunc Maxisorp) were coated with 100 μl johnin purified protein derivative (PPDj; 10 μg/ml) and kept at 4°C overnight in a humidified atmosphere. The plates were washed three times with PBS containing 0.05% Tween 20 (PBST) and blocked with 5% skim milk in PBST at 37°C for 1 h. After washing with PBST, 100 μl serum diluted 1:200 was added to the wells. The plates were incubated at 37°C for 2 h. After washing, 100 μl anti-goat IgG conjugated with horseradish peroxidase (1:3,000) was added, and plates were further incubated at 37°C for 45 min to 1 h. The plates were washed three times in PBST, 100 μl of tetramethylbenzidine substrate was added to each well, and plates were incubated in the dark at room temperature for 20 min. The enzymatic reaction was stopped by the addition of 1 M H2SO4, and the optical density was read at 450 nm by using an ELX 808 Ultra microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Suitable positive and negative sera and antigen and antibody controls were included in each plate. The results are expressed in ELISA units, calculated as follows: (sample OD − negative control OD) × 100.
Isolation and culturing of PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from the experimental goats as described previously (31). Briefly, 10 to 15 ml of peripheral blood was collected from the jugular vein into EDTA Vacutainer tubes (Becton, Dickinson and Co., Franklin Lakes, NJ). Blood was centrifuged, and after removing the buffy coat, lymphocytes were isolated by differential centrifugation using Histopaque 1.077 (Sigma-Aldrich, St. Louis, MO). The mononuclear cells were washed three times with PBS (pH 7.2). Washed cell pellets were resuspended in PBS and counted after staining with 0.4% trypan blue for viability determination. The lymphocytes were resuspended in RPMI 1640 medium containing 10% fetal bovine serum (Gibco, Grand Island, NY), 2 mM l-glutamine, 100 mM HEPES, 100 IU/ml of penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of gentamicin (Gibco) to a final concentration of 2 × 106 viable cells/ml. The cells were then seeded (200 μl/well) onto 96-well round- or flat-bottom plates, depending on the type of experiment.
Lymphoproliferation.
Lymphocyte proliferation assays were performed as previously described (31). Briefly, 2 × 105 PBMCs in 96-well flat-bottom plates were stimulated with 10 μg/ml johnin purified protein derivative (PPDj; DBL, National Veterinary Services Laboratory, Ames, IA) for 72 h. DNA synthesis in stimulated and unstimulated control cells was measured based on the incorporation of bromodeoxyuridine (BrdU) by using a cell proliferation ELISA and BrdU colorimetric kit (Roche Diagnostics, Indianapolis, IN) as per the manufacturer's protocol. Briefly, the cells were labeled for 2 h with 10 μl of BrdU labeling solution. The peroxidase-conjugated anti-BrdU antibody was added, and the mixture was incubated for 90 min. This was followed by the addition of the enzyme substrate solution and incubation at room temperature for 15 min. The enzymatic reaction was stopped by the addition of 1 M H2SO4, and the OD was read at 450 nm by using an ELX 808 Ultra microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). The tests were run in triplicate, and the results are expressed as the average stimulation index (SI), calculated as the ratio between the mean OD of cells cultured with the PPDj and the mean OD of cells cultured without PPDj.
Cytokine analysis by real-time PCR.
To compare the expression levels of selected immune response genes between PBMCs isolated from goats at various time points, 107 PBMCs in 6-well flat-bottom plates were stimulated with 10 μg/ml PPDj as described previously (20, 32). After 3 days of incubation, total RNA was extracted from the pooled PBMCs of both stimulated and unstimulated wells. Total RNA (3 μg) was reverse transcribed using oligo(dT) primers and the Superscript III first-strand synthesis system (Invitrogen) according to the manufacturer's specifications. The selected immune response genes, forward and reverse primers, product lengths, and GenBank accession numbers of the sequences used to design the primers are listed in Table 3. All primer pairs were designed to target areas with a minimal secondary structure, to work at an annealing temperature of 60°C, and where feasible, to span two exons. Real-time PCR (RT-PCR) was performed on an ABI 7500 Fast sequence detection system (Applied Biosystems) by using Power SYBR green master mix (Invitrogen) in a 20-μl reaction volume. Primers were used at a final concentration of 200 nM. Reactions were performed in 96-well MicroAmp Fast optical plates (Applied Biosystems) sealed with optical adhesive covers (Applied Biosystems). Thermal cycling conditions consisted of enzyme activation at 95°C for 15 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing and extension at 60°C for 60 s. No-template controls were included for each target on each plate (data not shown). Post-PCR dissociation melting curves were determined for every reaction mixture to confirm the specificity and melting temperature of the amplification products (data not shown). The resulting data were analyzed by the 2−ΔΔCT method, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as internal control and unstimulated sample as calibrator with 7500 Fast System SDS software version 1.4 (Applied Biosystems) (33).
Table 3.
Primers used for cytokine analysis
| Gene | Primer | Sequence (5′–3′) | Amplicon (bp) | Gene accession no. | |
|---|---|---|---|---|---|
| IL-2 | F | TGAAAGAAGTGAAGTCATTGCTGC | 138 | NM_001009806 | |
| R | GATGTTTCAATTCTGTAGCGTTAACC | ||||
| IL-4 | F | ACCTGTTCTGTGAATGAAGCCAA | 79 | NM_001009313 | |
| R | CCCTCATAATAGTCTTTAGCCTTTCC | ||||
| IL-6 | F | CGCTCCCATGATTGTGGTAGTT | 64 | NM_001009392 | |
| R | GCCCAGTGGACAGGTTTCTG | ||||
| IL-8 | F | CGAAAAGTGGGTGCAGAAGGT | 80 | NM_001009401 | |
| R | GGTTGTTTTTTCTTTTTCATGGA | ||||
| IL-10 | F | AGCAAGGCGGTGGAGCAG | 90 | NM_001009327 | |
| R | GATGAAGATGTCAAACTCACTCATGG | ||||
| IL-12 | F | GCTGGGAGTACCCTGACACG | 127 | NM_001009438 | |
| R | GTGACTTTGGCTGAGGTTTGGTC | ||||
| IL-13 | F | CAGTGTCATCCACAGGACCAAG | 90 | NM_001082594 | |
| R | TCTCGGACGTACTCACTGGAAAC | ||||
| IL-17 | F | CATCATCCCACAGAGTCCAGG | 201 | AF412040 | |
| R | CACTTGGCCTCCCAGATCAC | ||||
| IL-18 | F | ACTGTTCAGATAATGCACCCCAG | 100 | NM_001009263 | |
| R | TTCTTACACTGCACAGAGATGGTTAC | ||||
| IL-23 | F | CCTCCTTCTCCGTCTCAAGATC | 131 | XM_588269 | |
| R | CGGAGGTCTGGGTGTCATCCT | ||||
| IL-1b | F | CCTAACTGGTACATCAGCACTTCTCA | 95 | NM_001009465 | |
| R | TCCATTCTGAAGTCAGCACTTCTCA | ||||
| IFN-γ | F | GATAACCAGGTCATTCAAAGGAGC | 124 | NM_001009803 | |
| R | GATCATCCACCGGAATTTGAATC | ||||
| TNF-α | F | GCCCTGGTACGAACCCATCTA | 82 | NM_001024860 | |
| R | CGGCAGGTTGATCTCAGCAC | ||||
| TGF-β | F | CTGAGCCAGAGGCGGACTAC | 63 | NM_001009400 | |
| R | TGCCGTATTCCACCATTAGCA | ||||
| GAPDH | F | GAGAAGGCTGGGGCTCACC | 129 | AF030943 | |
| R | GCTGACAATCTTGAGGGTATTGTT |
IFN-γ assay.
A total of 2 × 105 PBMCs in each well of 96-well flat-bottom plates were stimulated with 10 μg/ml of PPDj for 72 h. Gamma interferon (IFN-γ) levels were measured in the culture supernatants by using a monoclonal antibody-based sandwich enzyme immunoassay (Bovigam; Biocor Animal Health, Omaha, NE), as per the manufacturer's instructions (31). The optical densities for plates were read at 450 nm using an ELx 808 Ultra microplate reader (Bio-Tek Instruments, Inc.). Results were considered positive (OD > positive control) or negative (OD < positive control) relative to the cutoff values suggested by the manufacturer and expressed as the mean OD in stimulated wells minus the OD in unstimulated wells.
FC analysis.
Flow cytometric (FC) analysis of PBMCs isolated from animals bled at different time points was performed as described previously (20). PBMCs (107) from the different groups were cultured for 6 days with or without PPDj (10 μg/ml) in 6-well tissue culture plates. Cells were recovered, and 106 cells were labeled with goat-specific monoclonal antibodies CD2-MUC2A-IgG2a, CD4-GC1A1-IgG2a, CD8-7C2B-IgG2a, CD25-CACT116A-IgG1, CD45R0-ILA116A-IgG3, and γδTCR-GB21A-IgG2b (Washington State University, Monoclonal Antibody Center, Pullman, WA) as previously described. The cells were stained with a three-color combination as CD4/CD25/CD45R0, CD8/CD25/CD45R0, or CD2/CD25/γδ T cells to analyze the activation status of memory CD4 and CD8 and γδ T cells. Briefly, the cells were washed three times with FC buffer (20% acid citrate-dextrose, 4% horse serum) and incubated with a cocktail of the primary antibodies (at 15 μg/ml [previously titrated for optimum reactivity]) for 15 min at 4°C. The lymphocytes were then washed three times and incubated for an additional 15 min at 4°C in cocktails of isotype-specific secondary antibodies: phycoerythrin (PE)-Cy5.5/PE-Cy5/fluorescein isothiocyanate for analyzing CD4/CD25 or CD4/CD45R0 T cells and CD8/CD25 or CD8/CD45R0 T cells and PE-Cy5.5/PE-Cy5/PE for analyzing CD2/CD25/γδ T cells (Invitrogen; Southern Biotechnology Associates, Birmingham, AL). Cells were washed three times and suspended in 200 μl of FC buffer. A total of 50,000 events were acquired on a BD LSR II apparatus housed in the Biomedical Sciences Flow Cytometer Core Laboratory at Cornell University. All the data were analyzed by using BD fluorescence-activated cell sorter (FACS) Diva software. The percentages of activated (CD25+) and memory phenotype (CD45R0+) CD4 and CD8 T cells in the total PBMC pool were determined by using electronic gates to isolate CD4 and CD8 populations for analysis.
Necropsy.
All animals were euthanized using a captive bolt stun gun. After exsanguination, the intestines were removed from below the abomasum through to the rectum. The small intestines were laid out to expose the jejunum, ileum, cecum, and lymph nodes. Samples were taken from serial sections of the mesenteric lymph nodes (MLN), the ileocecal lymph node (ICLN), descending duodenum (Dd), proximal, middle and distal portions of the jejunum (JP, JM, and JD, respectively), proximal, middle, and distal portions of the ileum (IP, IM, and ID, respectively), ileocecal orifice (ICO), and cecum (C).
Fecal and organ culture of M. avium subsp. paratuberculosis and M. avium subsp. paratuberculosis PCR.
Following challenge, attempts were made to isolate M. avium subsp. paratuberculosis organisms from feces by using Herald's egg yolk (HEY) medium (Becton, Dickinson and Co., Sparks, MD) following standard Cornell University protocols (34, 35). Fecal samples were collected from all animals at 2, 4, 6, 8, and 10 days after challenge and every month thereafter for M. avium subsp. paratuberculosis isolation. Similarly, 9 tissue samples collected from each of the 17 animals (goat 2214 died) at necropsy were also tested for M. avium subsp. paratuberculosis by culture. Tissues were homogenized separately in 10 ml of PBS in a stomacher for 5 min. Aliquots of the homogenate were removed for PCR analysis and for culture. Cultures were performed by the Bacteriology Section at the Cornell Animal Health Diagnostic Center on HEY slants containing Mycobactin J. PCR was performed by the Molecular Diagnostic Section at the Cornell Animal Health Diagnostic Center. DNA was extracted from glass bead-disrupted lysates by using an automated nucleic acid, magnetic bead-based 96-well purification system (Kingfisher 96; Thermo Fisher Scientific Inc., Pittsburg, PA), and DNA was amplified using a commercial assay (M. avium subsp. paratuberculosis reagents; Life Technologies, Grand Island, NY). All investigators involved in the M. avium subsp. paratuberculosis testing were blinded to the treatment group.
Gross pathology and histopathological examination.
All the goats were euthanized 30 weeks after primary vaccination and necropsied. A total of 9 tissue samples from each animal, which included mesenteric lymph nodes (3 sites), the ileocecal lymph node, descending duodenum, jejunum (three sites of approximately equal intervals from the proximal to distal end), ileum (two sites at the proximal end, two sites mid-ileum, and two sites at the distal end), ileocecal orifice, and cecum, were collected at the time of necropsy. Collected tissues were fixed by immersion in 10% neutral buffered formalin, embedded in paraffin wax, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E) and Ziehl-Neelsen for acid-fast bacteria, using conventional histological methods. Sections were examined by a board-certified veterinary pathologist (S. P. McDonough), who was blinded to the treatment group.
Statistical analyses.
The data were statistically analyzed using Excel software. Differences between groups were analyzed with a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison or Student t test. The numbers of M. avium subsp. paratuberculosis culture-positive animals between groups were compared with Fisher's exact test. In all tests, differences were considered significant when a probability value of <0.05 was obtained.
RESULTS
Humoral immune response.
Serum samples collected from goats at different time points were analyzed for antibodies against PPDj by using an indirect ELISA. Low levels of antibodies were detected in some animals at 3 weeks postimmunization in both the ΔleuD and Mycopar groups (Fig. 1). The antibody level was enhanced after booster vaccinations. The response varied among animals within a group. A few animals showed a steep rise in antibody response after challenge in both the ΔleuD and Mycopar groups, which was equivalent to the control group, except for goats 2154 and 2217, which generated significantly higher antibody levels. Although similar antibody levels were generated by Mycopar and leuD mutant vaccination 2 months after challenge, this level was only maintained long term by Mycopar, as there was a decline in antibody levels in the leuD mutant vaccine-immunized goats. In all groups, some animals did not generate a significant level of antibody at any time point (Fig. 1).
Fig 1.
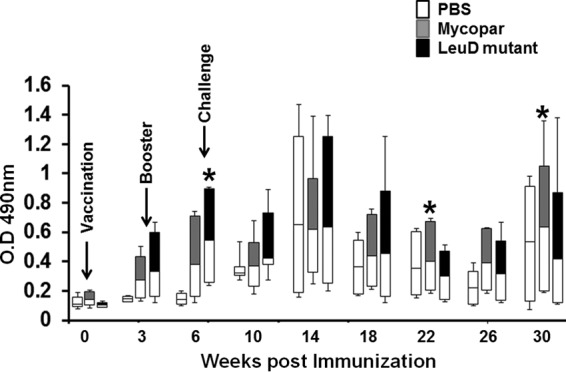
Antibody response. Sera isolated from blood collected at different time points were diluted (1:200) and analyzed for PPDj-specific IgG antibodies by indirect ELISA, as described in Material and Methods. The response was measured in individual goats, and data are presented in the form of whisker-box plots. The white box indicates the lower quartile (25% of data greater than this value), the shaded box indicates the upper quartile (25% of data less than this value), the middle line is the median (50% of data greater than this value), and error bars indicate the minimum and maximum values. *, P < 0.05, ΔleuD versus Mycopar.
IFN-γ response.
PBMCs from ΔleuD- or Mycopar-vaccinated goats generated considerable levels of IFN-γ, which was enhanced after the booster (Fig. 2). The response was further enhanced after challenge and was significantly higher than in the control group (P < 0.05). Although there was no significant difference in induction of IFN-γ by either the leuD mutant or Mycopar at the initial time points (except at 14 weeks, when Mycopar generated substantially higher levels of IFN-γ), this response was only maintained by the ΔleuD-vaccinated group and was significantly higher than for the Mycopar group at week 26 (P < 0.02) and week 30 (P < 0.05) (Fig. 2).
Fig 2.
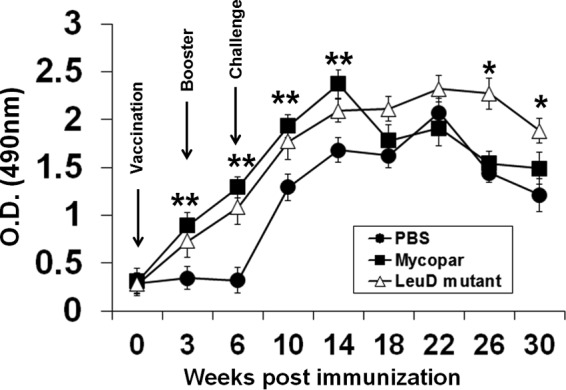
IFN-γ response. PBMCs isolated from animals bled at different time points were stimulated with 10 μg/ml PPDj in 200 μl RPMI for 72 h at 37°C in a humidified atmosphere supplemented with 5% CO2. Cells were centrifuged, culture supernatant was recovered, and IFN-γ levels were determined by using a BOVIGAM kit following the manufacturer's protocol. Results are expressed as the OD, and error bars indicate standard deviations from the means. *, P < 0.05 at week 30 and P < 0.02 at week 26 (ΔleuD versus Mycopar); **, P < 0.05 (ΔleuD versus PBS and Mycopar versus PBS).
T cell response.
PBMCs isolated from whole blood at different time points were analyzed after stimulation with PPDj for proliferation, activation, and generation of memory among CD4, CD8, and γδ T cells by lymphoproliferation and FC analyses. A enhanced proliferative response was detected after immunization with either Mycopar or ΔleuD, and this increased sharply after both booster and challenge and was significantly higher (P < 0.01) than in control animals treated with PBS at weeks 10 and 14 (Fig. 3). No significant difference in the proliferative capacity of PBMCs isolated from either the Mycopar- or ΔleuD-vaccinated groups was noted at earlier time points after challenge (14, 18, and 22 weeks), but this response was significantly higher in ΔleuD-vaccinated animals at 26 weeks (P < 0.05) and 30 weeks (P < 0.03) (Fig. 3). Analysis of the activation status of CD4, CD8, and γδ T cells by FC analysis at different time points showed that ΔleuD and Mycopar activated CD4 T cells at similar levels at most time points, except at weeks 14 and 18, when ΔleuD generated significantly higher levels (P < 0.05) of these cells (Fig. 4A). Similarly, activation of CD8 T cells was significantly higher in ΔleuD-vaccinated animals at weeks 3, 10, and 14 (P < 0.05) (Fig. 4B). Both the ΔleuD and Mycopar groups generated γδ T cells after challenge at similar levels, except at weeks 18, 22, and 26, when the levels of these cells were significantly higher (P < 0.05) in the ΔleuD group (Fig. 4C).
Fig 3.
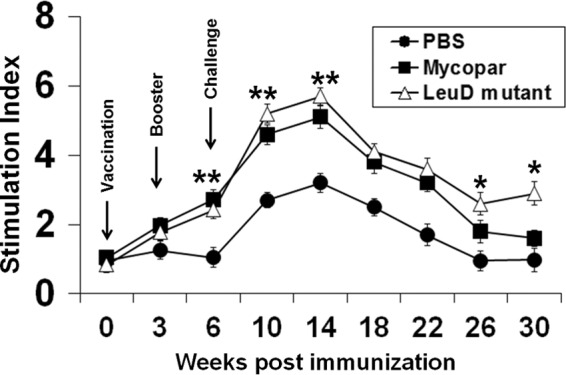
Lymphoproliferation. PBMCs isolated from animals bled at different time points were stimulated with 10 μg/ml PPDj in 200 μl RPMI for 72 h at 37°C in a humidified atmosphere supplemented with 5% CO2. The proliferative response was measured with a cell proliferation ELISA and BrdU colorimetric kit (Roche Diagnostics, Indianapolis, IN) as per the manufacturer's protocol. The results are expressed as the SI, and the error bars indicate standard deviations from the means. *, P < 0.05 at week 26 and P < 0.03 at week 30 (ΔleuD versus Mycopar); **, P < 0.05 (ΔleuD versus PBS and Mycopar versus PBS).
Fig 4.
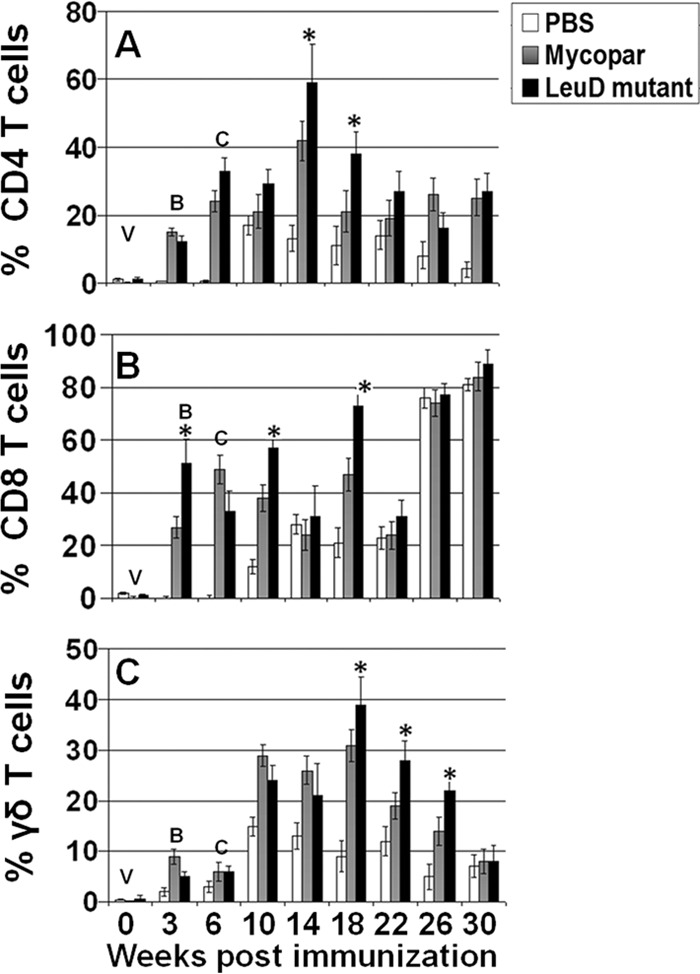
Analysis of activation status of T cells, based on comparison of activation status of T cells in PBMCs isolated from animals bled at various time points after immunization and challenge, stimulated with PPDj for 6 days, and subjected to FC analysis. The data were analyzed with the BD FACS Diva software. The data are presented as a bar graph for simplicity. Activation statuses of CD4 T cells (A), CD8 T cells (B), and γδ T cells (C) at different time points are shown. V, vaccination; B, booster; C, challenge; *, P < 0.05 (ΔleuD versus Mycopar).
Cytokine responses.
Relative changes in cytokine transcription in PBMCs stimulated for 3 days with PPDj were compared at different time points after vaccination and challenge, by using RT-PCR. The data were grouped to compare the cytokine profiles considered to define Th1, Th2, Th17, and regulatory T cells (Treg). Adherent and nonadherent cells were collected and processed to detect gene expression in lymphocytes and macrophages. Both Mycopar and the ΔleuD vaccines upregulated Th1 and proinflammatory (IL-6, IL-8, IL-18, and IL-1b) and Th17 (IL-17) cytokines and downregulated Th2/anti-inflammatory (IL-4, IL-10, IL-13) and regulatory (transforming growth factor-β [TGF-β]) cytokines (Fig. 5). Both vaccines induced similar levels of these cytokines at almost all time points; however, significantly higher level of IFN-γ (at weeks 26 and 30; P < 0.05), IL-2 (week 18; P < 0.05), IL-1b (weeks 14 and 22; P < 0.05), IL-17(weeks 18 and 22; P < 0.05), and IL-23 (week 18; P < 0.05) and significantly lower levels of IL-10 (weeks 14 and 18; P < 0.05) and TGF-β (week 18; P < 0.05) were detected in the ΔleuD-vaccinated group compared to the Mycopar group. A general trend for a drop in the level of all the cytokines after challenge was observed in all groups.
Fig 5.
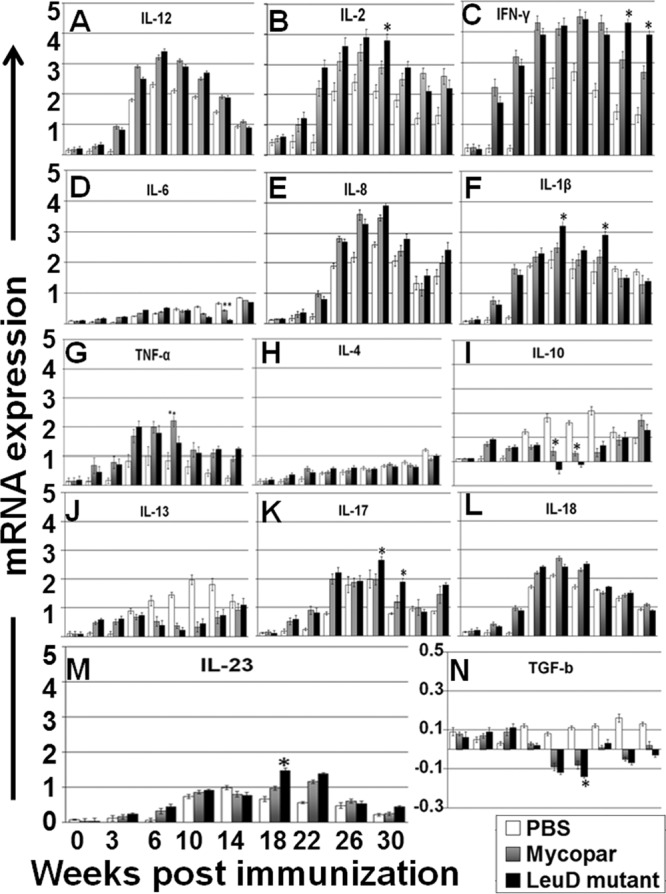
(A) Results of cytokine analysis by RT-PCR. Relative transcription of cytokine message was measured by RT-PCR in PBMCs isolated from animals bled at different time points after immunization and stimulation with PPDj for 3 days. The relative transcription level was calculated using the value of unstimulated cells as the calibrator, with the housekeeping gene GAPDH as an internal control. Data are presented as the relative mRNA expression level (mean fold change) of each group with error bars indicating the standard deviations. Panels are grouped together as follows: Th1 cytokines (A, B, and C); proinflammatory cytokines (D, E, F, and G); Th2/anti-inflammatory cytokines (H, I, and J); Th17/regulatory cytokines (K, L, M, N). *, P < 0.05 for ΔleuD versus Mycopar; **, P < 0.05 for Mycopar versus ΔleuD.
Protective efficacy of the leuD mutant vaccine against challenge in goats.
To evaluate the protective efficacy of the ΔleuD vaccine, M. avium subsp. paratuberculosis burdens in the 9 tissues (selected on the basis of previous studies) collected from each animal at necropsy were assessed by both bacterial culture and PCR. Histopathological analysis was also performed on these tissues to detect any lesions. Culture results showed that almost all the tissues from the control animals treated with PBS were culture positive and had higher bacterial loads (moderate [>50 CFU] to heavy [>300 CFU]). Animals immunized with Mycopar showed partial protection, as bacteria were recovered from some but not all tissues (Table 4). Some tissues had a moderate to low bacterial load (<50 or >50 CFU), and some were culture negative. In contrast, goats vaccinated with the ΔleuD vaccine had only a few tissues that were culture positive. Some of these animals demonstrated sterilizing immunity, as bacteria were not recovered from any of the cultured tissues. The bacterial loads in tissues from both control and vaccinated groups were further confirmed by PCR, and the results validated the culture results in most tissues, except in a few cases where M. avium subsp. paratuberculosis was not detected in culture but IS900 was detected by PCR. The protective efficacy was further demonstrated by bacterial shedding, where control animals maintained high to moderate levels of shedding and Mycopar-vaccinated animals showed reduced bacterial shedding, while only a few ΔleuD-immunized animals shed M. avium subsp. paratuberculosis at a low level in feces at some time points (Table 5). Histopathological examination of these tissues, including those obtained from the control group, revealed that there were no lesions in any tissue in any group.
Table 4.
M. avium subsp. paratuberculosis burden in goat tissues after necropsy
| Treatment group | Goat ID | Presence in indicated tissue, based on detection methoda |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MLN |
ICLN |
Dd |
J |
IP |
IM |
ID |
ICO |
C |
|||||||||||
| CT | PCR | CT | PCR | CT | PCR | CT | PCR | CT | PCR | CT | PCR | CT | PCR | CT | PCR | CT | PCR | ||
| ΔleuD mutant | 2152 | − | − | + | + | – | – | + | + | − | − | − | − | + | 2+ | – | − | − | − |
| 2154 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2156 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2158 | + | + | − | − | − | − | 2+ | 3+ | − | − | 2+ | 2+ | − | – | − | − | − | − | |
| 2161 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 2163 | − | − | − | + | – | – | − | + | − | − | − | − | + | + | − | − | 2+ | 2+ | |
| PBS | 2194 | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ |
| 2198 | + | 2+ | − | + | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ | |
| 2199 | − | + | 2+ | 3+ | - | + | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ | |
| 2200 | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | − | − | 2+ | 3+ | 2+ | 2+ | − | + | + | 3+ | |
| 2201 | 2+ | 2+ | − | − | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 2+ | 2+ | 2+ | 2+ | 3+ | |
| 2202 | 2+ | 2+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 3+ | 2+ | 2+ | + | + | 2+ | 3+ | |
| Mycopar | 2206 | 2+ | 2+ | − | − | + | + | 2+ | 2+ | 2+ | 2+ | − | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ |
| 2207 | 2+ | 2+ | − | − | + | + | 2+ | 2+ | 2+ | 2+ | − | − | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | |
| 2214 | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | |
| 2217 | − | − | − | − | 2+ | 2+ | − | + | + | 2+ | + | 2+ | 2+ | 2+ | + | + | + | 2+ | |
| 2218 | − | + | − | + | − | + | − | + | + | 2+ | − | 2+ | 2+ | 2+ | + | 2+ | + | 2+ | |
| 2221 | + | + | − | 2+ | − | + | + | 2+ | 2+ | 3+ | + | 2+ | 2+ | 2+ | − | 2+ | − | + | |
CT, culture method. −, no colonies of M. avium subsp. paratuberculosis detected; +, <50 CFU; 2+, >50 CFU; 3+, >300 CFU. ♣, goat 2214 was euthanized prior to study conclusion due to sickness unrelated to the study. MLN, mesenteric lymph nodes; ICLN, ileocecal lymph nodes; Dd, descending duodenum; J, jejunum; IP, proximal ilium; IM, middle ilium; ID, distal ilium; ICO, ileocecal orifice; C, cecum.
Table 5.
M. avium subsp. paratuberculosis fecal shedding postimmunization and after challenge
| Treatment group | Goat ID | Fecal sheddinga at indicated time postimmunization (wks) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 10 | 14 | 18 | 22 | 26 | 30 | ||
| ΔleuD mutant | 2152 | − | − | − | − | + | − | − | – | + |
| 2154 | − | − | − | − | + | + | − | + | − | |
| 2156 | − | − | − | − | − | + | + | – | − | |
| 2158 | − | − | − | − | + | − | − | + | − | |
| 2161 | − | − | − | − | − | + | − | − | − | |
| 2163 | − | − | − | − | + | − | + | − | − | |
| PBS | 2194 | − | − | − | − | 2+ | − | 3+ | 2+ | + |
| 2198 | − | − | − | − | − | 2+ | + | − | − | |
| 2199 | − | − | − | − | − | − | + | + | − | |
| 2200 | − | − | − | − | 2+ | – | 3+ | 2+ | 2+ | |
| 2201 | − | − | − | − | + | + | + | − | 2+ | |
| 2202 | − | − | − | − | 2+ | + | 3+ | 2+ | 3+ | |
| Mycopar | 2206 | − | − | − | − | + | – | – | + | − |
| 2207 | − | − | − | − | – | + | – | + | − | |
| 2214 | − | − | − | ♣ | ♣ | ♣ | ♣ | ♣ | ♣ | |
| 2217 | − | − | − | − | + | − | + | + | + | |
| 2218 | − | − | − | − | + | − | − | − | − | |
| 2221 | − | − | − | − | − | + | + | − | + | |
−, no colonies of M. avium subsp. paratuberculosis detected; +, <50 CFU; 2+, >50 CFU; 3+, >300 CFU. ♣, goat 2214 was euthanized prior to study conclusion due to sickness unrelated to the study.
DISCUSSION
M. avium subsp. paratuberculosis is an important animal pathogen that has a major economic impact on the dairy, beef, sheep, and goat industries in the United States and worldwide. The availability of an improved and cost-effective vaccine against paratuberculosis would provide an extremely beneficial tool for the control of JD. We decided to explore development of live attenuated candidate vaccines, because they are cost-effective and possess all the immunogenic proteins necessary to induce a strong, long-lasting immune response. Earlier attempts to produce M. avium subsp. paratuberculosis mutants as vaccine candidates proved effective in both goat and calf challenge models (20). As a major step toward this goal, in our previous study we produced an auxotroph LeuD mutant (ΔleuD) of M. avium subsp. paratuberculosis by allelic exchange. Studies in mice showed that ΔleuD elicited an immune response that limited colonization by virulent M. avium subsp. paratuberculosis (26), consistent with results reported with an M. bovis leuD mutant in cattle (25). We have also demonstrated that the attenuated phenotype of LeuD is associated with defects in transcription of several virulence genes important in cellular pathways, particularly fatty acid (mycolic acid) biosynthesis (27). In the present study, we compared the protective efficacy of M. avium subsp. paratuberculosis ΔleuD with that of a killed vaccine, Mycopar, in a goat model. A deeper understanding of the immune responses induced in goats by the ΔleuD vaccine may provide insights into the basis of protective immunity in ruminants.
Both the ΔleuD and Mycopar vaccines elicited immune responses that limited colonization, as assessed by a lower bacterial burden in tissues at the time of necropsy compared to the bacterial burdens in unvaccinated controls (Table 4). However, ΔleuD was more effective than Mycopar in limiting colonization, indicating an important difference in the type of response elicited by the two vaccines. Although both induced similar levels of IFN-γ at initial time points, there was a marked decline in the IFN-γ level in the Mycopar group after week 14. However, in the ΔleuD group this response was maintained and was significantly higher (P < 0.05) at weeks 26 and 30 (Fig. 2). These findings were in accordance with those of previous studies, where reduction in the IFN-γ level correlated to development of clinical disease (36, 37). Further, it is speculated that knockout of the leuD gene might abrogate the ability of M. avium subsp. paratuberculosis to disrupt Toll-like receptor 9 signaling and limit the responsiveness of infected macrophages to IFN-γ by inducing the expression of negative regulators of the IFN-γ receptors, called suppressors of cytokine signaling (SOCS) proteins, and decreasing the expression of IFN-γ receptors (38, 39). However, this needs to be tested. Both ΔleuD and Mycopar induced similar and low levels of antibodies (Fig. 1). This was expected, since the response was analyzed at the early phase of infection, and strong humoral immunity is usually observed in the final stages of infection (40–42). The high variation in antibody levels among individual goats within a group may have been due to infrequent M. avium subsp. paratuberculosis shedding, leading to continuous low-level stimulation of the humoral immune response (41, 43). Similarly, both vaccines demonstrated increased proliferative capacity and high-level activation (CD25 expression) of T cells (Fig. 3 and 4). However, a significantly enhanced proliferative capacity at weeks 26 and 30 (P < 0.05) and the activation status of T cells (CD4 and CD8) at some time points (weeks 14 and 18) (P < 0.05) observed in the ΔleuD group (Fig. 4A and B) indicate that these cells might be contributing to limiting the infection by a mechanism associated with secretion of IFN-γ, which activates bactericidal activity in macrophages (44). High levels of IFN-γ might inhibit production of IL-10, which is considered to have an inhibitory effect on the killing of mycobacteria and suppresses T cell functions (50). The clinical phase includes a marked reduction in circulating CD4+ T cell numbers, and cells become unresponsive to stimulation with M. avium subsp. paratuberculosis antigen (45, 46). Similarly, significantly higher levels (P < 0.05) of γδ T cells induced by ΔleuD at some time points correlate with its enhanced protective efficacy (Fig. 4C). Cytokine responses observed in the present study were consistent with the findings in previous studies (32, 47). Genes associated with a Th1 proinflammatory response (IFN-γ, IL-2, IL-1β, Il-6, IL-8, IL-17, IL-18, and TNF-α) and Th17 (IL-17) were upregulated, indicating that at least two T cell subsets were involved in the response to PPDj. Genes associated with a Th2 response (IL-4, IL-10, and IL-13) or Treg response (TGF-β) were expressed at low levels at most time points, consistent with a response dominated by Th1 and Th17 (Fig. 5). Although both vaccines induced similar levels of these cytokines at almost all time points, significant differences (P < 0.05) were noted in key cytokines, like IL-2, IFN-γ, IL-1b, IL-17, IL-23, and TGF-β, at some time points, which correlated with enhanced protection induced by ΔleuD (Fig. 5). Interestingly, high levels of IL-2 and Th17 cytokines in the current study were unexpected, as IL-2 has been shown to suppress Th17 cell development (48). Both vaccines downregulated and generated low levels of TGF-β; however, this effect was significantly lower for the ΔleuD group at week 18, which corresponds with protection, as this cytokine is a potent inhibitor of T cell proliferation, differentiation, and activation (42, 49). The difference in immune responses between the Mycopar and ΔleuD groups could be partly explained by the fact that Mycopar, a killed vaccine, is rapidly cleared from the host, whereas ΔleuD induces a low level of stimulation and maintains the immune response over a longer time period.
In summary, we demonstrated that deletion of the leuD gene in M. avium subsp. paratuberculosis produced a mutant with an effective balance between attenuation and immunogenicity. Vaccination of goats with ΔleuD elicited an immune response that cleared the mutant, limited colonization of virulent M. avium subsp. paratuberculosis, and provided sterilizing immunity in 50% of animals, albeit it did not prevent fecal shedding (Table 5). In contrast, the response elicited by Mycopar was only partially effective at limiting colonization. If this is the case, we have identified the gene (leuD) important to the survival of M. avium subsp. paratuberculosis in vivo. Deletion of this gene abrogates the capacity of M. avium subsp. paratuberculosis to establish a persistent infection. This is associated with the development of an immune response involving Th1 and Th17 subsets, which clearly differs from the response elicited by wild-type M. avium subsp. paratuberculosis or by Mycopar. We did not observe any histopathological lesions in any of the tissues analyzed in either the control or vaccinated groups, as the animals were kept for only a short period (6 months) after challenge, and lesions are only apparent at or after 1.5 years postchallenge. It would have been interesting to see if any lesions developed with corresponding changes in the immune profile at later time points. However, this was beyond the scope of the current study. Further studies are now needed to directly compare the immune response and protection elicited by ΔleuD in ruminants (goats or calves) over a longer time frame (2 to 3 years). Efforts to develop a model where disease progression can be studied over a shorter period of time have failed. This may be due to the fact that the initial immune response to M. avium subsp. paratuberculosis is resilient and not readily altered to trigger disease progression. Challenge with large and repeated doses of bacteria over time, immunosuppression by corticosteroids, or depletion of CD4 T cells neither accelerated colonization nor decreased the time to clinical disease (49, 51–53). Thus, studying the long-term immune response in a natural host is required and will permit a more complete analysis of how many T cell subsets are involved in the immune response to M. avium subsp. paratuberculosis, their roles in mediating protection, and the efficacy of ΔleuD in preventing clinical JD. However, the current study offers hope that a ΔleuD vaccine has potential for field use and warrants future studies that should focus on its efficacy under field conditions.
ACKNOWLEDGMENTS
This work was supported by the Biotechnology Research and Development Corporation (BRDC), Agriculture and Food Research Initiative competitive grants 2008-35204-04626, 2009-65119-05993, and 2008-55620-18710 (4535-CU-USDA-8710 and 3954-CU-USDA-8710), and the Animal Formula Fund (NY-478455 and NY-478437).
We thank Andrew Yen, Biomedical Sciences Flow Cytometry Core Facility, Cornell University, for FACS analysis.
We declare no financial or commercial conflicts of interest.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Sohal JS, Singh SV, Singh PK, Singh AV. 2010. On the evolution of ‘Indian Bison type’ strains of Mycobacterium avium subspecies paratuberculosis. Microbiol. Res. 165: 163–171 [DOI] [PubMed] [Google Scholar]
- 2. Sleeman JM, Manning EJ, Rohm JH, Sims JP, Sanchez S, Gerhold RW, Keel MK. 2009. Johne's disease in a free-ranging white-tailed deer from Virginia and subsequent surveillance for Mycobacterium avium subspecies paratuberculosis. J. Wildl. Dis. 45: 201–206 [DOI] [PubMed] [Google Scholar]
- 3. Clarke CJ. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116: 217–261 [DOI] [PubMed] [Google Scholar]
- 4. Johnson-Ifearulundu Y, Kaneene JB, Lloyd JW. 1999. Herd-level economic analysis of the impact of paratuberculosis on dairy herds. J. Am. Vet. Med. Assoc. 214: 822–825 [PubMed] [Google Scholar]
- 5. Kirkwood CD, Wagner J, Boniface K, Vaughan J, Michalski WP, Catto-Smith AG, Cameron DJ, Bishop RF. 2009. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn's disease. Inflamm. Bowel Dis. 15: 1643–1655 [DOI] [PubMed] [Google Scholar]
- 6. Naser SA, Ghobrial G, Romero C, Valentine JF. 2004. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet 364: 1039–1044 [DOI] [PubMed] [Google Scholar]
- 7. Davis WC, Madsen-Bouterse SA. 2012. Crohn's disease and Mycobacterium avium subsp. paratuberculosis: the need for a study is long overdue. Vet. Immunol. Immunopathol. 145: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pierce ES. 2010. Ulcerative colitis and Crohn's disease: is Mycobacterium avium subspecies paratuberculosis the common villain? Gut Pathog. 2: 21 doi:10.1186/1757-4749-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauby H, Saint Girons I, Picardeau M. 2003. Construction and complementation of the first auxotrophic mutant in the spirochaete Leptospira meyeri. Microbiology 149: 689–693 [DOI] [PubMed] [Google Scholar]
- 10. Rani PS, Sechi LA, Ahmed N. 2010. Mycobacterium avium subsp. paratuberculosis as a trigger of type-1 diabetes: destination Sardinia, or beyond? Gut Pathog. 2: 1 doi:10.1186/1757-4749-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emery DL, Whittington RJ. 2004. An evaluation of mycophage therapy, chemotherapy and vaccination for control of Mycobacterium avium subsp. paratuberculosis infection. Vet. Microbiol. 104: 143–155 [DOI] [PubMed] [Google Scholar]
- 12. Harris NB, Barletta RG. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14: 489–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalis CH, Hesselink JW, Barkema HW, Collins MT. 2001. Use of long-term vaccination with a killed vaccine to prevent fecal shedding of Mycobacterium avium subsp. paratuberculosis in dairy herds. Am. J. Vet. Res. 62: 270–274 [DOI] [PubMed] [Google Scholar]
- 14. Klawonn W, Cussler K, Drager KG, Gyra H, Kohler H, Zimmer K, Hess RG. 2002. The importance of allergic skin test with Johnin, antibody ELISA, cultural fecal test as well as vaccination for the sanitation of three chronically paratuberculosis-infected dairy herds in Rhineland-Palatinate. Dtsch. Tierarztl. Wochenschr. 109: 510–516 [PubMed] [Google Scholar]
- 15. Kohler H, Gyra H, Zimmer K, Drager KG, Burkert B, Lemser B, Hausleithner D, Cubler K, Klawonn W, Hess RG. 2001. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. J. Vet. Med. B Infect. Dis. Vet. Public Health 48: 185–195 [DOI] [PubMed] [Google Scholar]
- 16. Muskens J, van Zijderveld F, Eger A, Bakker D. 2002. Evaluation of the long-term immune response in cattle after vaccination against paratuberculosis in two Dutch dairy herds. Vet. Microbiol. 86: 269–278 [DOI] [PubMed] [Google Scholar]
- 17. Cavaignac SM, White SJ, de Lisle GW, Collins DM. 2000. Construction and screening of Mycobacterium paratuberculosis insertional mutant libraries. Arch. Microbiol. 173: 229–231 [DOI] [PubMed] [Google Scholar]
- 18. Park KT, Dahl JL, Bannantine JP, Barletta RG, Ahn J, Allen AJ, Hamilton MJ, Davis WC. 2008. Demonstration of allelic exchange in the slow-growing bacterium Mycobacterium avium subsp. paratuberculosis, and generation of mutants with deletions at the pknG, relA, and lsr2 loci. Appl. Environ. Microbiol. 74: 1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scandurra GM, de Lisle GW, Cavaignac SM, Young M, Kawakami RP, Collins DM. 2010. Assessment of live candidate vaccines for paratuberculosis in animal models and macrophages. Infect. Immun. 78: 1383–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park KT, Allen AJ, Bannantine JP, Seo KS, Hamilton MJ, Abdellrazeq GS, Rihan HM, Grimm A, Davis WC. 2011. Evaluation of two mutants of Mycobacterium avium subsp. paratuberculosis as candidates for a live attenuated vaccine for Johne's disease. Vaccine 29: 4709–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dosanjh NS, Rawat M, Chung JH, Av-Gay Y. 2005. Thiol specific oxidative stress response in Mycobacteria. FEMS Microbiol. Lett. 249: 87–94 [DOI] [PubMed] [Google Scholar]
- 22. Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60: 312–330 [DOI] [PubMed] [Google Scholar]
- 23. Bennett HJ, Pearce DM, Glenn S, Taylor CM, Kuhn M, Sonenshein AL, Andrew PW, Roberts IS. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63: 1453–1467 [DOI] [PubMed] [Google Scholar]
- 24. Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR, Jr, Bloom BR. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68: 2888–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khare S, Hondalus MK, Nunes J, Bloom BR, Garry Adams L. 2007. Mycobacterium bovis ΔleuD auxotroph-induced protective immunity against tissue colonization, burden and distribution in cattle intranasally challenged with Mycobacterium bovis Ravenel S. Vaccine 25: 1743–1755 [DOI] [PubMed] [Google Scholar]
- 26. Chen JW, Faisal SM, Chandra S, McDonough SP, Moreira MA, Scaria J, Chang CF, Bannantine JP, Akey B, Chang YF. 2012. Immunogenicity and protective efficacy of the Mycobacterium avium subsp. paratuberculosis attenuated mutants against challenge in a mouse model. Vaccine 30: 3015–3025 [DOI] [PubMed] [Google Scholar]
- 27. Chen JW, Scaria J, Chang YF. 2012. Phenotypic and transcriptomic response of auxotrophic Mycobacterium avium subsp. paratuberculosis leuD mutant under environmental stress. PLoS One 7: e37884 doi:10.1371/journal.pone.0037884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen LH, Kathaperumal K, Huang CJ, McDonough SP, Stehman S, Akey B, Huntley J, Bannantine JP, Chang CF, Chang YF. 2008. Immune responses in mice to Mycobacterium avium subsp. paratuberculosis following vaccination with a novel 74F recombinant polyprotein. Vaccine 26: 1253–1262 [DOI] [PubMed] [Google Scholar]
- 29. Park SU, Kathaperumal K, McDonough S, Akey B, Huntley J, Bannantine JP, Chang YF. 2008. Immunization with a DNA vaccine cocktail induces a Th1 response and protects mice against Mycobacterium avium subsp. paratuberculosis challenge. Vaccine 26: 4329–4337 [DOI] [PubMed] [Google Scholar]
- 30. Griffin JF, Cross JP, Chinn DN, Rodgers CR, Buchan GS. 1994. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N. Z. Vet. J. 42: 173–179 [DOI] [PubMed] [Google Scholar]
- 31. Kathaperumal K, Kumanan V, McDonough S, Chen LH, Park SU, Moreira MA, Akey B, Huntley J, Chang CF, Chang YF. 2009. Evaluation of immune responses and protective efficacy in a goat model following immunization with a coctail of recombinant antigens and a polyprotein of Mycobacterium avium subsp. paratuberculosis. Vaccine 27: 123–135 [DOI] [PubMed] [Google Scholar]
- 32. Coussens PM, Verman N, Coussens MA, Elftman MD, McNulty AM. 2004. Cytokine gene expression in peripheral blood mononuclear cells and tissues of cattle infected with Mycobacterium avium subsp. paratuberculosis: evidence for an inherent proinflammatory gene expression pattern. Infect. Immun. 72: 1409–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 34. Kathaperumal K, Park SU, McDonough S, Stehman S, Akey B, Huntley J, Wong S, Chang CF, Chang YF. 2008. Vaccination with recombinant Mycobacterium avium subsp. paratuberculosis proteins induces differential immune responses and protects calves against infection by oral challenge. Vaccine 26: 1652–1663 [DOI] [PubMed] [Google Scholar]
- 35. Shin SJ, Chang YF, Huang C, Zhu J, Huang L, Yoo HS, Shin KS, Stehman S, Shin SJ, Torres A. 2004. Development of a polymerase chain reaction test to confirm Mycobacterium avium subsp. paratuberculosis in culture. J. Vet. Diagn. Invest. 16: 116–120 [DOI] [PubMed] [Google Scholar]
- 36. Begg DJ, Griffin JF. 2005. Vaccination of sheep against M. paratuberculosis: immune parameters and protective efficacy. Vaccine 23: 4999–5008 [DOI] [PubMed] [Google Scholar]
- 37. Stabel JR. 2000. Cytokine secretion by peripheral blood mononuclear cells from cows infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 61: 754–760 [DOI] [PubMed] [Google Scholar]
- 38. Arsenault RJ, Li Y, Bell K, Doig K, Potter A, Griebel PJ, Kusalik A, Napper S. 2012. Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease. Infect. Immun. 80: 3039–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arsenault RJ, Li Y, Maattanen P, Scruten E, Doig K, Potter A, Griebel P, Kusalik A, Napper S. 31 October 2012. Altered Toll-like receptor 9 signaling in Mycobacterium avium subsp. paratuberculosis-infected bovine monocytes reveals potential therapeutic targets. Infect. Immun. 81: 226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juste RA, Garcia Marin JF, Peris B, Saez de Ocariz CS, Badiola JJ. 1994. Experimental infection of vaccinated and non-vaccinated lambs with Mycobacterium paratuberculosis. J. Comp. Pathol. 110: 185–194 [DOI] [PubMed] [Google Scholar]
- 41. Perez V, Tellechea J, Badiola JJ, Gutierrez M, Garcia Marin JF. 1997. Relation between serologic response and pathologic findings in sheep with naturally acquired paratuberculosis. Am. J. Vet. Res. 58: 799–803 [PubMed] [Google Scholar]
- 42. Stabel JR. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim. Health Res. Rev. 7: 61–70 [DOI] [PubMed] [Google Scholar]
- 43. Storset AK, Hasvold HJ, Valheim M, Brun-Hansen H, Berntsen G, Whist SK, Djonne B, Press CM, Holstad G, Larsen HJ. 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study. Vet. Immunol. Immunopathol. 80: 271–287 [DOI] [PubMed] [Google Scholar]
- 44. Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19: 93–129 [DOI] [PubMed] [Google Scholar]
- 45. Bassey EO, Collins MT. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect. Immun. 65: 4869–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burrells C, Clarke CJ, Colston A, Kay JM, Porter J, Little D, Sharp JM. 1998. A study of immunological responses of sheep clinically-affected with paratuberculosis (Johne's disease). The relationship of blood, mesenteric lymph node and intestinal lymphocyte responses to gross and microscopic pathology. Vet. Immunol. Immunopathol. 66: 343–358 [DOI] [PubMed] [Google Scholar]
- 47. Buza JJ, Mori Y, Bari AM, Hikono H, Aodon-geril , Hirayama S, Shu Y, Momotani E. 2003. Mycobacterium avium subsp. paratuberculosis infection causes suppression of RANTES, monocyte chemoattractant protein 1, and tumor necrosis factor alpha expression in peripheral blood of experimentally infected cattle. Infect. Immun. 71: 7223–7227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26: 371–381 [DOI] [PubMed] [Google Scholar]
- 49. Koo HC, Park YH, Hamilton MJ, Barrington GM, Davies CJ, Kim JB, Dahl JL, Waters WR, Davis WC. 2004. Analysis of the immune response to Mycobacterium avium subsp. paratuberculosis in experimentally infected calves. Infect. Immun. 72: 6870–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khalifeh MS, Stabel JR. 2004. Effects of gamma interferon, interleukin-10, and transforming growth factor beta on the survival of Mycobacterium avium subsp. paratuberculosis in monocyte-derived macrophages from naturally infected cattle. Infect. Immun. 72: 1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stabel JR, Palmer MV, Whitlock RH. 2003. Immune responses after oral inoculation of weanling bison or beef calves with a bison or cattle isolate of Mycobacterium avium subsp. paratuberculosis. J. Wildl. Dis. 39: 545–555 [DOI] [PubMed] [Google Scholar]
- 52. Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, Steadham EM, Hamilton MJ, Davis WC, Bannantine JP. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71: 5130–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sweeney RW, Uzonna J, Whitlock RH, Habecker PL, Chilton P, Scott P. 2006. Tissue predilection sites and effect of dose on Mycobacterium avium subs. paratuberculosis organism recovery in a short-term bovine experimental oral infection model. Res. Vet. Sci. 80: 253–259 [DOI] [PubMed] [Google Scholar]
- 54. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]


