Abstract
The immune protection initiated by γδ T cells plays an important role in mycobacterial infection. The γδ T cells activated by Mycobacterium tuberculosis-derived nonpeptidic, phosphorylated biometabolites (phosphoantigens) provide only partial immune protection against mycobacterium, while evidence has suggested that protein antigen-activated γδ T cells elicit effective protective immune responses. To date, only a few distinct mycobacterial protein antigens have been identified. In the present study, we screened protein antigens recognized by γδ T cells using cells transfected with the predominant pulmonary tuberculosis γδ T cell receptor (TCR) CDR3 fragment. We identified two peptides, TP1 and TP2, which not only bind to the pulmonary tuberculosis predominant γδ TCR but also effectively activate γδ T cells isolated from pulmonary tuberculosis patients. Moreover, 1-deoxy-d-xylulose 5-phosphate synthase 2 (DXS2), the TP1-matched mycobacterial protein, was confirmed as a ligand for the γδ TCR and was found to activate γδ T cells from pulmonary tuberculosis patients. The extracellular region (extracellular peptide [EP]) of Rv2272, a TP2-matched mycobacterial transmembrane protein, was also shown to activate γδ T cells from pulmonary tuberculosis patients. Both DXS2- and EP-expanded γδ T cells from pulmonary tuberculosis patients could secrete gamma interferon (IFN-γ) and monocyte chemoattractant protein 1 (MCP-1), which play important roles in mediating cytotoxicity against mycobacterium and stimulating monocyte chemotaxis toward the site of infection. In conclusion, our study identified novel mycobacterial protein antigens recognized by γδ TCR cells that could be candidates for the development of vaccines or adjuvants against mycobacterium infection.
INTRODUCTION
Tuberculosis (TB), caused by Mycobacterium tuberculosis, is one of the most prevalent serious infectious diseases worldwide. Approximately 30% of the world's population is affected by M. tuberculosis, which causes 1.7 million deaths every year. According to the 2009 WHO report on tuberculosis, there were an estimated 9.4 million incident cases of TB globally, which was equivalent to 137 cases per 100,000 people. In 2010, there were approximately 8.8 million incident cases of TB, 1.1 million deaths from TB among HIV-negative people, and an additional 0.35 million deaths from HIV-associated TB (1, 2). Although combination chemotherapy is effective in the treatment of tuberculosis, the treatment is arduous and requires stringent compliance to avoid the development of multidrug-resistant strains of M. tuberculosis. The attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin (BCG), is currently the only available vaccine against tuberculosis (3, 4). However, the efficacy of BCG in the control of tuberculosis has shown considerable variation in different clinical trials and geographically distinct populations (5). Moreover, BCG can cause disseminated disease in immunocompromised individuals (6, 7). Thus, current efforts are directed toward the development of a safer and more effective vaccine against M. tuberculosis.
In recent years, the vaccine for M. tuberculosis based on γδ T cells provided a novel approach to TB control due to its important role in preventing tuberculosis infection (8, 9). Originally, M. tuberculosis-derived nonpeptidic, phosphorylated biometabolites (phosphoantigens), such as isopentenyl pyrophosphate (IPP), were regarded as the main γδ T cell receptor (TCR)-recognized antigens. However, phosphoantigen-activated γδ T cells display a restricted TCR diversity, and only a subset of phosphoantigen-responsive γδ T cells mediate protective immunity against M. tuberculosis (10). In contrast, γδ T cells activated by M. tuberculosis-derived protein antigens were reported to be able to effectively induce innate and adaptive immunity against M. tuberculosis. Evidence also exists indicating that γδ T cells participate in the anti-TB immune response elicited by other immune cells (11–13). These immune cells play an important role in the control of M. tuberculosis. However, until now, only a few promising tuberculosis protein antigens that effectively activate γδ T cells against M. tuberculosis have been identified.
We have established a novel strategy to screen for BCG-specific, γδ TCR-recognized peptide and protein antigens through panning a 12-mer random peptide phage display library using BCG-specific γδ TCR CDR3-transfected cells as probes (14). A peptide (BP3) and a protein (oxidative stress response regulatory protein [OXY]) were identified that not only bound to the BCG-specific γδ TCR but also effectively activated γδ T cells isolated from human subjects inoculated with BCG. This strategy provides a novel means to screen mycobacterial vaccine candidates or adjuvants. Given that we have found a preponderant complementary determinant region (CDR3) sequence in pulmonary tuberculosis patients (15), it is rational to identify new tuberculosis protein antigens recognized by γδ TCR in pulmonary tuberculosis patients by extending the screening strategy previously used for BCG.
In this study, we first constructed γδ TCR-transfected cells expressing the predominant pulmonary tuberculosis CDR3 sequence and a healthy control CDR3 sequence. These cells then were used to carry out subtractive screening in vitro with a phage display 12-mer peptide library. Consequently, we obtained a group of peptides capable of binding specifically to γδ TCR-transfected cells and γδ T cells isolated from pulmonary tuberculosis patients. The biological function of these peptides and their matched proteins, identified through bioinformatics analysis, was further investigated and verified.
MATERIALS AND METHODS
Subjects.
This study was performed on 80 randomly selected pulmonary tuberculosis patients (mean age, 50.3 years; 56 men and 24 women) who had been admitted to the Beijing Tuberculosis and Thoracic Tumor Research Institute during a 12-month period. Pulmonary tuberculosis was diagnosed by the following clinical parameters: presence of cough/expectoration, chest X-ray showing infiltration and/or cavities, a minimum of one positive sputum smear, and a positive culture result for acid-fast bacilli. The exclusion criteria were human immunodeficiency virus positivity, diabetes mellitus, pregnancy, and immunological or autoimmune diseases. Detailed information of 20 pulmonary tuberculosis patients used in functional analysis is provided in the supplemental material. Forty healthy volunteer subjects (mean age, 40 years; 24 men and 16 women) were included as a control group. Healthy subjects did not have any changes on X-ray and tuberculosis history or other underlying disease. Exclusion criteria for the healthy control groups were smoking, medication, pregnancy, and any abnormalities in renal and liver function tests. This work received approval from the Clinical Ethics Committee of the Institute of Pathogen Biology, Chinese Academy of Medical Sciences, and Beijing Union Medical College. All subjects gave their informed consent to participate.
Reagents and cell lines.
The phD 12 phage display peptide library kit (New England Biolabs) was used to screen specific peptides binding to γδ TCR. J.RT3-T3.5 cells and THP-1 cells, a human myelomonocytic cell line, were obtained from the American Type Culture Collection (ATCC). γδ T cells, immobilized by anti-pan-γδ TCR monoclonal antibody (Immunotech), 1-deoxy-d-xylulose 5-phosphate synthase 2 (DXS2) protein, and extracellular peptide (EP) were obtained from fresh peripheral blood mononuclear cells (PBMC). In brief, PBMC were separated from peripheral blood by density gradient centrifugation on Ficoll-Hypaque (GE Healthcare). The cells were grown in RPMI 1640 medium supplemented with 12% fetal calf serum (FCS), 200 U/ml interleukin-2 (IL-2), penicillin, streptomycin, and 5 × 10−5 M β-mercaptoethanol in a 24-well cell culture plate containing immobilized anti-pan-γδ TCR monoclonal antibody, DXS2 protein, or EP at 37°C in 5% CO2. After 2 weeks of culture, the γδ T cells were sorted by flow cytometry (FACSAria I; BD). The purified cell population contained about 80% viable γδ T cells.
Construction of transfected cells expressing γδ TCR with predominant pulmonary tuberculosis CDR3 sequence and healthy control CDR3 sequence.
A full-length γ9 or δ2 chain was amplified from PBMC cDNA using specific primers containing KpnI and XhoI restriction sites. The predominant pulmonary tuberculosis CDR3 sequence was inserted into full-length γ9 and δ2 chains to substitute for the original CDR3 sequence using overlapping PCR. The full-length TCR chain was cloned into pREP7 and pREP9 expression vectors with hygromycin and neomycin resistance, respectively. Meanwhile, full-length pREP7-γ9 and pREP9-δ2 chains with healthy control γ9 and δ2 CDR3 sequences were also constructed in the same way and were used as healthy controls. The J.RT3-T3.5 cells (1.2 × 107) were cotransfected with 20 μg of pREP7-γ9 and pREP9-δ2 by electroporation at 260 V and 975 mF using a Bio-Rad Gene-Pulser. After 48 h, the transfected cells were cultured in selection medium with hygromycin and neomycin for 4 weeks. The resulting cells expressing surface γδ TCR were evaluated by flow cytometry with fluorescein isothiocyanate (FITC)-conjugated anti-human γ9 (clone B3; BD) and phycoerythrin (PE)-conjugated anti-human δ2 (clone B6; BD) monoclonal antibodies. The doubly positive cells were isolated by flow sorting for further experiments (FACSAria I). Thus, we developed two artificial cell lines expressing γδ TCR with dominant pulmonary tuberculosis CDR3 sequences and healthy control CDR3 sequences; they are designated PT-transfected cells and HC-transfected cells, respectively.
Peptide synthesis, labeling, protein expression, and preparation of H37Rv soluble extracts.
The peptides were synthesized in the peptide synthesis facility of the Academy of Military Medical Sciences, China. The purity of synthesized peptides was more than 90% according to high-performance liquid chromatography (HPLC) analysis. Half of the synthesized peptides were labeled with biotin at their N-terminal ends. Purified full-length 1-deoxy-d-xylulose 5-phosphate synthase (DXS2) amplified from H37Rv genomic DNA by PCR was digested with KpnI and XhoI enzymes and linked to prokaryotic expression plasmid pET30a. Freshly transformed Escherichia coli BL21(DE3) cells harboring plasmid pET30a-dxs2 were cultured in 500 ml of LB medium containing kanamycin at 37°C. When the optical density of the cell at 600 nm (OD600) reached 0.8 to 1.0, isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma) was added to a final concentration of 0.8 mM, and the bacteria were cultured for another 3 h at 37°C. The culture medium was then harvested and centrifuged at 5,000 × g for 15 min at 4°C. The inclusion body was dissolved in 8 M urea and then purified with a HisTrap column (Amersham Biosciences). The endotoxins were removed roughly using Triton X-114. The DXS2 protein molecular mass was 64 kDa and the purity was over 90% according to the results of SDS-PAGE on 12% acrylamide gels. LC3 protein, an autophagy detection marker used as the control protein, was obtained using the same expression system. The H37Rv bacteria were heat inactivated at 85°C for 20 min, and then the bacteria were subjected to 5 h of sonication at room temperature in a water bath sonicator. The extracts were then spun at 13,000 × g for 5 min and the supernatants collected.
Sequence of TCR Vγ9δ2 gene from DXS2- and EP-expanded γδ T cells.
To determine the amino acid sequence characteristics of CDR3 of DXS2- and EP-expanded T cells, we cloned and sequenced TCRVγ9δ2 cDNA of DXS2- and EP-expanded T cells. Total RNA was harvested by following the Qiagen RNeasy protocol. One microgram of total RNA was then converted into cDNA using a reverse transcription system kit (Qiagen, Germany). Primer sequences complementary to upstream V regions and downstream C regions were used to amplify CDR3 regions. The primer sequences are from the report of Xu et al. (16), and in the current study they are designated the following: TCRγ9CDR3-up, 5′-AATGTAGAGAAACAGGAC-3′; TCRγ9CDR3-down, 5′-ATCTGTAATGATAAGCTTT-3′; TCRδ2CDR3-up, 5′-GCACCATCAGAGAGAGATGAAGGG-3′; and TCRδ2CDR3-down, 5′-AAACGGATGGTTTGGTATGAGGC-3′. The purified PCR fragments were ligated into the pGEM-T Easy vector (Invitrogen), and the resulting plasmids were transfected into DH5a-competent Escherichia coli. The plasmid DNAs from the resulting clones were sequenced using an ABI Prism 3700 Genetic Analyzer with the T7 primer and analyzed by using DNAman software.
Flow cytometry.
For binding of TP1, TP2, and TP3 to cells, the transfected cells or γδ T cells were incubated with biotin-conjugated peptide at 4°C for 30 min. FITC-conjugated streptavidin (Pierce) was then added and incubated for another 30 min. The percentage of γδ T cells was measured 2 weeks later by fluorescence-activated cell sorting (FACS) using FITC-αβ TCR (clone WT31; BD) and PE-γδ TCR. Flow cytometry was performed using a FACSCantoII flow cytometer (BD), and data were analyzed by using FlowJo software (Treestar, San Carlos, CA).
ELISA.
For enzyme-linked immunosorbent assay (ELISA), different doses of biotin-conjugated peptides and control peptide were cocultured with HC-transfected cells, PT-transfected cells, and PT-transfected cells blocked with functional γδ TCR-blocking monoclonal antibody (10 mg/ml B1.1; eBioscience, San Diego, CA) at 37°C for 1 h. Horseradish peroxidase (HRP)-conjugated streptavidin was then added and incubated for another 1 h. The absorbance value at 450 nm was used to determine the binding of peptide to the cells. γδ T cells secreted cytokines and chemokines upon activation by DXS2 protein and EP were determined by ELISA (RD) according to the manufacturer's instructions.
CCK-8 assay.
The proliferation of γδ T cells was detected by the cell counting kit-8 (CCK-8) assay (KeyGEN). After the γδ T cells expanded by peptide and protein were isolated by flow sorting, 1 × 105 cells per 100 ml were inoculated into a 96-well plate, immobilized with different amounts of peptide/protein, and incubated for 4 h. CCK-8 reagent (10 ml) was added to each well, and the culture was continued for another 4 h. The absorbance value at 450 nm of each well was measured using a microplate reader (SpectraMax M5).
MTT assay.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric test was used to evaluate the cytotoxic effects of γδ T cells on THP-1 cells infected with BCG in vitro. The BCG strain was grown in Middlebrook 7H9 liquid medium (Invitrogen) with 10% albumin/dextrose-catalase (ADC) enrichment media (Sigma) supplemented with 0.2% glycerol. THP-1 cells were infected with BCG strains at a multiplicity of infection (MOI) of approximately 10 bacterial cells per macrophage. The BCG-infected THP-1 cells were used as target cells and seeded into 96-well plates at 104 cells per well. The γδ T cells, which were used as effector cells, were added to each well with ratios of effector cells to target cells of 1:1, 2.5:1, and 5:1. After the effector cells and the target cells were incubated at 37°C for 4 h, 15 ml of MTT solution (5 mg/ml) was added to each well, and they were incubated at 37°C for an additional 4 h. The reaction was stopped by the addition of 100 ml dimethyl sulfoxide to dissolve the tetrazolium crystals. The plate was examined at 570 and 630 nm on a microplate reader (SpectraMax M5), and the percentage of specific lysis was calculated.
Real-time PCR.
DXS2 protein and EP were precoated on microtiter plates. γδ T cells from pulmonary tuberculosis patients and healthy controls were added. Cells were collected after 24 h, and total RNA was harvested by following the Qiagen RNeasy protocol. One microgram of total RNA was then converted into cDNA using a reverse transcription system kit (Qiagen, Germany). One μl of the resulting cDNA was used in real-time PCR with Power Sybr green PCR master mix (Applied Biosystems). The following primers were used: CCR2 sense, 5′-AACATGCTGTCCACATCTCGTTCT-3′; CCR2 antisense, 5′-AACATGCTGTCCACATCTCGTTCT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense, 5′-TGGGCTACACTGAGCACCAG-3′; and GAPDH antisense, 5′-AAGTGGTCGTTGAGGGCAAT-3′. The reaction was carried out on a 7900HT Fast Real-Time PCR machine (Life Technologies, CA), and the result was analyzed with RQ manager software.
Bioinformatic analysis.
Homologous analysis and sequence alignment were done using the BLAST program to determine matched protein to TP1 and TP2. The NCBI protein BLAST program was selected for bioinformatic analysis (http://www.ncbi.nlm.nih.gov/blast). The first three matched proteins were listed. The online software HMMTOP (http://www.enzim.hu/hmmtop/) was used to analyze the transmembrane region of the unknown transmembrane protein (Rv2272).
Statistical analysis.
Statistical comparisons were performed using the Student t test. GraphPad Prism 5 (GraphPad software) was used to generate and perform statistical analyses on the data for cytokine secretion. A P value of <0.05 was considered statistically significant.
RESULTS
Biopanning of γδ TCR-recognized peptides using cells transfected with the dominant pulmonary tuberculosis CDR3 sequence.
Based on our previous findings (15), the specific CDR3 sequences CALWEVISELGKKIK and CACDTLVSTDKLIFGKG, for γ9 and δ2, respectively, are dominant in pulmonary tuberculosis patients. Thus, we developed two artificial cell lines expressing γδ TCRs from pulmonary tuberculosis patients, PT-transfected cells and HC-transfected cells (Fig. 1; also see the supplemental material). To identify the epitopes recognized by PT-transfected cells, we performed γδ TCR cell-mediated biopanning of a 12-mer random peptide phage display library. The peptides identified in this screen are listed in Table 1. Three prominent peptides were selected as the γδ TCR-recognized peptide candidates based on their high appearance frequency. These three peptides, designated TP1, TP2, and TP3, were then chemically synthesized for further analysis.
Fig 1.
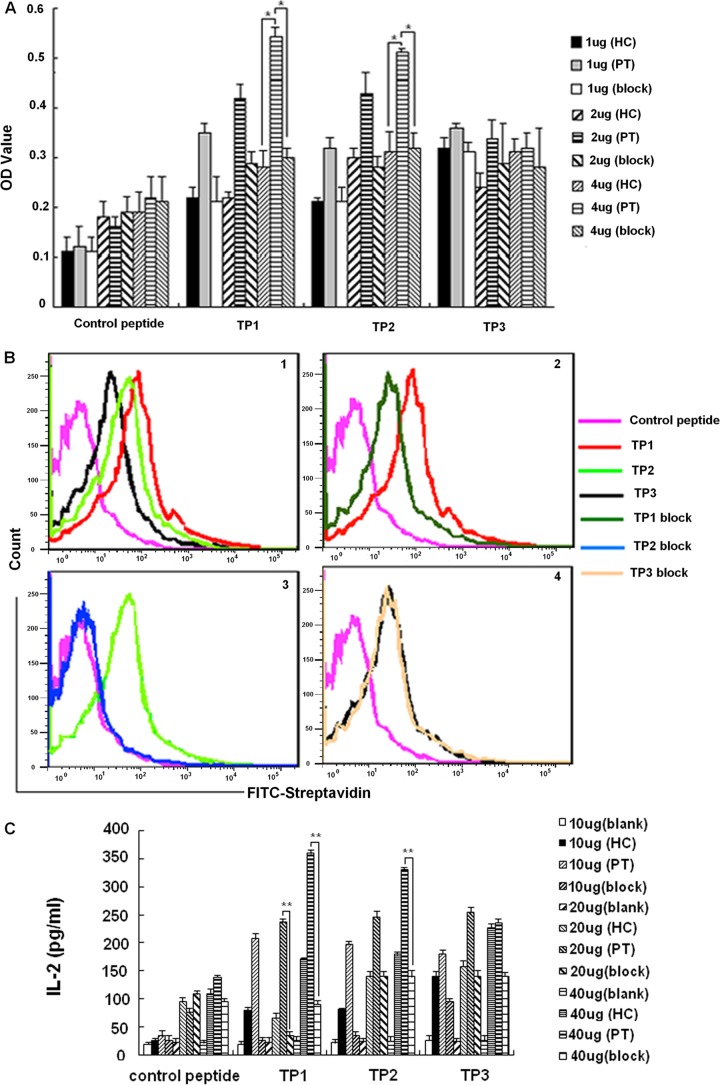
Confirmation of peptides capable of binding to PT-transfected cells. (A) Peptide binding and blocking with the transfected cells. Data shown are the means from three independent experiments. (B) Peptide binding and blocking assay by FACS. Graph 1 shows the binding percentage of control peptide and three peptides with PT-transfected cells (control peptide, 3%; TP1, 20.6%; TP2, 16.4%; TP3, 10.2%). Graphs 2 to 4 show the binding percentage of TP1, TP2, and TP3 with PT-transfected cells or preblocked PT-transfected cells, respectively (TP1 block, 11.4%; TP2 block, 4.8%; TP3 block, 10%). Results are representative of three independent experiments. (C) Peptide binding and blocking assay by detecting the production of IL-2. Data shown are the means from three independent experiments.
Table 1.
Amino acid sequences of 12 peptides
| Phage clone no. | Name | Sequencea | Frequency |
|---|---|---|---|
| 2/7/9/13/20/21/22/23/28 | TP1 | GDFDTGHQTTTR | 9/30 |
| 3/4/10/19/25/27 | TP2 | GLQAERTTAWTR | 6/30 |
| 5/6/18/30 | TP3 | TLILAHAPSGFQ | 4/30 |
| 1/8/11/15 | TP4 | AETVESCLAKSH | 4/30 |
| 12/24/26 | TP5 | LPHGYKIQRWRS | 3/30 |
| 14/29 | TP6 | HPETDASDDVDR | 2/30 |
| 16 | TP7 | YPFDKHPVPSRP | 1/30 |
| 17 | TP8 | GQSPHSYQPRTY | 1/30 |
The control peptide sequence was HGSQRPTRSYKI .
Confirmation of the peptides capable of binding to PT-transfected cells.
Binding assays and blocking assays were performed to determine whether the synthesized peptides bound specifically to PT-transfected cells. Different doses of biotin-conjugated peptides were cocultured with HC-transfected cells, PT-transfected cells, and PT-transfected cells blocked with a functional γδ TCR-blocking monoclonal antibody. The results showed that TP1 and TP2 specifically bound to the PT-transfected cells (Fig. 1A). The binding specificity of both TP1 and TP2 to the PT-transfected cells was further confirmed by flow-cytometric analysis (Fig. 1B). We also examined IL-2 secretion after the transfected cells were stimulated by the three putative peptides. All of the peptides stimulated PT-transfected cells to secrete IL-2 in a dose-dependent manner. Importantly, the IL-2 production by PT-transfected cells stimulated by TP1 and TP2 was significantly blocked by the functional γδ TCR-blocking monoclonal antibody at some doses (Fig. 1C). Collectively, these data suggested that the binding of TP1 and TP2 to PT-transfected cells was specific.
The artificial γδ TCR-identified peptides TP1 and TP2 bound and activated γδ T cells from pulmonary tuberculosis patients in vitro.
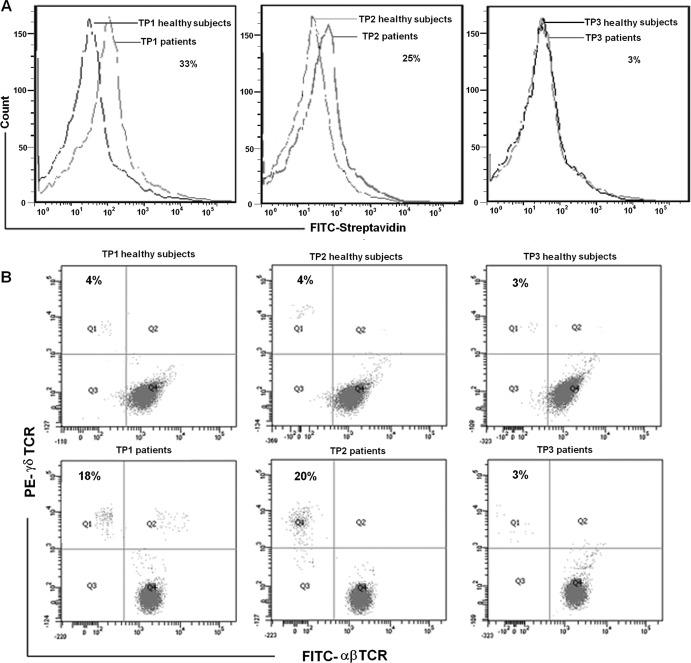
To investigate whether natural γδ T cells could recognize and become activated by the identified peptides, we investigated the binding capability of the peptides to γδ T cells isolated from pulmonary tuberculosis patients and healthy controls. The capacity of these peptides to induce the natural γδ T cell proliferation was also assessed. The results showed that 33% of γδ T cells from pulmonary tuberculosis patients bound TP1 and 25% of γδ T cells from pulmonary tuberculosis patients bound TP2 compared to the binding with γδ T cells from healthy controls (Fig. 2A). In addition, TP1 and TP2 were shown to induce the expansion of γδ T cells in PBMC from pulmonary tuberculosis patients to an average of 18 and 20%, respectively, which were significantly higher than the 4% expansion of γδ T cells in PBMC from healthy controls after coculture for 2 weeks (Fig. 2B).
Fig 2.
Artificial γδ TCR identified peptides of TP1- and TP2-bound and activated γδ T cells from pulmonary tuberculosis patients in vitro. (A) TP1- and TP2-bound γδ T cells from pulmonary tuberculosis patients in vitro. The cells were incubated with biotin-conjugated peptide or control peptide and FITC-conjugated streptavidin and then were subjected to FACS analysis. Results are representative of three independent experiments. (B) Immobilized TP1 and TP2 were able to induce expansion of γδ T cells from pulmonary tuberculosis patients. PBMC from pulmonary tuberculosis patients and healthy controls were cultured in 24-well plates with immobilized TP1, TP2, and TP3 in RPMI 1640 medium with 10% fetal bovine serum and IL-2. The percentage of γδ T cells was measured 2 weeks later by FACS using FITC-αβ TCR (clone WT31; BD) and PE-γδ TCR (clone 11F2; BD). Results are representative of three independent experiments.
BLAST analysis.
A protein BLAST search was performed to identify mycobacterial proteins containing the peptides of TP1 and TP2. The results are listed in Table 2. Upon sequence analysis, the peptide TP1 had the best matching characteristics to protein in Mycobacterium, with an E value of 26.1. The matching protein of TP1 was 1-deoxy-d-xylulose 5-phosphate synthase 2 (DXS2) in M. tuberculosis TB 02_1987, M. tuberculosis TB T46, and M. tuberculosis TB 98-R604. The peptide TP2 had the best matching characteristics to a protein in Mycobacterium, with an E value of 37.5. The matching protein of TP2 was the transmembrane protein Rv2272 in M. tuberculosis H37Rv.
Table 2.
BLAST analysis of candidate peptide
| No. | Reference | Protein | Species | E value | Matching part |
|---|---|---|---|---|---|
| TP1 | ZP_06506579.1 | 1-Deoxy-d-xylulose 5-phosphate synthase dxs2 | M. tuberculosis 02_1987 | 26.1 | --FDTGHQT--- |
| TP1 | ZP_05770037.1 | 1-Deoxy-d-xylulose 5-phosphate synthase dxs2 | M. tuberculosis T46 | 26.1 | --FDTGHQT--- |
| TP1 | ZP_05142929.1 | 1-Deoxy-d-xylulose 5-phosphate synthase dxs2 | M. tuberculosis 98-R604 INH-RIF-EM | 26.1 | --FDTGHQT--- |
| TP2 | ZP_05773066.1 | Hypothetical protein MtubK8_14869 | M. tuberculosis K85 | 37.5 | GLQAERTTAWTR |
| TP2 | ZP_04925745.1 | Hypothetical protein TBCG_02219 | M. tuberculosis C | 37.5 | GLQAERTTAWTR |
| TP2 | NP_216789.1 | Transmembrane protein | M. tuberculosis H37Rv | 37.5 | GLQAERTTAWTR |
The identified peptide TP1, matched to mycobacterium protein DXS2, bound and activated γδ T cells in vitro.
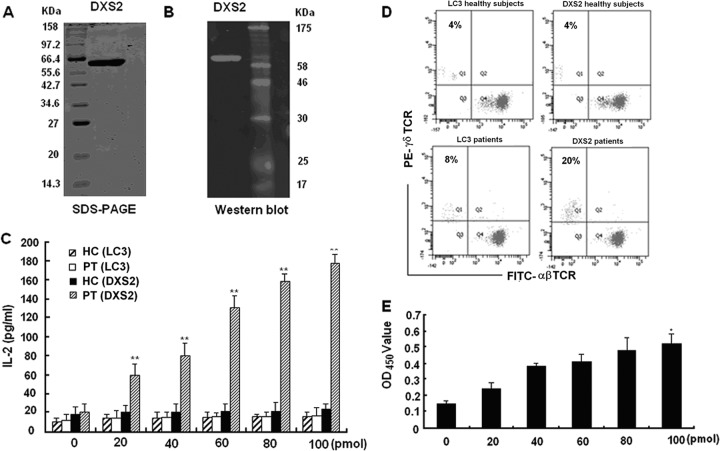
We examined the binding of DXS2 protein to transfected γδ T cells as well as its ability to activate natural γδ T cells. We expressed the full-length DXS2 protein with a His tag in E. coli. The purity of the DXS2 protein was determined by SDS-PAGE (Fig. 3A) and confirmed by Western blotting with an anti-His tag monoclonal antibody (Fig. 3B). Production of IL-2 in the medium of PT-transfected cells was enhanced in a dose-dependent manner after stimulation with DXS2 protein (Fig. 3C). Furthermore, addition of DXS2 protein induced expansion of γδ T cells in PBMC from pulmonary tuberculosis patients to an average of 20% after coculture for 2 weeks, which was significantly higher than the 8% expansion of γδ T cells in PBMC from healthy controls (Fig. 3D). The TCR of the γδ T cells expanded by DXS2 was Vγ9δ2 (see Fig. S2 in the supplemental material). The predominant sequence contained the original predominant CDR3 sequence from pulmonary tuberculosis patients (see Table S1 and S2). The proliferation of γδ T cells stimulated by DXS2 protein was also confirmed by the CCK-8 assay (Fig. 3E). Together, these data demonstrated that DXS2 protein bound to the γδ TCR and activated γδ T cells from pulmonary tuberculosis patients.
Fig 3.
DXS2 protein bound and activated γδ T cells in vitro. (A) The expression of DXS2 protein in E. coli was confirmed by 12% SDS-PAGE. (B) The expression of DXS2 protein was further confirmed by Western blotting using anti-His tag antibody. (C) DXS2 protein stimulated the PT-transfected cells to produce IL-2 in a dose-dependent manner. LC3 protein, an autophagy detection marker, was used as the control protein. The double asterisks denote significant differences (P < 0.01). Data are shown as the means from three independent experiments. (D) DXS2 protein induced the expansion of γδ T cells from pulmonary tuberculosis patients. The percentage of γδ T cells was measured 2 weeks later by FACS using FITC-αβ TCR (clone WT31; BD) and PE-γδ TCR (clone 11F2; BD). Results are representative of three independent experiments. (E) Detection of γδ T cell proliferation induced by DXS2 with CCK-8 assay. The single asterisk denotes significant difference (P < 0.05). Data shown are the means from three independent experiments.
The extracellular region of the identified peptide TP2, matched with mycobacterium protein Rv2272, bound and activated γδ T cells in vitro.
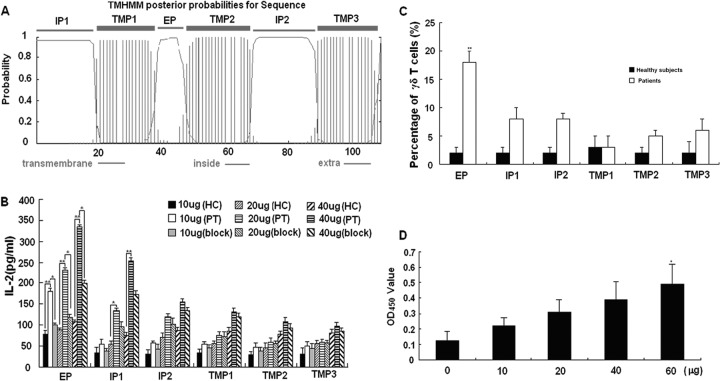
TP2 was the best match to Rv2272, but because of the high GC content, we were unable to express the full-length protein. In order to explore its function, we predicted the transmembrane region by using the online software HMMTOP (http://www.enzim.hu/hmmtop/), and every region of Rv2272 was synthesized. The prediction results are shown in Fig. 4A. The extramembrane, intramembrane, and transmembrane region peptides were designated EP (10 amino acids [aa]), IP1 (19 aa), IP2 (20 aa), TMP1 (19 aa), TMP2 (19 aa), and TMP3 (17 aa). The results showed that EP and IP1 stimulated PT-transfected cells to secrete IL-2 in a dose-dependent manner (Fig. 4B). EP also induced the expansion of γδ T cells in PBMC from pulmonary tuberculosis patients to an average of 18% after coculture for 2 weeks, which is significantly higher than the 2% expansion of γδ T cells in PBMC from healthy controls (Fig. 4C). The proliferation of γδ T cells stimulated by EP was further confirmed by the CCK-8 assay (Fig. 4D). The TCR of EP-expanded γδ T cells also was Vγ9δ2 (data not shown). The predominant sequence contained the original predominant CDR3 sequence in pulmonary tuberculosis patients (see Table S3 and S4 in the supplemental material). Taken together, these data demonstrated that the EP bound to γδ TCR and activated γδ T cells from pulmonary tuberculosis patients.
Fig 4.
Extracellular region of Rv2272 bound and activated γδ T cells in vitro. (A) The predicted transmembrane region of Rv2272. (B) The IL-2 production of PT-transfected cells stimulated by every region of Rv2272. EP and IP1 could stimulate PT-transfected cells to produce IL-2 in a dose-dependent manner. The single asterisk denotes significant differences (P < 0.05). The double asterisks denote significant differences (P < 0.01). Data are the means from three independent experiments. (C) EP of Rv2272 induced the expansion of γδ T cells from pulmonary tuberculosis patients. The percentage of γδ T cells was measured 2 weeks later by FACS using FITC-αβ TCR (clone WT31; BD) and PE-γδ TCR (clone 11F2; BD). The percentage of γδ T cells is shown in the form of a histogram. Data are shown as the mean percentages of γδ T cells ± standard deviations from two independent experiments. (D) Detection of γδ T cell proliferation induced by EP with CCK-8 assay. Data are the means from three independent experiments.
The effector function of γδ T cells from pulmonary tuberculosis patients induced by DXS2 and EP.
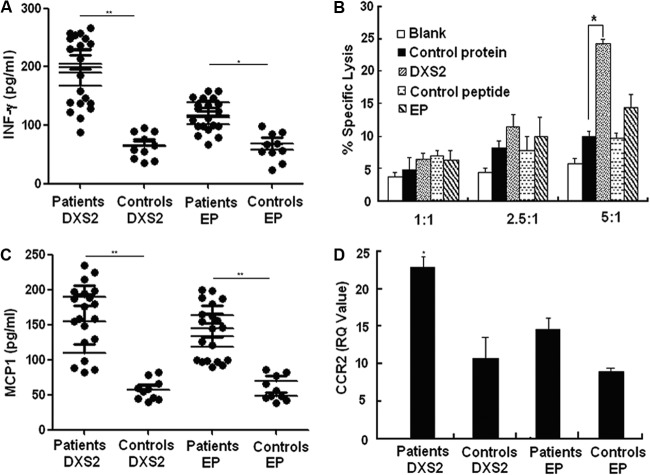
To investigate the immune function of DXS2- and EP-activated γδ T cells, we evaluated cytokine production, cytotoxicity, and chemotaxis. The results showed that the production of gamma interferon (IFN-γ) by γδ T cells from 20 pulmonary tuberculosis patients induced by DXS2 and EP was higher than that by γδ T cells from healthy controls (Fig. 5A). Furthermore, DXS2 protein-activated γδ T cells were shown to lyse BCG-infected THP-1 cells (Fig. 5B), but EP-activated γδ T cells did not show a similar effect. It was also found that the production of CCL-2 (MCP-1) by γδ T cells from 20 pulmonary tuberculosis patients induced by DXS2 and EP was also higher than that produced by γδ T cells from healthy controls (Fig. 5C). As MCP-1 binds to and activates leukocytes preferentially through interaction with CCR2, we examined the CCR2 expression on DXS2- and EP-stimulated γδ T cells from TB patients and healthy control subjects. We found that DXS2 protein significantly increased the expression of CCR2 on γδ T cells from TB patients compared to that expressed by γδ T cells from healthy subjects (Fig. 5D). Although the effect of EP on CCR2 expression was not as significant as that of DXS2, a trend of increased CCR2 expression on γδ T cells from TB patients was still apparent compared to that of healthy subjects. Collectively, these results suggested that γδ T cells activated by DXS2 protein and EP perform the function against M. tuberculosis through enhancing Th1 immune responses and/or cytolytic lymphocyte (CTL) activity and chemotaxis toward the site of infection.
Fig 5.
Effector function of γδ T cells induced by DXS2 and EP from pulmonary tuberculosis patients. (A) Immobilized DXS2 and EP were precoated on microtiter plates. γδ T cells from 20 pulmonary tuberculosis patients and 10 healthy controls were incubated. Supernatants collected after 48 h were measured for IFN-γ secretion. Data are shown as the means from three independent experiments. The single asterisk denotes significant difference at P < 0.05. The double asterisks denote significant difference at P < 0.01. (B) The cytotoxicity of γδ T cells induced by DXS2 to THP-1 infected with BCG. After sorting and culturing the γδ T cells from pulmonary tuberculosis patients inoculated with EP and DXS2, an MTT test was used to evaluate the cytotoxicity activities of γδ T cells to THP-1 cells infected with BCG in vitro. Data are the means from three independent experiments. (C) Immobilized DXS2 and EP were precoated on microtiter plates. γδ T cells from 20 pulmonary tuberculosis patients and 10 healthy controls were incubated. Supernatants collected after 48 h were measured for MCP-1 secretion. Data are the means from three independent experiments. (D) Immobilized DXS2 and EP were precoated on microtiter plates. γδ T cells from a mix of pulmonary tuberculosis patients and healthy controls were incubated. Cells were collected after 24 h, and real-time PCR was performed to detect the production of CCR2. Data are shown as the means from three independent experiments.
DISCUSSION
In this study, we identified one mycobacterium protein, DXS2, and one peptide, EP, recognized by γδ T cells. Both DXS2 and EP bound to the γδ TCR with the predominant pulmonary tuberculosis CDR3 sequence expressed by transfected cells and stimulated the expansion of γδ T cells from pulmonary tuberculosis patients. Furthermore, DXS2 protein and EP stimulated the production of IFN-γ and monocyte chemoattractant protein 1 (MCP-1) by γδ T cells from most pulmonary tuberculosis patients.
The known ligands for γδ T cells include nonpeptidic phosphoantigens, smaller peptides, major histocompatibility complex (MHC)-like molecules, and MHC-unrelated protein antigens. The diversity of these ligands likely precludes a single recognition mechanism akin to that of MHC-restricted αβ T cells. There has been accumulating evidence that phosphoantigens are presented on the surface of γδ T cells for recognition (17, 18). The recognition depends on all CDRs, including CDR1, CDR2, and CDR3 (19). For small-peptide and MHC-like molecules, γδ TCR could directly recognize them without the help of the antigen-presenting cells (APCs) (20, 21). The crystallographic structure of γδ TCR showed that CDR3δ might be in direct contact with peptide and MHC-like protein and served as a key determinant for the specificity of antigen recognition (22, 23). Our results regarding EP also indirectly proved the abovementioned recognition characteristics, as the EP was screened by using the γδ TCR-transfected cells containing the preponderant CDR3 sequence (CACDTLVSTDKLIFGKG) in pulmonary tuberculosis patients (15). Furthermore, the γδ T cells from pulmonary tuberculosis patients expanded by EP were confirmed to contain this preponderant CDR3 sequence (see Table S4, asterisk, in the supplemental material), demonstrating EP-expanded γδ T cell recognition dependent on this CDR3. An in-depth study is necessary to determine if direct contact exists between CDR3δ and EP. Recently, MHC-unrelated protein antigens recognized by γδ T cells have been identified, such as herpes simplex virus 1-glycoprotein I (24), heat shock proteins (25), F1-ATPase (26), and MHS-2 (27). He et al. demonstrated that the recognition of γδ T cells by MHS-2 depended on the γδ TCR and another activating receptor, NKG2D (28, 29). DXS2 protein is also a protein that is unrelated to MHC. To explore the relative contribution of γδ TCR and NKG2D in DXS2-induced activation of γδ T cells, the IFN-γ production of DXS2-activated γδ T cells was detected (see Fig. S3 in the supplemental material). We found that the γδ TCR-blocking antibody and a chemical inhibitor of γδ TCR (cyclosporine) caused a large reduction (60 to 70% inhibition) in IFN-γ production in DXS2-stimulated Vγ9δ2 cells. NKG2D blocking antibody and a chemical inhibitor of the NKG2D pathway (Wortmannin) have no blocking effect. In addition, like EP, γδ T cells from pulmonary tuberculosis patients expanded by DXS2 protein also contain original, preponderant CDR3 sequence (see Table S2, asterisk), suggesting that the activation of γδ TCR by DXS2 is γδ TCR dependent but not NKG2D dependent. Other activating receptors, such as Toll-like receptors (TLRs), CD16, CD266, and CD28, also may provide costimulator signals for γδ T cell activation by different non-MHC protein ligands (30). Whether these receptors also contribute to the recognition of DXS2 by γδ TCR is worth further investigation.
The major immunological effector functions of γδ T cells in response to M. tuberculosis are mediated mainly through secreted cytokines, effector CTL, and chemotaxis. Numerous studies have demonstrated that γδ T cells produce cytokines that function against tuberculosis infection, such as those categorized as T helper type 1 (Th1), Th2, Th17, or T-regulatory (IL-10)-type cytokines (31–33). Among these, IFN-γ is a key cytokine in the control of M. tuberculosis infection, and it activates further effector cells and cytotoxic T cells that are responsible for lysis or inhibition of M. tuberculosis (34, 35). Tumor necrosis factor alpha (TNF-α) induces a large number of immune cells to migrate toward an infection site and plays an important role in preventing the spread of M. tuberculosis outside the surrounding infection site (36). TNF-α also enhances immune cell migration and positioning in the presence of M. tuberculosis and influences the expression of adhesion molecules which facilitate the formation of nodular tuberculosis (37). The Th2 cytokine IL-4 can impair bactericidal activity and lead to toxicity of TNF-α and to pulmonary fibrosis (38). IL-10 has been associated with suppressive immunity, and overproduction of IL-10 increases susceptibility to mycobacterial infection (39). IL-10 has also been reported to inhibit proliferation and IL-2 production by activated T cells by downregulating the MHC molecules (40). IL-17-producing γδ T cells may be involved in immunity to M. tuberculosis infection or the pathological course of pulmonary tuberculosis (41). Our results demonstrated that IFN-γ production by γδ T cells from pulmonary tuberculosis patients was induced by DXS2, and the EP level was significantly higher than that produced by γδ T cells from healthy controls (Fig. 5A), although there were no significant differences in the production of other cytokines between pulmonary tuberculosis patients and healthy controls (data not shown). Thus, DXS2- and EP-stimulated Vγ9Vδ2 T cells have potential as Th1-biasing adjuvants for immunotherapy of pulmonary tuberculosis.
We then determined the cytotoxicity of γδ T cells activated by DXS2 protein and EP against M. tuberculosis. It was found that the γδ T cells expanded by DXS2 protein exerted cytotoxic effects on THP-1 cells infected with BCG (Fig. 5B), although EP-activated γδ T cells did not exert cytotoxic effects. Poccia et al. (42) reported that the cytokines participating in the cytotoxic effector function of γδ T cells against tuberculosis infection were IFN-γ, IL-12, and TNF-α. Although no significant differences were found in IL-12 and TNF-α production by γδ T cells from pulmonary tuberculosis patients and healthy controls, the IFN-γ produced by DXS2 protein-activated γδ T cells was much higher than that produced by EP-activated γδ T cells from pulmonary tuberculosis (Fig. 5A). This might, to some extent, account for the lack of cytotoxic activity against BCG-infected THP-1 cells exhibited by EP-activated γδ T cells despite the functional activity exhibited by DXS2-activated γδ T cells. This discrepancy in the activity exhibited by the two types of activated γδ T cells may be accounted for by EP being a short peptide that has fewer antigen recognition sites, leading to lower cytolytic capacity of EP-activated γδ T cells compared to that of cells activated by DXS2 protein (Fig. 3B and 4B). However, only 20 pulmonary tuberculosis patients were recruited into this study, and further investigations conducted with larger sample sizes are required to verify these hypotheses.
Finally, we investigated chemokine production by γδ T cells activated by DXS2 protein and EP. In this study, it was found that the production of MCP-1 by γδ T cells from 20 pulmonary tuberculosis patients stimulated with DXS2 and EP was higher than that by γδ T cells from healthy controls. MCP-1 has potent chemotactic and activating properties for monocytes, macrophages, dendritic cells, and CD4+ T cells. Most significantly, MCP-1 was consistently associated with severe disease (43) and thus is implicated as a novel tuberculosis biomarker. However, we found no correlation between MCP-1 production by γδ T cells and the severity of pulmonary tuberculosis among the 20 pulmonary tuberculosis patients included in this study. The further investigations conducted with larger sample sizes are required to evaluate MCP-1 as a tuberculosis biomarker.
MCP-1 binds to and activates leukocytes preferentially through CCR2 (44, 45). Our results confirmed that CCR2 was expressed on γδ T cells induced by DXS2 protein. Although the effect of EP on CCR2 expression was not as significant as that observed in response to DXS2, a trend of increased CCR2 expression by γδ T cells from TB patients was observed compared to expression by γδ T cells from healthy subjects. This finding indicated that γδ T cells respond to M. tuberculosis protein antigen by rapidly releasing high levels of chemokines involved in multitype immune cell recruitment and activation. This adds further support to the hypothesis that γδ T cells are specialized to form part of the early response to infectious agents through the rapid secretion of proinflammatory cytokines and chemokines.
In conclusion, in this study, a mycobacterial protein and peptide recognized by γδ T cells were identified. The activated γδ T cells exhibited cytolytic effector function against BCG-infected cells and played a role in the recruitment and activation of other immune cells involved in antimycobacterium responses. Our findings add to our understanding of the mechanism by which γδ TCR recognizes protein antigens and represent the basis of research into the screening and development of new antituberculosis vaccines or adjuvants.
ACKNOWLEDGMENTS
This work was sponsored by grants 2008ZX10003-012 and 2009ZX10004-303 from the Eleven-Fifth Mega-Scientific Project on prevention and treatment of AIDS, viral hepatitis, and other infectious diseases from China, grant 2012ZX10003002 from the Twelfth-Fifth Mega-Scientific Project on prevention and treatment of AIDS, viral hepatitis, and other infectious diseases from China, grant 30901314 from the National Natural Science Foundation of China, and a grant (2008IPB207) for basic research and development expenses from the Institute of Pathogen Biology, Chinese Academy of Medical Sciences.
Footnotes
Published ahead of print 6 February 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00584-12.
REFERENCES
- 1.World Health Organization 2008. Stop TB partnership. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.World Health Organization 2011. Global tuberculosis control. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Brewer TF, Colditz GA. 1995. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin. Infect. Dis. 20:126–135 [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702 [PubMed] [Google Scholar]
- 5.Ginsberg AM. 1998. The tuberculosis epidemic. Scientific challenges and opportunities. Public Health Rep. 113:128. [PMC free article] [PubMed] [Google Scholar]
- 6.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova JL. 1996. Interferon-γ receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956–1961 [DOI] [PubMed] [Google Scholar]
- 7.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra Williamson BAR, Levin M. 1996. A mutation in the interferon-g-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941–1949 [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Li BQ. 2009. Phenotype expression and function of antigen presenting cells in human γδ T cells activated by peptide antigen from Mycobacterium tuberculosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 25:588–591 [PubMed] [Google Scholar]
- 9.Zhang RJ, Zheng XD, Li BQ, Wei HM, Tian ZG. 2006. Human NK cells positively regulate γδ T cells in response to Mycobacterium tuberculosis. J. Immunol. 176:2610–2616 [DOI] [PubMed] [Google Scholar]
- 10.Spencer CT, Abate G, Blazevic A, Hoft DF. 2008. Only a subset of phosphoantigen-responsive γδ T cell mediated protective tuberculosis immunity. J. Immunol. 181:4471–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandes M, Willimann K, Bioley G, Levy N, Eberl M, Luo M, Tampé R, Lévy F, Romero P, Moser B. 2009. Cross-presenting human γδ T cells induce robust CD8+ αβ T cell responses. Proc. Natl. Acad. Sci. U. S. A. 106:2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Wu CY. 2008. CD4+CD25+Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M tuberculosis antigen ESAT-6. Blood 111:5629–5636 [DOI] [PubMed] [Google Scholar]
- 13.Price SJ, Hope JC. 2009. Enhanced secretion of interferon-γ by bovine γδ T cells induced by coculture with Mycobacterium bovis-infected dendritic cells: evidence for reciprocal activating signals. Immunology 126:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xi XY, Zhang XY, Wang B, Wang J, Huang He Han XQ, Li L, Cui LX, He W, Zhao ZD. 2011. A novel strategy to screen Bacillus Calmette-Guérin protein antigen recognized by γδ TCR. PLoS One 6(4):e18809 doi:10.1371/journal.pone.0018809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi XY, Han XQ, Li L, Zhao ZD. 2011. γδ T cells response to mycobacterium tuberculosis in pulmonary tuberculosis patients using preponderant complementary determinant region 3 sequence. Ind. J. Med. Res. 34:356–361 [PMC free article] [PubMed] [Google Scholar]
- 16.Xu CP, Zhang HY, Hu HB, He HB, Wang Z, Xu Y, Chen H, Cao W, Zhang S, Cui L, Ba D, He W. 2007. γδ T cells recognize tumor cells via CDR3 region. Mol. Immunol. 44:302–310 [DOI] [PubMed] [Google Scholar]
- 17.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR, Golan DE, Brenner MB. 1995. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity 3:495–507 [DOI] [PubMed] [Google Scholar]
- 18.Sarikonda G, Wang H, Puan KJ, Liu XH, Lee HK, Song Y, Distefano MD, Oldfield E, Prestwich GD, Morita CT. 2008. Photoaffinity antigens for human γδ T cells. J. Immunol. 181:7738–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Fang Z, Morita C. 2010. Vγ2Vδ2 T cell receptor recognition of prenyl pyrophosphates is dependent on all CDRs. J. Immunol. 184:6209–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien RL, Fu YX, Cranfill R, Dallas A, Reardon C, Lang J, Carding SR, Kubo R, Born W. 1992. Heat shock protein Hsp-60 reactive γδ cells: a large, diversified T lymphocyte subset with highly focused specificity. Proc. Natl. Acad. Sci. U. S. A. 89:4348–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien YH, Hampl J. 2000. Antigen-recognition properties of murine gamma delta T cells. Springer Semin. Immunopathol. 22:239–250 [DOI] [PubMed] [Google Scholar]
- 22.Adams EJ, Chien Garcia Y-HKC. 2005. Structure of a cd T cell receptor in complex with the nonclassical MHC T22. Science 308:227–231 [DOI] [PubMed] [Google Scholar]
- 23.Chien YH, Konigshofer Y. 2007. Antigen recognition by γδ T cells. Immunol. Rev. 215:46–58 [DOI] [PubMed] [Google Scholar]
- 24.Sciammas R, Bluestone JA. 1998. HSV-1 glycoprotein I-reactive TCR gamma delta cells directly recognize the peptide backbone in a conformationally dependent manner. J. Immunol. 161:5187–5192 [PubMed] [Google Scholar]
- 25.Zhang H, Hu H, Jiang X, He H, Cui L, He W. 2005. Membrane HSP70: the molecule triggering gammadelta T cells in the early stage of tumorigenesis. Immunol. Investig. 34:453–468 [DOI] [PubMed] [Google Scholar]
- 26.Scotet E, Martinez LO, Grant E, Barbaras R, Jenö P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Estève JP, Lopez F, Perret B, Collet X, Bonneville M, Champagne E. 2005. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22:71–80 [DOI] [PubMed] [Google Scholar]
- 27.Chen H, He X, Wang Z, Wu D, Zhang H, Xu C, He H, Cui L, Ba D, He W. 2008. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J. Biol. Chem. 283:12528–12537 [DOI] [PubMed] [Google Scholar]
- 28.Dai Y, Chen H, Mo C, Cui L, He W. 2012. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human γδ T cells to induce innate anti-tumor/virus immunity. J. Biol. Chem. 287:16812–16819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo C, Dai Y, Kang N, Cui L, He W. 2012. Ectopic expression of human MutS homologue 2 on renal carcinoma cells is induced by oxidative stress with interleukin-18 promotion via p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways. J. Biol. Chem. 287:19242–19254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne E. 2011. γδ T cell receptor ligands and modes of antigen recognition. Arch. Immunol. Ther. Exp. 59:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García VE, Sieling PA, Gong J, Barnes PF, Uyemura K, Tanaka Y, Bloom BR, Morita CT, Modlin RL. 1997. Single-cell cytokine analysis of γδ T cell responses to nonpeptide mycobacterial antigens. J. Immunol. 159:1328–1335 [PubMed] [Google Scholar]
- 32.Tsukaguchi K, De Lange B, Boom WH. 1999. Differential regulation of IFN-γ, TNF-α, and IL-10 production by CD4+αβTCR+ T cells and Vδ2 γδ T cells in response to monocytes infected with Mycobacterium tuberculosis-H37Ra. Cell. Immunol. 194:12–20 [DOI] [PubMed] [Google Scholar]
- 33.Wesch D, Glatzel A, Kabelitz D. 2001. Differentiation of resting human peripheral blood γδ T cells toward Th1- or Th2-phenotype. Cell. Immunol. 212:110–117 [DOI] [PubMed] [Google Scholar]
- 34.Dillon SM, Griffin JF, Hart DN, Watson JD, Baird MA. 1998. A long lasting interferon gamma response is induced to a single inoculation of antigen pulsed dendritic cells. Immunology 95:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to mycobacterium tuberculosis infection. J. Exp. Med. 185:2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engele M, Stössel E, Castiglione K, Schwerdtner N, Wagner M, Bölcskei P, Röllinghoff M, Stenger S. 2002. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J. Immunol. 168:1328–1337 [DOI] [PubMed] [Google Scholar]
- 37.Price NM, Gilman RH, Uddin J, Recavarren S, Friedland JS. 2003. Unopposed matrix metalloproteinase-9 expression in human tuberculosis granuloma and the role of TNF alpha dependent monocyte networks. J. Immunol. 171:5579–5586 [DOI] [PubMed] [Google Scholar]
- 38.Rook GA, Hernandez-Pando R, Dheda K, Seah GT. 2004. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 25:483–488 [DOI] [PubMed] [Google Scholar]
- 39.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore KW, de Wall Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765 [DOI] [PubMed] [Google Scholar]
- 41.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 178:3786–3796 [DOI] [PubMed] [Google Scholar]
- 42.Martino A, Casetti R, Sacchi A, Poccia F. 2007. Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guérin-infected dendritic cells kill mycobacterial-infected monocytes. J. Immunol. 179:3057–3064 [DOI] [PubMed] [Google Scholar]
- 43.Hasan Z, Cliff JM, Dockrell HM, Jamil B, Irfan M, Ashraf M, Hussain R. 2009. CCL2 responses to Mycobacterium tuberculosis are associated with disease severity in tuberculosis. PLoS One 4:e8459 doi:10.1371/journal.pone.0008459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cipriani B, Borsellino G, Poccia F, Placido R, Tramonti D, Bach S, Battistini L, Brosnan CF. 2000. Activation of C-C b-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood 95:39–47 [PubMed] [Google Scholar]
- 45.Penido C, Vieira-de-Abreu A, Bozza MT, Castro-Faria-Neto HC, Bozza PT. 2003. Role of monocyte chemotactic protein-1/CC chemokine ligand 2 on γδ T lymphocyte trafficking during inflammation induced by lipopolysaccharide or Mycobacterium bovis bacille Calmette-Guerin. J. Immunol. 171:6788–6794 [DOI] [PubMed] [Google Scholar]