Abstract
Escherichia coli O157:H7 is an enteric pathogen of animals and humans that can result in deadly sequelae. Cattle are asymptomatic carriers and shedders of the bacteria and serve as an important reservoir of human infection. E. coli O157:H7 colonizes the gastrointestinal tract, most frequently at the rectoanal junction mucosa in cattle. Vaccination is a potentially highly effective means of decreasing cattle colonization and shedding and thereby decreasing human infections. Currently available vaccines are administered subcutaneously or intramuscularly, and immune responses have been evaluated solely by systemic immunoglobulin responses. This study evaluated local and systemic lymphoproliferative responses in addition to immunoglobulin responses following subcutaneous or mucosal (rectal) immunization with E. coli O157:H7 outer membrane protein intimin over three trials. In all three trials, significant local and systemic lymphoproliferative responses (P < 0.05) occurred following immunization in the majority of animals, as well as significant immunoglobulin responses (P < 0.001) in all animals. Surprisingly, local responses in the mesorectal lymph nodes were very similar between the subcutaneous and mucosal immunization groups. Moreover, the responses in mesorectal lymph nodes appeared targeted rather than generalized, as minimal or no significant responses were observed in the associated prescapular lymph nodes of subcutaneously immunized animals. The results indicate that both subcutaneous and mucosal immunizations are effective methods of inducing immune responses against E. coli O157:H7 in cattle.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) is an important zoonotic pathogen in humans, causing self-limiting to severe bloody or nonbloody diarrhea which in some cases progresses to life-threatening hemolytic uremic syndrome (HUS) (1–3), the most common cause of acute renal failure in children in the United States (4). There are currently no effective therapies for EHEC in humans beyond supportive care, and antibiotics may exacerbate HUS development (5–8). E. coli serotype O157:H7 causes the majority of EHEC infections in North America (9). With a lack of effective treatment, prevention of infection in humans is critical.
Cattle are the main reservoir of E. coli O157:H7 (10), and contaminated beef, unpasteurized dairy products, and produce such as spinach have all been implicated as sources of human infection (11–16). Healthy cattle transiently carry E. coli O157:H7 with no associated clinical signs and can shed it in their feces for a few days or up to a month or more (17), causing contamination of hides, udders, and the environment. Studies evaluating prevalence of E. coli O157:H7 have shown 0.2 to 40% in dairy cattle, 0.3 to 27.3% in beef cattle, and up to 54% in ground beef (18, 19). The primary site of colonization in cattle is the mucosa of the recto-anal junction (RAJ) in both naturally exposed and experimentally challenged cattle (20, 21) but can be distributed throughout the gastrointestinal tract (20). While human infection with E. coli O157:H7 has decreased in recent years, due in part to active education and intervention methods, it is still a significant problem (22). Human infection is preventable, and decreasing the colonization of E. coli O157:H7 at the RAJ in cattle is vital to decreasing the prevalence of food-borne infection in humans. Many methods of reducing hide and environmental contamination, including carcass washing, intestinal exclusion, antibiotics, and vaccines, have been tried with variable results (23). An effective vaccine could drastically reduce or eliminate colonization and shedding of E. coli O157:H7 in cattle, contamination of food products, and infection in humans.
Colonization of E. coli O157:H7 in both humans and cattle is mediated by the interaction of intimin, a bacterial outer membrane protein, and the translocated intimin receptor (TIR) (24, 25). Intimin is necessary but not sufficient for colonization of E. coli O157:H7 (26, 27). Intimin has been shown to be a target of long-lived humoral immune responses in mice (28, 29), and antibodies against intimin decrease fecal shedding of E. coli O157:H7 or are protective against colonization by E. coli O157:H7 and related bacteria in some animal models (30–34).
Cattle experimentally challenged or naturally exposed to E. coli O157:H7 have measurable levels of mucosal immunoglobulin G (IgG) and IgA to intimin and secreted O157 proteins, (35, 36) implying that mucosal immunization of cattle with intimin may stimulate an effective mucosal immune response and be valuable as a potential vaccination method. The majority of commercially available vaccines, however, are administered subcutaneously or intramuscularly, and the systemic and mucosal lymphoproliferative responses in cattle following vaccination have not been characterized. To this end, we designed this study to evaluate both systemic and regional lymphoproliferative responses following subcutaneous or mucosal immunization with recombinant intimin and ovalbumin as a control protein.
MATERIALS AND METHODS
Animals.
Holstein steers, 4 to 9 months of age, were purchased from regional dairy producers. Twenty-five animals total were used over 3 trials. All animals were healthy throughout the duration of the study. Animals were monitored several times daily, and a veterinarian was on call in case of emergencies. Animals were housed and handled on Washington State University campus in accordance with IACUC-approved protocols.
Recombinant intimin production.
All salts and chemicals used in producing recombinant intimin were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Top10 E. coli cells (Invitrogen, Grand Island, NY) were transfected with a pBAD vector (Invitrogen) coding His-tagged full-length intimin, generously provided by Carolyn Hovde at University of Idaho. Transfected E. coli cells were cultured overnight on LB agar containing 50 μg/ml of carbenicillin. Twenty milliliters of low-salt LB broth with 50 μg/ml carbenicillin was then inoculated with a single colony and incubated overnight at 37°C with shaking at 225 rpm. The next day, this broth was added to 500 ml LB broth with 50 μg/ml carbenicillin and incubated at 37°C with shaking at 225 rpm for 2 h. Protein expression was then induced by adding arabinose to a final concentration of 0.5% and incubating another 5 h. Culture broth was then centrifuged at 3,000 × g for 30 min. The supernatant was discarded, and the bacterial pellet was stored at −20°C overnight. The bacterial pellet was resuspended in 25 ml of lysis buffer (6 M guanidine-HCl, 20 mM sodium phosphate, 500 mM NaCl, pH 7.8), transferred to a 50-ml conical vial, and sonicated at 350 W for 45-s pulses three times. The solution was then centrifuged at 3,000 × g for 30 min and the pellet discarded. Protein was purified by mixing with 12.5 ml Probond resin (Invitrogen) for 45 min and then centrifuged at 910 × g for 30 s. Resin was rinsed twice with 20 ml of binding buffer (8 M urea, 20 mM sodium phosphate, 500 mM NaCl, pH 7.8) and centrifuged at 910 × g for 1 min. Resin was then washed twice for 2 min each with each wash buffer (8 M urea, 20 mM sodium phosphate, 500 mM NaCl, pH 6.0 and pH 5.3). Resin was resuspended in 7 ml of elution buffer (8 M urea, 20 mM sodium phosphate, 500 mM NaCl, pH 4.0) and added to a Probond column (Invitrogen). Eluant was collected and concentrated to 2 ml using Amicon Ultra 10-kDa molecular-mass-cutoff protein concentrator centrifugation tubes (Millipore, Billerica, MA) and dialyzed against 1× phosphate-buffered saline (PBS) (pH 7.3) overnight in Silde-A-Lyzer G2 dialysis cassettes (Thermo Scientific, Rockford, IL). Protein purity was verified by Coomassie stain and Western blotting, and protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA).
Immunization schedule.
The immunization schedule for all trials is summarized in Table 1. Trial 1 was designed to compare systemic lymphoproliferative responses using peripheral blood mononuclear cells (PBMC) and local lymphoproliferative responses using cells from the mesorectal lymph nodes (MRLN) that drain the RAJ in animals immunized either subcutaneously or rectally with intimin. To this end, a known potent subcutaneous adjuvant (Freund's) and mucosal adjuvant (cholera toxin B subunits [CTB]) were used. Two groups of three animals each were immunized on weeks 0, 3, and 6. Rectally immunized animals received 100 μg each of recombinant intimin and ovalbumin. Intimin was conjugated to CTB (Sigma-Aldrich, St. Louis, MO) in a 2:1 intimin-CTB mixture by weight, suspended in 1.0 ml of sterile 1× PBS, and applied approximately 3 to 5 cm inside the rectum. Subcutaneously immunized animals received 33 μg each of intimin and ovalbumin in a 1.5-ml total volume. Freund's adjuvant (Sigma-Aldrich) was used as the adjuvant at a ratio of 1:1 (complete for initial immunization, incomplete for subsequent immunizations), administered under the skin of the neck on alternate sides. Trial 2 was designed to more directly compare systemic and local lymphoproliferative responses following immunization with intimin using the same adjuvant both subcutaneously and rectally. Two groups of three animals each were immunized at weeks 0, 3, and 6. Rectally immunized animals received 100 μg each of recombinant intimin and ovalbumin with Toll-like receptor 7/8 agonist, R848, Resiquimod (InvivoGen, San Diego, CA) at 5 mg/ml combined in a vanishing cream applied approximately 3 to 5 cm inside the rectum. Subcutaneously immunized animals received 33 μg each of intimin and ovalbumin in a 1.5-ml total volume oil emulsion with 50 μg/ml of R848 administered on the neck. An additional three animals were given 1.5 ml of R848 agonist at 5 μg/ml with no protein, subcutaneously at weeks 0, 3, and 6. Trial 3 was designed to more closely approximate the time between calfhood vaccines and vaccines given upon arrival to a feedlot. Again we examined both systemic and local lymphoproliferative responses as well as peripheral responses in the prescapular lymph nodes (PLN). Two groups of five animals each received an initial subcutaneous immunization of 100 μg each of intimin and ovalbumin in 1.5 ml R848 at 5 μg/ml at week 0 on the left side of the neck. Then, at weeks 8 and 11, one group received subcutaneous immunizations and the other group was immunized rectally. Subcutaneously immunized animals received 100 μg each of intimin and ovalbumin in 1.5 ml R848 at 50 μg/ml on the left side of the neck. Rectally immunized animals received 300 μg each of intimin and ovalbumin combined in a vanishing cream formula with R848 at 5 mg/ml as the adjuvant. The cream was applied to the mucosa approximately 3 to 5 cm inside the rectum.
Table 1.
Trial groups and treatmentsa
| Trial | Trial group | No. of animals/group | Vaccination protocol |
||
|---|---|---|---|---|---|
| Proteins | Adjuvant | Vaccination weeks | |||
| 1 | Rectal | 3 | 100 μg intimin, 100 μg ovalbumin | 50 μg CTB | 0, 3, 6 |
| SQ | 3 | 33 μg intimin, 33 μg ovalbumin | 1.5 ml Freund's | 0, 3, 6 | |
| 2 | Rectal | 3 | 100 μg intimin, 100 μg ovalbumin | 5 mg R848 | 0, 3, 6 |
| SQ | 3 | 33 μg intimin, 33 μg ovalbumin | 50 μg R848 | 0, 3, 6 | |
| Adjuvant (SQ) | 3 | None | 50 μg R848 | 0, 3, 6 | |
| 3 | Rectal | 5 | 100 μg and 300 μg intimin, 100 μg and 300 μg ovalbumin | 5 mg R848 | 0, 8, 11 |
| SQ | 5 | 100 μg intimin, 100 μg ovalbumin | 50 μg R848 | 0, 8, 11 | |
Summary of treatments for each trial group. In trial 1, complete Freund's adjuvant was used in the first immunization, and incomplete adjuvant was used for subsequent immunizations. In the rectal groups of trial 3, all animals received one subcutaneous immunization (100 μg protein), and subsequent immunizations were mucosal applications of 300 μg of each protein.
Sample collection.
Samples were collected weekly, including at least 1 week before immunization and at the time of euthanasia and necropsy. Blood samples were collected weekly from the jugular vein to obtain serum and PBMC. Following the full course of immunization (9 weeks for trials 1 and 3 and 11 weeks for trial 2), animals were euthanatized and necropsied. At necropsy, multiple MRLN were collected. Cells were isolated from fresh lymph nodes and used immediately. In trial 3, the draining and contralateral PLN were also collected, and cells were harvested from fresh tissue.
Serum collection.
Blood was collected and allowed to sit at room temperature overnight. Serum was collected and stored at −20°C.
Isolation of PBMC.
PBMC were collected as previously described using the buffy coat method (37). Briefly, 60 ml of blood was collected into a syringe containing 2.0 ml 0.5 M EDTA (Invitrogen, Grand Island, NY). The whole blood was centrifuged at 1,200 × g for 30 min at 10°C. The buffy coat was isolated and diluted at least 1 to 3 with phenol red-free Hanks' balanced salt solution (HBSS) with 2 mM EDTA. Cells were underlayed with Histopaque (Sigma-Aldrich) and centrifuged at 900 × g for 30 min at 10°C. PBMC were removed from the interface, diluted with HBSS solution, and centrifuged at 500 × g for 15 min at 10°C. The cell pellet was washed with 1× PBS solution 3 to 4 times and finally resuspended in complete Roswell Park Memorial Institute (RPMI) 1640 medium and kept on ice until used or frozen.
Isolation of lymphocytes from lymph nodes.
Mononuclear cells were isolated from lymph nodes as previously described (38). Briefly, lymph nodes were removed at necropsy and placed immediately in ice-cold PBS. Lymph nodes were washed, and the capsule and large vessels were removed. Lymph nodes were manually broken down into small pieces, placed in RPMI 1640 with collagenase II (Invitrogen), and incubated for 30 min at room temperature for collection of lymphocytes. Lymph nodes were swirled in medium to loosen cells from the tissue to create a slurry. Large debris was removed, and slurries were centrifuged for 8 min at 220 × g at 10°C. Cells were washed 2 to 3 times with HBSS, resuspended in complete RPMI 1640 medium, and kept on ice until used or frozen.
Measurement of anti-intimin and anti-ovalbumin antibodies in serum via enzyme-linked immunosorbent assay (ELISA).
Each well of 96-well microtitration plates (Nunc; Thermo Fischer Scientific, Rochester, NY) was coated with 50 μl of either intimin or ovalbumin at 10 μg/ml in a 7.5 mM Na2CO3, 17.8 mM NaHCO3 coating buffer overnight at 4°C. Wells were washed with1× PBS with 0.05% Tween 20 (Sigma-Aldrich) followed by 1× PBS and blocked with 0.5% casein (nonfat dry milk) and 0.05% Tween 20 for 1 h at room temperature. After being washed with 1× PBS with 0.05% Tween 20 followed by 1× PBS, 50 μl of serially 2-fold-diluted serum samples and corresponding negative controls were added in triplicate and incubated for 1 h. Following washing with 1× PBS with 0.05% Tween 20 followed by 1× PBS, wells were incubated with 50 μl of either recombinant G protein (rGP) (Invitrogen), sheep anti-bovine IgG, or IgA conjugated to horseradish peroxidase (HRP) (AbD Serotec) at a dilution of 1:500 for 1 h at 37°C. After a final wash with 1× PBS, color reactions were developed by adding 100 μl/well of TMB substrate (SureBlue TMB microwell peroxidase substrate; Kirkegaard and Perry Lab, Gaithersburg, MD). After 15 min, reactions were terminated by adding 100 μl of 1% HCl. Optical densities were then measured at 450 nm. Titers were determined based on the highest consecutive dilution to be greater than average of the corresponding dilution of the negative control plus 2 standard deviations (based on a per-plate negative control).
Proliferation of PBMC and lymph node cells after in vitro stimulation.
Proliferation assays were performed as described previously (39). Briefly, cell suspensions were adjusted to 4 × 106 cells/ml and seeded (50 μl/well) in U-bottom 96-well cell culture plates (Nunc) and were incubated with 50 μl/well intimin or ovalbumin at 10 μg/ml in triplicate for 6 days. A total of 50 μl/well of concanavalin A (ConA) (Sigma-Aldrich) at 5 μg/ml and T-cell growth factor (TCGF) at a final dilution of 10% were used as positive controls, and an irrelevant recombinant protein from Babesia bovis, merozoite surface antigen 1 (MSA1), at 10 μg/ml was used as a negative control. To measure proliferation, cells were radiolabeled for the last 8 to 18 h of culture with 0.25 μCi [3H]thymidine (Dupont New England Nuclear, Boston, MA). Radiolabeled cells were harvested onto glass filters by using a cell harvester (Tomtech, Hamden, CT), and radionucleotide incorporation was measured with a Betaplate 1205 liquid scintillation counter (Wallac, Gaithsburg, MD). Results are presented as the stimulation index (SI), representing the average count per minute (cpm) of sample divided by mean cpm of antigen-free medium control.
Statistical analysis.
Statistical analyses were performed using the one-tailed Student t test for lymphoproliferation data from PBMC or MRLN cells, comparing cells stimulated with antigen to cells stimulated with medium. Responses were considered significant at a P value of <0.05 if the SI was greater than 2 and the average cpm was at least 1,000. The Mann-Whitney rank-sum test was used for the comparison of groups of nonparametric serum titers. Differences were considered significant at P values of <0.05, using the Bonferroni correction for multiple comparisons when appropriate. Pearson's correlation coefficient was calculated to evaluate correlation of systemic antibody and lymphoproliferative responses over all trials.
RESULTS
Trial 1.
In trial 1, the goal was to compare the induced immune responses to intimin between subcutaneous immunization and rectal immunization (Table 1). All animals were seronegative to intimin and ovalbumin at week 0 (not detected by ELISA at a 4-fold dilution) (Table 2). No animals had significant PBMC responses to either protein at week 0 or 1, whereas responses to ConA and TCGF indicate T cells were viable (Fig. 1A and data not shown).
Table 2.
Anti-intimin and anti-ovalbumin antibody titers pre- and postimmunization
| Trial no. | Animal | Titera |
|||
|---|---|---|---|---|---|
| Intimin |
Ovalbumin |
||||
| Preimmunization | At euthanasia | Preimmunization | At euthanasia | ||
| Trial 1 rectal | 30865 | ND | 320* | ND | ND |
| 30877 | ND | 20* | ND | ND | |
| 30893 | ND | 320* | ND | ND | |
| Trial 1 SQ^^ | 30915 | ND | 256,000* | ND | 64,000* |
| 31017 | ND | 256,000* | ND | 1,000* | |
| 31021 | ND | 256,000* | ND | 8,000* | |
| Trial 2 rectal | 31897 | ND | 6,400* | ND | 12,800* |
| 31905 | ND | 8,000* | ND | 100* | |
| 31928 | ND | 16,000* | ND | 800* | |
| Trial 2 SQ | 31914 | ND | 8,000* | ND | ND |
| 31929 | ND | 16,000* | ND | 800* | |
| 31931 | ND | 2,000* | ND | ND | |
| Trial 2 adjuvant | 37822 | ND | ND | ND | ND |
| 37859 | ND | ND | ND | ND | |
| 37866 | ND | ND | ND | ND | |
| Trial 3 rectal | 34545 | ND | 100* | ND | 200* |
| 34549 | 40 | 100* | ND | 200* | |
| 34551 | ND | 100* | ND | 320* | |
| 34553 | ND | 6,400* | ND | 200* | |
| 34590 | 40 | 100* | ND | 200* | |
| Trial 3 SQ^ | 32888 | 40 | 1,280* | ND | 8,000* |
| 32896 | 40 | 1,280* | ND | 8,000* | |
| 34548 | ND | 1,280* | ND | 320* | |
| 34570 | ND | 1,280* | ND | 32,000* | |
| 34577 | 40 | 1,280* | ND | 32,000* | |
Serum titers for intimin and ovalbumin were measured at week 0 and at the time of euthanasia in each trial (ND, not detected). Titers were determined by ELISA by comparing serial dilutions of the serum samples to corresponding dilutions of a known negative serum. Titers were determined as the last consecutive sample dilution to be greater than the same negative sample dilution plus two standard deviations. Asterisks indicate a significant titer increase postimmunization (P < 0.001). Carats and double carats indicate that titers from subcutaneously immunized groups are significantly greater than those from the rectally immunized groups (P < 0.05 and P < 0.001, respectively).
Fig 1.

Trial 1 local and systemic lymphoproliferative responses: PBMC or cells from the MRLN were incubated with intimin (medium-gray bars), ovalbumin (dark-gray bars), concanavalin A (white bars), and TCGF (light-gray bars) as positive controls to determine lymphoproliferative responses to proteins. (A) PBMC from week 0, prior to immunization; (B) cells from MRLN at week 9; (C) PBMC from week 9. Asterisks indicate significant responses of cells incubated with antigen compared to cells incubated with medium by a one-tailed Student t test at P values of <0.05 and SI greater than 2.0.
Trial 1 local lymphoproliferative responses.
Since the RAJ is the primary site of colonization of E. coli O157:H7 in cattle (20), and the RAJ drains to the MRLNs, cells freshly harvested from these lymph nodes at euthanasia at week 9 postimmunization were cultured with intimin, ovalbumin, and positive and negative controls for 6 days, and local lymphoproliferative responses were measured. PBMC from all animals responded to at least one of the positive controls, and surprisingly, the responses of the MRLN cells to intimin and ovalbumin were comparable between groups immunized either subcutaneously or rectally. In the subcutaneously immunized group, MRLN cells from two of three animals had significant responses to intimin, and MRLN cells from one animal had a significant response to ovalbumin (Fig. 1B). In the rectally immunized group, MRLN cells from two of three animals had significant responses to intimin, and the same two animals had significant responses to ovalbumin (Fig. 1B). In both groups, the responses to intimin and ovalbumin followed similar trends, although overall responses to ovalbumin were less robust. The similar MRLN responses to the two proteins suggest the immunization was successful, and responses to intimin are due to immunization and not natural exposure. The responses observed in the MRLN in the mucosal group were not surprising; however, the comparable responses in the subcutaneous group were unexpected.
Trial 1 systemic lymphoproliferative responses.
To evaluate systemic induced immune responses in vaccinated animals and compare them with local responses, PBMC were harvested from blood at week 9 to measure responses to the proteins following immunization. PBMC from all animals responded to at least one of the positive controls, and all animals showed increasing proliferative responses over the course of immunization, with five of six animals showing significant responses to intimin starting at week 7, and responses to ovalbumin followed a similar trend (data not shown). At the time of euthanasia at week 9, all three animals in the subcutaneously immunized group had significant responses to intimin, and two of the three had significant responses to ovalbumin (Fig. 1C). In the rectally immunized group at week 9, two of the three animals had significant responses to intimin, and two of the three had significant ovalbumin responses (Fig. 1C). As expected, subcutaneous immunization resulted in systemic responses, while the comparable systemic responses to intimin and ovalbumin following mucosal immunization were not anticipated. The similar local and systemic responses following rectal or subcutaneous immunization suggest either method may be a viable way to induce immune responses to E. coli O157:H7 in cattle that may reduce shedding when challenged.
Trial 1 serum titers.
All animals in trial 1 were seronegative for both intimin and ovalbumin at the time of immunization (Table 2). At the time of euthanasia at week 9, all animals in the subcutaneously immunized group had intimin-specific total Ig titers that were significantly increased from week 0. Similar trends, but overall less robust responses to ovalbumin, were seen in these animals as well. The anti-ovalbumin antibody titers were also all statistically significant increases from week 0 (Table 2). Rectally immunized animals had lower but still statistically significant final anti-intimin total Ig titers but undetectable anti-ovalbumin serum titers. Serum titers from subcutaneously immunized animals were overall significantly higher than those of the rectally immunized group, as expected.
Trial 2.
Following trial 1, where systemic and local immune responses were induced by both rectal and subcutaneous immunization using CTB and Freund's adjuvant, respectively, we compared responses to the two vaccination routes (Table 1) using the Toll-like receptor 7/8 agonist (R848) as the adjuvant in both groups, as this adjuvant has been shown to be effective in humans when used mucosally or subcutaneously (40, 41). An additional three animals received adjuvant only (Table 1). All animals were seronegative for intimin and ovalbumin at week 0 (Table 2). PBMC from the six calves immunized with proteins did not have significant responses to intimin or ovalbumin at week 0 (Fig. 2A). In contrast, PBMC from all three animals receiving adjuvant only had low but statistically significant responses to intimin but not to ovalbumin at this time point (Fig. 2A), suggesting these three animals may have been naturally exposed to E. coli O157:H7 prior to immunization, even though the animals were seronegative (Table 2).
Fig 2.

Trial 2 local and systemic lymphoproliferative responses: PBMC or cells from the MRLN were incubated with intimin (medium-gray bars), ovalbumin (dark-gray bars), concanavalin A (white bars), or TCGF (light-gray bars) as positive controls to assess lymphoproliferative responses to proteins. (A) PBMC from week 0, prior to immunization; (B) cells from MRLN at week 9; C) PBMC from week 9. Asterisks indicate significant responses of cells incubated with antigen compared to cells incubated with medium by a one-tailed Student t test at P values of <0.05 and SI greater than 2.0.
Trial 2 local lymphoproliferative responses.
As in trial 1, the responses to intimin and ovalbumin in regional lymph nodes were surprisingly comparable between the subcutaneously and rectally immunized groups. MRLN cells from all animals responded to at least one of the positive controls, and in the subcutaneously immunized group, MRLN cells from two of the three animals had significant responses to intimin, and cells from all three animals had significant responses to ovalbumin. In the rectally immunized group, MRLN cells from all animals had significant responses to intimin, and cells from two of the three animals had significant responses to ovalbumin (Fig. 2B). In animals receiving adjuvant only, MRLN cells from one of the three had significant responses to intimin, which is likely due to natural exposure, and no animals had cells that responded to ovalbumin (Fig. 2B), confirming that ovalbumin responses are due to immunization while intimin responses have the potential to be due to exposure.
Trial 2 systemic lymphoproliferative responses.
Animals in trial 2 show response trends similar to those of animals from trial 1. Cells from all animals responded to at least one of the positive controls. In the subcutaneously immunized group, cells from all three animals had significant responses to intimin, which were generally similar to or less robust than MRLN cell responses at the same time point, and cells from one animal had a significant response to ovalbumin (Fig. 2C). In the rectally immunized group, cells from all three animals had significant responses to intimin, and cells from one animal had a significant response to ovalbumin (Fig. 2C). In animals receiving adjuvant only, cells from two of the three animals had significant responses to intimin, most likely from natural exposure, and none had significant responses to ovalbumin (Fig. 2C), again confirming that responses to ovalbumin are due to immunization, while responses to intimin can be due to natural exposure.
In these first two trials, subcutaneous immunization resulted in more consistent systemic responses, as expected; however, at the time of euthanasia, the MRLN lymphoproliferative responses tended to be equal to or greater than the systemic PBMC responses. This raised the question of whether this was a targeted response or a generalized response throughout all lymph nodes. This question was addressed in trial 3.
Trial 2 serum titers.
All animals were seronegative to both intimin and ovalbumin at the time of immunization (Table 2). At the time of euthanasia at week 9, all animals in both immunization groups had significant anti-intimin and anti-ovalbumin antibody titers. While serum antibody responses were stronger in the subcutaneous group as expected, overall, titers between subcutaneous and mucosal groups were not significantly different. In animals receiving adjuvant only, all animals were seronegative at weeks 0 and 9 (Table 2).
Trial 3.
In trial 3, the immunization schedule was altered to more closely mimic the time between calfhood vaccines and vaccine administration on feedlots (Table 1). Also, since in both previous trials the proliferative responses from the MRLN were similar to or more robust than the systemic responses, peripheral and MRLN responses were evaluated to better elucidate if this was a generalized lymph node response or possibly targeted to the MRLN. To this end, all subcutaneous immunizations were administered on the left side of the neck, and PLN, both draining the immunization site (left) and contralateral (right), were collected at the time of euthanasia to compare to MRLN proliferative responses. All animals were seronegative for ovalbumin at week 0 (not detected by ELISA at a 4-fold dilution) (Table 2). Five of 10 animals were also seronegative for intimin at week 0, while the other five animals had anti-intimin antibody titers of 40 or were undetected (Table 2). PBMC from two other animals had statistically significant responses to intimin at the time of the first immunization (Fig. 3A), suggesting possible natural exposure to E. coli O157:H7 prior to immunization.
Fig 3.
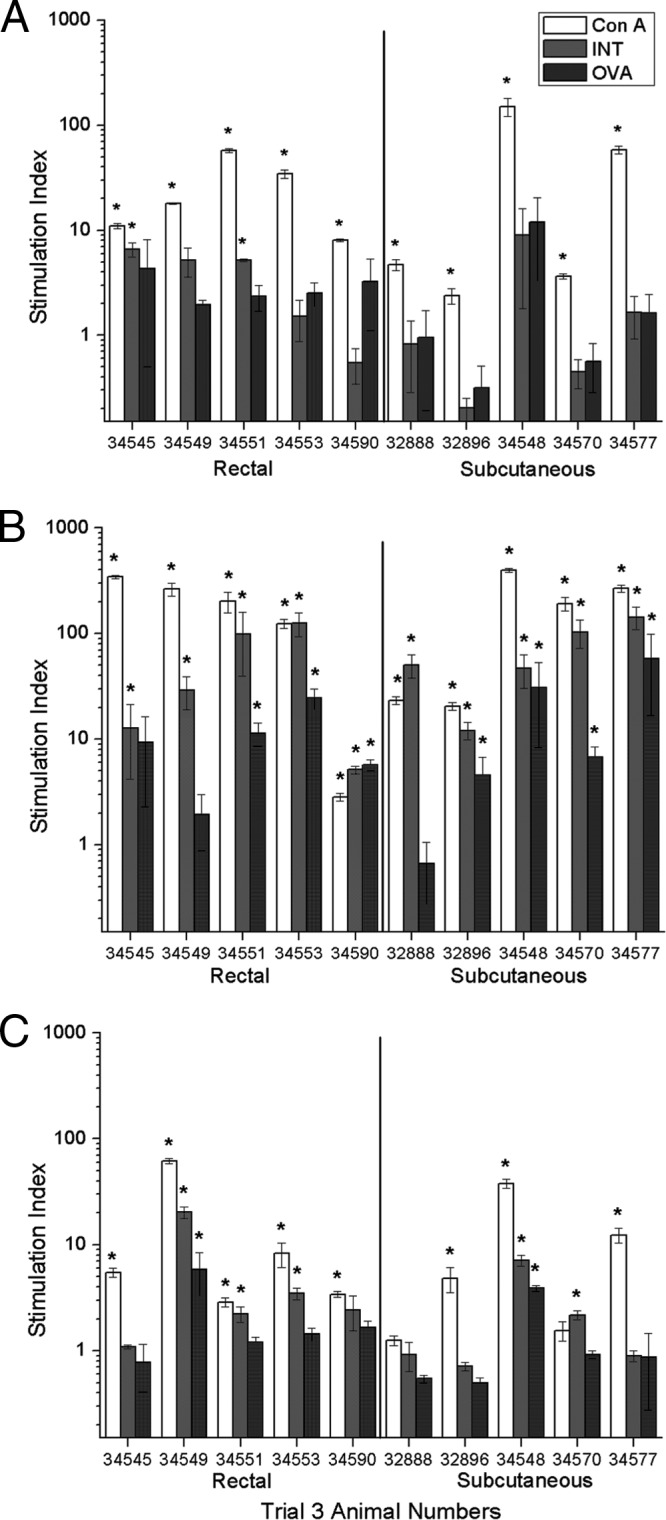
Trial 3 local and systemic lymphoproliferative responses: PBMC or cells from the MRLN were incubated with intimin (medium-gray bars), ovalbumin (dark-gray bars), or concanavalin A (white bars) as a positive control. Lymphoproliferative responses were then measured. (A) PBMC from week 0, prior to immunization; (B) cells from MRLN at week 11; (C) PBMC from week 11. Asterisks indicate significant responses of cells incubated with antigen compared to cells incubated with medium by a one-tailed Student t test at P values of <0.05 and SI greater than 2.0.
Trial 3 local lymphoproliferative responses.
MRLN cells from all animals harvested 3 days following the final immunization at week 11 responded to ConA, and MRLN cells from all animals in both groups had significant responses to intimin (Fig. 3B). In the subcutaneously immunized group, MRLN cells from four of five animals had significant responses to ovalbumin, and in the rectally immunized group, MRLN cells from three animals had significant responses to ovalbumin (Fig. 3B).
Trial 3 systemic lymphoproliferative responses.
In the subcutaneously immunized group, cells from two of five animals had significant responses to intimin, and cells from one animal had a significant response to ovalbumin (Fig. 3C). This was one of the animals that also had MRLN responses to ovalbumin. In the rectally immunized group, cells from three of five animals had significant responses to intimin, and cells from one animal had significant responses to ovalbumin (Fig. 3C). This animal also had a significant response to ovalbumin in the MRLN.
Trial 3 peripheral lymphoproliferative responses.
Since in trials 1 and 2 both subcutaneous and rectal immunization resulted in local lymphoproliferative responses, we wanted to determine if this was a generalized response or targeted to the MRLN. Toward this end, at the time of euthanasia (3 days following the final immunization at week 11), cells were freshly harvested from the draining and contralateral PLN, and lymphoproliferative responses to proteins were measured. Overall, responses to intimin by MRLN cells in both groups (Fig. 3) were much more robust than the responses in PLN, either draining or nondraining (Fig. 3). In the subcutaneously immunized group, draining PLN cells from two of five animals had a significant response to intimin (Fig. 4A), while draining PLN cells from only one animal had a significant response to ovalbumin. This was not one of the two animals with a significant response to ovalbumin in the MRLN. The nondraining PLN cells did not respond to ovalbumin (Fig. 4B). In the rectally immunized group, draining PLN cells from one of five animals had a significant response to intimin, and no cells from any animals had significant responses to ovalbumin (Fig. 4A). There were no significant responses to intimin or ovalbumin by nondraining PLN cells (Fig. 4B). It was unexpected that only 3 days after the final immunization, there were few animals that had statistically significant responses by draining PLN cells from the subcutaneously immunized group since previous studies have shown vigorous lymphoproliferative responses in draining PLN 3 days following subcutaneous immunization (42). It was also surprising that there were consistently significant responses in the MRLN, even to ovalbumin. In addition, there were no responses by cells from contralateral lymph nodes, suggesting that this was a targeted rather than generalized response. The MRLN responses to intimin may have developed subsequent to natural exposure; however, the ovalbumin responses indicate that immunization was effective and at least partly responsible for the apparently targeted MRLN lymphoproliferative responses.
Fig 4.

Trial 3 lymphoproliferative responses in draining and contralateral PLN: cells from the lymph node draining the subcutaneous immunization site (left PLN) and contralateral lymph node (right PLN) were harvested 3 days after final immunization at week 11, incubated with intimin (medium-gray bars), ovalbumin (dark-gray bars), or concanavalin A (white bars) as a positive control to determine lymphoproliferative responses to proteins. (A) Cells from draining PLN; (B) cells from contralateral PLN. Asterisks indicate significant responses of cells incubated with antigen compared to cells incubated with medium by a one-tailed Student t test at P values of <0.05 and SI greater than 2.0.
As in both previous trials, both groups had comparable responses to intimin in the MRLN, and these responses were generally more robust than the systemic PBMC responses. These findings, along with the minimal responses to both proteins in the PLN, were consistent with this being a targeted response to MRLN and possibly the RAJ.
Trial 3 serum titers.
Three animals in the subcutaneously immunized group and two in the mucosally immunized group had anti-intimin antibody titers of 40 at the time of immunization, and all animals were seronegative to ovalbumin at this time (Table 2). At the time of euthanasia 3 days after the final immunization at week 11, all animals in the subcutaneously and rectally immunized groups had significantly increased anti-intimin and anti-ovalbumin antibody titers compared to those at week 0 (Table 2). Overall, titers from the subcutaneously immunized group were significantly higher than those of the rectally immunized group (Table 2).
In all trials, intimin-specific antibodies were induced by both rectal and subcutaneous immunization and were found in some animals that received adjuvant only, showing that natural exposure also led to a humoral immune response. Antibody responses had a very weak positive correlation with systemic PBMC responses (Pearson's correlation coefficient R = 0.1024), so cellular responses cannot be extrapolated by evaluating humoral immune responses only. Ovalbumin-specific antibodies were less consistent but developed only in immunized animals.
DISCUSSION
This study has shown that subcutaneous and mucosal immunization with intimin and ovalbumin induced comparable lymphoproliferative responses in the MRLN in cattle and induced systemic humoral and cellular responses in both groups. The response patterns to the different antigens were fairly consistent across all three trials, using different immunization timelines, adjuvants, and formulations, and in animals that may have been previously exposed to E. coli O157:H7, based on antibody titers and lymphoproliferative responses. The animals with low antibody titers to intimin at the start of trial 3 may have been previously exposed or currently colonized with E. coli O157:H7, providing a secondary means of inducing immune responses. However, the observed responses were attributed at least in part to experimental immunizations due to the animals' responses to ovalbumin, which they were not exposed to at any other time before or during the trial. Also, immunization resulted in humoral, local, and systemic lymphoproliferative responses, whereas apparent natural exposure in the group receiving adjuvant resulted in local and systemic lymphoproliferative responses only. While, in general, responses to ovalbumin in these trials showed similar although less robust trends compared to responses to intimin, they confirmed that immunization was successful in some animals. The lack of response to ovalbumin in animals receiving adjuvant only showed that adjuvant alone did not lead to any response that cross-reacted with ovalbumin. Concurrent or previous exposure to E. coli O157:H7 may be considered a confounding variable in an immunization study but, when dealing with this highly prevalent bacteria (18, 19), likely represents a real-world scenario. Besides E. coli O157:H7, other organisms that express γ-intimin, such as enteropathogenic E. coli and Citrobacter rodentium (43–45), may also induce immune responses in exposed animals. Given the conserved nature of the protein, the ideal vaccine would be one that induces significant responses in both naïve animals and acts as an effective booster in animals previously exposed to intimin by natural infection.
The MRLN were chosen to represent the local mucosal immune response because of the difficultly of reliably measuring mucosal antibodies or lymphoproliferative responses from the RAJ (unpublished data). The RAJ contains dense lymphoid tissue, but our laboratory has had limited success with consistently harvesting enough viable cells to use for lymphoproliferative studies, and cultures were often contaminated. Similarly, while there is support for mucosal swabs being effective for measuring mucosal antibody levels (46), our laboratory found swabs to be inconsistent and greatly influenced by the amount of fecal material in the rectum at the time of swabbing. As the draining lymph nodes for the RAJ, the MRLN are the next closest site to measure the local lymphoproliferative responses. Similarly, serum antibody levels may be a viable method of predicting mucosal antibody levels, especially IgG1, which has been shown to be secreted into the intestine and contribute to clearance of rotavirus in cattle (47–49). Further studies would be needed to evaluate the potential protective role of secreted IgG1 against E. coli O157:H7 and how serum titers correlate with intestinal concentrations following vaccination in cattle.
Interestingly, in this study, the responses in the cells from the MRLN appear to be targeted following immunization rather than being a generalized response in all lymph nodes. Only 3 days after the final subcutaneous immunization, animals had consistent lymphoproliferative responses in the MRLN, while only two of 10 animals had significant responses in the PLN draining the immunization site, and no animals had significant responses in the contralateral PLN. While not all animals had significant PLN responses to the positive-control antigens (concanavalin A and TCGF), which was consistent over multiple assays, the majority of animals that did respond to the positive controls did not have significant responses to intimin or ovalbumin. This may have to do with the different populations of cells in the MRLN and PLN, and further studies would be needed to fully characterize the potential differences.
While mucosal immunization has been well documented to induce both local and systemic immunity, it is generally accepted that parenteral immunization does not reliably result in strong mucosal responses. However, some studies show that parenteral immunization with certain adjuvants, typically vitamin D3, induces strong, protective mucosal immune responses toward bacterial agents in mice (50). Also in mice, TLR7/8 agonists, including R848, induce mucosa-directed responses to parenterally delivered antigen when applied topically (51). To the authors' knowledge, this is the first time this TLR7/8 agonist has been investigated as an adjuvant in cattle.
Studies show that cattle develop antibodies to E. coli O157:H7 proteins following vaccination with E. coli O157:H7-secreted proteins (33) or experimental challenge (52). Anti-E. coli O157:H7 antibodies have also been shown to decrease colonization and protect against experimental challenge in animal models (30). Additionally, a recent study by our group has shown that systemic lymphoproliferative responses to E. coli O157:H7 proteins, including intimin, EspA, EspB, and TIR, are induced by natural exposure and that in both experimental challenge and natural exposure, lymphoproliferative responses in the MRLN are comparable to or more robust than systemic responses (unpublished data). The animals in these three immunization studies showed similar antibody production and systemic and local lymphoproliferative responses as those seen in challenged or naturally exposed animals in previous trials in this lab. These findings indicate that mucosal or subcutaneous immunization or a combination of the two with E. coli O157:H7 proteins results in local immune responses with the potential to decreased colonization and shedding in cattle if used as a vaccine, as has been demonstrated in multiple studies using a subcutaneous vaccine composed of E. coli O157:H7-secreted proteins that decreased probability of colonization and shedding in cattle in feedlot settings (33, 53–56). However, intimin was not included in this formulation, and only systemic antibody responses were measured in these studies, so the levels of systemic and lymphoproliferative responses are unknown. Future studies evaluating the role of lymphoproliferative responses as well as humoral responses in protection against colonization may lead to an even more effective vaccine formulation, which could reduce or ideally eliminate the reservoir of E. coli O157:H7 in cattle and greatly increase food safety and decrease disease in humans.
ACKNOWLEDGMENTS
This work was supported by the Life Science Discovery Fund Grant 08-02. K. G. Boland is supported by a fellowship through American College of Veterinary Pathologists/Society for Toxicologic Pathologists Coalition for Veterinary Fellows and Amgen.
We thank Susan Smart for the technical assistance as well as Emma Karel, Eric Sutten, Lonie Austin, and Kathleen White for their assistance with animal care and handling.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1.Boyce TG, Swerdlow DL, Griffin PM. 1995. Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N. Engl. J. Med. 333:364–368 [DOI] [PubMed] [Google Scholar]
- 2.Cohen MB. 1996. Escherichia coli O157:H7 infections: a frequent cause of bloody diarrhea and the hemolytic-uremic syndrome. Adv. Pediatr. 43:171–207 [PubMed] [Google Scholar]
- 3.CDC 1985. Hemolytic-uremic syndrome associated with Escherichia coli O157:H7 enteric infections—United States, 1984. MMWR Morb. Mortal. Wkly. Rep. 34:20–21 [PubMed] [Google Scholar]
- 4.Noel JM, Boedeker EC. 1997. Enterohemorrhagic Escherichia coli: a family of emerging pathogens. Dig. Dis. 15:67–91 [DOI] [PubMed] [Google Scholar]
- 5.Dundas S, Todd WT, Stewart AI, Murdoch PS, Chaudhuri AK, Hutchinson SJ. 2001. The central Scotland Escherichia coli O157:H7 outbreak: risk factors for the hemolytic uremic syndrome and death among hospitalized patients. Clin. Infect. Dis. 33:923–931 [DOI] [PubMed] [Google Scholar]
- 6.Grif K, Dierich MP, Karch H, Allerberger F. 1998. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 17:761–766 [DOI] [PubMed] [Google Scholar]
- 7.Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6:458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. 2000. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong GL, Hollingsworth J, Morris JG., Jr 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 18:29–51 [DOI] [PubMed] [Google Scholar]
- 11.CDC 2008. Escherichia coli 0157:H7 infections in children associated with raw milk and raw colostrum from cows—California, 2006. MMWR Morb. Mortal. Wkly. Rep. 57:625–628 [PubMed] [Google Scholar]
- 12.CDC 2006. Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach—United States, September 2006. MMWR Morb. Mortal. Wkly. Rep. 55:1045–1046 [PubMed] [Google Scholar]
- 13.CDC 2005. Escherichia coli O157:H7 infections associated with ground beef from a U.S. military installation—Okinawa, Japan, February 2004. MMWR Morb. Mortal. Wkly. Rep. 54:40–42 [PubMed] [Google Scholar]
- 14.CDC 2000. Outbreak of Escherichia coli O157:H7 infection associated with eating fresh cheese curds—Wisconsin, June 1998. MMWR Morb. Mortal. Wkly. Rep. 49:911–913 [PubMed] [Google Scholar]
- 15.CDC 1997. Escherichia coli O157:H7 infections associated with eating a nationally distributed commercial brand of frozen ground beef patties and burgers—Colorado, 1997. MMWR Morb. Mortal. Wkly. Rep. 46:777–778 [PubMed] [Google Scholar]
- 16.CDC 1997. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. MMWR Morb. Mortal. Wkly. Rep. 46:4–8 [PubMed] [Google Scholar]
- 17.Cray WC, Jr, Moon HW. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussein HS. 2007. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J. Anim. Sci. 85(Suppl 13):E63–E72 [DOI] [PubMed] [Google Scholar]
- 19.Hussein HS, Sakuma T. 2005. Prevalence of Shiga toxin-producing Escherichia coli in dairy cattle and their products. J. Dairy Sci. 88:450–465 [DOI] [PubMed] [Google Scholar]
- 20.Naylor SW, Low JC, Besser TE, Mahajan A, Gunn GJ, Pearce MC, McKendrick IJ, Smith DG, Gally DL. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JY, Li J, Sheng H, Besser TE, Potter K, Hovde CJ. 2007. Escherichia coli O157:H7 colonization at the rectoanal junction of long-duration culture-positive cattle. Appl. Environ. Microbiol. 73:1380–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, 2009. MMWR Morb. Mortal. Wkly. Rep. 59:418–422 [PubMed] [Google Scholar]
- 23.Loneragan GH, Brashears MM. 2005. Pre-harvest interventions to reduce carriage of E. coli O157 by harvest-ready feedlot cattle. Meat Sci. 71:72–78 [DOI] [PubMed] [Google Scholar]
- 24.DeVinney R, Gauthier A, Abe A, Finlay BB. 1999. Enteropathogenic Escherichia coli: a pathogen that inserts its own receptor into host cells. Cell. Mol. Life Sci. 55:961–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVinney R, Stein M, Reinscheid D, Abe A, Ruschkowski S, Finlay BB. 1999. Enterohemorrhagic Escherichia coli O157:H7 produces Tir, which is translocated to the host cell membrane but is not tyrosine phosphorylated. Infect. Immun. 67:2389–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornick NA, Booher SL, Moon HW. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean-Nystrom EA, Bosworth BT, Moon HW, O'Brien AD. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cataldi A, Yevsa T, Vilte D, Schulze K, Castro-Parodi M, Larzabal M, Ibarra C, Mercado E, Guzman CA. 2008. Efficient immune responses against intimin and EspB of enterohaemorragic Escherichia coli after intranasal vaccination using the TLR2/6 agonist MALP-2 as adjuvant. Vaccine 26:5662–5667 [DOI] [PubMed] [Google Scholar]
- 29.Ghaem-Maghami M, Simmons CP, Daniell S, Pizza M, Lewis D, Frankel G, Dougan G. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agin TS, Zhu C, Johnson LA, Thate TE, Yang Z, Boedeker EC. 2005. Protection against hemorrhagic colitis in an animal model by oral immunization with isogeneic rabbit enteropathogenic Escherichia coli attenuated by truncating intimin. Infect. Immun. 73:6608–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dean-Nystrom EA, Gansheroff LJ, Mills M, Moon HW, O'Brien AD. 2002. Vaccination of pregnant dams with intimin (O157) protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun. 70:2414–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judge NA, Mason HS, O'Brien AD. 2004. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Immun. 72:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potter AA, Klashinsky S, Li Y, Frey E, Townsend H, Rogan D, Erickson G, Hinkley S, Klopfenstein T, Moxley RA, Smith DR, Finlay BB. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362–369 [DOI] [PubMed] [Google Scholar]
- 34.Gansheroff LJ, Wachtel MR, O'Brien AD. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 67:6409–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nart P, Holden N, McAteer SP, Wang D, Flockhart AF, Naylor SW, Low JC, Gally DL, Huntley JF. 2008. Mucosal antibody responses of colonized cattle to Escherichia coli O157-secreted proteins, flagellin, outer membrane proteins and lipopolysaccharide. FEMS Immunol. Med. Microbiol. 52:59–68 [DOI] [PubMed] [Google Scholar]
- 36.Bretschneider G, Berberov EM, Moxley RA. 2008. Enteric mucosal antibodies to Escherichia coli O157:H7 in adult cattle. Vet. Rec. 163:218–219 [DOI] [PubMed] [Google Scholar]
- 37.Von Beust BR, Brown WC, Estes DM, Zarlenga DS, McElwain TF, Palmer GH. 1999. Development and in vitro characterization of recombinant vaccinia viruses expressing bovine leukemia virus gp51 in combination with bovine IL4 or IL12. Vaccine 17:384–395 [DOI] [PubMed] [Google Scholar]
- 38.Solano-Aguilar GI, Vengroski KG, Beshah E, Lunney JK. 2000. Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. J. Immunol. Methods 241:185–199 [DOI] [PubMed] [Google Scholar]
- 39.Brown WC, Zhu D, Shkap V, McGuire TC, Blouin EF, Kocan KM, Palmer GH. 1998. The repertoire of Anaplasma marginale antigens recognized by CD4(+) T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect. Immun. 66:5414–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KJ, Hamza S, Skelton H. 2003. The imidazoquinolines and their place in the therapy of cutaneous disease. Expert Opin. Pharmacother. 4:1105–1119 [DOI] [PubMed] [Google Scholar]
- 41.Gupta AK, Cherman AM, Tyring SK. 2004. Viral and nonviral uses of imiquimod: a review. J. Cutan. Med. Surg. 8:338–352 [DOI] [PubMed] [Google Scholar]
- 42.Tuo W, Palmer GH, McGuire TC, Zhu D, Brown WC. 2000. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect. Immun. 68:270–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jerse AE, Kaper JB. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J, Kaper JB. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411–417 [DOI] [PubMed] [Google Scholar]
- 45.Schauer DB, Falkow S. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeilly TN, Naylor SW, Mitchell MC, McAteer S, Mahajan A, Smith DG, Gally DL, Low JC, Huntley JF. 2007. Simple methods for measurement of bovine mucosal antibody responses in vivo. Vet. Immunol. Immunopathol. 118:160–167 [DOI] [PubMed] [Google Scholar]
- 47.Besser TE, Gay CC, McGuire TC, Evermann JF. 1988. Passive immunity to bovine rotavirus infection associated with transfer of serum antibody into the intestinal lumen. J. Virol. 62:2238–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besser TE, McGuire TC, Gay CC, Pritchett LC. 1988. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J. Virol. 62:2234–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parreno V, Bejar C, Vagnozzi A, Barrandeguy M, Costantini V, Craig MI, Yuan L, Hodgins D, Saif L, Fernandez F. 2004. Modulation by colostrum-acquired maternal antibodies of systemic and mucosal antibody responses to rotavirus in calves experimentally challenged with bovine rotavirus. Vet. Immunol. Immunopathol. 100:7–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enioutina EY, Visic D, McGee ZA, Daynes RA. 1999. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine 17:3050–3064 [DOI] [PubMed] [Google Scholar]
- 51.Chang BA, Cross JL, Najar HM, Dutz JP. 2009. Topical resiquimod promotes priming of CTL to parenteral antigens. Vaccine 27:5791–5799 [DOI] [PubMed] [Google Scholar]
- 52.Bretschneider G, Berberov EM, Moxley RA. 2007. Isotype-specific antibody responses against Escherichia coli O157:H7 locus of enterocyte effacement proteins in adult beef cattle following experimental infection. Vet. Immunol. Immunopathol. 118:229–238 [DOI] [PubMed] [Google Scholar]
- 53.Peterson RE, Klopfenstein TJ, Moxley RA, Erickson GE, Hinkley S, Rogan D, Smith DR. 2007. Efficacy of dose regimen and observation of herd immunity from a vaccine against Escherichia coli O157:H7 for feedlot cattle. J. Food Prot. 70:2561–2567 [DOI] [PubMed] [Google Scholar]
- 54.Peterson RE, Klopfenstein TJ, Moxley RA, Erickson GE, Hinkley S, Bretschneider G, Berberov EM, Rogan D, Smith DR. 2007. Effect of a vaccine product containing type III secreted proteins on the probability of Escherichia coli O157:H7 fecal shedding and mucosal colonization in feedlot cattle. J. Food Prot. 70:2568–2577 [DOI] [PubMed] [Google Scholar]
- 55.Smith DR, Moxley RA, Peterson RE, Klopfenstein TJ, Erickson GE, Bretschneider G, Berberov EM, Clowser S. 2009. A two-dose regimen of a vaccine against type III secreted proteins reduced Escherichia coli O157:H7 colonization of the terminal rectum in beef cattle in commercial feedlots. Foodborne Pathog. Dis. 6:155–161 [DOI] [PubMed] [Google Scholar]
- 56.Moxley RA, Smith DR, Luebbe M, Erickson GE, Klopfenstein TJ, Rogan D. 2009. Escherichia coli O157:H7 vaccine dose-effect in feedlot cattle. Foodborne Pathog. Dis. 6:879–884 [DOI] [PubMed] [Google Scholar]


