Abstract
The immune function test is an integrated measure of total mitogen-inducible CD4+ T cell metabolic activity in the peripheral blood, and it is used to guide the dosing of immunosuppressive medications after solid organ transplantation. Recently, low CD4+ T cell metabolic activity due to pharmacologic immunosuppression has been linked to rapidly progressive cirrhosis in hepatitis C virus (HCV)-infected liver transplant recipients. We speculate that either cirrhosis or HCV might adversely affect the CD4+ T cell reactivity even in the absence of immunosuppressive medications. We thus performed this assay on a cohort of untransplanted hepatology patients who were not taking immunomodulatory drugs. Low mitogen-stimulated CD4+ T cell metabolic reactivity was more commonly seen in untransplanted patients with HCV cirrhosis or with cirrhosis due to other causes but not in control patients or in those with chronic HCV in the absence of cirrhosis. The lowest mean CD4+ T cell reactivities were seen in patients with both cirrhosis and HCV. Caution should be exercised when immune function test results are used to guide immunomodulatory therapy in transplant recipients with suspected cirrhosis, as low immune function test results may be a consequence of hepatic cirrhosis or of pharmacologic immunosuppression.
INTRODUCTION
In 2002, the immune function test (IFT) was approved by the U.S. Food and Drug Administration for clinical use as guide to pharmacologic immunosuppressive therapies in transplant patients. This assay is an integrated measure of CD4+ T cell number and total CD4+ T cell mitogen-inducible metabolic activity in peripheral blood. In liver transplant recipients, appropriate immunosuppression is achieved when mitogen-inducible CD4+ T cell ATP values are in the lower end of the moderate immune response zone (226 to 524 ng/ml), as patients are at increased risk of opportunistic infection when they have CD4+ T cell ATP values of <226 ng/ml (1). Clinically, IFT is used to guide immunotherapy in order to simultaneously minimize the risks of transplant rejection and opportunistic infection (2).
Cirrhosis and hepatocellular carcinoma due to chronic infection with hepatitis C virus (HCV) are the most common indications for orthotopic liver transplantation (OLT) in the United States (3). The transplanted liver invariably becomes reinfected in patients with chronic HCV, and HCV cirrhosis develops in 30% of these patients within 5 years of transplantation (4). Progressive cirrhosis in posttransplant HCV is poorly understood but has been associated with altered host immune responses (5). A strong, multispecific CD4+ and CD8+ T cell response is necessary for the immunologic control of HCV infection, and the pharmacologic suppression of cell-mediated immunity is likely to contribute to the accelerated progression of HCV-induced liver damage after OLT (6).
An association between progressive hepatic fibrosis and low CD4+ T cell ATP values has been reported in liver transplant recipients (7). Low-normal assay values are more frequent in transplant patients with chronic HCV infection than in uninfected transplant recipients (8). In addition, low mitogen-inducible CD4+ T cell metabolic activity has been associated with the rapid progression of hepatic fibrosis and cirrhosis in the setting of posttransplant HCV recurrence (7, 8). Whereas pharmacologic immunosuppression may accelerate HCV fibrosis in transplanted patients, the presence of both cirrhosis and the hepatitis C virus may diminish host immune responses even in the absence of immunosuppressive therapy (9, 10). In order to quantify the effects of HCV and/or cirrhosis on mitogen-induced CD4+ T cell potential, we examined IFT results for a convenience cohort of untransplanted hepatology patients who were not taking immunomodulatory medications.
MATERIALS AND METHODS
Patient recruitment.
We recruited a convenience sample of patients and healthy volunteers from the San Francisco Veterans Administration (SFVA) medical center's liver clinic to compare CD4+ T cell reactivities, as determined by the IFT assay (ImmuKnow; Cylex, Columbia, MD), in control patients and in those with cirrhosis and HCV infection. Patients consecutively seen at the SFVA liver clinic over a 15-month period were asked for consent for the study. These included patients with chronic HCV infection (defined as HCV seropositivity with two or more consecutively positive HCV RNA tests taken at least 12 months apart) or liver disease secondary to nonalcoholic steatohepatitis (NASH) or excessive alcohol consumption. We also sought consent from healthy volunteers, patients who had spontaneously resolved HCV infection (defined as at least two negative HCV RNA results more than 6 months apart after seroconversion), and patients with treatment-induced HCV resolution (defined as one negative HCV RNA test at least 6 months after cessation of therapy) as the control group. We excluded patients with conditions typically associated with low CD4+ T cell counts, including those having prior organ transplants, HIV-1 seropositivity, and treatment with alpha interferon-based HCV therapy or immunosuppressive medications within the prior 9 months. Clinical and laboratory data, including age, gender, race/ethnicity, alcohol use history, body mass index (BMI), and HCV genotype, were collected from the patient's electronic medical record and from source documents maintained for an ongoing longitudinal cohort study of HCV-infected patients. The protocol was approved by the institutional review boards at the SFVA and at UCSF, and the study was conducted in accordance with international guidelines on good clinical practice (11).
Cirrhosis determination.
Fibrosis staging was ascertained through retrospective chart reviews. Cirrhosis diagnoses were based upon liver imaging, liver biopsy, or both using the following criteria: (i) stage 4 scarring on a liver biopsy specimen according to the Batts-Ludwig system or (ii) the presence on ultrasound, computed tomography (CT) scan, or magnetic resonance imaging (MRI) of the abdomen of nodular liver contour and one or more of the three abnormalities (i) portal hypertension/enlarged portal vein/presence of venous collaterals, (ii) splenomegaly, or (iii) ascites. Determination of whether HCV, alcohol, or nonalcoholic steatohepatitis (NASH) was the primary influence on the development of cirrhosis was made based upon the clinical assessment of the attending hepatologist. We defined clinically significant alcohol use with the following criteria: self reports of problems with alcoholism, a self report of alcohol intake of more than 80 g daily during a period of greater than 5 years, or evidence of social, legal, psychiatric, or physical consequences of heavy drinking as described in the medical record.
Virology.
All HCV RNA characterizations were performed using Roche Amplicor PCR-based quantitation assays according to the manufacturer's instructions (Roche Molecular Diagnostics, Pleasanton, CA).
Immune function test.
At the study enrollment visit, whole blood was collected from patients in sodium heparin tubes and processed on the same day. Briefly, 250 μl of anticoagulated whole blood was diluted with the provided sample diluents to make a final volume of 1 ml. Samples were added to the wells of a 96-well plate and incubated overnight with phytohemagglutinin at 37°C and 5% CO2. After enrichment in CD4+ T cells by the addition of magnetic particles coated with anti-human CD4 monoclonal antibody, cells were washed and then lysed to release intracellular ATP. ATP was measured fluorimetrically in triplicate and normalized to known ATP standards in accordance with the manufacturer's instructions. A quantitative measure of each patient's immune response was expressed as the amount of ATP (ng/ml) in CD4+ T cells after mitogen stimulation. Low ATP values were defined as equal to or below 226 ng/ml.
Statistical analysis.
Patients were divided into four groups: (i) chronic HCV without cirrhosis, (ii) chronic HCV with cirrhosis, (iii) alcohol/NASH with cirrhosis, and (iv) HCV uninfected without cirrhosis (includes patients with spontaneous or treatment-induced viral clearance). Descriptive analysis was performed for all clinical and biochemical characteristics, generating means with standard deviations or medians with interquartile ranges for continuous variables and frequencies with percentages for categorical variables. All continuous variables were tested for normality of distribution. Continuous variables were tested for differences with analysis of variance (ANOVA) and Fisher's exact tests, and categorical variables were tested with the chi-square test of independence using 95% confidence intervals (CIs). To determine significant associations between patient group and low ATP values, univariate logistic analysis was used to calculate odds ratios (ORs), and 95% CIs were generated. All analyses were conducted using SAS software version 9.2 (SAS Institute, Cary, NC).
RESULTS
The clinical and demographic characteristics of the 4 patient groups, including the proportion of patients with low CD4+ T cell ATP levels (≤226 ng/ml), are presented in Table 1. Among the four groups, there was an increased frequency of hepatocellular carcinoma in patients with cirrhosis and an increased frequency of reported heavy alcohol use among patients with cirrhosis, as expected. Absolute CD4 lymphocyte counts were not available for study patients, but the majority of patients had absolute lymphocyte counts in the normal range (not shown). Notably, low CD4+ T cell reactivity was seen in 18/61 patients (29.5%) with cirrhosis compared to 3/57 noncirrhotic patients (5.3%) (P < 0.003). Compared to the other groups, the HCV cirrhosis group contained the greatest proportion of patients (32.5%) with low CD4+ T cell reactivity. Cirrhosis was significantly associated (OR = 7.5; CI, 2.1 to 27.3) with low (≤226 ng/ml) CD4+ T cell ATP values, as presented in Table 2. In subgroup analyses for patients with cirrhosis secondary to HCV, the odds of having lower CD4+ T cell ATP values were marginally significant (OR = 4.8; CI, 1.0 to 23.8). Patients with alcohol- or NASH-associated cirrhosis were more likely to have low CD4+ ATP values; however, this result was not significant compared to values for uninfected patients without cirrhosis. There was no significant association between low CD4+ ATP values and chronic HCV infection in patients without cirrhosis (Table 1).
Table 1.
Demographic and clinical characteristics
| Variable | Value for group |
P valuea | |||
|---|---|---|---|---|---|
| Chronic HCV, no cirrhosis (n = 35) | Chronic HCV, cirrhosis (n = 40) | Alcohol/NASH cirrhosis (n = 21) | Uninfected, no cirrhosis (n = 22) | ||
| Age, yr (mean ± SD) | 57.0 ± 8.8 | 60.8 ± 4.7 | 60.0 ± 6.2 | 58.4 ± 7.6 | 0.06c |
| Gender, no. (%) | |||||
| Female | 2 (5.7) | 1 (2.5) | 0 (0.0) | 1 (4.5) | 0.76b |
| Male | 33 (94.3) | 39 (97.5) | 21 (100.0) | 21 (95.5) | |
| Ethnicity, no. (%) | |||||
| Caucasian | 19 (54.3) | 26 (65.0) | 17 (81.0) | 15 (68.2) | 0.49 |
| Black | 10 (28.6) | 8 (20.0) | 1 (4.8) | 4 (18.2) | |
| Other | 6 (17.1) | 6 (15.0) | 3 (14.3) | 3 (13.6) | |
| Hepatocellular carcinoma, no. (%) | |||||
| Yes | 0 (0.0) | 7 (17.9) | 3 (14.3) | 0 (0.0) | 0.006b |
| No | 35 (100.0) | 32 (82.1) | 18 (85.7) | 22 (100.0) | |
| BMI (mean ± SD) | 27.2 ± 4.6 | 28.4 ± 4.36 | 29.2 ± 6.3 | 29.3+-5.2 | 0.40c |
| Alcohol, no. (%) | |||||
| Yes | 2 (5.9) | 16 (41.0) | 17 (81.0) | 5 (22.7) | <0.0001 |
| No | 32 (94.1) | 23 (59.0) | 4 (19.0) | 17 (77.3) | |
| ATP (ng/ml), median (IQR) | 432 (366.2–552.1) | 256.7 (176.5–425.5) | 392.1 (280.3–519.4) | 456.3 (301.6–726.8) | 0.0001c |
| ALT (U/liter), median (IQR) | 40 (26–64) | 67 (39–96.5) | 23 (15–35) | 27 (20–52) | <0.0001c |
P values were calculated from the chi-square test for category variables and ANOVA for continuous variables unless otherwise indicated.
P values were calculated from Fisher's exact test.
The variable was rank transformed.
Table 2.
Association between patient group and CD4+ T cell reactivity
| Variable | Value for group |
Relative risk (95% CI) | P value | |
|---|---|---|---|---|
| ATP ≤ 226 (n = 21) | ATP > 226 (n = 97) | |||
| Cirrhosis, no. (%) | ||||
| Yes | 18 (29.5) | 43 (70.5) | 7.5 (2.1–27.3) | 0.002 |
| No | 3 (5.3) | 54 (94.7) | Reference | |
| HCV/cirrhosis group, no. (%) | ||||
| Chronic HCV and cirrhosis | 13 (32.5) | 27 (67.5) | 4.8 (1.0–23.8) | 0.05 |
| Alcohol/NASH cirrhosis | 5 (23.8) | 16 (76.2) | 3.1 (0.5–18.3) | 0.21 |
| Chronic HCV, no cirrhosis | 1 (2.9) | 34 (97.1) | 0.3 (0.03–3.5) | 0.33 |
| Uninfected, no cirrhosis | 2 (9.1) | 20 (90.9) | Reference | |
The effects of serum alanine aminotransferase (ALT) and of HCV viral load (defined as less than or greater than 800,000 copies/ml) on CD4+ T cell reactivity were also examined. Univariate linear regression analysis suggested that incremental increases in ALT correlated with minimally lower CD4+ ATP levels in patients with cirrhosis due to alcohol/NASH and with minimally higher CD4+ ATP levels among HCV-infected patients, but these differences were not statistically significant (data not shown). A high HCV viral load had no appreciable effect on CD4+ T cell reactivities in patients with HCV cirrhosis, and CD4+ T cell reactivities were numerically, but not significantly, higher in HCV patients without cirrhosis (not shown).
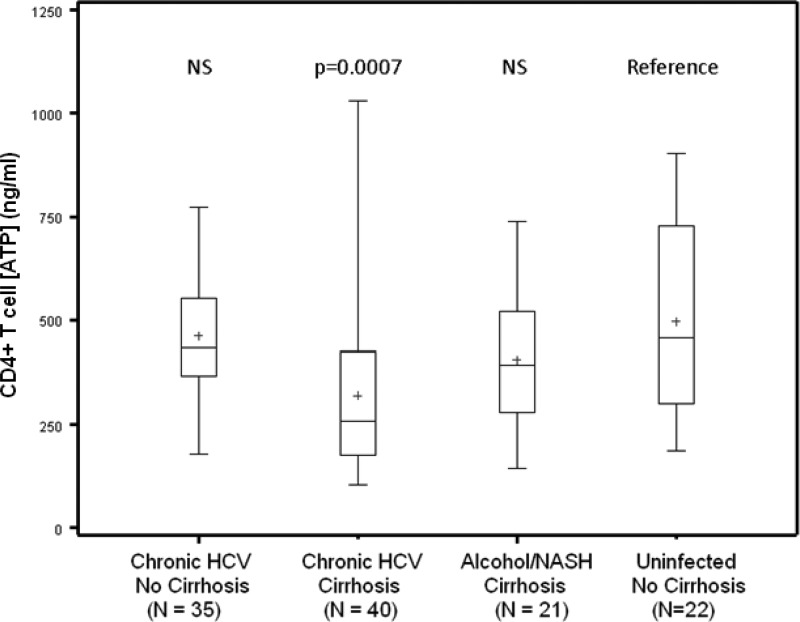
Interquartile analysis of CD4+ T cell ATP levels in each patient group is presented in Fig. 1. Compared with those in uninfected patients without cirrhosis, mean CD4+ T cell ATP values were significantly reduced in patients with HCV-induced cirrhosis (mean = 320.3 ng/ml; P < 0.0001) but were not significantly lower in patients with alcohol- or NASH-induced cirrhosis (mean = 406.6 ng/ml). There were no significant differences between CD4+ T cell ATP values among chronically infected HCV patients (mean ATP = 461.2 ng/ml) and uninfected liver clinic patients without cirrhosis (mean ATP = 495.6 ng/ml).
Fig 1.
Cumulative analysis of mitogen-inducible CD4+ T cell ATP values as measured by the IFT assay. Values are displayed as boxes representing interquartile (25th to 75th percentile) groups. The horizontal bar within each box represents the median value, the plus sign (+) represents the mean, and whiskers represent the full range of values in each patient group.
DISCUSSION
The rapid progression to cirrhosis in HCV-infected patients has been linked to strong immunosuppression (5). Notably, the development of cirrhosis in HCV patients has been linked to immune dysfunction in HIV-1 infection or due to pharmacologic immunosuppression in HCV-infected solid organ transplant recipients (5). In this study, we show that cirrhosis itself is associated with low mitogen-induced CD4+ T cell metabolic activity as determined by the IFT assay. Specifically, 13/40 (32.5%) patients with HCV cirrhosis and 5/21 (23.8%) patients with alcohol- or NASH-induced cirrhosis had IFT values below the normal moderate immune response zone (≤226 ng/ml). In contrast, only 1 of 34 patients with chronic HCV without cirrhosis had low IFT values. Among patients with cirrhosis, those with chronic HCV infection had lower mean CD4+ T cell reactivity than uninfected cirrhotic patients.
An association between hepatic fibrosis and low IFT values has been observed in liver transplant patients (7). Mendler et al. showed that low ImmuKnow values were more frequently seen in immunosuppressed transplant recipients with higher hepatic fibrosis stages on liver biopsy (8). In addition, lower overall IFT values were seen in patients experiencing transplant rejection, although these tests were felt to reflect more aggressive immunosuppression in the setting of suspected or proven rejection (8). Interestingly, low IFT values were also seen in patients with bile duct injury, a mechanical stimulus for hepatic fibrosis (8). Alkhouri et al. found that the rapid progression to HCV-induced cirrhosis after transplantation was associated with lower IFT values (7). They speculated that these low values reflected profound pharmacologic immunosuppression and that this immunosuppression was instrumental in the rapid development of HCV-induced cirrhosis in the graft (7).
Our results suggest that even in the absence of pharmacologic immunosuppression, patients with cirrhosis can have low mitogen-inducible CD4+ T cell reactivity. The lowest mean CD4+ T cell ATP values were seen in patients with both cirrhosis and HCV. It is unclear whether this low immunoreactivity causes liver fibrosis or whether the cirrhotic liver can directly cause immunologic defects. Both HCV and cirrhosis likely have influences on host immune function. Virulence factors encoded by the hepatitis C virus can cause focused defects in immune signaling pathways, and patients with cirrhosis are more susceptible to numerous infectious agents (9, 10). The mechanisms of immune dysfunction in cirrhosis are poorly understood, but they likely involve at least four theoretical mechanisms. First, cirrhosis may result in defective complement activation, as many components of the complement cascade are produced by the normal liver (12). Second, portal hypertension may lead to anatomic portosystemic shunting, bypassing the filtration of bacterial products by the liver (13). Third, patients with cirrhosis exhibit depressed neutrophil and phagocytic killing of cellular pathogens (10). Finally, disordered cellular architecture in the cirrhotic liver may lead to defective lymphocyte stimulation or proliferation (14). Typically, professional antigen-presenting cells (APCs) in the liver include stellate cells and hepatic dendritic cells (15). In cirrhosis, stellate cells differentiate into fibrocytes, resulting in hepatic fibrosis (16). Thus, in the cirrhotic liver, professional APCs are likely diminished in number, or they may be inaccessible to lymphocytes due to altered hepatic architecture. Inadequate T cell costimulation might then result in tolerogenic signals and T cell anergy (17). Limited data from cirrhotic mice and from patients with HCV-induced cirrhosis suggest that regulatory CD4+ T cells (Tregs) may be increased in the peripheral blood in cirrhosis (18). Since Tregs are a specialized subpopulation of T cells that can suppress activation of the immune system, they might contribute to global defects in mitogen-induced immune responsiveness (18).
Whatever the mechanism, our finding that low IFT values are commonly seen in patients with cirrhosis has important clinical implications in the posttransplant setting. The report by Alkhouri et al. implied that changes in immunosuppressive therapy might be guided by results of the IFT assay and that these changes might possibly prevent progressive HCV cirrhosis following OLT (7). The data presented in our study, however, suggest that low mitogen-inducible CD4+ T cell ATP levels can be seen in the setting of cirrhosis even in the absence of immunosuppression. If immunocompromise is a secondary effect of cirrhosis, IFT-guided medication adjustments might have lesser effects on the course of hepatic fibrosis in HCV-infected liver transplant patients. In addition, cirrhosis due to HCV infection is frequently seen in renal transplant recipients (19). If immunosuppression is a secondary effect of cirrhosis, the IFT assay might not be a reliable guide to the dosing of immunosuppressive medications in cirrhotic recipients of transplanted kidneys or other organs. Rather, our data suggest that clinicians should interpret IFT results with caution in the setting of established or suspected cirrhosis.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants U19 AI 40034 and R01 AI 083113, grant P30 DK026743 (UCSF Liver Center), and the U.S. Veterans Administration.
We have no conflicts of interest to disclose.
Footnotes
Published ahead of print 6 February 2013
REFERENCES
- 1. Sottong PR, Rosebrock JA, Britz JA, Kramer TR. 2000. Measurement of T-lymphocyte responses in whole-blood cultures using newly synthesized DNA and ATP. Clin. Diagn. Lab. Immunol. 7:307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cadillo-Chavez R, de Echegaray S, Santiago-Delpin EA, Rodriguez-Trinidad AT, Camacho-Carrazo B, Alfaro T, Saavedra-Pozo M, Carrasquillo L, Gonzalez-Caraballo ZA, Morales-Otero LA. 2006. Assessing the risk of infection and rejection in Hispanic renal transplant recipients by means of an adenosine triphosphate release assay. Transplant. Proc. 38:918–920 [DOI] [PubMed] [Google Scholar]
- 3. Alter HJ, Seeff LB. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17–35 [DOI] [PubMed] [Google Scholar]
- 4. Neumann UP, Berg T, Bahra M, Seehofer D, Langrehr JM, Neuhaus R, Radke C, Neuhaus P. 2004. Fibrosis progression after liver transplantation in patients with recurrent hepatitis C. J. Hepatol. 41:830–836 [DOI] [PubMed] [Google Scholar]
- 5. Demetris AJ. 2009. Evolution of hepatitis C virus in liver allografts. Liver Transpl. 15(Suppl. 2):S35–S41 [DOI] [PubMed] [Google Scholar]
- 6. Neumann UP, Berg T, Bahra M, Puhl G, Guckelberger O, Langrehr JM, Neuhaus P. 2004. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation 77:226–231 [DOI] [PubMed] [Google Scholar]
- 7. Alkhouri N, Hanouneh IA, Lopez R, Zein NN. 2010. Monitoring peripheral blood CD4+ adenosine triphosphate activity in recurrent hepatitis C and its correlation to fibrosis progression. Liver Transpl. 16:155–162 [DOI] [PubMed] [Google Scholar]
- 8. Mendler M, Kwok H, Franco E, Baron P, Weissman J, Ojogho O. 2008. Monitoring peripheral blood CD4+ adenosine triphosphate activity in a liver transplant cohort: insight into the interplay between hepatitis C virus infection and cellular immunity. Liver Transpl. 14:1313–1322 [DOI] [PubMed] [Google Scholar]
- 9. Gale M, Jr, Foy EM. 2005. Evasion of intracellular host defence by hepatitis C virus. Nature 436:939–945 [DOI] [PubMed] [Google Scholar]
- 10. Rajkovic IA, Williams R. 1986. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 6:252–262 [DOI] [PubMed] [Google Scholar]
- 11. Bruce-Chwatt LJ. 1965. Declaration of Helsinki. Recommendations guiding doctors in clinical research. WHO Chron. 19:31–32 [PubMed] [Google Scholar]
- 12. Whaley K, Schwaeble W. 1997. Complement and complement deficiencies. Semin. Liver Dis. 17:297–310 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, Rodes J. 2002. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 35:140–148 [DOI] [PubMed] [Google Scholar]
- 14. Bonacini M, Govindarajan S, Kohla M, Lai MM, Lindsay KL. 2007. Intrahepatic lymphocyte phenotypes in hepatitis C virus infection: a comparison between cirrhotic and non-cirrhotic livers. Minerva Gastroenterol. Dietol. 53:1–7 [PubMed] [Google Scholar]
- 15. Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. 2007. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26:117–129 [DOI] [PubMed] [Google Scholar]
- 16. Friedman SL. 2010. Evolving challenges in hepatic fibrosis. Nat. Rev. Gastroenterol. Hepatol 7:425–436 [DOI] [PubMed] [Google Scholar]
- 17. Winau F, Quack C, Darmoise A, Kaufmann SH. 2008. Starring stellate cells in liver immunology. Curr. Opin. Immunol. 20:68–74 [DOI] [PubMed] [Google Scholar]
- 18. Yoshizawa K., Abe H, Kubo Y, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y. 2010. Expansion of CD4(+)CD25(+)FoxP3(+) regulatory T cells in hepatitis C virus-related chronic hepatitis, cirrhosis and hepatocellular carcinoma. Hepatol. Res. 40:179–187 [DOI] [PubMed] [Google Scholar]
- 19. Bloom RD, Lake JR. 2006. Emerging issues in hepatitis C virus-positive liver and kidney transplant recipients. Am. J. Transplant. 6:2232–2237 [DOI] [PubMed] [Google Scholar]