Abstract
It is difficult to distinguish infections with different Bartonella species using commercially available immunofluorescence (indirect immunofluorescent antibody [IFA]) assay kits. To identify appropriate proteins for serodiagnosis of Bartonella quintana infections, we investigated the antigenicity of B. quintana proteins using sera from homeless people with high B. quintana IgG titers in IFA assay. These sera reacted strongly to an outer membrane protein, hemin-binding protein D (HbpD). Further, serum from an endocarditis patient infected with B. quintana reacted to HbpB and HbpD. To locate the antigenic sites within the proteins, we generated deletion mutants of HbpB and HbpD. Amino acid residues 89 to 220 of HbpB and 151 to 200 of HbpD were identified as the minimum regions required for recognition by these sera. Several oligopeptides comprising parts of the minimum regions of HbpB and HbpD were synthesized, and their immunoreactivity with the above-mentioned sera was tested by enzyme-linked immunosorbent assay (ELISA). Serum from the endocarditis patient reacted similarly to synthetic peptides HbpB2 (amino acid residues 144 to 173 of HbpB) and HbpD3 (151 to 200 residues of HbpD), while sera from the other subjects reacted to HbpD3. These results indicate that synthetic peptides HbpB2 and HbpD3 might be suitable for developing serological tools for differential diagnosis of B. quintana infections from other Bartonella infections.
INTRODUCTION
Bartonella is a genus of ubiquitous, fastidious, slow-growing, and hemotropic Gram-negative bacteria, of which 24 species are known to date. Among them, Bartonella henselae and B. quintana are common microbes responsible for human infections (1). B. henselae is the causative pathogen of cat scratch disease (CSD) and is present in various animals, including wild and domestic cats, which act as a natural reservoir. Although B. henselae-infected cats show no typical disease symptoms, the bacterium may cause CSD, bacillary angiomatosis (BA), peliosis hepatis, chronic bacteremia, and endocarditis after transmission to humans (2). On the other hand, B. quintana was the causative agent of epidemic trench fever (also called 5-day fever) in World Wars I and II. It grows extracellularly in the midgut of human body lice, and the bacterium in crushed lice or in louse feces is transmitted to humans via broken skin (3, 4). In the 1990s, trench fever reemerged in refugee camps and prisons and also in developed countries among homeless people and drug addicts. These outbreaks are referred to as urban trench fever, to distinguish them from classical trench fever (5). B. quintana also causes BA, chronic bacteremia, and endocarditis in humans, as B. henselae does (6–11).
Since culture of Bartonella is time-consuming, complicated, and often unsuccessful, serological methods are considered preferable for diagnosis of Bartonella infections. Several serodiagnostic methods, including Western blotting, enzyme-linked immunosorbent assay (ELISA), and indirect immunofluorescent antibody (IFA) assay, have been proposed (12, 13). IFA assay is most commonly used for routine clinical diagnosis of Bartonella infections, since a quality-controlled commercial kit is available, using fixed bacterial cells cocultured with Vero cells on a slide as the antigen, for semiquantitative measurements of human serum IgG against B. henselae and B. quintana. The availability of independent IgG titers for both B. henselae and B. quintana at once is one of the advantages of this kit. However, it is difficult to distinguish B. henselae and B. quintana infections reliably by using the IFA test, since the kit utilizes whole cells and the genomes of the two species show a high degree of overall similarity.
We have conducted an epidemiological survey of trench fever in Japan in conjunction with a rescue outreach program for homeless persons in Tokyo. In the survey, blood samples from nonhospitalized homeless people were prepared and examined for IgG antibodies against B. quintana (7). Because the subjects may be infected with various bacteria, including B. henselae, it is important that the diagnostic tools used for serological differentiation should exhibit high species specificity without cross-reaction. Recently, several bacterial outer membrane proteins, including Pap31 and BH11510 from B. henselae (14–20) and VompA, VompB, PpI, and hemin-binding protein E (HbpE) from B. quintana (21), have been proposed as candidate proteins for development of serodiagnostic tools for Bartonella infections. In this study, we attempted to identify species-specific antigenic proteins from B. quintana, using sera from homeless people and a B. quintana-infected endocarditis patient. We also synthesized fragment peptides of two of the identified proteins as candidates for development of a novel serodiagnostic assay.
MATERIALS AND METHODS
Bacterial strains.
Bartonella and Escherichia coli strains used in this study are listed in Table 1. B. quintana Oklahoma was kindly provided by P. Brouqui (WHO Collaborative Center for Rickettsial Reference and Research, Marseilles, France) in 2003. B. quintana and B. henselae were cultured on Columbia agar with 5% sheep's blood (Sysmex-bioMérieux, Tokyo, Japan) for 14 days at 37°C in 5% CO2.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| B. henselae | ATCC 49882 | ATCC |
| B. quintana Oklahoma | Human isolate | P. Brouqui |
| E. coli TOP10 | Host strain for cloning | Invitrogen |
| E. coli BL21Star(DE3) | Host strain for gene expression | Invitrogen |
| Plasmids | ||
| pCR4-TOPO | TA-cloning vector | Invitrogen |
| pET100D/TOPO | Expression vector | Invitrogen |
| pHbpA | pET100D/TOPO containing hbpA | This study |
| pHbpB | pET100D/TOPO containing hbpB | This study |
| pHbpC | pET100D/TOPO containing hbpC | This study |
| pHbpD | pET100D/TOPO containing hbpD | This study |
| pHbpE | pET100D/TOPO containing hbpE | This study |
| pHbpB1 | pET100D/TOPO containing hbpB (1–354) | This study |
| pHbpB2 | pET100D/TOPO containing hbpB (265–660) | This study |
| pHbpB3 | pET100D/TOPO containing hbpB (601–900) | This study |
| pHbpB4 | pET100D/TOPO containing hbpB (901–1362) | This study |
| pHbpD1 | pET100D/TOPO containing hbpD (1–300) | This study |
| pHbpD2 | pET100D/TOPO containing hbpD (151–450) | This study |
| pHbpD3 | pET100D/TOPO containing hbpD (301–600) | This study |
| pHbpD4 | pET100D/TOPO containing hbpD (451–750) | This study |
| pHbpD5 | pET100D/TOPO containing hbpD (601–885) | This study |
Numbers indicate the positions in the nucleotide sequence of the appropriate gene.
Plasmids.
Primers used in this study are listed in Table 2. Genomic DNA of B. quintana was prepared, and genes for Hbp were amplified by standard PCR methods. Purified fragments were then cloned into pCR4-TOPO vector (Invitrogen, Carlsbad, CA), and DNA sequences were verified. To generate Xpress-tagged full-length versions and deletion mutants of Hbp, a Champion pET Directional TOPO Expression kit (Invitrogen) was used according to the manufacturer's instructions. His6-tagged proteins were purified by using His-Bind kits (Novagen, Darmstadt, Germany).
Table 2.
Primers for construction of hemin-binding proteins in Bartonella quintana
| Construct | Sequence (5′→3′) |
|
|---|---|---|
| Forward primera | Reverse primer | |
| HbpA | CACCATGAATATAAAATCTTTAATAACG | TTAGAATTTATAAGCTACACCAAAACGG |
| HbpB | CACCATGAATACGAAACGTTTAATAAC | TTAGAATTTATAAGCTACACCAATACG |
| HbpC | CACCATGAATATGAAATGGTTAATAAC | TTAAAATTTGTAAGCCACACCAACGC |
| HbpD | CACCATGGCTAAAAAATATTTAATCAC | TTAAAATTTGTACGCTACACCAACACGG |
| HbpE | CACCATGAATATGAAATTTATAATAGG | TTAGAATTTGTAAGCTACACCCACACG |
| HbpB1 | CACC ATG AATACGAAACGTTTAATA | TTATGTGTATTTTTGTCCAGACCA |
| HbpB2 | CACCGGTCTTTACGCCGGTTCTAAT | TTATGTTAATGATGGTCGGGCTAC |
| HbpB3 | CACCCCAGCAGCACCAGCGTCGGTA | TTACTCACCTTGTGCACTCCGTCC |
| HbpB4 | CACCCAAACGGTGAATGAAAAAAGT | TTAGAATTTATAAGCTACACCAAT |
| HbpD1 | CACCATGGCTAAAAAATATTTAATC | TTAGAAATCAAAATTGAAACCGCC |
| HbpD2 | CACCATAGGTAATTTTTCAAGTAAG | TTACCCTGGAATCTCTATCCCTGA |
| HbpD3 | CACCGGCAAGGGTTTTGTTATAGGT | TTACAAGCGCTCCCCAGAAAAACC |
| HbpD4 | CACCACAGAGTTTCCAGGAGGGGTA | TTAAAAATCAACACCGCCTCCAAG |
| HbpD5 | CACCATGCCTTATATTGCTGGAGGT | TTAAAATTTGTACGCTACACCAAC |
CACC at the 5′ end is a sequence added to allow pET directional cloning.
Sera and antibodies.
Serum from nonhospitalized homeless people had been obtained during an epidemiological investigation of trench fever in Japan. Informed consent had been obtained, and data were anonymized. Demographic, clinical, and other information about the subjects of the present study was described previously (7). Serum from an anonymized endocarditis patient infected with B. quintana had been obtained at three time points (22). The IFA titers of these sera were determined with a Bartonella IFA IgG kit (Focus Diagnostics, Cypress, CA). Pooled serum from 30 healthy donors (Dako, Glostrup, Denmark) was used as a control in ELISA and Western blotting. Anti-Xpress antibody was purchased from Invitrogen. Alkaline phosphatase (AP)- and peroxidase (PO)-conjugated secondary antibodies for human and mouse IgG were obtained from Dako.
Western blotting.
Bacterial cells of B. quintana and B. henselae were collected from plates, and whole cells were lysed by sonication. B. quintana or B. henselae proteins were separated by SDS-PAGE and immunoblotted with sera from homeless people. For detection of Xpress-tagged Hbp, E. coli lysates suspended in lysis buffer (50 mM potassium phosphate, pH 7.8, 400 mM NaCl, 100 mM KCl, 10% glycerol, 0.5% Triton X-100, 10 mM imidazole) were separated by SDS-PAGE and immunoblotted with anti-Xpress antibody or human serum as indicated.
Amino acid sequencing.
Whole-cell lysate of B. quintana was separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was stained with Coomassie brilliant blue (CBB) staining solution, and the 35-kDa band was cut out. Protein was extracted, and the N-terminal amino acid sequence was determined.
ELISA.
Peptides used as ELISA antigens are listed in Table 3. One-microgram samples of peptides synthesized by TaKaRa Bio Inc. (Shiga, Japan) were applied as a coating on immunoplates (Maxisorp; Nunc, Roskilde, Denmark) with coating buffer (15 mM Na2CO3, 35 nM NaHCO3, 3 mM NaN3, pH 9.6). Serum diluted to 1:100 was added, and the plates were incubated for 1 h at room temperature and then further incubated with AP-conjugated anti-human IgG (1:1,000) and p-nitrophenyl phosphate disodium (Sigma, St. Louis, MO). The absorbance at 405 nm was measured.
Table 3.
Sequences of peptides synthesized as antigens for ELISAa
| Hbp | Position of ORF (no. of amino acids) | Amino acid sequence (from N terminus to C terminus) |
|---|---|---|
| HbpB1 | 144–173 (20) | LENQLLGKSPKRKVQRTASA |
| HbpB2 | 144–163 (30) | LENQLLGKSPKRKVQRTASAAPAAPAAPAP |
| HbpD1 | 151–180 (30) | TEFPGGVEFPDPVPGPERPPFEYDRIIQAT |
| HbpD2 | 171–200 (30) | FEYDRIIQATLKQKWSGATRVRVGFSGERL |
| HbpD3 | 151–200 (50) | TEFPGGVEFPDPVPGPERPPFEYDRIIQATLKQKWSGATRVRVGFSGERL |
Statistical analysis.
Mean values of Bartonella IFA titer were calculated, and the numbers of sera with titers of ≤1/128 and ≥1/256 were compared using Student's t test. Statistical analysis was performed using Stata software, version 11 (StataCorp. LP, College Station, TX).
RESULTS
Identification of HbpD from B. quintana as an antigen strongly reactive to sera from homeless people.
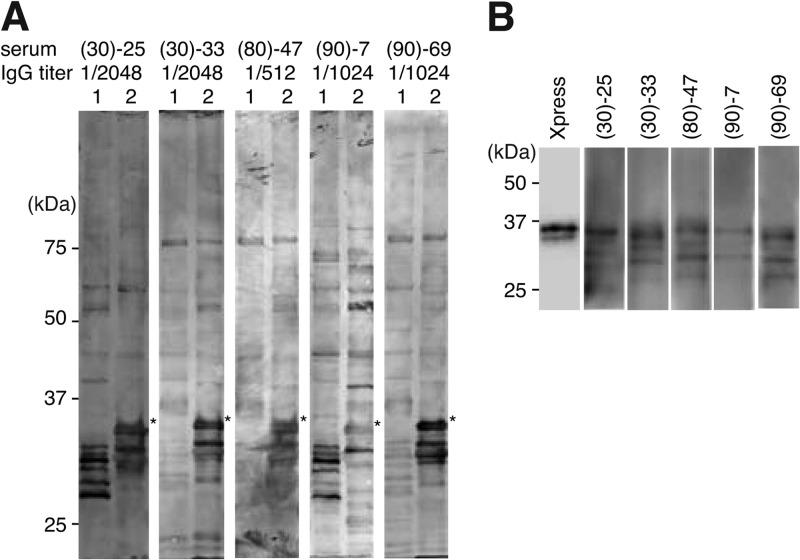
To identify B. quintana proteins that react to sera from B. quintana-infected subjects, whole-cell lysates of B. quintana and B. henselae were separated by means of SDS-PAGE and subjected to Western blotting with 50 selected sera from homeless people, in which the IgG titer to B. quintana measured with the Bartonella IFA IgG kit was ≥1/512. As shown in Fig. 1A, several proteins with molecular masses below 37 kDa were detected in both B. quintana and B. henselae lysates. It is noteworthy that a strong signal at approximately 35 kDa was detected only in B. quintana lysate (asterisks in Fig. 1A), with almost all sera (five representative sera that showed a strong signal are shown). These results indicated that antibody to this 35-kDa protein is strongly induced by B. quintana infection, and therefore, this is a potential candidate for developing a diagnostic tool. To identify the 35-kDa protein, its N-terminal amino acid sequence was determined by the Edman degradation method. We obtained two N-terminal amino acid sequences corresponding to malate dehydrogenase and hemin-binding protein D (HbpD). Since HbpD was reported to be an outer membrane protein located at the bacterial surface (23), we considered that the strong signal observed at 35 kDa in B. quintana lysate represented HbpD. To investigate whether antibodies against HbpD were actually increased in these sera, Xpress-tagged HbpD was subjected to Western blotting with individual sera. As shown in Fig. 1B, Xpress-tagged HbpD was detected at approximately 37 kDa with anti-Xpress antibody (Fig. 1B, leftmost lane). A signal at the same position was detected with all the sera. These results suggest that antibody against HbpD of B. quintana is typically increased in sera from homeless people.
Fig 1.
Western blots of sera from homeless people with HbpD of B. quintana. (A) Western blots of B. henselae and B. quintana with sera from homeless people. Whole-cell lysates of B. henselae (lanes 1) and B. quintana (lanes 2) were separated on a 12.5% SDS-PAGE gel and subjected to Western blotting with five sera (top). IgG titers for Bartonella, measured with a Bartonella IFA IgG kit, are indicated under the serum numbers. Asterisks indicate a protein of approximately 35 kDa in B. quintana lysate that reacts strongly to these sera. (B) E. coli lysates expressing Xpress-tagged HbpD were subjected to Western blotting with anti-Xpress or sera from homeless people as indicated at the top.
Antibodies reacting to the second extracellular domain of HbpD are increased by B. quintana infection.
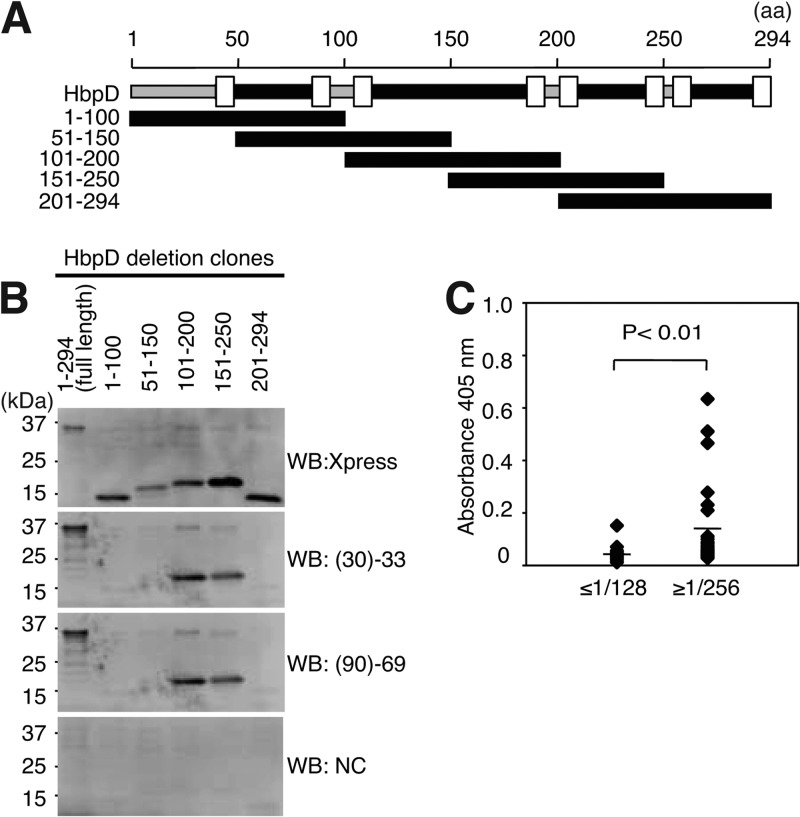
To identify the antigenic site in HbpD, we prepared a series of deletion mutants, which contain approximately 100 amino acids each and cover the entire region of HbpD (Fig. 2A). As shown in Fig. 2B, Xpress-tagged full-length proteins and deletion mutants were detected by anti-Xpress antibody at the expected position (Fig. 2B, top panel). Full-length HbpD was also detected with two sera, (30)-33 and (90)-69, whereas this signal was not detected with control serum. Among five deletion mutants, only two, HbpD/101–200 and HbpD/151–250, were detected with both (30)-33 and (90)-69 (Fig. 2B, middle panels). These results indicated that antibodies against the 101–200 and 151–250 sequences of HbpD are present in these two sera, which exhibited a high IFA titer for B. quintana. We next tested whether or not antibody against HbpD/101–200 was increased in sera from 65 homeless people by using ELISA with the purified HbpD/101–200 fragment. These sera covered various ranges of IFA titers and were divided into two groups (30 sera with IFA titers of ≤1/128 and 35 sera with IFA titers of ≥1/256). As shown in Fig. 2C, the geometric mean of the ≥1/256 group was 3-fold higher than that of the ≤1/128 group (mean absorbance values at 405 nm were 0.127 and 0.042, respectively).
Fig 2.
Antibody against the second extracellular domain of HbpD is increased in sera from homeless people. (A) Schematic structure of HbpD of B. quintana. Amino acid (aa) numbering is indicated at the top. The regions corresponding to the five HbpD deletion mutants are also indicated. The intracellular domain (gray bar), the extracellular domain (black bar), and the transmembrane domain (TM; open box) are shown. The transmembrane domains are located at residues 41 to 49, 85 to 93, 105 to 113, 187 to 195, 200 to 208, 242 to 250, 256 to 264, and 286 to 294 in HbpD. (B) Full-length forms and deletion mutants of HbpD were separated on a 15 to 25% SDS-PAGE gel and subjected to Western blotting with anti-Xpress antibody (top panel), sera from two homeless people (middle panels), and control serum (NC; bottom panel). Positions of molecular mass markers are indicated on the left. (C) Purified HbpD/101–200 was applied as a coating to ELISA plates, and antibody titers of sera from 65 homeless people were measured. The sera were separated into two groups based on the IFA IgG titer, as indicated at the bottom. Geometric means of the ≤1/128 and ≥1/256 groups were 0.042 and 0.127, respectively, as indicated by lines (P < 0.01).
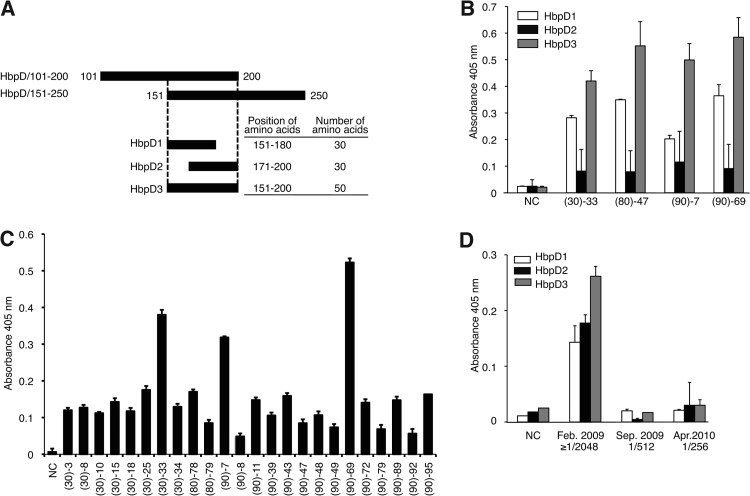
HbpD is a membrane protein with eight β-stranded transmembrane domains and four extracellular domains (23). The reactive HbpD-derived fragments found here include the second or the third extracellular domain. Amino acids 151 to 200, which are shared by these two fragments, correspond to the latter half of the second extracellular domain (Fig. 2A). We then investigated whether synthetic peptides corresponding to this region could be potential epitopes for antibodies in sera with high IFA titers for B. quintana. To this end, three peptides, HbpD1, HbpD2, and HbpD3 (corresponding to amino acid residues 151 to 180, 171 to 200, and 151 to 200 of HbpD, respectively), were synthesized (Fig. 3A; Table 3), and the avidities of four sera from homeless people, (30)-33, (80)-47, (90)-7, and (90)-69, for these targets were measured by ELISA (Fig. 3B). In terms of absorbance at 405 nm, the titers of these sera were 8- to 15-fold for HbpD1, 3- to 5-fold for HbpD2, and 20- to 28-fold for HbpD3, relative to the control serum. All four sera showed the highest avidity for HbpD3, though the avidity for HbpD1 was only slightly lower. Avidity for HbpD2 was weak compared to that for HbpD1 or HbpD3. The lower avidity for HbpD2 was not unexpected, since the C-terminal half of HbpD2 corresponds to transmembrane and intracellular domains, which may be unlikely to act as epitopes. HbpD3 peptide was further tested with another 24 sera belonging to the ≥1/256 IFA titer group. As shown in Fig. 3C, all sera tested showed high avidity for HbpD3 compared to the control serum. Among them, four sera, (30)-25, (30)-33, (90)-7, and (90)-69, which gave a strong signal in Western blot assays with the 35-kDa protein of B. quintana lysate (Fig. 1), showed relatively high avidity for HbpD3 in ELISA. These results also indicated that sera showing high IgG titers for B. quintana in the standard IFA test contain antibodies against HbpD, and the epitope of these antibodies is probably located at the C-terminal region of the second extracellular domain, corresponding to HbpD3 peptide.
Fig 3.
Reaction of sera from homeless people and the B. quintana-infected patient with HbpD synthetic peptides. (A) Schematic diagram of the HbpD synthetic peptides. HbpD1, HbpD2, and HbpD3 were designed to cover overlapping regions of HbpD/101–200 and HbpD/151–250. The positions and numbers of amino acids of the three peptides are indicated on the right. (B) HbpD1 (white bars), HbpD2 (black bars), and HbpD3 (gray bars) were applied as a coating to plates, and the avidities of four sera from homeless people and control serum (NC) were measured by ELISA. Data are the means of three independent experiments. (C) Sera from 24 homeless people and control serum were examined by ELISA with an HbpD3-coated plate. Data are the means of three independent experiments. (D) Serum from an endocarditis patient infected with B. quintana was measured by ELISA with HbpD1 (white bars), HbpD2 (black bars), and HbpD3 (gray bars). Sera were prepared at indicated time points during the course of the infection. IgG titers for B. quintana measured by IFA are shown. Data are the means of three independent experiments.
Given that the avidity in HbpD ELISA and the IFA titer of these sera were well correlated, we next investigated serum from a patient with prosthetic valve endocarditis caused by B. quintana infection, by means of ELISA with the HbpD synthetic peptides. Serum samples had been obtained at three different time points during the course of infection, i.e., on admission (February 2009), on discharge (September 2009), and at an outpatient clinic (April 2010), and showed IFA titers for B. quintana of ≥1/2,048, 1/512, and 1/256, respectively. As shown in Fig. 3D, increased avidity for all three peptides was observed with the serum obtained in February 2009. The avidity of sera obtained at the other time points was similar to that of the control serum. These results suggested that HbpD3 is the most suitable peptide for an ELISA antigen among the three peptides.
Antibodies against HbpB, but not HbpA, HbpC, or HbpE, are increased in sera of B. quintana-infected subjects.
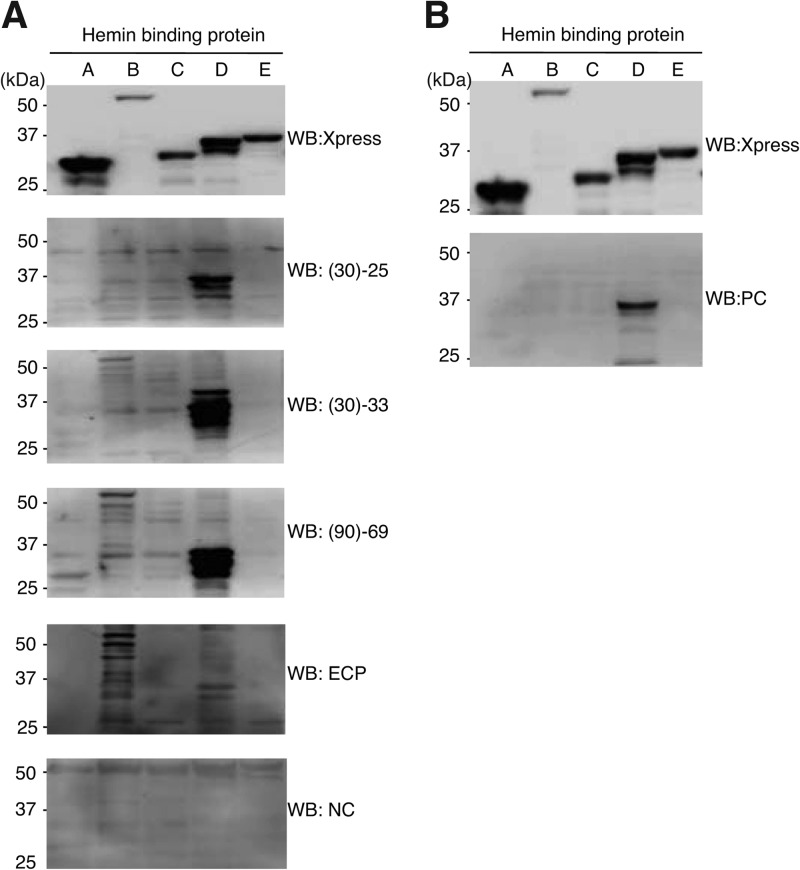
We next tested whether or not antibodies against other Hbp family proteins are also increased in sera from homeless people and the B. quintana-infected patient by using five Xpress-tagged Hbps. As shown in Fig. 4A, expression of all Hbps was confirmed by Western blotting with anti-Xpress antibody, although the expression level of HbpB was slightly lower than those of the others (Fig. 4A, top panel). When these extracts were subjected to Western blotting with sera from homeless people [Fig. 4A, panels (30)-25, (30)-33, and (90)-69], strong signals corresponding to HbpD were observed as expected, while no signals corresponding to HbpA, HbpC, and HbpE were detected. In the cases of (30)-33 and (90)-69, very weak signals for HbpB were detected. Serum from the endocarditis patient showed signals with HbpB and HbpD but not HbpA, HbpC, or HbpE. No Hbp was detected with control serum. These results indicated that, in addition to antibody against HbpD, antibody against HbpB is also increased by B. quintana infection. The five Hbps were also subjected to Western blotting with the polyvalent murine antibody supplied with the Bartonella IFA IgG kit as a positive control. Only a signal corresponding to HbpD was detected (Fig. 4B). This result suggested that the positive-control antibody in the IFA kit predominantly recognizes HbpD among the Hbp family members, and thus, the standard diagnosis of B. quintana infection by IFA might depend predominantly on the presence of HbpD.
Fig 4.
Antibody against HbpB was increased in serum from the B. quintana-infected patient. (A) Five Xpress-tagged Hbps (as indicated at the top) were separated on a 7.5 to 15% SDS-PAGE gel and subjected to Western blot analysis with anti-Xpress antibody (top panel), three sera from homeless people [(30)-25, (30)-33, and (90)-69], serum from the B. quintana-infected endocarditis patient (ECP), and control serum (NC). Positions of molecular mass markers are indicated on the left. (B) The same membrane as that in panel A was subjected to Western blotting with the positive control (PC) from the Bartonella IFA kit.
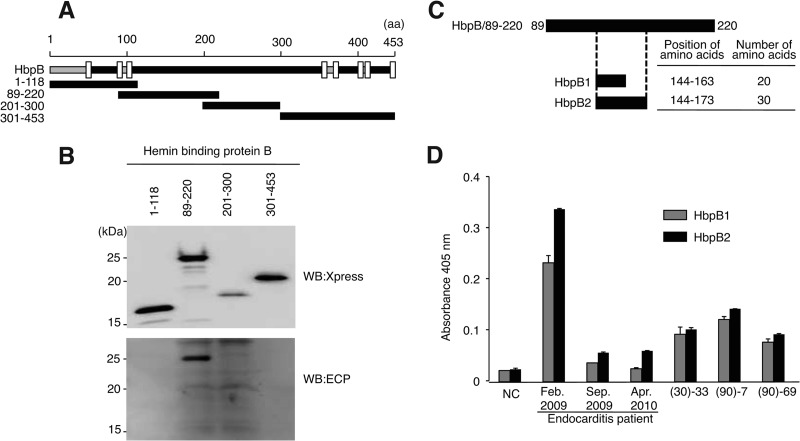
Given that anti-HbpB antibody was detected in serum from the B. quintana-infected patient, we next investigated the antigenic region of HbpB in order to design a suitable synthetic peptide for use as an ELISA antigen. Four Xpress-tagged HbpB fragments covering the entire region of HbpB were generated (Fig. 5A), and bacterial extracts including these HbpB fragments were subjected to Western blotting. As shown in Fig. 5B, signals corresponding to the four fragments were detected by anti-Xpress antibody. Among these four fragments, HbpB/89–220 reacted to serum from the B. quintana-infected patient. HbpB contains four extracellular domains, and HbpB/89–220 corresponds to the second extracellular domain. These results indicated that, in addition to anti-HbpD antibody, anti-HbpB antibody is also induced by B. quintana infection, and the epitope may be located at the N-terminal region of the second extracellular domain of HbpB. Given that antibody against HbpB/89–220 was predominant in serum of the B. quintana-infected patient, we synthesized two peptides corresponding to HbpB/89–220 for use in ELISA (Fig. 5C). HbpB1 and HbpB2 correspond to amino acid residues 144 to 163 and 144 to 173 of HbpB, respectively. As shown in Fig. 5D, the avidity to HbpB1 and HbpB2 of serum obtained on admission from the B. quintana patient was increased 11- and 14-fold, respectively, compared to the control serum. In the case of sera from homeless people, (90)-7 showed the highest avidity, and the avidity for HbpB1 and HbpB2 was increased 5.5- and 5.7-fold versus the control, respectively. These results suggest that detection of antibodies against HbpB1 and HbpB2 may be an effective basis for diagnosis of B. quintana infections.
Fig 5.
The sequence of amino acid residues 89 to 220 of HbpB is recognized by serum from the B. quintana-infected patient. (A) Schematic structure of HbpB of B. quintana. Regions corresponding to four HbpB deletion mutants are also shown. The intracellular domain (gray bar), the extracellular domain (black bar), and the transmembrane domain (TM; open box) are indicated. The number of amino acids is shown at the top. Transmembrane domains are located at residues 43 to 52, 83 to 91, 103 to 111, 364 to 372, 377 to 385, 401 to 409, 415 to 423, and 445 to 453. (B) Xpress-tagged HbpB mutants (indicated at the top) were separated on a 15% SDS-PAGE gel and subjected to Western blotting by using anti-Xpress antibody (top panel) and serum from the B. quintana-infected endocarditis patient (ECP; bottom panel). Positions of molecular mass markers are indicated on the left. (C) Schematic diagram of two HbpB synthetic peptides. HbpB1 and HbpB2 were designed based on the sequence of the extracellular domain of HbpB (residues 89 to 220). Positions and numbers of amino acid residues of the two synthetic peptides are indicated on the right. (D) HbpB1 and HbpB2 were applied as a coating to ELISA plates, and sera from the endocarditis patient and homeless people were examined. Data are the means of three independent experiments.
DISCUSSION
We previously used a commercial IFA assay to examine the incidence of B. quintana infections in homeless people in urban areas of Japan (7), and our results suggested an unexpectedly high rate of exposure to B. quintana. Such a high rate of putative B. quintana infection might have several possible explanations. In particular, the IFA system used might generate a significant number of false positives, since it employs bacterial whole cells as the antigen for detecting anti-B. quintana antibodies. For example, antibodies to other infectious agents may cross-react to B. quintana, or nonspecific antibodies reacting to this microbe might be present in the population. The possibility of asymptomatic infection with B. quintana should also be taken into account.
We considered that the use of a single, potent peptide antigen instead of whole bacterial cells might virtually eliminate false positives caused by cross-reaction of other antibodies. In the present study, therefore, we first examined the antigenicity of B. quintana proteins. As a result, we identified hemin-binding protein B (HbpB) and HbpD of B. quintana as potential candidates for developing a novel serological tool for diagnosis of B. quintana infections. Importantly, HbpB and HbpD of B. quintana reacted to B. quintana-infected sera but not sera from B. henselae-infected CSD patients in either Western blotting or ELISA (data not shown). Consequently, it should be possible to distinguish B. quintana and B. henselae infections by using these antigens. To further characterize the specificity of the B. quintana ELISA, it would be necessary to examine patients infected with Bartonella species other than B. quintana. Also, it would be desirable to confirm that the 35-kDa protein, which we identified as HbpD by N-terminal sequence analysis, is truly specific for B. quintana by testing sera from homeless people against antigens from other Bartonella species. We hope to address these issues in association with the next epidemiological survey of trench fever in Japan.
Hbp proteins are located at the bacterial surface and bind to environmental heme (23–27). The hbp gene family in B. quintana consists of five members (hbpA to hbpE), and all are expressed under usual bacterial culture conditions (14). For in vitro culture of Bartonella species, erythrocytes, hemoglobin, or hemin is usually supplied as a source of iron, because Bartonella lacks genes for heme biosynthesis. Genes hbpB and hbpC appear to be expressed predominantly under high-environmental-hemin conditions, based on the environment in lice, while hbpA, hbpD, and hbpE appear to be expressed predominantly under low-environmental-hemin conditions, based on the environment in humans (24). In this study, we found that sera from homeless people reacted to HbpD, while serum from the endocarditis patient reacted to HbpB. This finding may be attributable to differences in the expression levels of HbpB and HbpD in the pathogens in response to interindividual differences in patients' background factors or differences in the sites of infections or other factors. To examine this possibility, sera from a larger number of B. quintana-infected patients should be examined. The five Hbps in B. quintana show a high degree of identity at the amino acid sequence level, and the positions and the amino acid sequences at transmembrane domains are particularly well conserved. They are also similar in size except for HbpB, which has a 170-amino-acid extension in the second extracellular domain. Although we found anti-HbpB and anti-HbpD antibodies in individuals infected with B. quintana, no antibodies against HbpA, HbpC, or HbpE were detected, despite the high degree of identity among these proteins. Therefore, we considered that the target epitopes of antibodies directed to HbpB and HbpD may be located at unique regions in these molecules, probably at regions exposed on the bacterial outer membrane. It is possible that only HbpB and HbpD form immunogenic complexes in the bacterial outer membrane, though the interacting partners of Hbp proteins have not been elucidated yet.
As a next step, we established the locations of the antigenic sites within these proteins by generating a series of deletion mutants of HbpB and HbpD and identified residues 89 to 220 of HbpB and 151 to 200 of HbpD as the minimum regions required for recognition by the sera. Several oligopeptides comprising parts of the minimum regions of HbpB and HbpD were synthesized, and their immunoreactivity with the above sera was tested by ELISA. In this way, we identified synthetic peptides HbpB2 (amino acid residues 144 to 173 of HbpB) and HbpD3 (residues 151 to 200 of HbpD) as candidates for the development of novel serological tools for differential diagnosis of B. quintana infections from other Bartonella infections. Further work is under way to develop and validate these assays.
ACKNOWLEDGMENTS
This work was supported by Grants for Research on Emerging and Reemerging Infectious Diseases from the Japanese Ministry of Health, Labor and Welfare (H21-Shinko-Ippan-005 and H24-Shinko-Ippan-010).
We thank Yuki Yamada, Department of Laboratory Medicine, Iwate Medical University School of Medicine, Morioka, Japan, for donating serum from an endocarditis patient infected with B. quintana.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1.Greub G, Raoult D. 2002. Bartonella: new explanations for old diseases. J. Med. Microbiol. 51:915–923 [DOI] [PubMed] [Google Scholar]
- 2.Jacomo V, Kelly PJ, Raoult D. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin. Diagn. Lab. Immunol. 9:8–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet. Res. 40:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurin M, Raoult D. 1996. Bartonella (Rochalimaea) quintana infections. Clin. Microbiol. Rev. 9:273–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohl ME, Spach DH. 2000. Bartonella quintana and urban trench fever. Clin. Infect. Dis. 31:131–135 [DOI] [PubMed] [Google Scholar]
- 6.Brouqui P, Houpikian P, Dupont HT, Toubiana P, Obadia Y, Lafay V, Raoult D. 1996. Survey of the seroprevalence of Bartonella quintana in homeless people. Clin. Infect. Dis. 23:756–759 [DOI] [PubMed] [Google Scholar]
- 7.Seki N, Sasaki T, Sawabe K, Matsuoka M, Arakawa Y, Marui E, Kobayashi M. 2006. Epidemiological studies on Bartonella quintana infections among homeless people in Tokyo, Japan. Jpn. J. Infect. Dis. 59:31–35 [PubMed] [Google Scholar]
- 8.Foucault C, Barrau K, Brouqui P, Raoult D. 2002. Bartonella quintana bacteremia among homeless people. Clin. Infect. Dis. 35:684–689 [DOI] [PubMed] [Google Scholar]
- 9.Drancourt M, Mainardi JL, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. 1995. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N. Engl. J. Med. 332:419–423 [DOI] [PubMed] [Google Scholar]
- 10.Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. 2004. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents Chemother. 48:1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spach DH, Kanter AS, Dougherty MJ, Larson AM, Coyle MB, Brenner DJ, Swaminathan B, Matar GM, Welch DF, Root RK, Stamm WE. 1995. Bartonella (Rochalimaea) quintana bacteremia in inner-city patients with chronic alcoholism. N. Engl. J. Med. 332:424–428 [DOI] [PubMed] [Google Scholar]
- 12.Tea A, Alexiou-Daniel S, Arvanitidou M, Diza E, Antoniadis A. 2003. Occurrence of Bartonella henselae and Bartonella quintana in a healthy Greek population. Am. J. Trop. Med. Hyg. 68:554–556 [DOI] [PubMed] [Google Scholar]
- 13.Vermeulen MJ, Herremans M, Verbakel H, Bergmans AM, Roord JJ, van Dijken PJ, Peeters MF. 2007. Serological testing for Bartonella henselae infections in The Netherlands: clinical evaluation of immunofluorescence assay and ELISA. Clin. Microbiol. Infect. 13:627–634 [DOI] [PubMed] [Google Scholar]
- 14.Li DM, Liu QY, Zhao F, Hu Y, Xiao D, Gu YX, Song XP, Zhang JZ. 2011. Proteomic and bioinformatic analysis of outer membrane proteins of the protobacterium Bartonella henselae (Bartonellaceae). Genet. Mol. Res. 10:1789–1818 [DOI] [PubMed] [Google Scholar]
- 15.Saisongkorh W, Kowalczewska M, Azza S, Decloquement P, Rolain JM, Raoult D. 2010. Identification of candidate proteins for the diagnosis of Bartonella henselae infections using an immunoproteomic approach. FEMS Microbiol. Lett. 310:158–167 [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt C, Engelmann S, Kusch H, Albrecht D, Hecker M, Autenrieth IB, Kempf VA. 2009. Proteomic analysis of the bacterial pathogen Bartonella henselae and identification of immunogenic proteins for serodiagnosis. Proteomics 9:1967–1981 [DOI] [PubMed] [Google Scholar]
- 17.McCool TL, Hoey JG, Montileone F, Goldenberg HB, Mordechai E, Adelson ME. 2008. Discovery and analysis of Bartonella henselae antigens for use in clinical serologic assays. Diagn. Microbiol. Infect. Dis. 60:17–23 [DOI] [PubMed] [Google Scholar]
- 18.Rhomberg TA, Karlberg O, Mini T, Zimny-Arndt U, Wickenberg U, Rottgen M, Jungblut PR, Jeno P, Andersson SG, Dehio C. 2004. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of the bacterial pathogen Bartonella henselae. Proteomics 4:3021–3033 [DOI] [PubMed] [Google Scholar]
- 19.Chenoweth MR, Greene CE, Krause DC, Gherardini FC. 2004. Predominant outer membrane antigens of Bartonella henselae. Infect. Immun. 72:3097–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loa CC, Mordechai E, Tilton RC, Adelson ME. 2006. Production of recombinant Bartonella henselae 17-kDa protein for antibody-capture enzyme-linked immunosorbent assay. Diagn. Microbiol. Infect. Dis. 55:1–7 [DOI] [PubMed] [Google Scholar]
- 21.Boonjakuakul JK, Gerns HL, Chen YT, Hicks LD, Minnick MF, Dixon SE, Hall SC, Koehler JE. 2007. Proteomic and immunoblot analyses of Bartonella quintana total membrane proteins identify antigens recognized by sera from infected patients. Infect. Immun. 75:2548–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada Y, Ohkusu K, Yanagihara M, Tsuneoka H, Ezaki T, Tsuboi J, Okabayashi H, Suwabe A. 2011. Prosthetic valve endocarditis caused by Bartonella quintana in a patient during immunosuppressive therapies for collagen vascular diseases. Diagn. Microbiol. Infect. Dis. 70:395–398 [DOI] [PubMed] [Google Scholar]
- 23.Minnick MF, Sappington KN, Smitherman LS, Andersson SG, Karlberg O, Carroll JA. 2003. Five-member gene family of Bartonella quintana. Infect. Immun. 71:814–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battisti JM, Sappington KN, Smitherman LS, Parrow NL, Minnick MF. 2006. Environmental signals generate a differential and coordinated expression of the heme receptor gene family of Bartonella quintana. Infect. Immun. 74:3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Battisti JM, Smitherman LS, Sappington KN, Parrow NL, Raghavan R, Minnick MF. 2007. Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect. Immun. 75:4373–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll JA, Coleman SA, Smitherman LS, Minnick MF. 2000. Hemin-binding surface protein from Bartonella quintana. Infect. Immun. 68:6750–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrow NL, Abbott J, Lockwood AR, Battisti JM, Minnick MF. 2009. Function, regulation, and transcriptional organization of the hemin utilization locus of Bartonella quintana. Infect. Immun. 77:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]