Abstract
During the 2009-2010 H1N1 influenza pandemic, an adjuvanted monovalent vaccine containing ∼25% of the normal antigen dose and AS03 adjuvant was widely used in Canada. This vaccine was found to be well-tolerated and immunogenic in young children (D. W. Scheifele et al., Pediatr. Infect. Dis. J. 30:402–407, 2011). We report here additional analyses to further characterize the humoral response to this vaccine. We measured standard hemagglutination inhibition (HAI) and microneutralization (MN) titers, as well as influenza virus-specific IgG avidity and subclass distribution by enzyme-linked immunosorbent assay in 73 subjects. Sera were collected before (day 0) and 3 weeks after each dose of vaccine (days 21 and 42). Most children (55/73) had undetectable HAI and MN titers at day 0 (presumed to be antigen naive) and mounted good responses at days 21 and 42. The majority of these children (43/55) had the expected pattern of an increasing IgG avidity index (AI) after each dose of vaccine (not detected [ND], 0.30, and 2.97 at days 0, 21, and 42, respectively). The avidity responses in the remaining children (12/55) were quite different, with AIs increasing abruptly after the first dose and then declining after the second dose of vaccine (ND, 8.83, and 7.15, respectively). These children also had higher concentrations of influenza virus-specific IgG1 and IgG3 antibodies at day 21. Although the antibody titers were similar, some antigen-naive children demonstrated an unusual pattern of avidity maturation after two immunizations with AS03-adjuvanted, low-dose influenza virus vaccine. These data suggest the presence of subtle differences in the quality of the antibodies produced by some subjects in response to this vaccine.
INTRODUCTION
The 2009-2010 A/California pandemic H1N1 (pdmH1N1) influenza pandemic sharply refocused the world's attention on the need to provide safe and effective influenza virus vaccines on a global scale. Although the available vaccines are generally safe, they are far from ideal. In particular, they tend to be least effective in the very young and the elderly and do not elicit long-lasting immunity. To address such concerns, industry is increasingly turning its attention to adjuvants. During the 2009-2010 H1N1 pandemic, an Adjuvant System 03 (AS03)-adjuvanted monovalent vaccine (Arepanrix; GlaxoSmithKline, Laval, Quebec, Canada) was recommended and widely used in Canada. The inclusion of this oil-in-water adjuvant allowed the vaccine to be formulated with 25% of the normal antigen dose (∼3.7 μg of hemagglutinin [HA] protein/dose) while still achieving adequate hemagglutination inhibition (HAI) antibody titers (1, 2). The World Health Organization recommended the use of such antigen sparing vaccines during the 2009 pandemic (3).
AS03 is composed of alpha-tocopherol (a form of vitamin E) and squalene (an oil naturally occurring in humans) in an oil-in-water emulsion. This adjuvant induces innate immune responses (e.g., cytokine production) at the site of injection and in draining lymph nodes (4). It is thought that this effect leads to the recruitment of monocytes contributing to enhanced adaptive immune responses (4). In the context of influenza virus vaccines, the use of AS03 in preclinical and clinical studies showed that the adjuvant can enhance the induction of memory B cell and polyfunctional CD4 T cell responses (5) and promote the generation of cross-reactive and cross-clade antibody responses (6). However, before vaccine rollout, little was known about the effects of AS03-adjuvanted vaccines in children. The vaccine regimen chosen for children was largely based on the safety and effectiveness profile of AS03-adjuvanted vaccines in adults. In Canada, children aged 6 to 36 months were recommended to receive two immunizations at least 3 weeks apart with half the adult vaccine dosage of the AS03-adjuvanted pdmH1N1 monovalent vaccine (1.9 μg of HA/dose) (7).
Under the auspices of the Public Health Agency of Canada-Canadian Institute for Health Research Influenza Research Network (PCIRN), an observational study of this vaccine in healthy young children (aged 6 to 35 months) was performed in five urban centers across Canada. The study was completed between November 2009 to January 2010 and found the adjuvanted vaccine to be well tolerated and highly immunogenic (8). After one or two doses, HAI titers thought to be seroprotective were achieved in 80 and 100% of the children, respectively (8).
In the present study, we performed additional serological analyses to more fully investigate the development of the humoral immune response to this novel vaccine. In addition to the HAI titers already measured, we assessed the ability of the antibodies to prevent viral entry in a standard microneutralization (MN) format and both influenza virus-specific IgG avidity and IgG subclass distribution after immunization.
MATERIALS AND METHODS
Serum collection.
The PCIRN RT-03 study was designed to investigate the effect of one versus two doses of the adjuvanted pdmH1N1 vaccine in healthy young children (8). Blood samples were collected before vaccination (day 0, visit 1 [V1]) and 3 weeks after each dose (V2 at day 21, V3 at day 42). Sera were stored at −80°C in the PCIRN archive located at the Research Institute of the McGill University Health Centre until used. HAI titers of all samples were previously determined (8). HAI titers below the limit of detection (<10) are identified in graphs as not detected (ND) and assigned a value of 5 for statistical analysis. Based on the availability of serum aliquots at all three time points, we selected 73 subjects for further analysis.
MN assay.
The microneutralization (MN) assay was adapted from the method recommended by the World Health Organization (9). Virus stock (Influenza A/California/07/2009 H1N1; supplied by Y. Li, National Microbiology Laboratory, Winnipeg, Manitoba, Canada) was propagated in Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34). MDCK cells were grown in Eagle minimal essential medium (Wisent, St. Bruno, Quebec, Canada) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 μg/ml gentamicin, and 10 mM HEPES (all from Wisent). Sera were heat-inactivated (56°C for 30 min) and stored at −20°C until processing. MDCK cells were seeded into flat-bottom 96-well plates (30,000 to 45,000/well) in HyClone SFM4MegaVir medium (Thermo Scientific, Waltham, MA) supplemented with 10 μg/ml gentamicin (Gibco Life Technologies, Burlington, Ontario, Canada), 0.25 μg/ml amphotericin B (Gibco Life Technologies), 100,000 U/ml penicillin G (Sigma, St. Louis, MO), and 10 μg/ml glutamine (Wisent) to achieve confluent cell monolayers, which were used within 3 days of confluence. Twofold serial dilutions of serum starting at 1:10 in MegaVir were incubated with 100 50% tissue culture infective doses (TCID50) of virus for 2 h at 37°C with 5% CO2. The serum/virus was then added to MDCK cells in MegaVir medium with 1× TPCK (tolylsulfonyl phenylalanyl chloromethyl ketone)-treated trypsin. After 3 h at 37°C with 5% CO2, the medium in each well was refreshed with MegaVir medium with 0.75× TPCK-treated trypsin. The cells were observed for the presence of cytopathic effect (3 to 5 days), and the MN titer was defined as the highest dilution to retain a confluent cell monolayer. The assay was repeated if the sample replicates differed by more than one dilution. MN titers below the limit of detection (<10) are identified in graphs as not detected (ND) and assigned a value of 5 for statistical analysis.
Avidity index ELISA.
Based on the work of de Bruijn et al. (10), we developed an IgG avidity enzyme-linked immunosorbent assay (ELISA) that measures the binding strength of influenza virus-specific antibodies. Briefly, ELISA plates were coated overnight with 0.5 μg/ml recombinant HA protein from A/California/07/2009 H1N1 (catalog no. IT-003-SW12ΔTMp [Immune Technology, New York, NY]) in 100 mM bicarbonate/carbonate buffer at pH 9.5 (50 μl/well). Wells were washed four times with phosphate-buffered saline (PBS) and then blocked with 2% bovine serum albumin (BSA; Sigma) in PBS-Tween 20 (0.05%; Fisher Scientific, Ottawa, Ontario, Canada) at 150 μl/well for 1 h at 37°C. Each serum sample was pretested to determine a dilution (1/50 or lower) that would yield a final optical density between 0.4 and 1.5. Samples that did not reach 0.4 were processed at a dilution of 1:50. Wells were washed four times with PBS, and then each sample was diluted to the appropriate extent in block solution and added to eight antigen-coated wells (four duplicates; 50 μl/well) and allowed to bind for 1 h at 37°C. Increasing concentrations of the chaotropic reagent urea (0, 2, 6, and 8 M in PBS at 100 μl/well) were added to duplicate wells, followed by incubation for 30 min at room temperature with urea. Wells were then washed with PBS and were reblocked with PBS-Tween 20–2% BSA for 1 h at 37°C (150 μl/well). Finally, anti-human IgG-horseradish peroxidase secondary antibodies (Sigma) diluted in blocking solution was added (100 μl/well), followed by incubation for 1 h at 37°C. After six washes with PBS, plates were detected with TMB substrate (Millipore, Billerica, MA) and then stopped after 20 min with 0.5 M H2SO4. The plates were read at a wavelength of 450 nm with an EL800 universal microplate reader (BioTek Instruments, Inc., Winooski, VT). The avidity index (AI) was calculated as the concentration of urea needed to displace 50% of the antigen-specific antibodies. This process was repeated if the optical density at 0 M urea was outside of 0.4 to 1.6 and if the r2 value of the linear line of best fit was <0.8. Serum samples with an optical density reading below 0.4 at a dilution of 1:50 were considered to have undetectable AI; these are identified in the graphs as not detected (ND) and assigned a value of 0.1 for statistical analysis. Samples for each subject were run in duplicate with all three time points of the same subject run at the same time. The interassay variation was calculated to be 0.92.
Antigen-specific IgG subclass ELISA.
We determined the concentrations of influenza virus-specific IgG subclasses (IgG1, IgG2, IgG3, and IgG4) in serum. ELISA plates were coated with purified HA protein as described above (Immune Technology) and with standard concentrations of purified human IgG1, IgG2, IgG3, and IgG4 antibodies (Sigma) in 100 mM bicarbonate/carbonate buffer at pH 9.5. Wells were washed and blocked as described above for 1 h at 37°C. Serum samples were diluted 1/50 in blocking buffer and added in duplicate to HA-coated wells (50 μl/well), while additional blocking buffer was added to IgG subclass standards. Serum samples were allowed to bind for 2 h at 37°C. Wells were washed four times as described above and then biotinylated-IgG subclass antibodies (Sigma) (50 μl/well) were added to wells, which was incubated for 1 h at 37°C. After washes as described above, peroxidase-conjugated Extravidin (Sigma) tertiary antibody diluted in blocking solution was added (75 μl/well), followed by incubation for 1 h at 37°C. After six washes with PBS, plates were detected with TMB substrate (Millipore) and then stopped after 20 min with 0.5 M H2SO4. The concentration of influenza virus HA-specific IgG subclass antibodies was determined using a standard curve of purified human antibodies included on each plate. The percent contribution of subclass antibodies was calculated individually for each subject at each time point, and then the geometric mean and confidence interval were calculated for each group. As a result, the total subclass percentage does not equal exactly 100%.
Statistical analysis.
To determine significance between the same subjects at different time points, or between subgroups at the same time point, the paired t test with two-tailed P values and the Mann-Whitney U test were used, respectively. All calculations for statistical significance were performed using GraphPad Prism 5.0 software.
RESULTS
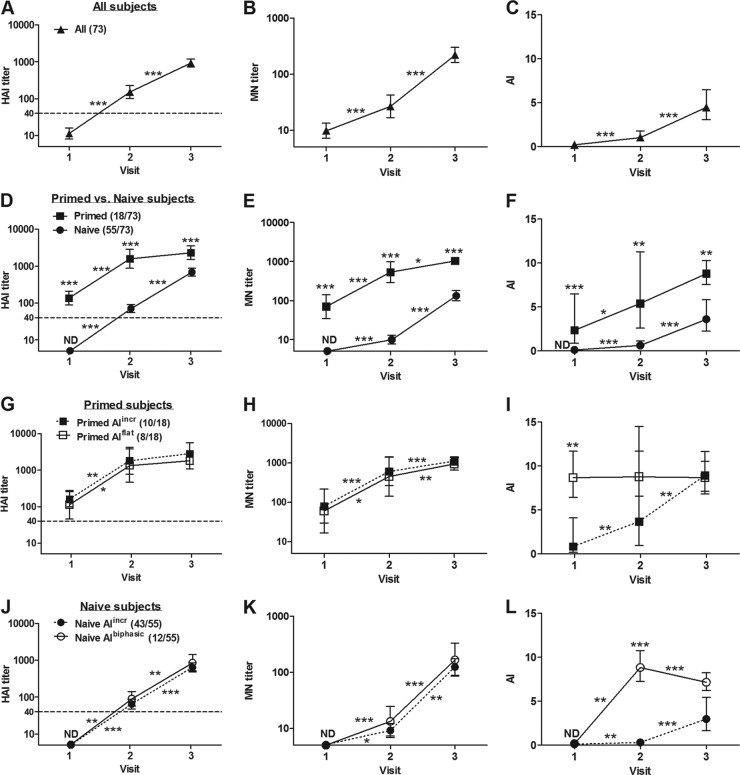
The HAI results shown in Fig. 1A, D, G, and J were plotted using data reported in Table 1 of our previous publication in the Pediatric Infectious Disease Journal (8). All children demonstrated increasing HAI and MN titers following the two doses of vaccine (Fig. 1A and B). Overall, the MN titers at all time points correlated well with HAI titers (Spearman rank correlation coefficient = 0.97). However, titers tended to be lower overall, as measured by MN than as measured by HAI. Based on HAI results prior to the first dose, some of the children (18/73 [25%]) had likely been exposed to wild-type pdmH1N1 and were considered antigen primed (HAI > 40 at V1 [day 0]) (Fig. 1D). The remaining children had undetectable HAI titers at V1 and mounted good responses at V2 (day 21) and V3 (day 42). These children (55/73 [75%]) had HAI titers of <10, 71, and 690 at V1, V2, and V3, respectively (Fig. 1D, naive). MN titers for this group at the same time points were <10, 10, and 133 (Fig. 1E, naive). As expected, the children who appeared to have been exposed to the pandemic virus prior to immunization (primed) had much higher HAI (137, 1,582, and 2,325) and MN (71, 533, and 1,036) titers at all time points (Fig. 1D and E). As would be expected, the difference between these two groups was particularly marked at day 21; HAI titers were 22-fold higher and MN titers were 53-fold higher in primed versus naive subjects. By day 42, HAI and MN titers were 3.3- and 8-fold higher, respectively, in primed compared to naive children.
Fig 1.
Humoral responses in young children immunized with two doses of AS03-adjuvanted pdmH1N1 split vaccine. Serum samples were collected before and 3 weeks after each immunization, which were administered at visit 1 and visit 2. The HAI titers in panels A, D, G, and J of the serum samples were previously determined and are adapted from Scheifele et al. (8) with the permission of the publisher. MN titer (B, E, H, and K) and AI by avidity ELISA (C, F, I, and L) of serum samples were determined in the present study. (A, B, and C) All subjects analyzed; (D, E, and F) subjects divided into primed or naive subgroups based on HAI titer at visit 1; (G, H, and I) primed subjects further subgrouped based on increasing (primed AIincr) or stable (Prime AIflat) avidity; (J, K, and L) naive subjects further subgrouped based on increasing (naive AIincr) or biphasic (naive AIbiphasic) avidity. Geometric means and 95% confidence intervals are shown. Numbers in brackets beside group names denote the numbers of subjects in each subgroup. Asterisks on connecting lines indicate significant changes between time points of the same group. Asterisks on data points indicate significant differences between two subgroups. ND, not detected; *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
All children also had readily apparent IgG avidity maturation (0.24, 1.06, and 4.44) with statistically significant increases in AI following each dose of vaccine (Fig. 1C). Compared to the naive children, the children previously primed by presumed exposure to wild-type virus had higher AI values (primed, 2.36, 5.40, and 8.81 versus naive, ND, 0.62, and 3.60, respectively) (Fig. 1F). Within the primed group, the majority (10/18 [56%]) showed the expected pattern of progressively increasing IgG avidity (0.83, 3.67, and 8.92) (Fig. 1I, primed AIincr) in parallel with increasing HAI and MN titers (Fig. 1G and H, primed AIincr) after each dose of vaccine. The AI was essentially constant in the remaining children from day 0 to day 42 (8.67, 8.76, and 8.67), suggesting that IgG avidity had already fully matured prevaccination (Fig. 1I, primed AIflat). Despite constant AI values in these children, the HAI and MN titers continued to increase (Fig. 1G and H, primed AIflat).
Overall, the naive group had increasing AI after each dose of vaccine (Fig. 1F, naive). The majority (43/55 [78%]) behaved in a fashion similar to the primed children with steadily increasing antibody titers (by HAI and MN) and increasing IgG avidity (ND, 0.3, and 2.97) after each dose of vaccine (Fig. 1J, K, and L, naive AIincr). However, the avidity response in the remaining children (12/55 [22%]) was quite different, with AI increasing steeply after the first dose and then declining significantly after the second dose of vaccine (ND, 8.83, and 7.15) (Fig. 1L, naive AIbiphasic). This is a very unusual pattern of IgG avidity maturation, made even more unusual by an essentially normal pattern of increasing HAI and MN titers with each dose (Fig. 1J and K, naive AIbiphasic).
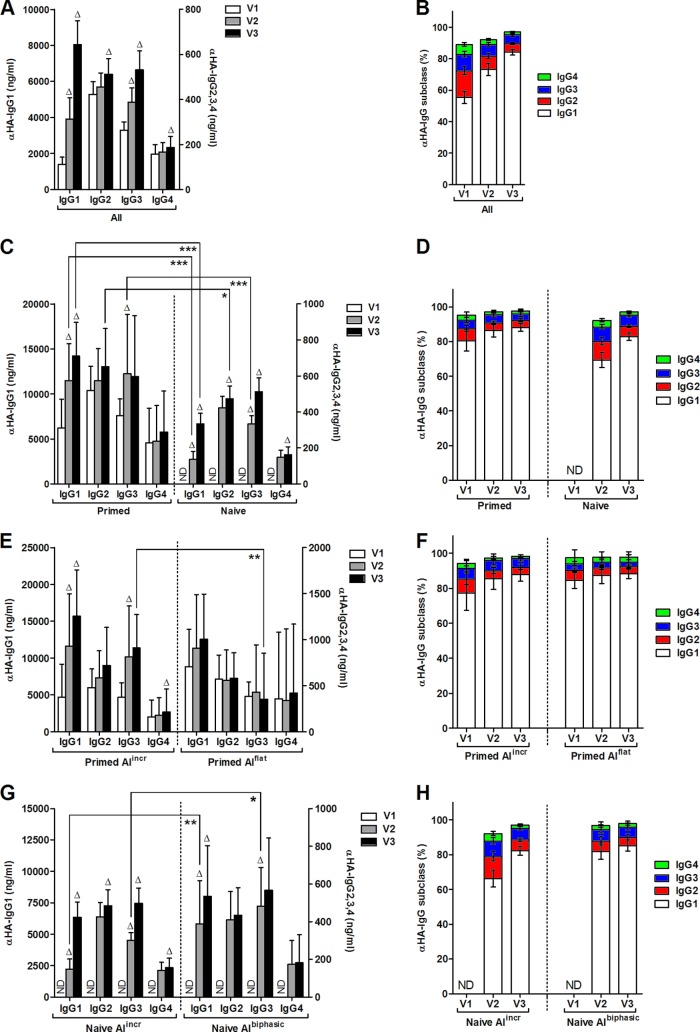
Because IgG subclasses have different serum half-lives (11) and may have different kinetics of avidity maturation, we measured influenza virus-specific IgG antibody subclasses in all samples. In human sera, IgG antibodies are present as four subclasses—IgG1, IgG2, IgG3, and IgG4—with the average subclass concentration in serum at 8, 4, 0.8, and 0.4 mg/ml, respectively (12). Since IgG1 serum concentrations are typically the highest (12), it is not surprising that the majority of influenza virus-specific antibodies were subclass IgG1; all four subclasses of IgG antibodies were present in all children before the vaccination (Fig. 2A and B; note that the scales for the IgG1 versus other subclasses are on different y axes). This observation is consistent with previous studies of IgG subclasses after natural infection and vaccination (13, 14). After the first dose of vaccine, there were significant increases in the concentrations of HA-specific IgG1 and IgG3 in all subjects (Fig. 2A), whereas after the second dose of vaccine, the concentrations of all IgG subclasses of HA-specific antibodies increased significantly (Fig. 2A). After two immunizations, there was a steady increase in the percentage of HA-specific IgG1 antibodies, while the proportions of the remaining subclasses tended to decrease (Fig. 2B).
Fig 2.
Influenza virus-specific IgG subclass analysis in young children immunized with two doses of AS03-adjuvanted pdmH1N1 split vaccine. Serum samples were collected before and 3 weeks after each immunization, which were given at visit 1 and visit 2. The concentrations of influenza virus HA-specific IgG subclass antibodies were determined by ELISA and the percent contribution of subclass antibodies was calculated individually for each subject at each time point and then averaged for each group. Subjects were divided into subgroups as described in Fig. 1. All subjects (A and B) were divided into primed and naive subgroups (C and D) and then further divided into primed groups with increasing or stable AI (primed AIincr and primed AIflat) (E and F) or naive groups with increasing or biphasic AI (naive AIincr or naive AIbiphasic) (G and H). Geometric means and 95% confidence intervals are shown. For subclass concentrations, the IgG1 concentration is graphed on the left y axis, while the IgG2, IgG3, and IgG4 concentrations are graphed on the right y axis. Delta symbols (Δ) at the tops of bars indicate a significant increase (P < 0.05) of the same subclass antibody from the previous time point. Significant differences of the same subclass antibody between subgroups are indicated by brackets. ND, not detected; *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
Considering the same naive and primed subgroups based on prevaccination HAI titers, primed subjects had increases in the concentrations of HA-specific IgG1 and IgG3 after the first dose of vaccine (Fig. 2C), while after a second dose, only the concentration of HA-specific IgG1 antibodies increased significantly. The general pattern of IgG subclass response among the naive subjects was essentially identical to the primed children with significant increases in HA-specific IgG1 and IgG3 after the first dose but increases in all subclasses after the second dose (Fig. 2C). Compared to the naive subjects, the primed subjects had higher concentrations of HA-specific IgG1 and IgG3 antibodies at V2 and higher concentrations of IgG1 and IgG2 antibodies at V3 (Fig. 2C). The concentration of HA-specific IgG4 antibodies was similar in both groups. The primed subjects had a similar distribution of IgG subclasses pre- and postvaccination (Fig. 2D, primed). Naive subjects had a broader distribution of IgG subclasses than primed subjects but also tended to increase the proportion of IgG1 antibodies after a second immunization (Fig. 2D, naive).
The primed subjects with constant high avidity (primed AIflat) had a trend of increasing HA-specific IgG1 antibodies after vaccination, although not significant; there were no progressive increases with the other antibody subclasses (Fig. 2E, primed AIflat). In contrast, primed subjects with increasing avidity (primed AIincr) had significant increases in the concentrations of HA-specific IgG1 and IgG3 after the first dose of vaccine and of IgG1 and IgG4 after the second dose of vaccine (Fig. 2E, primed AIincr). The concentration of HA-specific IgG3 at V3 was significantly higher in the primed AIincr group compared to the primed AIflat group (Fig. 2E). Furthermore, the proportion of IgG3 antibodies tended to be higher in the primed AIincr subjects compared to the primed AIflat group (Fig. 2F). The primed AIincr subjects also showed a trend to increase the proportion of IgG1 antibodies and decrease IgG4 antibodies after immunization, while the ratio of subclass antibodies was essentially unchanged in the primed AIflat subjects (Fig. 2F).
All of the naive subjects had increasing concentrations of HA-specific IgG1 after the two doses of vaccine (Fig. 2G). Both naive subgroups also generated significant concentrations of HA-specific IgG3 antibodies after the first dose of vaccine (Fig. 2G). In contrast, only subjects in the naive AIincr group showed increasing levels of IgG2, IgG3, and IgG4 antibodies after a second dose of the vaccine (Fig. 2G, naive AIincr). Interestingly, at V2 (after one dose of vaccine), the naive subjects with the unusual avidity pattern (naive AIbiphasic) also had significantly higher concentrations of IgG1 and IgG3 antibodies, which may have contributed to the higher antibody avidity at this time point (Fig. 2G). Finally, the naive AIincr subjects had a broader subclass response compared to the remaining naive subjects and showed a clear trend toward increases in the proportion of IgG1 antibodies with decreases in the proportion of IgG2 and IgG4 antibodies following a booster immunization (Fig. 2H). In contrast, the naive AIbiphasic subjects only had a slight increase in the relative amount of IgG1, and the remaining subclasses were largely unchanged after immunization (Fig. 2H). Interestingly, both subgroups of naive and primed subjects showed similar patterns of IgG subclass proportions. Primed AIincr and naive AIincr subjects had increases in the proportion of IgG1 antibodies with decreases in the proportion of the other antibodies over time, while relative proportions of the IgG subclass antibodies remained largely unchanged in the primed AIflat and naive AIbiphasic groups (Fig. 2F and H).
DISCUSSION
The primary goal of this study was to increase our understanding of the antibody response elicited in young children by the AS03-adjuvanted pdmH1N1 influenza virus vaccine. Although experience with squalene-based vaccine adjuvants was substantial in adults and the elderly, very few young children had received any product containing either squalene or squalene/tocopherol adjuvants prior to the 2009-2010 pandemic. Using samples from a large study of the AS03-adjuvanted pdmH1N1 influenza vaccine conducted within the framework of the PHAC-CIHR Influenza Research Network (PCIRN), we determined the titers of functional antibodies using a standard MN assay and developed assays to examine both influenza-specific IgG avidity and IgG subclass distribution.
As has been previously reported, we found a strong correlation between the HAI and MN results overall, which is reassuring given the technical complexities of these assays (15). Although the HAI and single-radial hemolysis (SRH) assays are the only standardized tests used in vaccine licensure (HAI in North America and SRH in Europe), other serological assays can be powerful tools to further characterize vaccine-induced immune responses. For example, deficiency in IgG2 antibodies was implicated in severe disease during the 2009-2010 H1N1 pandemic (16). As expected, we observed a broad IgG subclass response that was dominated by IgG1 in virtually all of the children included in our study. Our analysis of influenza virus-specific IgG subclass antibodies is in line with previous studies, which showed that in children, the influenza virus-specific antibody response to unadjuvanted split vaccine was mainly of the IgG1 subclass (13, 14, 17). The main objective of the present study, however, was to investigate antibody avidity maturation after administration of the AS03-adjuvanted vaccine. Affinity/avidity maturation occurs during the development of the immune response and leads to the production of high-affinity/avidity antibodies (18). Once again, the overall pattern of increasing AI following each dose of vaccine that we observed was expected. Despite general conformity to expectations, there were still intriguing differences in both the magnitude and pattern of serologic responses among significant subsets of these children.
Since this study was completed during the 2009-2010 H1N1 pandemic, a proportion of subjects were found to have encountered wild-type virus prior to vaccination as indicated by positive HAI titers at recruitment (i.e., the primed group). In addition to the preexisting antibody titers, the rapidity, magnitude, and quality (IgG avidity and subclass distribution) of the humoral response in these children after the first dose of vaccine are all strong evidence of prior exposure to wild-type virus. Although structural similarities between the HA of pdmH1N1 and strains circulating in the early part of the 20th century have been implicated in the unusual pattern of age susceptibility during the pandemic (19), the only plausible prior exposure for the young children in the current study is the pdmH1N1 virus itself. As a result, the rapidly rising titers in these children likely reflect a “mixed” response to wild-type virus and vaccination. At day 42 (V3), these primed children generally had higher antibody titers, as measured by both routine serologic assays but the differences were most striking using the functional MN assay (an 8-fold-higher MN titer in primed versus naive children and a 3-fold-higher HAI titer). Such a result would be expected following exposure to all of the pdmH1N1 antigens from the wild-type virus instead of a more limited number of antigens in the split-virus vaccine (primarily HA with much smaller amounts of other viral proteins).
All of the primed subjects had similar HAI and MN titers, which were relatively low at V1, but increased following each dose of vaccine. Just over half of these children (55%) had the expected pattern of avidity maturation after vaccination, with low AI at V1 that increased after each dose (primed AIincr). This group may have had a very recent or even concurrent infection and therefore had yet to reach maximal antibody avidity. The antibody titers and AI profiles in these children are therefore consistent with a “mixed” response to vaccination plus a recent WT virus exposure. Antibody avidity has been used to differentiate between recent versus more distant infection with other viruses, as well as primary versus secondary vaccine failure (20–22).
It is harder to find a satisfying explanation for the remainder of the primed children (44%) who had very high or maximal AI prevaccination (V1) despite uniformly low HAI and MN titers at V1. The AI remained essentially unchanged in these children at V2 and V3 despite rapidly rising HAI and MN titers (primed AIflat). Despite the similarities in HAI and MN titers, as well as IgG subclass distribution between the primed AIincr and the primed AIflat groups at V1, the AI profiles in these children were so dissimilar that their prevaccination serological status must have been different as well. The antibody profile in the primed AIflat children strongly suggests prior but more distant exposure to the pdmH1N1 virus. The earliest possible exposure for these children was April 2009, while recruitment for the study was completed between November 2009 and January 2010, a 7- to 9-month period between exposure and vaccination. The 2009-2010 H1N1 pandemic in Canada occurred in two distinct waves: the first wave peaked between June and July 2009, and the second wave peaked between October and November 2009, with some variability by province (23–26). The most plausible explanation for this pattern of response is that the primed AIflat children were exposed to the pdmH1N1 strain in the first wave of the pandemic, developed a fully mature AI response after this exposure, and then completely lost their HAI and MN titers in the intervening 7- to 9-month period. Such a rapid loss of antibodies has been reported following both vaccination and natural disease (27, 28). Indeed, rapid loss of antibodies appears to be particularly common in young children experiencing their first or second influenza virus infections (27, 28). Although preexisting, cross-reactive antibodies were found in some elderly subjects, presumably due to distant exposure to H1N1 strains circulating in the first part of the 20th century, it seems highly unlikely that the high AI in these children can be explained by cross-reacting antibodies.
Our analysis of influenza virus-specific IgG subclasses in young children following AS03-adjuvanted vaccination was consistent with the findings of previous studies in which the unadjuvanted split vaccine also elicited a predominant IgG1 response (13, 14, 17). In the naive children, however, the HAI and MN titers and the influenza virus-specific IgG subclass concentrations never reached the same levels as those of children in the primed groups, even after the second dose of vaccine. The IgG subclass ratios were eventually similar in naive and primed subjects, but only after two doses of vaccine. After the first dose, the naive children tended to have a broader subclass response, most notably with higher relative proportions of IgG2 and IgG4 antibodies. This observation is similar to that of Hocart et al., who noted that natural influenza virus infection in adults almost exclusively elicits IgG1 antibodies, whereas the response to inactivated vaccine is broader, with IgG1, IgG2, and IgG3 antibodies generated (17). Based on this observation, the subgroups with unusual AI profiles (prime AIflat and naive AIbiphasic) appeared to mimic the response to natural infection with mainly IgG1 produced and unchanged percentages of other IgG subclasses. On the other hand, the subgroups with normal AI profiles (primed and naive AIincr) appear to follow the response to inactivated vaccine with a broader initial IgG subclass response, followed by an increasing proportion of IgG1 antibodies after vaccination.
Even after two doses of vaccine, antibody avidity was generally lower in the naive groups compared to the primed subjects. Collectively, all of the naive subjects demonstrated increasing HAI and MN titers, as well as increasing AI after each dose of vaccine. However, the naive subjects could be subdivided according to their pattern of avidity maturation. The majority (43/55 [78%]) had steadily increasing AI with each dose of vaccine, which is the expected pattern of antibody avidity maturation. Although the increase in AI between V1 and V2 in the naive AIincr subgroup was statistically significant, the pace of avidity maturation was slow, and the AI remained quite low in this group of children at V2, achieving only 25 to 30% of the anticipated avidity of the fully mature response at V3. Unfortunately, we do not know how the AI profile evolved beyond the V3 time point in these subjects.
In the remaining naive subjects (naive AIbiphasic), there was a totally unexpected pattern of IgG avidity with an abrupt and substantial increase after the first dose of vaccine, followed by a significant decrease after the second dose. This pattern was observed in 22% of the naive children (12/55) and not in any of the primed subjects. Given the lack of cross-reactivity between pdmH1N1 and recently circulating H1N1 viruses, it is also unlikely that cross-reacting antibodies from another strain of influenza virus could have contributed to this unusual pattern of AI evolution (29). This “boom and bust” pattern is quite distinct from reported AI responses following one and two doses of unadjuvanted monovalent pdmH1N1 vaccines or trivalent seasonal vaccines in adult and elderly subjects (10, 30) and, furthermore, this biphasic AI profile was never previously reported in the response to any inactivated vaccine with or without adjuvant.
A recent study with an influenza vaccine adjuvanted with MF59 (squalene alone) showed that this vaccine produces more and higher-avidity antibodies than unadjuvanted vaccine in subjects across age groups, including toddlers, adults, and elderly individuals (31). The authors of that study found that the MF59 adjuvant had the greatest effect in naive subjects (toddlers aged 12 to 35 months) and hypothesized that a major effect of the adjuvant was to enhance the level of somatic hypermutation in naive B cells (31). In our study, it is possible that the presence of the AS03 adjuvant also enhanced somatic hypermutation in some children, leading to accelerated antibody avidity at V2. However, our study had several important limitations, including the absence of control groups that had received unadjuvanted vaccine or that had been exposed only to natural disease. We cannot be certain that children we classified as “primed” had truly been exposed to natural infection or the timing of such hypothetical exposure. Finally, the children in the present study were only monitored for 6 weeks after vaccination, so we do not know how the measures of humoral response that we targeted evolved after this time point. Later time points would have been of particular interest for the children who appeared to be losing IgG avidity with time (naive AIbiphasic). To our knowledge, there are two other reports of loss of IgG avidity over time. Recently, waning IgG avidity was demonstrated in children immunized with MMR vaccine (32) and, interestingly, declining IgG avidity was observed in healthy adults immunized with a recombinant polysaccharide vaccine that was adjuvanted with MF59 (33). In both of those studies, however, waning avidity was observed in time frames (20 years and 10 to 24 months, respectively) that were much longer than the loss of IgG avidity observed here (6 weeks).
Unfortunately, sera from children immunized with unadjuvanted pdmH1N1 vaccine were not available for our study; all children in Canada received the AS03-adjuvanted vaccine. Although sera from children immunized with one or two doses of the unadjuvanted 2011 TIV containing the pdmH1N1 antigen are available to us, no AS03-adjuvanted TIV is licensed in Canada for comparison. Since unadjuvanted TIV is not really a good control for our study of children exposed to the monovalent, adjuvanted pdmH1N1 formulation, there is really no ideal control group for our study. Efforts are under way to obtained age-matched sera from children in other countries (e.g., Australia, some parts of the United States, and Europe) who received two doses of unadjuvanted monovalent inactivated pdmH1N1 split vaccine on roughly the same schedule as the children in our study. However, even if we succeed in acquiring such sera, there will still be important limitations (e.g., different demographics, vaccine formulations, schedules, etc.). In addition, we are currently pursuing this observation in elderly subjects for whom both unadjuvanted and adjuvanted formulations are available and in a mouse model of low-dose immunization to better understand the long-term implication for humoral immunity after adjuvanted influenza vaccination.
It is possible that the “boom and bust” pattern of antibody avidity we observed also occurs following the administration of other vaccines. As mentioned above, previous reports have demonstrated long-term declines in IgG avidity after vaccination with other types of vaccines (32, 33). In our study, when all of the naive subjects were considered together, the overall pattern of avidity was as expected, with increasing AI after each dose of vaccine. Our aggregate data are therefore similar to “normal” AI observations following live-attenuated vaccination (33–35) and studies following inactivated vaccines with or without adjuvants (30, 36–38). However, it is interesting that, in a study of antibody avidity to multiple doses of inactivated poliovirus vaccine in infants, the AI profile was essentially flat after the first two doses. Furthermore, although only aggregate data were reported, the range of avidities seen suggest that at least some of these children may have had up-and-down avidity patterns after vaccination (39). These few studies and our observations certainly support the analysis of individual avidity patterns rather than aggregate data only.
In the present study, we found that ca. 20% of antigen-naive children had an unusual evolution of their influenza virus-specific IgG avidity after two doses of AS03-adjuvanted pdmH1N1 vaccinations despite normal HAI and MN responses. In these children, IgG avidity rose abruptly after the first dose of vaccine but dropped significantly after the second dose. After one dose, these children also had higher levels of influenza virus-specific IgG1 and IgG3 antibodies with a broader overall distribution of IgG subclass antibodies compared to other children. These data clearly demonstrate that there can be major qualitative differences in the antibody response in certain children after vaccination despite the induction of very similar HAI and MN titers. Additional studies are needed to determine whether this response is unique to AS03-adjuvanted vaccines or whether this is an essentially normal response in some individuals following immunization with inactivated vaccines.
ACKNOWLEDGMENTS
We thank Louis P. Dufour for technical assistance.
This study was supported by the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN), and K.K.Y. was the recipient of a PCIRN scholarship.
PCIRN Rapid Trials Network contributing investigators included the following individuals: Gaston De Serres and Vladimir Gilca, Unité de Recherche en Santé Publique (CHUQ) and Laval University, Québec City, Québec, Canada; Shelly McNeil and Bruce Smith (statistician), Canadian Center for Vaccinology and Dalhousie University, Halifax, Nova Scotia, Canada; James Kellner and Judy MacDonald, Alberta Children's Hospital and University of Calgary, Calgary, Alberta, Canada; Laura Sauvé and Tobias Kollmann, Vaccine Evaluation Center, BC Children's Hospital and University of British Columbia, Vancouver, British Columbia, Canada; and Nathalie Bastien, National Microbiology Laboratory, Winnipeg, Manitoba, Canada.
Footnotes
Published ahead of print 23 January 2013
REFERENCES
- 1. Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. 2007. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 370:580–589 [DOI] [PubMed] [Google Scholar]
- 2. Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, Leroux-Roels G, Pichon S, Kusters I. 2008. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J. Infect. Dis. 198:642–649 [DOI] [PubMed] [Google Scholar]
- 3. Technical Advisory Group on Vaccine-Preventable Diseases 2009. Final recommendations of pandemic influenza. Pan American Health Organization, Regional Office of the World Health Organization, Geneva, Switzerland: http://www.paho.org/english/ad/fch/im/PandemicFlu_TAGReco_Aug2009_e.pdf [Google Scholar]
- 4. Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, Kielland A, Chomez P, Garcon N, Van MM. 2011. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29:2461–2473 [DOI] [PubMed] [Google Scholar]
- 5. Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van MM. 2011. H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J. Clin. Immunol. 31:443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. 2008. Broad clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 3:e1665 doi:10.1371/journal.pone.0001665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GlaxoSmithKline, Inc 2009. Arepanrix H1N1 (package insert). GlaxoSmithKline, Inc., Mississauga, Ontario, Canada [Google Scholar]
- 8. Scheifele DW, Ward BJ, Dionne M, Vanderkooi O, Langley JM, Dobson S, Li Y, Law B, Halperin SA. 2011. Evaluation of adjuvanted pandemic H1N1(2009) influenza vaccine after one and two doses in young children. Pediatr. Infect. Dis. J. 30:402–407 [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization 2010. Serological diagnosis of influenza by microneutralization assay. World Health Organization, Geneva, Switzerland: http://www.who.int/influenza/gisrs_laboratory/2010_12_06_serological_diagnosis_of_influenza_by_microneutralization_assay.pdf [Google Scholar]
- 10. de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp RG, Knook DL. 1999. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J. Infect. Dis. 179:31–36 [DOI] [PubMed] [Google Scholar]
- 11. Jefferis R. 2012. Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 526:159–166 [DOI] [PubMed] [Google Scholar]
- 12. Hamilton RG, Mohan C. 2001. The human IgG subclasses. Calbiochem, Baltimore, MD [Google Scholar]
- 13. El-Madhun AS, Cox RJ, Haaheim LR. 1999. The effect of age and natural priming on the IgG and IgA subclass responses after parenteral influenza vaccination. J. Infect. Dis. 180:1356–1360 [DOI] [PubMed] [Google Scholar]
- 14. Ferrante A, Beard LJ, Feldman RG. 1990. IgG subclass distribution of antibodies to bacterial and viral antigens. Pediatr. Infect. Dis. J. 9:S16–S24 [PubMed] [Google Scholar]
- 15. Wagner R, Gopfert C, Hammann J, Neumann B, Wood J, Newman R, Wallis C, Alex N, Pfleiderer M. 2012. Enhancing the reproducibility of serological methods used to evaluate immunogenicity of pandemic H1N1 influenza vaccines-an effective EU regulatory approach. Vaccine 30:4113–4122 [DOI] [PubMed] [Google Scholar]
- 16. Gordon CL, Johnson PD, Permezel M, Holmes NE, Gutteridge G, McDonald CF, Eisen DP, Stewardson AJ, Edington J, Charles PG, Crinis N, Black MJ, Torresi J, Grayson ML. 2010. Association between severe pandemic 2009 influenza A (H1N1) virus infection and immunoglobulin G(2) subclass deficiency. Clin. Infect. Dis. 50:672–678 [DOI] [PubMed] [Google Scholar]
- 17. Hocart MJ, Mackenzie JS, Stewart GA. 1990. Serum IgG subclass responses of humans to inactivated and live influenza A vaccines compared to natural infections with influenza A. J. Med. Virol. 30:92–96 [DOI] [PubMed] [Google Scholar]
- 18. McHeyzer-Williams LJ, McHeyzer-Williams MG. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487–513 [DOI] [PubMed] [Google Scholar]
- 19. Xu R, Ekiert DC, Krause JC, Hai R, Crowe JE, Jr, Wilson IA. 2010. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 328:357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamkar R, Jalilvand S, Mokhtari-Azad T, Nouri JK, Dahi-Far H, Soleimanjahi H, Nategh R. 2005. Assessment of IgM enzyme immunoassay and IgG avidity assay for distinguishing between primary and secondary immune response to rubella vaccine. J. Virol. Methods 130:59–65 [DOI] [PubMed] [Google Scholar]
- 21. Fox JL, Hazell SL, Tobler LH, Busch MP. 2006. Immunoglobulin G avidity in differentiation between early and late antibody responses to West Nile virus. Clin. Vaccine Immunol. 13:33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prince HE, Yeh C, Lape-Nixon M. 2011. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin. Vaccine Immunol. 18:1951–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Institut National de Santé Publique du Québec 2010. Bilan épidémiologique de la pandeemie d'influenza A (H1N1). Government of Quebec, Montreal, Quebec, Canada: http://www.inspq.qc.ca/pdf/publications/1212_BilanAH1N12009.pdf [Google Scholar]
- 24. Influenza and Emerging Respiratory Pathogens Team 2010. Pandemic influenza A/H1N1 virus (pH1N1) case surveillance update, British Columbia. BC Centre for Disease Control, Vancouver, British Columbia, Canada: http://www.bccdc.ca/NR/rdonlyres/EB9B5651-1251-4E16-8AFE-D4819DADBEB2/0/BC_pH1N1_Surveillance_Update_2010Feb25.pdf. [Google Scholar]
- 25. Alberta Health and Wellness and Alberta Health Services 2010. Pandemic H1N1 (2009): the Alberta experience. Government of Alberta, Calgary, Alberta, Canada: http://www.health.alberta.ca/documents/H1N1-Alberta-Experience-2010.pdf [Google Scholar]
- 26. Department of Health Promotion and Protection and Department of Health 2010. Nova Scotia's response to H1N1: summary report. Government of Nova Scotia, Halifax, Nova Scotia, Canada: http://www.gov.ns.ca/hpp/publications/H1N1-Summary-Report.pdf [Google Scholar]
- 27. Grilli EA, Davies JR, Smith AJ. 1986. Infection with influenza A H1N1. 1. Production and persistence of antibody. J. Hyg. (Lond.) 96:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schild GC, Newman RW, McGregor IA, Williams K. 1977. The use of transportable single-radial-diffusion immunoplates in seroepidemiological studies of influenza in the Gambia: the occurrence and persistence of antibody to influenza A/Hong Kong/68 (H3N2) virus in selected inhabitants of two rural villages. Bull. World Health Organ. 55:3–13 [PMC free article] [PubMed] [Google Scholar]
- 29. Skowronski DM, Hottes TS, De SG, Ward BJ, Janjua NZ, Sabaiduc S, Chan T, Petric M. 2011. Influenza Beta/Victoria antigen induces strong recall of Beta/Yamagata but lower Beta/Victoria response in children primed with two doses of Beta/Yamagata. Pediatr. Infect. Dis. J. 30:833–839 [DOI] [PubMed] [Google Scholar]
- 30. Khurana S, Verma N, Talaat KR, Karron RA, Golding H. 2012. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J. Infect. Dis. 205:610–620 [DOI] [PubMed] [Google Scholar]
- 31. Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del GG, Rappuoli R, Golding H. 2011. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci. Transl. Med. 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. 2012. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. J. Infect. Dis. 206:1542–1548 [DOI] [PubMed] [Google Scholar]
- 33. Marshall BC, Adler SP. 2003. Avidity maturation following immunization with two human cytomegalovirus (CMV) vaccines: a live attenuated vaccine (Towne) and a recombinant glycoprotein vaccine (gB/MF59). Viral Immunol. 16:491–500 [DOI] [PubMed] [Google Scholar]
- 34. Atrasheuskaya AV, Neverov AA, Rubin S, Ignatyev GM. 2006. Horizontal transmission of the Leningrad-3 live attenuated mumps vaccine virus. Vaccine 24:1530–1536 [DOI] [PubMed] [Google Scholar]
- 35. Hedman K, Hietala J, Tiilikainen A, Hartikainen-Sorri AL, Raiha K, Suni J, Vaananen P, Pietilainen M. 1989. Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (avidity-ELISA) and by haemolysis typing. J. Med. Virol. 27:293–298 [DOI] [PubMed] [Google Scholar]
- 36. Lavoria MA, Di-Giacomo S, Bucafusco D, Franco-Mahecha OL, Perez-Filgueira DM, Capozzo AV. 2012. Avidity and subtyping of specific antibodies applied to the indirect assessment of heterologous protection against foot-and-mouth disease virus in cattle. Vaccine 30:6845–6850 [DOI] [PubMed] [Google Scholar]
- 37. Bergen MJ, Pan CH, Greer CE, Legg HS, Polo JM, Griffin DE. 2010. Comparison of the immune responses induced by chimeric alphavirus-vectored and formalin-inactivated alum-precipitated measles vaccines in mice. PLoS One 5:e10297 doi:10.1371/journal.pone.0010297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nair N, Gans H, Lew-Yasukawa L, Long-Wagar AC, Arvin A, Griffin DE. 2007. Age-dependent differences in IgG isotype and avidity induced by measles vaccine received during the first year of life. J. Infect. Dis. 196:1339–1345 [DOI] [PubMed] [Google Scholar]
- 39. Mellander L, Bottiger M, Hanson LA, Taranger J, Carlsson B. 1993. Avidity and titers of the antibody response to two inactivated poliovirus vaccines with different antigen content. Acta Paediatr. 82:552–556 [DOI] [PubMed] [Google Scholar]