Abstract
Dense granule antigen proteins derived from Toxoplasma gondii (TgGRAs) are potential antigens for the development of diagnostic tools. TgGRA7 and TgGRA14 were detected in the peritoneal fluid of T. gondii-infected mice, suggesting that TgGRAs may be highly antigenic proteins. Here, TgGRA7 and TgGRA14 were evaluated as candidates for the development of a marker for a rapid diagnostic test. The specificity and sensitivity of purified recombinant proteins of TgGRA7 and TgGRA14 were compared in an indirect enzyme-linked immunosorbent assay (iELISA) using a series of serum samples from T. gondii-experimentally infected mice and using recombinant T. gondii major surface antigen 2 (TgSAG2) as a reference control. The iELISA with TgGRA7 showed the greatest diagnostic accuracy and could detect anti-TgGRA7 antibody in acute and chronic infections. A total of 59 field samples from pigs were also examined by the iELISAs, and the results compared with those of the latex agglutination test (LAT). Among the three recombinant antigens, TgGRA7 had the highest rates of positivity, with significant concordance (88.14) and kappa value (0.76) in comparison with the results using LAT. Furthermore, an immunochromatographic test (ICT) based on recombinant TgGRA7 was developed for rapid detection of antibodies to the infection. The ICT differentiated clearly between sera from T. gondii-infected mice and uninfected or Neospora caninum-infected mice. Pig sera were examined with the ICT, and the results compared favorably with those of LAT and iELISA for TgGRA7, with kappa values of 0.66 and 0.70 to 0.79, respectively. These data suggest that the ICT based on TgGRA7 is a promising diagnostic tool for routine testing in the clinic and mass screening of samples in the field.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite that is widely prevalent in humans and animals. The infection is characterized by significant morbidity and mortality in immunocompromised patients, such as those with AIDS and serious congenital birth defects, among both humans and livestock (1). Cats and other felids are the only definitive hosts of T. gondii that excrete oocysts. These appear 10 days after ingestion of infected tissue and continue to be excreted for 2 weeks. Transmission to intermediate hosts can occur through ingesting infectious oocysts from the environment or cysts in undercooked or uncooked meat (2). Transplacental transmission may also occur when the tachyzoites pass to the fetus via the placenta (3). The infection has a major economic impact on the livestock industry, resulting in reproductive losses, abortion, fetal resorption, and stillbirths (1, 3, 4). Accurate diagnosis of Toxoplasma gondii infection is crucial for the proper management of the infection in humans and animals.
Diagnosis of toxoplasmosis can be achieved using a number of different approaches, including microscopic examination, animal inoculation, in vitro culture, and molecular and serological methods. Animal inoculation, in vitro culture, and microscopic examination of parasites by inspection of blood smears, tissue specimens, feces, lymph node aspirates, and cerebrospinal fluid are not appropriate for routine mass screening (5). The latex agglutination test (LAT) is to date considered the gold standard method for diagnosis of T. gondii infection; however, the test has poor specificity (5, 6). Enzyme-linked immunosorbent assays (ELISAs) with purified recombinant proteins are widely used for routine diagnostic testing and in seroepidemiological investigations (7–9). However, the test is expensive, needs specialized facilities in the laboratory, and cannot be used in the field and clinic. The immunochromatographic test (ICT) is rapid and does not require expensive equipment and expertise (10, 11). A previous study has shown a promising application of ICT based on recombinant T. gondii major surface antigen 2 (TgSAG2) for the detection of antibody to the infection in cats (10). A more recent report has documented limitations of TgSAG2 in the detection of antibody in early and acute stages of infection (12). Therefore, the development of a new ICT with a more sensitive and specific antigen for detecting the infection in animals and humans is required for preventive and control programs for toxoplasmosis.
Dense granule antigen proteins of T. gondii (TgGRAs) are identified as secretory proteins found abundantly within the parasitophorous vacuole (PV) surrounding the parasites (13). Members of this family are potential antigens for the development of a diagnostic tool for toxoplasmosis. TgGRA7 is a member of this family that was earlier identified by serological immunoscreening of a T. gondii cDNA library using infected human sera (14). After host cell invasion, this protein is secreted into the vacuolar network, the parasitophorous vacuole membrane (PVM), and extensions protruding into the cytoplasm. TgGRA7 accumulates within the PV and PVM in tachyzoite-infected cells and in the cytoplasm of bradyzoite-infected cells (13, 15). Several reports have demonstrated the usefulness of TgGRA7 as a serodiagnostic marker of toxoplasmosis. Indeed, the indirect ELISA (iELISA) based on TgGRA7 showed overall sensitivity of 81 to 88% and specificity of 98 to 100% with sera from patients (16, 17). Moreover, the iELISA with TgGRA7 had the highest positivity rates in comparison with the positivity rates of other T. gondii proteins, including the rhoptry (TgROP1) and matrix antigens (TgMAG1), the major surface antigen (TgSAG1), and TgGRA8 (18). Another member of the dense granule proteins, TgGRA14, was recently identified within the PV and PVM surrounding the parasite, although its antigenicity was unknown (19).
In the present study, the diagnostic performances of recombinant proteins of TgGRA7 were evaluated in the iELISA and ICT using sera from experimentally and naturally infected animals. Our data show that the ICT based on TgGRA7 is a better diagnostic tool for routine testing of T. gondii infection in the clinic and mass screening of samples in the field.
MATERIALS AND METHODS
Parasites.
T. gondii (PLK and RH strains) and Neospora caninum tachyzoites (Nc-1 strain) were maintained in African green monkey kidney (Vero) cells cultured in Eagle's minimum essential medium (EMEM; Sigma, St. Louis, MO) supplemented with 8% heat-inactivated fetal bovine serum. For purification of tachyzoites, the infected cells were washed with cold phosphate-buffered saline (PBS) to remove host cell debris. Cell pellets were resuspended in medium and passed through a 27-gauge needle and then through a 5.0-μm-pore filter (Millipore, Bedford, MA).
Mice.
C57BL/6, BALB/c, and ICR female mice (6 to 7 weeks of age) were obtained from Clea Japan (Tokyo, Japan) and housed in the animal facility of the National Research Center for Protozoan Diseases under the Guiding Principles for the Care and Use of Research Animals promulgated by the Obihiro University of Agriculture and Veterinary Medicine.
Parasite infection of mice.
For preparation of peritoneal fluid from the infected mice, C57BL/6 mice were intraperitoneally infected with 1 × 103 tachyzoites of T. gondii PLK or RH strain. Mice were then sacrificed 0, 3, or 6 days postinfection. The peritoneal cells were collected from the peritoneal cavity of naïve or parasite-infected mice by peritoneal washing with 5 ml of cold PBS. After harvesting, cells were centrifuged at 800 × g for 10 min and suspended in cold PBS. The supernatant was stored at −30°C for the further use of measuring TgGRA7 and TgGRA14 by the double-antibody sandwich ELISA (DAS-ELISA). For collection of serum samples from mice, BALB/c mice were intraperitoneally infected with 1 × 103 tachyzoites of PLK or Nc-1. Serum (20 μl) was obtained from mice at 7, 14, 21, and 28 days after the infection via tail vein for measuring antigen-specific antibodies by the iELISA. To confirm the lack of an antibody response in uninfected mice, control sera were taken from all animals on day zero before the parasite infection.
Preparation of the recombinant proteins.
Total RNA extracted from the RH strain of T. gondii using TRI reagent (Sigma) was reverse transcribed using the SuperScript first strand synthesis system for reverse transcription (RT)-PCR (Invitrogen, Carlsbad, CA) and then used as a template to amplify the target genes. The cDNAs encoding TgGRA7 (corresponding to amino acids 27 to 236, GenBank accession number JX045574.1) and TgGRA14 (corresponding to amino acids 36 to 282, GenBank accession number FJ015061) were amplified using PCR with specific primer sets designed from the available sequences in GenBank, as follows: 5′ GCG GAT CCG CCA CCG CGT CAG ATG 3′ includes a BamH I restriction enzyme site and 5′ GTG AAT TCC TAC TGG CGG GCA TCC TC 3′ includes an EcoRI restriction enzyme site for TgGRA7, and 5′ CGA ATT CGC CAG TTT GGA GCA GAC AAT T 3′ includes a EcoRI restriction enzyme site and 5′ TCT CGA GTT ACT TCG TGG TTT CGG AGG T 3′ includes a XhoI restriction enzyme site for TgGRA14 (restriction enzyme sites are in boldface). PCR products were digested with the appropriate restriction enzymes and then ligated into the glutathione S-transferase (GST) fusion protein in the Escherichia coli expression vector pGEX (GE Healthcare, Buckinghamshire, United Kingdom), which had been digested with the same set of restriction enzymes. Plasmid nucleotide sequences were determined using an ABI 3100 DNA sequencer (Applied Biosystems, Foster City, CA). The cloning of truncated TgSAG2 was performed as described previously (20). Recombinant proteins of TgGRA7, TgGRA14, and TgSAG2 were expressed as glutathione S-transferase (GST) fusion proteins in the E. coli DH5α strain (TaKaRa Bio, Inc., Japan). GST tags of the recombinant proteins were removed with thrombin protease (GE Healthcare) according to the manufacturer's instructions. As shown by the results in Figure 1, the purity and quantity of the recombinant proteins of TgGRA7 (29 kDa), TgGRA14 (30 kDa), and TgSAG2 (20 kDa) were confirmed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie brilliant blue R250 staining (MP Biomedicals, Inc., France). The protein concentrations were measured using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Inc., Rockford, IL).
Fig 1.
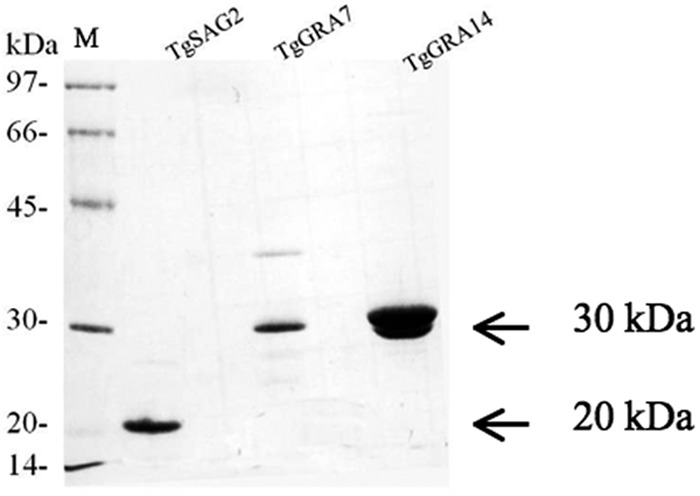
Expression of recombinant proteins in E. coli. The results of 15% SDS-PAGE of recombinant TgGRA7 (29 kDa), TgGRA14 (30 kDa), and TgSAG2 (20 kDa) stained with Coomassie blue are shown. M, molecular markers.
Production of anti-TgGRA7 or anti-TgGRA14 serum and purification of IgG.
One hundred micrograms of each purified recombinant protein in Freund's complete adjuvant (Sigma) were intraperitoneally injected into 6-week-old female ICR mice (n = 4) on day zero. The same protein in Freund's incomplete adjuvant (Sigma) was also intraperitoneally injected into the mice on days 14, 28, and 42. The sera were collected from the mice at day 56. Serum that exhibited a higher sensitivity and specificity to T. gondii from all the inoculated mice was used in this study. One milligram of each purified recombinant protein in Freund's complete adjuvant was also intradermally injected into a female Japanese white rabbit on day zero. The same protein in Freund's incomplete adjuvant was further intradermally injected into the rabbit on days 14, 28, and 42. Immunoglobulin G (IgG) was purified from 2 ml of the rabbit serum that was collected 7 days after the last immunization, using protein A chromatography columns (Bio-Rad Laboratories, CA), following the manufacturer's instructions. Fractions containing IgG were pooled and resolved by SDS-PAGE to test protein purity and quantity. The protein concentrations of purified rabbit polyclonal IgG were measured using a BCA protein assay kit (Thermo Fisher Scientific, Inc.).
iELISA.
Fifty-microliter amounts of purified TgGRA7 (29 kDa), TgGRA14 (30 kDa), and TgSAG2 (20 kDa) at a final concentration of 0.1 μM were coated onto the ELISA plates (Nunc, Denmark) overnight at 4°C with a carbonate-bicarbonate buffer (pH 9.6). Plates were washed once with PBS containing 0.05% Tween 20 (PBS-T) and blocked with PBS containing 3% skimmed milk (PBS-SM) for 1 h at 37°C before the plates were washed once with PBS-T, and 50-μl amounts of serum samples diluted at 1:100 with PBS-SM were added to duplicate wells. The plates were incubated at 37°C for 1 h. After washing six times with PBS-T, the plates were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG or anti-pig total IgG or IgM (Bethyl Laboratories, United States) diluted at 1:4,000 with PBS-SM at 37°C for 1 h. Plates were further washed six times before the substrate solution [0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, and 0.3 mg/ml 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); Sigma-Aldrich] was added to each well in 100-μl aliquots. The absorbance at 415 nm after 1 h of incubation at room temperature was measured using an ELISA reader (Corona microplate reader MTP-120; Corona, Tokyo, Japan). The cutoff point for pig sera was determined as the mean value at an optical density of 415 nm (OD415) for specific pathogen-free (SPF) pig sera kept in our laboratory (n = 10) plus three standard deviations (IgG, 0.201 for anti-TgGRA7, 0.181 for anti-TgGRA14, and 0.107 for anti-TgSAG2, and IgM, 0.087 for anti-TgGRA7, 0.095 for anti-TgGRA14, and 0.112 for anti-TgSAG2). All samples were examined at least twice to obtain reproducible data.
DAS-ELISA.
Rabbit anti-TgGRA7 or TgGRA14 IgG was diluted to 20 μg/ml in a 0.05 M carbonate buffer (pH 9.6). One microgram of the IgG per well was used as the capture antibody to coat microtiter plates at 4°C overnight. Blocking was performed with a blocking solution (3% skimmed milk in PBS) at 37°C for 1 h. Plates were incubated at 37°C for 30 min with test samples in duplicate. After washing six times with a washing solution (0.05% Tween 20 in PBS), anti-TgGRA7 or anti-TgGRA14 mouse serum diluted at 1:2,000 in blocking solution was added to each well and the plate was incubated at 37°C for 1 h. After a further six washes, the plates were incubated with HRP-conjugated anti-mouse IgG diluted at 1:5,000 in blocking solution at 37°C. Binding was visualized with the substrate solution [0.1 M citric acid, 0.2 M sodium phosphate, 0.003% H2O2, and 0.3 mg/ml 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)]. The absorbance at 415 nm was measured using an MTP-500 microplate reader. The concentration of TgGRA7 or TgGRA14 was calculated for each sample by standardization with the purified recombinant protein of TgGRA7 or TgGRA14. Standard curves based on recombinant TgGRA7 and TgGRA14 were generated to quantify the secreted proteins in peritoneal fluids and showed coefficients of determination (R2) of 0.9948 and 0.9974, respectively.
LAT.
The latex agglutination test (LAT) was performed according to the manufacturer's instructions (Toxocheck-MT; Eiken Chemical, Tokyo, Japan). Samples were considered positive when agglutination was observed at dilutions of 1:32 and greater.
Field sera.
Blood samples from 59 pigs with hemorrhagic or necrotic lesions were collected at an abattoir in Japan. The serum samples were then prepared and stored at −30°C for further use.
ICT.
The ICT strips, based on gold colloid-conjugated TgGRA7, were prepared as described previously (10). Briefly, 0.5 mg/ml recombinant TgGRA7 was gently mixed with gold colloids (1:10, vol/vol) and incubated at room temperature for 15 min. The conjugated particles were then blocked with 0.05% polyethylene glycol (PEG) 20,000 (Sigma) and 1% bovine serum albumin (BSA; Sigma). The conjugated gold colloid was pelleted at 18,000 × g for 30 min, and the precipitates were suspended with 0.05% PEG and 0.5% BSA in PBS. The mixture was centrifuged again at 18,000 × g for 30 min, and the pellet was suspended in an appropriate volume of solution containing 10 mM Tris-HCl (pH 8.2) with 5% sucrose. The conjugate was then soaked in borosilicate fiberglass paper (Schleicher and Schuell, Keene, NH) and dried in a vacuum overnight. Recombinant protein was immobilized onto a nitrocellulose membrane (Schleicher and Schuell) using a BioDot BioJet 3050 quanti-dispenser (BioDot, Inc., Irvine, CA) to assess its efficiency as a capture reagent for the test. The purified rabbit anti-TgGRA7 IgG antibody (1.5 mg/ml) was sprayed onto the nitrocellulose membrane at a 4-mm distance from the test line to serve as a procedural control. The strips were assembled on a plastic card (Schleicher and Schuell) and then cut into 2-mm-wide strips using a BioDot cutter (BioDot Inc.). The assay was performed by adding 30 μl of the optimal dilution of the serum samples (1:1 in PBS) to the sample pad. The results were judged within 15 min on the basis of the presence or absence of the reaction at the test line. All samples were examined at least twice to obtain reproducible data.
Statistical analysis.
The results of LAT, iELISA, and ICT were estimated by the percentage of agreement, the sensitivity and specificity, and the kappa values with 95% confidence interval (http://vassarstats.net/). The strength of agreement was graded with kappa values of fair (0.21 to 0.40), moderate (0.41 to 0.60), and substantial (0.61 to 0.80). The results under various assay conditions were evaluated using Student's t test or analysis of variance (ANOVA) followed by Tukey's multiple comparison. P values of <0.05 were considered significant.
RESULTS
Detection of TgGRA7 and TgGRA14 in peritoneal fluids of infected mice.
To examine the release of TgGRA7 and TgGRA14, the peritoneal fluids of mice infected with a type I strain (RH) or type II strain (PLK) of T. gondii were analyzed by DAS-ELISA (Fig. 2). The DAS-ELISAs demonstrated no reaction with the noninfected mouse samples but reacted strongly with samples from T. gondii-infected mice. However, the levels of TgGRA7 and TgGRA14 in PLK-infected mice were significantly lower than those in RH-infected mice. More importantly, the quantities of TgGRA7 released were significantly higher (P < 0.0001) than those of TgGRA14 at days 3 and 6 postinfection. These data suggested that the quantity of TgGRA7 released from the parasites is more abundant than that of TgGRA14 in the body fluids during the course of the infection.
Fig 2.
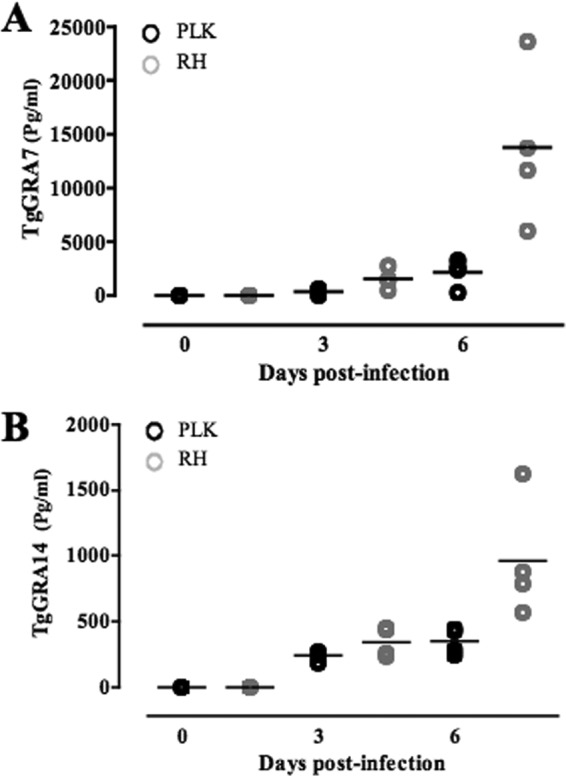
Detection of TgGRA7 and TgGRA14 in peritoneal fluid of T. gondii-infected mice by DAS-ELISA. Amounts of TgGRA7 (A) and TgGRA14 (B) in peritoneal fluid of infected mice on days 0, 3, and 6 postinfection were quantified (n = 4). The concentration of each protein was determined based on the standard curves generated by different concentrations of recombinant proteins.
Evaluation of the diagnostic performance of TgGRA7 and TgGRA14.
Recombinant proteins were used as antigens for the iELISAs and then validated with sera from experimentally infected mice. A previously established iELISA with TgSAG2 was used as a reference control (20). The iELISAs based on these recombinant proteins succeeded in clearly differentiating between T. gondii-infected mouse sera and both the SPF and Neospora caninum-infected mouse sera (Fig. 3A). Although the recombinant proteins showed good specificity with experimentally infected mouse sera, TgGRA7 performed best, as evidenced by the higher OD values of sera from T. gondii-infected mice (Fig. 3A). Sera from T. gondii-infected mice were also sampled serially postinfection to determine the sensitivity of the iELISAs with recombinant proteins (Fig. 3B). Interestingly, the iELISA with TgGRA7 could detect specific antibody as early as 14 days after the infection with levels that were significantly higher than those of anti-TgGRA14 or anti-TgSAG2 antibody. These data demonstrate that TgGRA7 is a better diagnostic marker than TgGRA14 and TgSAG2 in detecting T. gondii antibody in acute and chronic infections. Furthermore, the diagnostic performance of the iELISAs with recombinant proteins was evaluated with 59 field sera from pigs. Among the antigens, iELISA with TgGRA7 showed the highest positive rates for the detection of T. gondii-specific IgG and IgM antibodies (Table 1). Next, the results of the iELISA were compared with those of LAT as a reference test (Table 2). Of the 59 field samples, 32 samples were positive by LAT and 33, 28, and 25 were positive by iELISA with TgGRA7, TgGRA14, and TgSAG2, respectively. As shown by the results in Table 3, the iELISA with TgGRA7 had the highest level of performance, demonstrating a higher kappa value (0.76). Although TgGRA7 showed the highest concordance among the recombinant proteins, four samples detected as negative by LAT were positive by iELISA-TgGRA7, exhibiting low OD values (<0.3), and three samples that were positive by LAT were negative by iELISA-TgGRA7, exhibiting low OD values near the cutoff. These results support the conclusion that TgGRA7 is a promising diagnostic antigen for the detection of specific antibodies against T. gondii.
Fig 3.
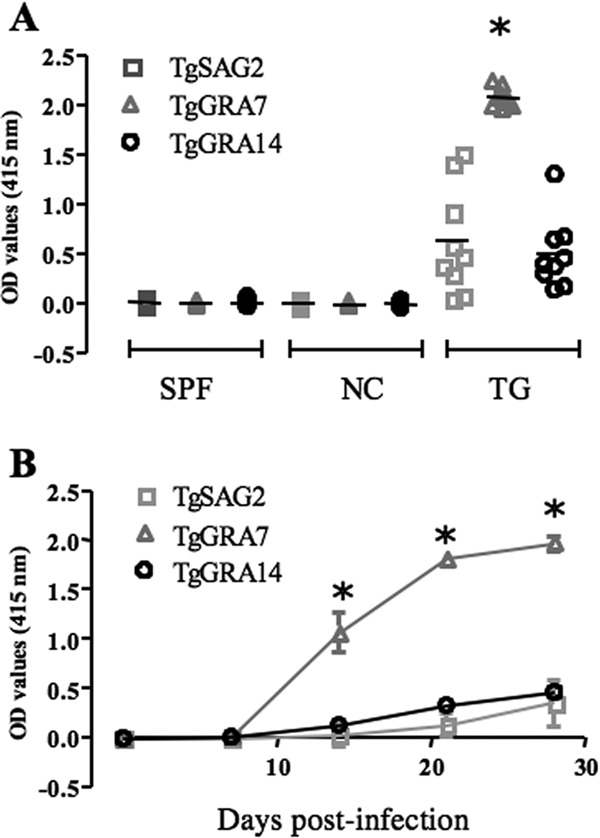
Results of iELISA of sera from T. gondii-infected mice using recombinant antigens. (A) The reactivities of recombinant antigens with sera from SPF mice, experimentally N. caninum-infected mice (NC), and experimentally T. gondii-infected (TG) mice at 30 days after infection were examined by iELISA with TgGRA7, TgGRA14, and TgSAG2 as the antigens (n = 10). (B) Reactivities of recombinant antigens with sera collected serially from experimentally T. gondii-infected mice (n = 12). Each asterisk indicates a significant difference by ANOVA test followed by Tukey's multiple comparison. Differences of P < 0.05 were considered significant.
Table 1.
Results of field pig samples examined by the iELISA using recombinant proteins for detection of IgG and IgM antibodies
| ELISA | No. (%) of samples positive using: |
||
|---|---|---|---|
| TgSAG2 | TgGRA7 | TgGRA14 | |
| IgG | 21 (33.6) | 30 (50.8) | 24 (40.7) |
| IgM | 5 (8.5) | 21 (35.6) | 12 (20.3) |
| Combined | 25 (42.4) | 33 (55.9) | 28 (47.4) |
Table 2.
Comparison of the results for field pig samples examined by the iELISA (IgG and/or IgM antibodies) and LAT using recombinant proteins
| LAT | No. of samples with result in iELISA using: |
Total | |||||
|---|---|---|---|---|---|---|---|
| TgSAG2 |
TgGRA7 |
TgGRA14 |
|||||
| Negative | Positive | Negative | Positive | Negative | Positive | ||
| Negative | 26 | 1 | 23 | 4 | 25 | 2 | 27 |
| Positive | 8 | 24 | 3 | 29 | 6 | 26 | 32 |
| Total | 34 | 25 | 26 | 33 | 31 | 28 | 59 |
Table 3.
Specificity and sensitivity of the iELISA using recombinant proteins in detecting the IgG and/or IgM antibodies to infection in field pig sera compared with the results in the reference test LAT
| Parameter | Value for iELISA compared to LAT result usinga: |
||
|---|---|---|---|
| TgSAG2 | TgGRA7 | TgGRA14 | |
| Sensitivity (%) | 75.00 | 90.63 | 81.25 |
| Specificity (%) | 96.29 | 85.19 | 92.59 |
| Concordance (%) | 84.75 | 88.14 | 86.44 |
| Kappa value | 0.69 | 0.76 | 0.73 |
IgG and/or IgM antibody was used.
Development of ICT based on recombinant TgGRA7.
The ICT based on the gold colloid-conjugated TgGRA7 antigen was then developed and validated with sera from experimentally infected mice and pig field samples (Fig. 4A). Consistent with the iELISA results, positive reactions were observed only in the T. gondii-infected mouse sera and not in the sera from SPF mice or N. caninum-infected mice (data not shown). Next, the 59 pig serum samples were tested with the developed ICT and the results were compared with the results of LAT and iELISA with TgGRA7. The ICT detected a total of 24 positive samples, including 1 sample that was negative by LAT but positive by iELISA. Nine samples that were negative by ICT were positive by LAT (Table 4). The positivity of ICT was significantly correlated with high OD values in iELISA (Fig. 4B). The ICT thus demonstrated substantial and acceptable concordance with LAT and iELISA results, as evidenced by kappa values of 0.66 and 0.70 to 0.79, respectively (Table 5). These data suggest that the developed ICT is a practical and reliable test for the diagnosis of toxoplasmosis.
Fig 4.
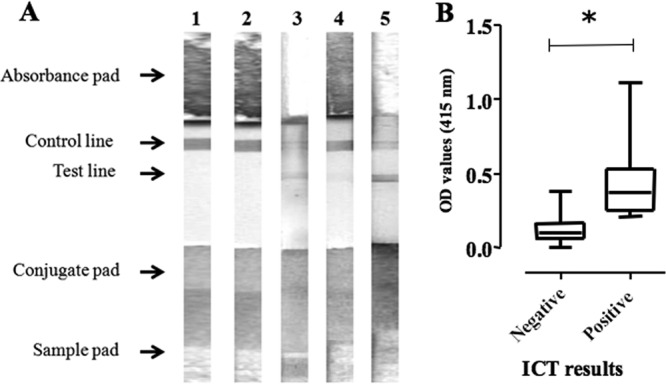
The diagnostic performance of ICT with TgGRA7. (A) Representative images of the ICT reaction with mouse and swine sera are shown. Lane 1, SPF mouse; lane 2, N. caninum-infected mouse; lane 3, T. gondii-infected mouse; lane 4, iELISA with TgGRA7-negative and LAT-negative pig; lane 5, iELISA with TgGRA7-positive and LAT-positive pig. (B) Comparison of the ICT results and the OD values from iELISA with TgGRA7. The interquartile range values (25-50-75%) are shown as boxes and whiskers. The asterisk indicates a significant difference by Student's t test (P < 0.05).
Table 4.
Results for the field pig samples examined by the ICT, iELISA-TgGRA7, and LAT
| ICT | No. of samples with result in: |
Total | |||||
|---|---|---|---|---|---|---|---|
| iELISA with: |
LAT |
||||||
| IgG |
IgG and/or IgM |
||||||
| Negative | Positive | Negative | Positive | Negative | Positive | ||
| Negative | 29 | 6 | 26 | 9 | 26 | 9 | 35 |
| Positive | 0 | 24 | 0 | 24 | 1 | 23 | 24 |
| Total | 29 | 30 | 26 | 33 | 27 | 32 | 59 |
Table 5.
Specificity and sensitivity of the current ICT in detection of specific antibody to the infection in field pig sera compared with the results in the iELISA-TgGRA7 and LAT
| Parameter | Value for ICT compared to: |
||
|---|---|---|---|
| LAT | iELISA (IgG) | iELISA (IgG and/or IgM) | |
| Sensitivity (%) | 71.87 | 80 | 72.7 |
| Specificity (%) | 96.29 | 100 | 100 |
| Concordance (%) | 83.05 | 89.83 | 84.74 |
| Kappa value | 0.66 | 0.79 | 0.70 |
DISCUSSION
The performances of recombinant TgGRA7, TgGRA14, and TgSAG2 were evaluated by iELISA using sera from experimentally T. gondii-infected mice and field pigs. Strikingly, the iELISA with TgGRA7 succeeded in the detection of antibody in acute and chronic stages of the infection. Moreover, TgGRA7 demonstrated the best diagnostic performance in detecting infection in the pig samples, exhibiting high specificity, sensitivity, and agreement with the results of LAT. Our results were consistent with previous reports showing that TgGRA7 is a good serological marker for the detection of IgGs in acute and chronic infection (7, 14, 15, 17). Moreover, Pfrepper and colleagues (18) have documented that IgG antibodies against TgGRA7 appeared significantly earlier than those against TgSAG1 and TgMAG1 in human sera. Likewise, other reports have shown that TgGRA7 is more sensitive with serum samples from recently and acutely infected individuals than with samples from those with chronic infection (21, 22). The antigenic properties of TgGRA7 can be explained by the facts that this protein is expressed by all infectious stages of T. gondii and is abundant on the surface of host cells and within the PV lumen, the PVM, and the host cell cytosol (23, 24). The TgGRA7 released from tachyzoites and bradyzoites after the rupture of their host cells allows the antigen to have direct contact with the host immune system; this can trigger strong antibody response in both early and late stages of infection (14, 17, 23). On the other hand, the fact that the antigenicity of the recombinant TgGRA14 is lower than that of TgGRA7 is most probably due to its lower level of release from the infected host. Thus, the high expression levels and exposure to the host immune system result in greater antigenicity of TgGRA7.
The excellent antigenic properties of recombinant TgGRA7 and its easy preparation from E. coli in purified form were encouraging for the development of a rapid ICT based on this antigen. Using field pig sera, the ICT results with TgGRA7 were substantially concordant with the results of LAT and iELISA with TgGRA7. However, samples that showed low OD values (<0.3) in iELISA with TgGRA7 were negative in the ICT because the low titer of antibody could not capture sufficient conjugated antigen to cause a colored reaction on the membrane. Our data indicate that the current ICT with TgGRA7 is a reliable test for rapid diagnosis of swine toxoplasmosis, producing results similar to those of traditional serological tests.
Toxoplasmosis is one of the most important food-borne diseases in the world. The major sources of infection for humans are undercooked meat, especially pork and lamb, and contaminated fruit and vegetables (2, 3, 8, 9). Accurate diagnostic assays for the detection of the T. gondii infection in infected animals are crucial as a step toward management and control of the disease. So far, a large number of different recombinant antigens have been produced in E. coli and their potentials as diagnostic markers have been validated using ELISA in both humans and animals (7, 22, 25, 26). Although this approach is very sensitive and can be incorporated into an automated procedure, it is time consuming, and it requires laboratory procedures, skilled technicians, and special facilities. The current lack of a routine test for screening animals in the field and slaughterhouse makes the development of a rapid, simple, and more practical assay for detection of the infection highly desirable. In this study, we have successfully developed a highly specific and sensitive ICT with TgGRA7 for the detection of antibody against T. gondii infection in pigs. The current ICT has several advantages over traditional serological tests, including its speed, simplicity, and low cost of preparation. These advantages show an important diagnostic potential for ICT in clinical practice, providing a primary screening tool for routine tests and mass screening programs in areas where toxoplasmosis is endemic. Application of the ICT in the field and slaughterhouse prior to meat processing should be considered a control program to decrease the spread of the infection, as well as the risk of infection to humans.
ACKNOWLEDGMENTS
This research was supported by the Japan Society for the Promotion of Science through the Funding Program for Next Generation World-Leading Researchers (NEXT Program), initiated by the Council for Science and Technology Policy (grant 2011/LS003).
The authors declare no conflicts of interest.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30: 1217– 1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones JL Dubey JP. 2012. Foodborne toxoplasmosis. Clin. Diagn. Lab. Immunol. 55: 845– 851 [DOI] [PubMed] [Google Scholar]
- 3. Dubey JP. 2009. Toxoplasmosis in pigs—the last 20 years. Vet. Parasitol. 164: 89– 103 [DOI] [PubMed] [Google Scholar]
- 4. Dubey JP. 2008. The history of Toxoplasma gondii—the first 100 years. J. Eukaryot. Microbiol. 55: 467– 475 [DOI] [PubMed] [Google Scholar]
- 5. Montoya JG. 2002. Laboratory diagnosis of Toxoplasma gondii infection and toxoplasmosis. J. Infect. Dis. 185(Suppl 1):S73–S82 [DOI] [PubMed] [Google Scholar]
- 6. Dannemann BR, Vaughan WC, Thullies P, Remington JS. 1990. Differential agglutination test for diagnosis of recently acquired infection with Toxoplasma gondii. J. Clin. Microbiol. 28: 1928– 1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, Brojanac S, Chovan LE, Nowlan SF, Pinon JM. 2000. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J. Clin. Microbiol. 38: 1144– 1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gamble HR, Andrews CD, Dubey JP, Webert DW, Parmley SF. 2000. Use of recombinant antigens for detection of Toxoplasma gondii infection in swine. J. Parasitol. 86: 459– 462 [DOI] [PubMed] [Google Scholar]
- 9. Gamble HR, Brady RC, Dubey JP. 1999. Prevalence of Toxoplasma gondii infection in domestic pigs in the New England states. Vet. Parasitol. 82: 129– 136 [DOI] [PubMed] [Google Scholar]
- 10. Huang X, Xuan X, Hirata H, Yokoyama N, Xu L, Suzuki N, Igarashi I. 2004. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 42: 351– 353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajerison M, Dartevelle S, Ralafiarisoa LA, Bitam I, Dinh TN, Andrianaivoarimanana V, Nato F, Rahalison L. 2009. Development and evaluation of two simple, rapid immunochromatographic tests for the detection of Yersinia pestis antibodies in humans and reservoirs. PLoS Negl. Trop. Dis. 3: e421 doi:10.1371/journal.pntd.0000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Béla SR, Oliveira Silva DA, JPCunha-Júnior Pirovani CP, Chaves-Borges FA, Reis de Carvalho F, Carrijo de Oliveira T, Mineo JR. 2008. Use of SAG2A recombinant Toxoplasma gondii surface antigen as a diagnostic marker for human acute toxoplasmosis: analysis of titers and avidity of IgG and IgG1 antibodies. Diagn. Microbiol. Infect. Dis. 62:245– 254 [DOI] [PubMed] [Google Scholar]
- 13. Nam HW. 2009. GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane. Korean J. Parasitol. 47(Suppl):S29–S37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs D, Dubremetz J-F, Loyens A, Bosman F, Saman E. 1998. Identification and heterologous expression of a new dense granule protein (GRA7) from Toxoplasma gondii. Mol. Biochem. Parasitol. 91: 237– 249 [DOI] [PubMed] [Google Scholar]
- 15. Bonhomme A, Maine GT, Beorchia A, Burlet H, Aubert D, Villena I, Hunt J, Chovan L, Howard L, Brojanac S, Sheu M, Tyner J, Pluot M, Pinon JM. 1998. Quantitative immunolocalization of a P29 protein (GRA7), a new antigen of Toxoplasma gondii. J. Histochem. Cytochem. 46: 1411– 1422 [DOI] [PubMed] [Google Scholar]
- 16. Beghetto E, Spadoni A, Bruno L, Buffolano W, Gargano N. 2006. Chimeric antigens of Toxoplasma gondii: toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 44: 2133– 2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs D, Vercammen M, Saman E. 1999. Evaluation of recombinant dense granule antigen 7 (GRA7) of Toxoplasma gondii for detection of immunoglobulin G antibodies and analysis of a major antigenic domain. Clin. Diagn. Lab. Immunol. 6: 24– 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfrepper KI, Enders G, Gohl M, Krczal D, Hlobil H, Wassenberg D, Soutschek E. 2005. Seroreactivity to and avidity for recombinant antigens in toxoplasmosis. Clin. Diagn. Lab. Immunol. 12: 977– 982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rome ME, Beck JR, Turetzky JM, Webster P, Bradley PJ. 2008. Intervacuolar transport and unique topology of GRA14, a novel dense granule protein in Toxoplasma gondii. Infect. Immun. 76: 4865– 4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang X, Xuan X, Kimbita EN, Battur B, Miyazawa T, Fukumoto S, Mishima M, Makala LH, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Mikami T, Igarashi I. 2002. Development and evaluation of an enzyme-linked immunosorbent assay with recombinant SAG2 for diagnosis of Toxoplasma gondii infection in cats. J. Parasitol. 88: 804– 807 [DOI] [PubMed] [Google Scholar]
- 21. Nigro M, Gutierrez A, Hoffer AM, Clemente M, Kaufer F, Carral L, Martin V, Guarnera EA, Angel SO. 2003. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47: 609– 613 [DOI] [PubMed] [Google Scholar]
- 22. Pietkiewicz H, Hiszczynska-Sawicka E, Kur J, Petersen E, Nielsen HV, Stankiewicz M, Andrzejewska I, Myjak P. 2004. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J. Clin. Microbiol. 42: 1779– 1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer H-G, Stachelhaus S, Sahm M, Meyer HE, Reichman G. 1998. GRA7, an excretory 29 kDa Toxoplasma gondii dense granule antigen released by infected host cells. Mol. Biochem. Parasitol. 91: 251– 262 [DOI] [PubMed] [Google Scholar]
- 24. Neudeck A, Stachelhaus S, Nischik N, Striepen B, Reichmann G, Fischer HG. 2002. Expression variance, biochemical and immunological properties of Toxoplasma gondii dense granule protein GRA7. Microbes Infect. 4: 581– 590 [DOI] [PubMed] [Google Scholar]
- 25. Kotresha D, Noordin R. 2010. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 118: 529– 542 [DOI] [PubMed] [Google Scholar]
- 26. Li S, Maine G, Suzuki Y, Araujo FG, Galvan G, Remington JS, Parmley S. 2000. Serodiagnosis of recently acquired Toxoplasma gondii infection with a recombinant antigen. J. Clin. Microbiol. 38: 179– 184 [DOI] [PMC free article] [PubMed] [Google Scholar]


