Abstract
Hydatidosis is a public health problem in many parts of the world, and improvement in diagnosis of the disease is still being pursued. Protoscoleces of Echinococcus granulosus were isolated from hydatid cysts collected from naturally infected sheep slaughtered in abattoirs in Iran. Sonicated extract of protoscolex was subjected to two-dimensional gel electrophoresis and Western blot analysis. Primary antibodies were from serum samples from 130 hydatidosis patients, 38 individuals infected with other parasitic infections, and 30 healthy people, whereas peroxidase (HRP)-conjugated anti-human IgG and IgG4 were used as secondary antibodies. The recombinant form of the identified protein was produced and tested for its sensitivity and specificity for the detection of human hydatidosis. An antigenic band of ∼60 kDa was found to be sensitive (82%) and specific (100%) for the detection of hydatidosis when probed with anti-human IgG4-HRP, while the sensitivity and specificity were 33 and 100%, respectively, with anti-human IgG-HRP. By mass spectrometry, the band was identified as protoscolex tegument paramyosin. The sensitivity and specificity of full-length paramyosin-recombinant protein in IgG4 blots were found to be 86 and 98%, respectively. In conclusion, IgG4 detection of Echinococcus granulosus paramyosin was found to be useful for the diagnosis of human hydatidosis.
INTRODUCTION
Hydatid cyst, or hydatidosis, is caused by the larval stage (metacestode) of the tapeworm Echinococcus granulosus in human and livestock. As the intermediate host, humans acquire the disease by accidental ingestion of vegetables or water contaminated with the ova of adult worms that live in the small intestines of canids, the definitive host. After exposure to gastrointestinal enzymes, the infective ovum hatches into an oncosphere, which is able to reach organs such as the liver and lungs through the vascular and lymphatic systems. The larva then develops into a hydatid cyst, which is gradually filled with fluid and protoscoleces. Hydatid cysts not only cause severe illness but also cause economic losses due to costs related to diagnosis and surgery (1). This disease is scattered throughout the world, with an emerging or reemerging status in several countries (2) and is also highly prevalent in Iran (3). It is estimated that 2 to 3 million people are infected with this neglected disease worldwide (4). Early diagnosis is based on clinical signs, which is followed by imaging of suspected organs. Clinical signs in humans are not specific, and the imaging methods cannot differentiate between hydatid cysts, tumors, and other lesions (5, 6). Therefore, immunodiagnosis remains an important tool in the diagnosis of the disease. Chordi and Kagan were the first to use immunoelectrophoresis to identify the antigenic components of sheep hydatid cyst fluid (HCF) and subsequently determined which antigenic components were active in detecting antibodies in the sera of patients with hydatid cysts (7). A successful immunodiagnostic test depends on the use of highly specific and sensitive antigens, as well as the detection of the appropriate antibody class or subclass (8, 9). Detection of circulating antigens in serum was reported to be less sensitive than detection of Echinococcus-specific antibodies (10). In this study, two-dimensional electrophoresis (2-DE) and Western blots using IgG and IgG4 as secondary antibodies were performed to identify antigen of E. granulosus protoscolex with good diagnostic potential.
MATERIALS AND METHODS
Serum samples.
Group I serum samples were collected from 81 patients with cysts in their livers or lungs who were diagnosed based on clinical symptoms and serodiagnosis and/or with magnetic resonance imaging. The samples were obtained from serum banks from pathology laboratories in Tehran, Iran. Nine pools of sera, containing nine samples per pool, were created. Group II serum samples (obtained prior to surgery) were individual samples from 49 confirmed hydatidosis patients from Imam Hospital, Tehran, Iran, who had cysts in their livers or lungs and who underwent surgical removal of the cysts. Consent was obtained from the patients for collection of the serum samples, and the Tarbiat Modarres University research ethics committee approved the study. Group III control samples (n = 68) were from sera from healthy people (n = 30) and patients infected with other parasites (n = 38), including Ascaris lumbricoides (n = 5), Fasciola hepatica (n = 2), Taenia saginata (n = 1), Toxocara canis (n = 2), Toxoplasma gondii (n = 4), Trichuris trichiura (n = 5), Strongyloides stercoralis (n = 2), Brugia malayi (n = 11), Gnathostoma spinigerum (n = 1), Taenia solium (cysticercosis) (n = 1), Necator americanus (n = 2), and Ancylostoma duodenale (n = 2).
Preparation of the protoscolex antigens.
Protoscoleces were aseptically isolated from hydatid cysts collected from infected sheep slaughtered in abattoirs in Iran. The protoscoleces were washed three times with sterile phosphate-buffered saline (PBS [pH 7.2]) by centrifugation at 3,000 × g for 10 min. The final pellet was suspended in an equal volume of sterile PBS containing a cocktail of protease inhibitors (Roche Diagnostics, Germany) at 40 μl/ml. Three freeze-thaw cycles (water bath and liquid nitrogen) were performed, followed by sonication on ice (model XL2020; Liquid Pressor, USA). The homogenate was centrifuged (10,000 × g, 1 h), and the pellet was discarded. The supernatant was washed twice with 10 mM Tris and then concentrated by centrifugation at 3,000 × g for 30 min using a Vivaspin spin filter (Sartorius, Germany) with a 5-kDa molecular mass cutoff. The protein concentration of the supernatant was determined using an RCDC protein assay kit (Bio-Rad, USA), and then the supernatant was stored in aliquots at −80°C.
One-dimensional electrophoresis.
One-dimensional electrophoresis was performed using an OFFGEL Fractionator (Agilent, USA) to separate the protein according to isoelectric point. Ready-made 13-cm IPG dry strip gels with a pH range of 3 to 10 were used. In total, 1,000 μg of desalted protein was fractionated into 12 wells.
SDS-PAGE.
The second dimension of electrophoresis was performed on a SDS–12% PAGE gel Mini-Protean 3 cell apparatus (Bio-Rad). The amount of protein loaded was optimized at 20 μg per well. Standard molecular mass markers were loaded in the first lane. Samples were run at 100 V for 110 min.
Western blot analysis of native antigens.
Protein was electro-transferred from gel to a nitrocellulose paper (NCP; 0.45-μm pore size) using a Trans-Blot Cell (Bio-Rad) at 12 A for 30 min. The NCP was stained with Ponceau-S solution (Sigma, USA) and washed with TBS-T (Tris-HCl-buffered saline, 0.05% Tween 20) wash buffer. It was blocked with blocking buffer (Roche Diagnostics, Germany) for 1 h at room temperature. The NCP was then washed four times at 1-, 5-, 10-, and 15-min intervals consecutively and cut into 3-mm strips. The strips were then incubated with human serum diluted 1:100 in blocking buffer for 2 h at room temperature and then overnight at 4°C. The next day, after a washing step, anti-human IgG4 or IgG (Invitrogen, USA) conjugated to horseradish peroxidase (HRP) at dilutions of 1:2,000 or 1:1,000, respectively, was incubated with the strips for 1 h at room temperature. The strips were then washed and developed with chemiluminescence substrate (Roche Diagnostics, Germany).
Initially, the potential diagnostic band was identified from results of Western blots performed with each of the nine pools from the group I sera and pooled healthy sera, using anti-human IgG4-HRP as the secondary antibody. Subsequently, individual sera from group II and group III samples were tested to determine the sensitivity and specificity of the identified band, using both HRP-conjugated anti-human IgG and IgG4 as secondary antibodies.
Staining of gels.
The gels were stained with Coomassie blue or with mass spectrometry (MS)-compatible silver stain. The latter was performed according to the method described by Shevchenko et al. (11). The identified band for tandem MS (MS/MS) analysis was manually excised and transferred to a microfuge tube.
In-gel digestion of a silver-stained gel.
A 50-μl portion of acetonitrile was added into each microfuge tube containing the sliced band, followed by incubation for 15 min at room temperature. The supernatant was removed, and 25 μl of ammonium bicarbonate (25 mM) was added, followed by incubation for 10 min. The supernatant was again removed, and the gel pieces were air dried for 1 h. Resuspension solution containing 10 ng of trypsin/μl was added, and the gel pieces were incubated on ice for 5 min. Subsequently, the gel pieces were immersed in 25 μl of ammonium bicarbonate (25 mM) and incubated at 30°C overnight. Two volumes of acetonitrile were added for digestion, and then the samples were vortexed and incubated for 20 min at room temperature. Finally, the supernatant was transferred into a fresh 1.5-ml microfuge tube. Sample cleanup for matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) MS analysis was performed using Zip-Tip pipette tips (Zip-Tip U-C18; Millipore, USA).
MS analysis.
In-gel-digested protein from a silver-stained gel was sent for MS/MS analysis using a MALDI-TOF-TOF 4800 (ABSCIEX, USA) at the Protein and Proteomics Centre, National University of Singapore. Meanwhile, a Coomassie blue-stained, sliced gel band was sent for processing and analysis using a MALDI-TOF-TOF 5800 (ABSCIEX) at Proteomics International, Australia.
Preparation of recombinant antigens.
The gene sequence that corresponded to the MS/MS-identified protein was custom cloned into a pET28 expression vector by Epoch Life Science (USA). The recombinant plasmid received from the company was transformed into competent Escherichia coli BL21(DES) expression host cells. A single colony containing the recombinant plasmid was inoculated into 100 ml of Terrific broth containing 100 μg of kanamycin/ml, followed by incubation overnight at 37°C with agitation at 180 rpm. Then, 50 μl of the overnight culture was inoculated into 500 ml of Terrific broth with kanamycin, followed by incubation at 37°C with agitation at 200 rpm until the optical density at 600 nm reached 0.4 to 0.5. Protein expression was induced by incubation in 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 5 h at 37°C. The cells were harvested by centrifugation at 10,000 × g and at 4°C for 10 min. The cell pellet was resuspended in 1.5 (vol/wt) lysis buffer containing protease inhibitors and 0.5 mg of lysozyme/ml and then incubated on ice for 30 min. The cell suspension was next lysed using a sonicator, followed by centrifugation at 10,000 × g and 4°C for 30 min. The supernatant was transferred to a new tube, and DNase (2,500 μg/ml) was added, followed by incubation on ice for 10 to 15 min and then centrifugation of the tube at 10,000 × g and 4°C for 30 min. The resultant supernatant was filtered by using a syringe filter (0.45-μm pore size). The filtered supernatant was mixed with Ni-NTA resin (Qiagen, USA), followed by incubation at 4°C (rotating) for 30 min, and then the mixture was loaded into a chromatography column. Gradient washings of the column were performed using 10 ml each of four kinds of washing buffers containing 500 mM NaCl (10, 20, 30, and 40 mM imidazole, respectively). The protein fractions were eluted using elution buffer containing 250 mM imidazole.
Western blot of recombinant antigens.
Protein-containing fractions from the affinity column were pooled and buffer exchanged into PBS with 1 M urea (pH 7). The fractions were concentrated by centrifugation at 3,000 × g for 30 min with a spin filter as described above. After the protein was transferred onto the NCP, it was blocked with 5% alkali-soluble casein (Novagen, USA) for 1 h at room temperature. After blocking, the NCP was washed with TBS-T washing buffer and incubated with His tag antibody-HRP at a 1:1,000 dilution for 1 h at room temperature. Western blotting was also performed using human sera as described above. The same set of serum samples was used; however, due to the limited volumes of some samples, a reduced number of control sera were used.
RESULTS
In the initial set of experiments using pooled serum samples, a band of ∼60 kDa was observed in IgG4 blots using group I sera from patients with hydatidosis. This band was not observed with pooled healthy serum (Fig. 1). Figure 2 shows representative IgG4 blots with individual serum samples; the ∼60-kDa band was found to be present when blotted with the sera of hydatidosis patients but not when blotted with control sera.
Fig 1.
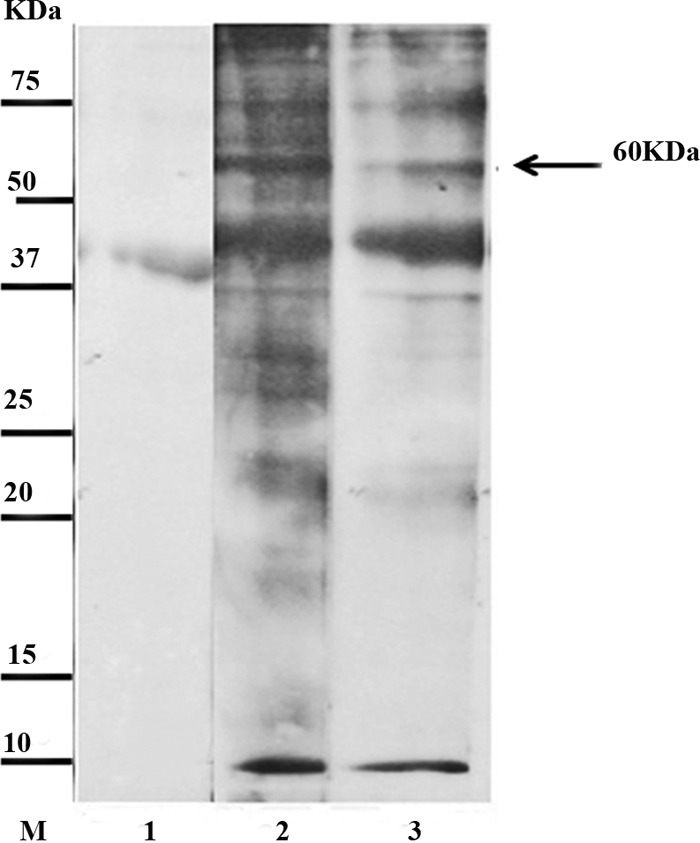
Representative Western blot of protoscolex antigen incubated with pooled sera and probed with anti-human IgG4-HRP. Lane M, low-molecular-mass markers; lane 1, pooled sample of healthy people (pooled group III); lanes 2 and 3, pooled samples from hydatid cyst patients (group I).
Fig 2.
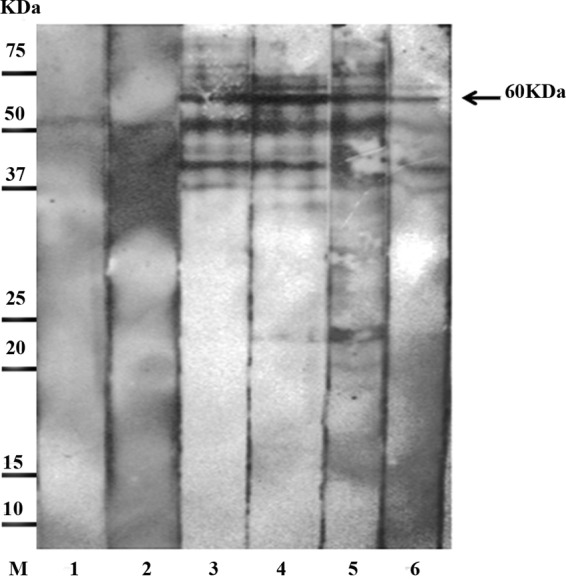
Representative Western blot of protoscolex antigen incubated with individual serum samples and probed with anti-human IgG4-HRP. Lane M, low-molecular-mass markers; lane 1, serum of healthy individual; lane 2, serum of patient infected with Ascaris lumbricoides; lanes 3 to 6, individual serum samples from group II patients.
As shown in Table 1, the 60-kDa band in IgG4 blots of the native protoscolex antigen showed 82% sensitivity (40/49) with group II serum samples. Of the 40 serum samples that reacted with the 60-kDa band, 18 were from patients with liver cysts, 19 were from patients with lung cysts, and 3 were from patients with mixed liver and lung cysts. Of the 9 false-negative results, 5 were from patients with liver cysts, and 4 were from patients with lung cysts. A 100% specificity (67/67) was seen with group III sera. In IgG blots of the native protoscolex antigen, the 60-kDa band showed 33% sensitivity of (10/30) and 100% specificity with the same set of sera.
Table 1.
Reactivities of the 60-kDa band in IgG and IgG4 blots of native protoscolex antigen probed with sera from hydatid cyst patients and with control sera
| HRP-conjugated secondary antibody | No. of reactive or nonreactive sera/total no. of sera examined (%)a |
|
|---|---|---|
| Group II, sensitivity | Group III, specificity | |
| IgG4 | 42/49 (86) | 68/68 (100) |
| IgG | 10/30 (33) | 68/68 (100) |
Sensitivity was determined as the number of sera reactive with the band/the total number of sera tested. Specificity was determined as the number of sera nonreactive with the band/the total number of sera tested. Group II was composed of sera from patients with hydatid cysts confirmed by surgery. Group III was composed of sera from healthy people and from patients with other parasitic infections (controls).
A database search was performed using Platyhelminthes database from UniProtKB. Both proteomic service centers identified the band as Echinococcus tegument paramyosin (accession number gi|547974) with protein scores of >200 and peptide scores well above the cutoff values. No protein other than paramyosin was identified. Two tryptic peptides were reported which covered 3% of the paramyosin protein sequence. It showed 100% homology to E. granulosus and 97% to Taenia solium.
Custom cloning into the pET28 expression vector was made based on the coding sequence (cds) of the previously submitted GenBank sequence, accession Z21787.1. The recombinant paramyosin showed 86% sensitivity (42/49) with group II serum samples from hydatid cyst patients and 98% specificity (58/59) with group III control sera from 24 healthy people and 35 patients with other parasitic infections. Figure 3 shows a representative Western blot performed with the recombinant paramyosin.
Fig 3.
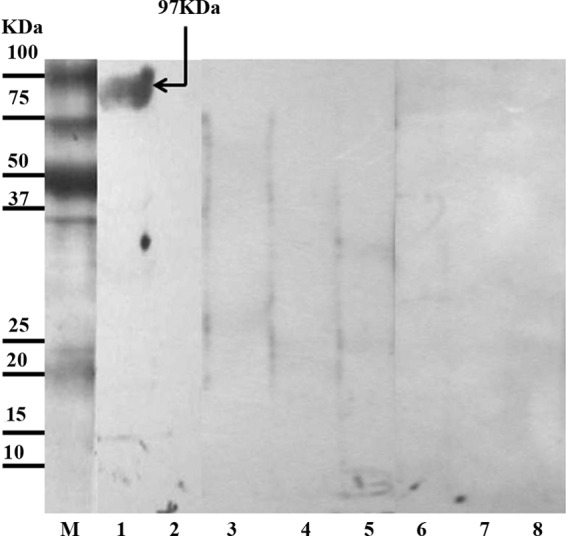
Representative Western blots of recombinant paramyosin protein incubated with individual serum samples and probed with anti-human IgG4-HRP. Lane M, low-molecular-mass markers; lane 1, serum of hydatidosis patient from group II; lanes 2 and 3, sera of healthy individuals from group III; lanes 4 to 8, sera of patients from group III infected with Taenia solium, Fasciola hepatica, Brugia malayi, Toxocara canis, and Toxoplasma gondii, respectively.
DISCUSSION
Clinical signs of human hydatid cysts are variable and nonspecific. Primary diagnosis is based on imaging methods such as ultrasound, computed tomography, and magnetic resonance imaging (12). To complement the radiological findings, immunological tests such as enzyme-linked immunosorbent assay (ELISA), indirect immunofluorescence, immunoelectrophoresis, and immunoblotting are used in diagnostic laboratories. However, false-negative (up to 25%) and false-positive results remain a problem with the available immunodiagnostic assays (13). To date, there is no standardized, highly sensitive, and specific test available for the immunodiagnosis of human cystic echinococcosis (14). Therefore, during the last 3 decades, extensive efforts have been made to characterize antigenic components of the protoscolex, germinal layer, and hydatid cyst fluid (HCF) of E. granulosus for use in serodiagnostic assays. HCF is one of the main antigen sources for the serodiagnosis of hydatid disease. Two major components of HCF, thermostable antigen B (AgB) and thermolabile antigen 5 (Ag5), are the most widely used antigens in reported assays for the disease (15). However, there are difficulties related to their lack of sensitivity and to their cross-reactivity with antigens from other parasites, notably other taeniid cestodes, as well as problems with the standardization of the antigens. Thus far, the use of these antigens is predominantly restricted to scientific applications, and neither is available for general use (5).
The number of reports on the use of protoscolex as an antigen for detecting antibodies in human hydatidosis is limited (16–19). Chemale et al. (20) described the analysis of E. granulosus metacestode protein extract by 2-DE and the identification of prominent proteins by peptide mass fingerprinting (PMF). A total of 100 prominent protein spots from three 2-DE gels were analyzed by MALDI-TOF MS; 15 of them were identified by their PMFs, which include protoscoleces tegument paramyosin (20). Monteiro et al. (21) performed a proteomic analysis of E. granulosus metacestodes in a bovine host. They used two complementary proteomic approaches, 2-DE/MALDI-TOF MS/MS and LC-MS/MS, to analyze proteins expressed in E. granulosus protoscoleces. The 2-DE IgG immunoblots using a pool of six sera from hydatid disease patients showed 14 protoscolex proteins recognized by hydatid cyst patients' sera. These proteins were reported to contribute to immunoregulatory events at the host-parasite interface during infection. According to the investigators, some of them, such as paramyosin and tetraspanin, may be useful for vaccine development (21).
In the present study, protoscoleces isolated from hydatid cysts from the livers of sheep were analyzed by 2-DE, using OFFGEL for the first dimension of separation, followed by mini SDS-PAGE. This approach has the advantage of using less volume of the serum samples in the subsequent Western blots (22). The clear band on the Western blots allowed the band on the corresponding SDS-PAGE gels to be readily identified and excised for MS analysis. The high protein and peptide scores obtained in MS/MS analyses of the in-gel digested protein provided a confident identification of the protein as paramyosin.
Paramyosin is a 97-kDa muscle protein present in the tegument of protoscoleces. It is an α-helical protein and was first noted in invertebrate muscle as a structural component (23). The report of paramyosin-based vaccination against schistosomes led to the characterization of the protein in other organisms (24). In the platyhelminths Taenia, Echinococcus, and Schistosoma, an extramuscular localization of paramyosin has been reported (25). Muhlschlegel et al. described the cloning of a paramyosin-homologous protein of E. granulosus, and by immunofluorescence they demonstrated its presence in the tegument, subtegument of the body wall, and muscles of the four oral suckers of the E. granulosus larvae (26). Paramyosin has also been identified as an immunogenic protein in several parasitic infections, such as Schistosoma japonicum, Schistosoma mansoni, Taenia solium, and Taenia saginata infections (24, 27).
In the present study, the identified E. granulosus paramyosin amino acid sequence was 97% homologous to the paramyosin sequence of T. solium. Thus, it is not surprising that the recombinant paramyosin protein showed cross-reactivity with serum from a cysticercosis patient. Interestingly, the same serum was not cross-reactive with the 60-kDa protein of the native protoscolex antigen. One possible reason for this difference is that the recombinant paramyosin comprised the full-length paramyosin (∼97 kDa), whereas the native paramyosin band on SDS-PAGE was not the full-length protein since its molecular mass was ∼60 kDa. The paramyosin antigenic epitope may reside in the nonhomologous part of the sequence. However, before performing further studies to identify the epitope fragment, several more serum samples from cysticercosis patients should be tested to confirm the Western blot results.
The levels of serum IgG4 antibodies were reported to have increased and remained at high levels in patients with active cysts or in patients with relapsing disease (28, 29). Patients with relapsing disease were reported to maintain high IgG4 titers in ELISA, whereas the levels of IgG4 decreased in patients with infiltration or calcified hydatid cysts and became negative in patients after the removal of cyst(s) by surgery or pharmacological treatment (10, 30). These results suggest that the IgG4 subclass is a good marker for follow-up of hydatidosis cases. Previous studies related to paramyosin used IgG in the seroanalysis; however, our study showed that the use of IgG4 as secondary antibody led to much higher sensitivity and similar specificity compared to the use of IgG. Both native and recombinant antigens demonstrated similar sensitivities and specificities. However, the latter would be more useful for patient diagnosis because it can be produced as a standardized and reproducible diagnostic reagent (31).
One limitation of the present study is the lack of samples from patients posttreatment; thus, further studies should be performed on these types of samples. In addition, a lateral flow format of the IgG4 test using the paramyosin recombinant antigen would be useful in facilitating a multicenter evaluation of the test. In conclusion, we showed here that sensitive and specific diagnosis of human hydatidosis could be achieved by performing an IgG4 assay using either the native or the recombinant form of E. granulosus paramyosin.
ACKNOWLEDGMENTS
This study was funded by the Ministry of Higher Education HICoE research grant no. 311/CIPPM/4401005 and the Universiti Sains Malaysia (USM) Postgraduate Student research grant 1001/CIPPM/844079. The first author received financial support from a USM fellowship program.
We thank Tan Sin Yee for technical assistance.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Budke CM, Deplazes P, Torgerson PR. 2006. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 12: 296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eckert J, Conraths FJ, Tackmann K. 2000. Echinococcosis: an emerging or re-emerging zoonosis? Int. J. Parasitol. 30: 1283–1294 [DOI] [PubMed] [Google Scholar]
- 3. Sadjjadi SM, Ardehali S, Noman-Pour B, Kumar V, Izadpanah A. 2001. Diagnosis of cystic echinococcosis: ultrasound imaging or counter current immunoelectrophoresis? East Mediterr. Health J. 7: 907–911 [PubMed] [Google Scholar]
- 4. Craig PS, Rogar MT, Allan CC. 1996. Detection, screening, and community epidemiology of taeniid cestode zoonoses: cystic echinococcosis, alveolar echinococcosis, and neurocysticercosis. Adv. Parasitol. 36: 169–250 [DOI] [PubMed] [Google Scholar]
- 5. Babba H, Messedi A, Masmoudi S, Zribi M, Grillot R, Ambriose-Thomas P, Beyrouti I, Sahnoun Y. 1994. Diagnosis of human hydatidosis: comparison between imagery and 6 serologic techniques. Am. J. Trop. Med. Hyg. 50: 64–68 [DOI] [PubMed] [Google Scholar]
- 6. Hira PR, Shweiki HM, Francis I. 1993. Cystic hydatid disease: pitfalls in diagnosis in the Middle-East endemic area. Trop. Med. Hyg. 96: 363–369 [PubMed] [Google Scholar]
- 7. Chordi A, Kagan IG. 1965. Identification and characterization of antigenic components of sheep hydatid cyst fluid by immunoelectrophoresis. J. Parasitol. 51: 63–71 [PubMed] [Google Scholar]
- 8. Matossian RM, Anami SY, Salti I, Araj GF. 1976. Serum immunoglobulin levels in human hydatidosis. Int. J. Parasitol. 6: 367–371 [DOI] [PubMed] [Google Scholar]
- 9. Sbihi Y, Janssen D, Osuna A. 1997. Specific recognition of hydatid cyst antigens by serum IgG, IgE, and IgA using Western blot. J. Clin. Lab. Anal. 11: 154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Li J, McManus DP. 2003. Concepts in immunology and diagnosis of hydatid disease. Clin. Microbiol. Rev. 16: 18–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shevchenko A, Wilm M, Vorm O, Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68: 850–858 [DOI] [PubMed] [Google Scholar]
- 12. Lightowlers MW, Gottstein B. 1995. Echinococcosis/hydatidosis: antigens, immunological and molecular diagnosis, p 355–410 In Thompson RCA, Lymbery AJ. (ed), The biology of Echinococcus and hydatid disease. CAB International, Wallingford, United Kingdom [Google Scholar]
- 13. Ortona E, Rigano R, Buttari B, Delunardo F, Ioppolo S, Margutti P, Profumo E, Teggi A, Vaccari S, Siracusano A. 2003. An update on immunodiagnosis of cystic echinococcosis. Acta Trop. 85: 165–171 [DOI] [PubMed] [Google Scholar]
- 14. Li J, Zhang-Wilson W-BM, Ito A, McManus DP. 2003. A novel recombinant antigen for immunodiagnosis of human cystic echinococcosis. J. Infect. Dis. 188: 1951–1960 [DOI] [PubMed] [Google Scholar]
- 15. Carmena D, Benito A, Eraso E. 2006. Antigens for the immunodiagnosis of Echinococcus granulosus infection: an update. Acta Trop. 98: 74–86 [DOI] [PubMed] [Google Scholar]
- 16. Ben Nouir N, Nunez S, Gianinazzi C, Gorcii M, Muller N, Nouri A, Babba H, Gottstein B. 2008. Assessment of Echinococcus granulosus somatic protoscoleces antigens for serological follow-up of young patients surgically treated for cystic echinococcosis. J. Clin. Microbiol. 46: 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carmena D, Martinez J, Benito A, Guisantes JA. 2005. Shared and non-shared antigens from three different extracts of the metacestode of Echinococcus granulosus. Mem. Inst. Oswaldo Cruz 100: 861–867 [DOI] [PubMed] [Google Scholar]
- 18. Carmena D, Martinez J, Benito A, Guisantes JA. 2004. Characterization of excretory-secretory products from protoscoleces of Echinococcus granulosus and evaluation of their potential for immunodiagnosis of human cystic echinococcosis. Parasitology 129: 371–378 [DOI] [PubMed] [Google Scholar]
- 19. Rafiei A, Craig PS. 2002. The immunodiagnostic potential of protoscoleces antigens in human cystic echinococcosis and the possible influence of parasite Strain. Ann. Trop. Med. Parasitol. 96: 383–389 [DOI] [PubMed] [Google Scholar]
- 20. Chemale G, van Rossum AJ, Jefferies JR, Barrett J, Brophy PM. 2003. Proteomic analysis of the larval stage of the parasite Echinococcus granulosus: causative agent of cystic hydatid disease. Proteomics 3: 1633–1636 [DOI] [PubMed] [Google Scholar]
- 21. Monteiro KM, de Carvalho MO, Zaha A, Ferreira HB. 2010. Proteomic analysis of the Echinococcus granulosus metacestode during infection of its intermediate host. Proteomics 10: 1985–1999 [DOI] [PubMed] [Google Scholar]
- 22. Saadatnia G, Mohamed Z, Ghaffarifar F, Osman E, Moghadam ZK, Noordin R. 2012. Toxoplasma gondii excretory secretory antigenic proteins of diagnostic potential. APMIS 120: 47–55 [DOI] [PubMed] [Google Scholar]
- 23. Cohen C. 1982. Matching molecules in the catch mechanism. Proc. Natl. Acad. Sci. U. S. A. 79: 3176–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gobert GN, McManus DP. 2005. Update on paramyosin in parasitic worms. Parasitol. Int. 54: 101–107 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt J, Bodor O, Gohr L, Kunz W. 1996. Paramyosin isoforms of Schistosoma mansoni are phosphorylated and localized in a large variety of muscle types. Parasitology 112(Pt 5): 459–467 [DOI] [PubMed] [Google Scholar]
- 26. Muhlschlegel F, Sygulla L, Frosch P, Massetti P, Frosch M. 1993. Paramyosin of Echinococcus granulosus: cDNA sequence and characterization of a tegumental antigen. Parasitol. Res. 79: 660–666 [DOI] [PubMed] [Google Scholar]
- 27. Lanar DE, Pearce EJ, James SL, Sher A. 1986. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science 234: 593–596 [DOI] [PubMed] [Google Scholar]
- 28. Rigano R, Profumo E, Teggi A, Siracusano A. 1996. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin. Exp. Immunol. 105: 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rigano R, Profumo E, Ioppolo S, Notargiacomo S, Ortona E, Teggi A, Siracusano A. 1995. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin. Exp. Immunol. 102: 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guerri ML, Davila M, Rodriguez M, Nieto FJ, Ladron de Guevara C. 2000. Utility of IgG subclasses in the diagnosis and follow up of hydatidosis. Enferm. Infecc. Microbiol. Clin. 18: 262–2666 [PubMed] [Google Scholar]
- 31. Siracusano A, Teggi A, Ortona E. 2009. Human cystic echinococcosis: old problems and new perspectives. Interdiscip. Perspect. Infect. Dis. 2009: 474368 doi:10.1155/2009/474368 [DOI] [PMC free article] [PubMed] [Google Scholar]


