Abstract
Cryopreservation of peripheral blood mononuclear cells (PBMC) allows assays of cellular function and phenotype to be performed in batches at a later time on PBMC at a central laboratory to minimize assay variability. The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of the natural and treated history of human immunodeficiency virus (HIV) infection that stores cryopreserved PBMC from participants two times a year at four study sites. In order to ensure consistent recovery of viable PBMC after cryopreservation, a quality assessment program was implemented and conducted in the MACS over a 6-year period. Every 4 months, recently cryopreserved PBMC from HIV-1-infected and HIV-1-uninfected participants at each MACS site were thawed and evaluated. The median recoveries of viable PBMC for HIV-1-infected and -uninfected participants were 80% and 83%, respectively. Thawed PBMC from both HIV-1-infected and -uninfected participants mounted a strong proliferative response to phytohemagglutinin, with median stimulation indices of 84 and 120, respectively. Expression of the lymphocyte surface markers CD3, CD4, and CD8 by thawed PBMC was virtually identical to what was observed on cells measured in real time using whole blood from the same participants. Furthermore, despite overall excellent performance of the four participating laboratories, problems were identified that intermittently compromised the quality of cryopreserved PBMC, which could be corrected and monitored for improvement over time. Ongoing quality assessment helps laboratories improve protocols and performance on a real-time basis to ensure optimal cryopreservation of PBMC for future studies.
INTRODUCTION
Repositories of biological specimens from clinical trials and cohort studies are useful for investigating the pathogenesis and treatment of various diseases. In addition to the storage of biological fluids, viable peripheral blood mononuclear cells (PBMC) are often cryopreserved for future assays of immunological function. PBMC that have been cryopreserved at different time points can then be analyzed together, thereby minimizing assay variability. Cryopreserved PBMC from multiple centers can also be shipped to a single testing laboratory, eliminating potential interlaboratory assay variability. As new technologies and novel biomarkers emerge, they can be applied to cryopreserved PBMC for additional insights into immune mechanisms of disease progression and therapeutic interventions. Studies using banked specimens have helped to elucidate the pathogenesis of HIV infection (1, 2).
The Multicenter AIDS Cohort Study (MACS) is an ongoing study of the natural and treated history of HIV-1 infection in men who have sex with men that was started in 1984 and maintains centers in Baltimore, MD, Chicago, IL, Pittsburgh, PA, and Los Angeles, CA (3, 4). MACS centers isolate and cryopreserve PBMC twice a year from study participants. In a retrospective study, cryopreserved PBMC from the MACS repository yielded a median recovery of 50% for viable PBMC after thawing, with no significant change in this percentage over a 12-year period (5). To ensure the adequate recovery of functionally viable cells from each vial, the MACS established a prospective quality assessment (QA) program in July 2004 to evaluate the PBMC cryopreserved in the four MACS laboratories. In this program, randomly selected vials of recently cryopreserved PBMC from all MACS laboratories are evaluated three times a year, not only for viable cell recovery but also for phenotype and function of the recovered cells. Lymphocyte surface markers on recovered cells are compared to those measured in real time on fresh blood samples obtained during participant study visits. Here, we report the results from this QA program over a 6-year period.
MATERIALS AND METHODS
Isolation of PBMC.
Seventy to 90 ml of whole blood was collected in sodium heparin-containing Vacutainer tubes (Becton, Dickinson Inc., Rutherford, NJ) from MACS participants at each semiannual study visit. The blood was centrifuged and the plasma carefully removed. Whenever possible, the blood samples were processed within 8 h of collection in order to preserve optimal functional activity (6). The leukocyte-containing buffy coat layer above the erythrocytes was transferred to a polypropylene tube, and the volume was doubled with phosphate-buffered saline. The leukocyte suspension was overlaid onto a Ficoll-Hypaque (GE Healthcare Bio-Science Corp., Piscataway, NJ; Baltimore, Chicago, and Pittsburgh sites) or Percoll (GE Healthcare Bio-Science Corp.; Los Angeles site) density gradient and centrifuged. The PBMC at the interface of the density gradient were isolated, washed twice with phosphate-buffered saline, and resuspended in 2 ml of 100% cold, heat-inactivated fetal bovine serum (FBS). The Baltimore, Chicago, Los Angeles, and Pittsburgh sites used FBS obtained from HyClone (HyClone Laboratories, South Logan, UT), Gibco (Life Technologies, Carlsbad, CA), Sigma (Sigma-Aldrich Corporation, St. Louis, MO), and Gemini (Gemini Bio-Products, Sacramento, CA), respectively. Cell concentrations were determined using an automated particle counter (Beckman Coulter Z1 at the Baltimore and Los Angeles sites; Beckman Coulter Vi-Cell at the Pittsburgh site) or a hemacytometer (Chicago site). Calibration beads (Beckman Coulter, Brea, CA) were analyzed regularly on the automated particle counters to confirm accurate cell counting. At weekly intervals (Baltimore and Los Angeles sites), two selected blood samples were analyzed using a second instrument (Sysmex XT-1800 in Los Angeles and Vi-Cell in Baltimore) and the cell counts were required to agree within 10% as an additional check for instrument performance. After counting, the cells were resuspended in 100% FBS at a concentration of 2 × 107 cells/ml. In a few cases when low numbers of PBMC were isolated, the cells were resuspended at concentrations less than 2 × 107 cells/ml. The volume was doubled with RPMI 1640 containing 20% dimethyl sulfoxide (DMSO; Sigma-Aldrich Corporation), and 1 ml aliquots were placed into 2-ml cryogenic vials (107 PBMC/vial). The final freezing solution consisted of RPMI 1640 (Sigma-Aldrich Corporation) containing 50% FBS and 10% DMSO. Vials were immediately placed into a controlled-rate freezer (Gordinier Cryogenics Electronics, Roseville, MI) programmed to lower the temperature one degree per minute (Los Angeles and Pittsburgh sites) or an isopropanol-containing freezing container (Nalgene Mr. Frosty) at −80°C (Baltimore and Chicago sites). The isopropanol-containing freezing container is designed to produce a rate of cooling close to one degree per minute. The vials were transferred the same day (Los Angeles and Pittsburgh sites) or the next day (Baltimore and Chicago sites) into liquid nitrogen or −135°C freezers for long-term storage.
Viability and recovery of cryopreserved PBMC.
Each MACS site randomly selected one vial of cryopreserved PBMC from 2 participants (one HIV-1-uninfected participant and one HIV-1-infected participant), with concentrations of 9.3 × 106 to 10.8 × 106 cells/vial (Baltimore) or 10 × 10 6 cells/vial (Chicago, Pittsburgh, and Los Angeles) every 4 months for the QA program. The vials were stored locally for 1 to 3 months before being selected and only cryopreserved PBMC were shipped for functional and phenotypic evaluation as part of the QA program. The Baltimore, Chicago, and Pittsburgh sites shipped selected vials in dry-nitrogen shippers to the Los Angeles laboratory, where all testing was performed. Cryopreserved vials were immediately transferred to a liquid nitrogen freezer upon arrival at the Los Angeles laboratory until testing. For testing, frozen PBMC were rapidly thawed by immersion in a 37°C water bath with gentle agitation. Each vial was thawed individually until only a small amount of ice crystals remained. The contents of each vial were immediately transferred to a 14-ml round-bottom tissue culture tube, and 10 ml of RPMI 1640 containing 50% FBS was added to each tube in a dropwise fashion (∼4 min), with gentle agitation. The tubes were centrifuged at 300 × g for 10 min, and the supernatant was carefully removed. The PBMC were then washed using RPMI 1640 containing 10% FBS. Cell counts were determined using a Coulter Z1 automated particle counter (Beckman Coulter). Viability was determined by the trypan blue dye exclusion technique.
Proliferative response of cryopreserved PBMC.
Starting in 2009, the functional capacity of the cryopreserved PBMC was determined by evaluating the proliferative response to the T cell mitogen phytohemagglutinin (PHA; Sigma-Aldrich Corporation). Fourteen PBMC samples from each MACS site were evaluated. PBMC were resuspended in RPMI 1640 containing 10% human AB serum (Gemini Bio-Products) and were plated at 105 cells/well in triplicate in Corning round-bottom 96-well plates (Fisher Scientific, Tustin, CA), with and without 5 μg/ml of PHA. Plates were incubated for 4 days in a humidified incubator at 37°C with 5% CO2. Tritiated thymidine (PerkinElmer Life & Analytical Sciences, Inc., Wellesley, MA) was added to the cell cultures on day 3 (1 μCi/well), and cell cultures were harvested on day 4. Tritiated thymidine incorporation was measured in a beta scintillation counter, and mean counts per minute of triplicate wells were determined for each sample. The proliferative response was expressed as a stimulation index (counts per minute with PHA divided by counts per minute without PHA).
Phenotypic analysis.
EDTA whole-blood samples were examined for T cell markers at each MACS site within 24 h of phlebotomy, as described previously (7). Flow cytometric studies were performed using a FACSCalibur flow cytometer and CellQuest software (BD Biosciences, San Jose, CA) at the Los Angeles and Chicago sites or a FACSCanto flow cytometer with DIVA software (BD Biosciences) at the Pittsburgh and Baltimore sites. Starting in 2009, cryopreserved PBMC were evaluated for T cell markers at the Los Angeles laboratory. Briefly, 5 × 106 thawed PBMC were incubated with Tritest reagents (CD3-FITC/CD4-PE/CD45-PerCP or CD3-FITC/CD8-PE/CD45-PerCP from BD Biosciences, San Jose, CA) according to the manufacturer's instructions. Samples were analyzed immediately after staining using a FACSCalibur flow cytometer with CellQuest software. CD45bright cells with low right angle light scatter were gated, and the percentages of CD3+, CD3+ CD4+, and CD3+ CD8+ cells were determined. All thawed PBMC were evaluated for viability prior to flow cytometric studies, and all were ≥85% viable.
Statistical analysis.
The unpaired t test was used to determine the statistical significance of differences between HIV-1-uninfected and HIV-1-infected groups for PBMC recovery, proliferative response to PHA, and the surface phenotypes of fresh and cryopreserved cells. The correlations between fresh whole-blood and cryopreserved PBMC for T cell markers and between HIV-1-uninfected and -infected PBMC for proliferation to PHA were evaluated using Spearman correlation and Deming regression analyses. Statistical tests were performed using SigmaStat (Jandel Scientific, San Rafael, CA) and Analyze-It software (Leeds, United Kingdom). A P value of <0.05 was considered statistically significant.
RESULTS
Viable cell recovery.
A total of 151 vials, each nominally containing 1 × 107 cryopreserved PBMC, from all four MACS sites were analyzed for viability and viable cell recovery at the Los Angeles laboratory. Median cell viabilities were 96% (range, 86 to 98%) and 95% (range, 87 to 98%) for HIV-uninfected and -infected participants, respectively (Fig. 1), with interquartile ranges >92% for both. The median recoveries of viable PBMC were 83% (range, 55 to 147%) and 80% (range, 36 to 132%) for HIV-uninfected and -infected participants, respectively (Fig. 1), and this did not differ significantly based on HIV-1 serological status. Thirty-five (23%) of the samples yielded fewer viable PBMC than our target value of 75%, and this proportion did not differ significantly based on HIV status.
Fig 1.
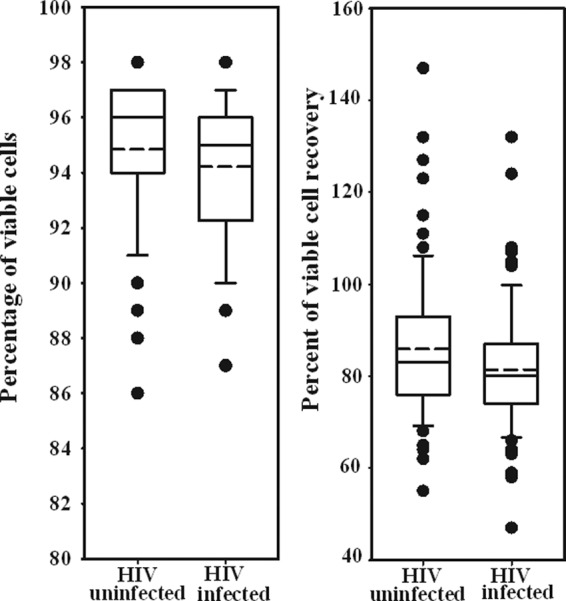
Viability and recovery of cryopreserved PBMC from 75 HIV-uninfected and 76 HIV-infected participants across the four MACS sites. The solid lines represent the median, and the dashed lines represent the mean. The box represents the 25th to 75th percentiles. The lower and upper horizontal bars represent the 10th and 90th percentiles, respectively.
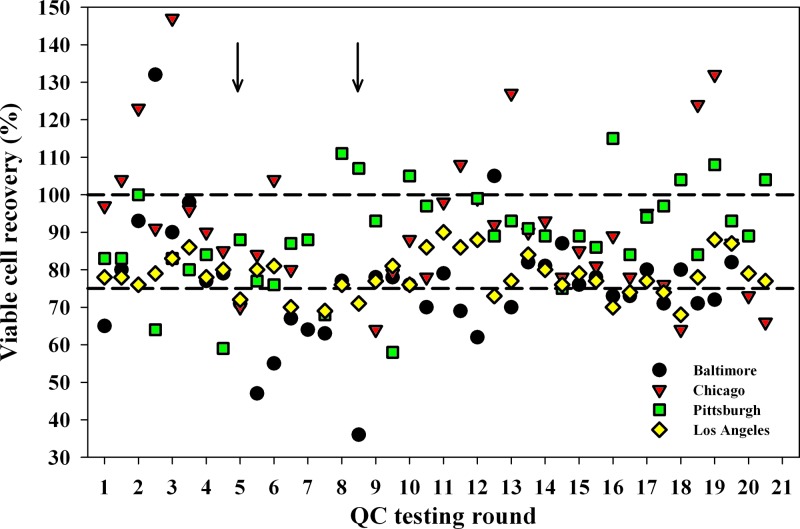
It is noteworthy that viable cell recovery differed among sites. As shown in Fig. 2, the Baltimore, Chicago, Pittsburgh, and Los Angeles sites had 17, 5, 4, and 9 samples, respectively, with viable cell recoveries lower than 75%. In addition, 17 samples yielded viable cell recoveries higher than 100%, with the majority coming from Chicago (n = 8) and Pittsburgh (n = 7) (Fig. 2). These higher-than-expected viable cell recoveries were most likely due to cell counting errors prior to cryopreservation. The use of a hemocytometer rather than an automated cell counter at the Chicago site may have contributed to this, but the Pittsburgh site was using an automated counter. After the patterns of higher cell counts at these two sites, and the lower viable cell recoveries at Baltimore and Los Angeles became apparent and were pointed out to the sites, there was noticeable improvement in results from subsequent monitoring rounds. Specifically, there were fewer PBMC samples with cell recoveries greater than 107 from the Chicago site after round 13 (Fig. 2). In addition, only 2 samples from the Los Angeles site had cell recoveries below 74% after round 13, with 1 occurring at round 16 (70%) and the other at round 18 (68%).
Fig 2.
Viable cell recovery from each vial of cryopreserved PBMC from 75 HIV-uninfected and 76 HIV-infected participants. Numbers on the x axis represent sequential times when cryopreserved PBMC from the four MACS sites were tested. The QA testing program was started in July 2004 and was performed every 4 months. The first symbol in each testing round for each of the MACS sites is the value for the HIV-uninfected participants, and the second symbol is the value for the HIV-infected participants. The dashed lines represent 75% and 100% viable cell recoveries.
Another problem was identified and fixed by virtue of the QA program. Regular monitoring of viable cell recovery identified one laboratory (Baltimore) that had low recoveries ranging from 36% to 73% per vial on 4 consecutive QA monitoring rounds for both HIV-1-uninfected and -infected participants (Fig. 2, QA rounds 5 to 8; see arrows). This suboptimal performance was traced to an automated particle counter that was out of calibration and required servicing by the manufacturer. After the instrument was repaired, there were only 2 samples from this laboratory with viable cell recoveries that were <70% (Fig. 2, rounds 11 and 12).
Proliferative response.
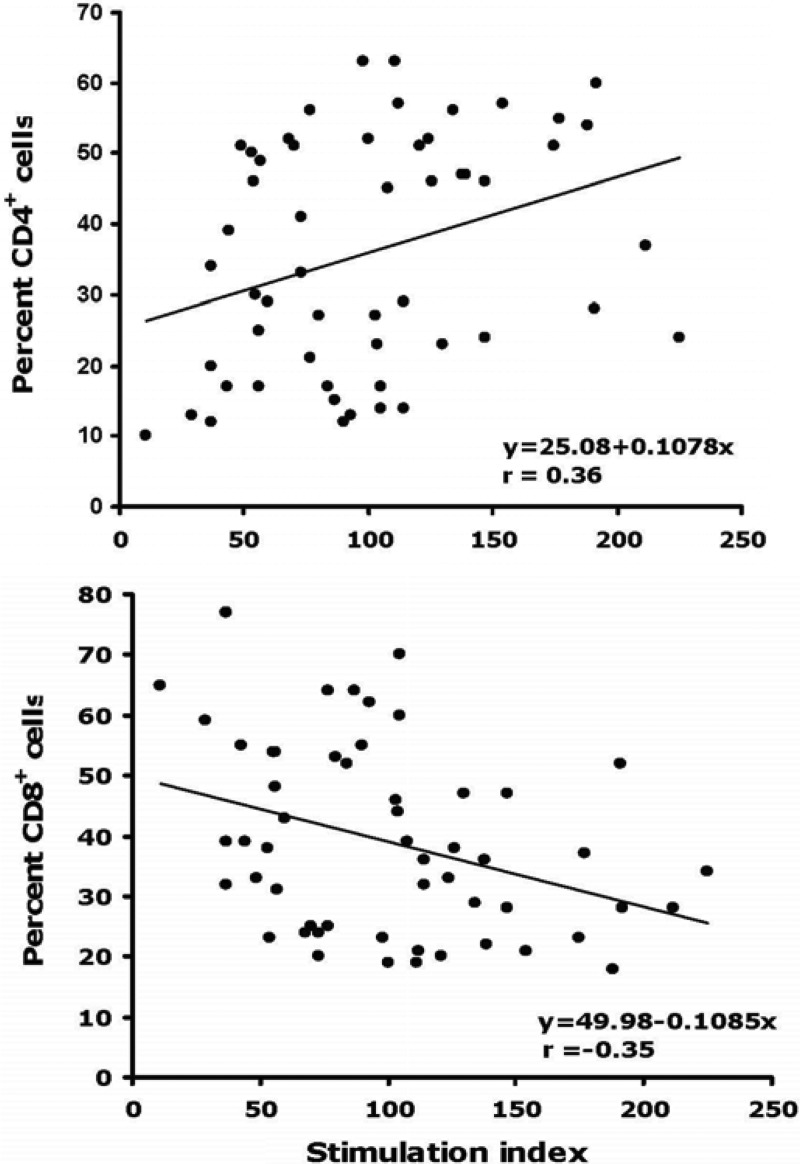
Proliferative responses to PHA were assessed in thawed PBMC from 28 HIV-uninfected and 28 HIV-infected participants (Fig. 3). PBMC from both groups proliferated vigorously in response to PHA, with median stimulation indices of 120 and 84, respectively, although the stimulation index for HIV-infected PBMC was significantly lower (P < 0.001). The proliferative responses correlated positively with the percentage of CD4+ T lymphocytes (Fig. 4; r = 0.36) and negatively with the percentage of CD8+ T cells (Fig. 4; r = −0.35). Participants with the two lowest stimulation indices (11 and 29) were HIV-1-infected and had CD4+ cell percentages of 10% and 13%, respectively. The results were consistent with previous reports that cryopreserved PBMC from HIV-1-infected participants respond less well to PHA than PBMC from HIV-1-uninfected participants and that proliferation is correlated positively and negatively with CD4+ and CD8+ T cell percentages, respectively (8).
Fig 3.
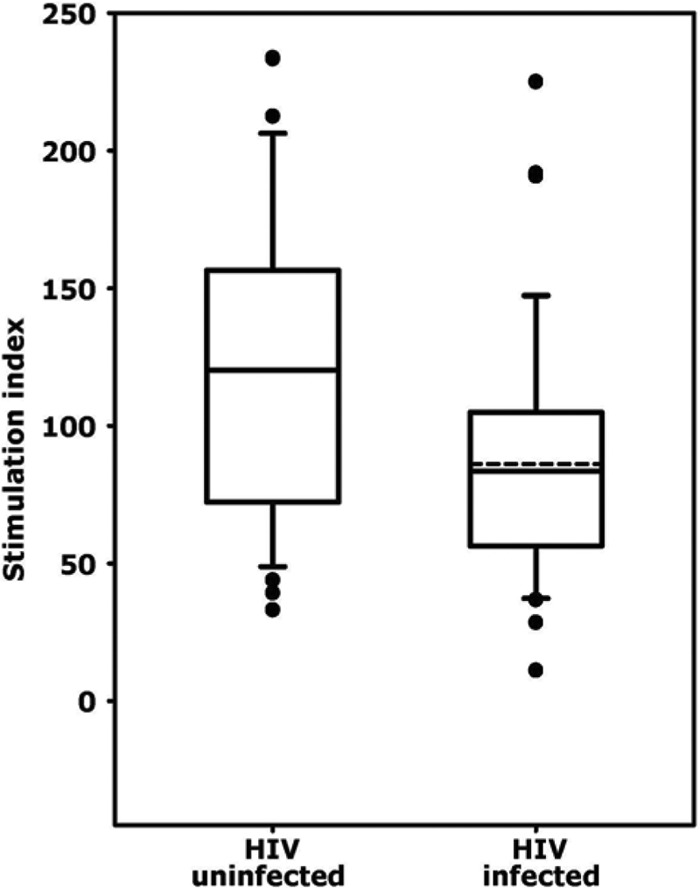
Proliferative responses of cryopreserved PBMC (n = 28 per group) to phytohemagglutinin (PHA). The stimulation index was defined as the counts per minute in the presence of PHA divided by the counts per minute in the absence of PHA. The solid lines represent medians, and the dashed lines represent the means of the distributions. For HIV-uninfected samples the median and mean were the same. The boxes represent the 25th to 75th percentiles. The lower and upper horizontal bars indicate the 10th and 90th percentiles, respectively.
Fig 4.
Correlation between the PHA-induced proliferative response of cryopreserved PBMC and the percentage of CD4+ (top panel) and CD8+ (bottom panel) T lymphocytes for 27 HIV-uninfected and 27 HIV-infected participants. Least-squares regression lines are indicated.
Phenotypic analyses.
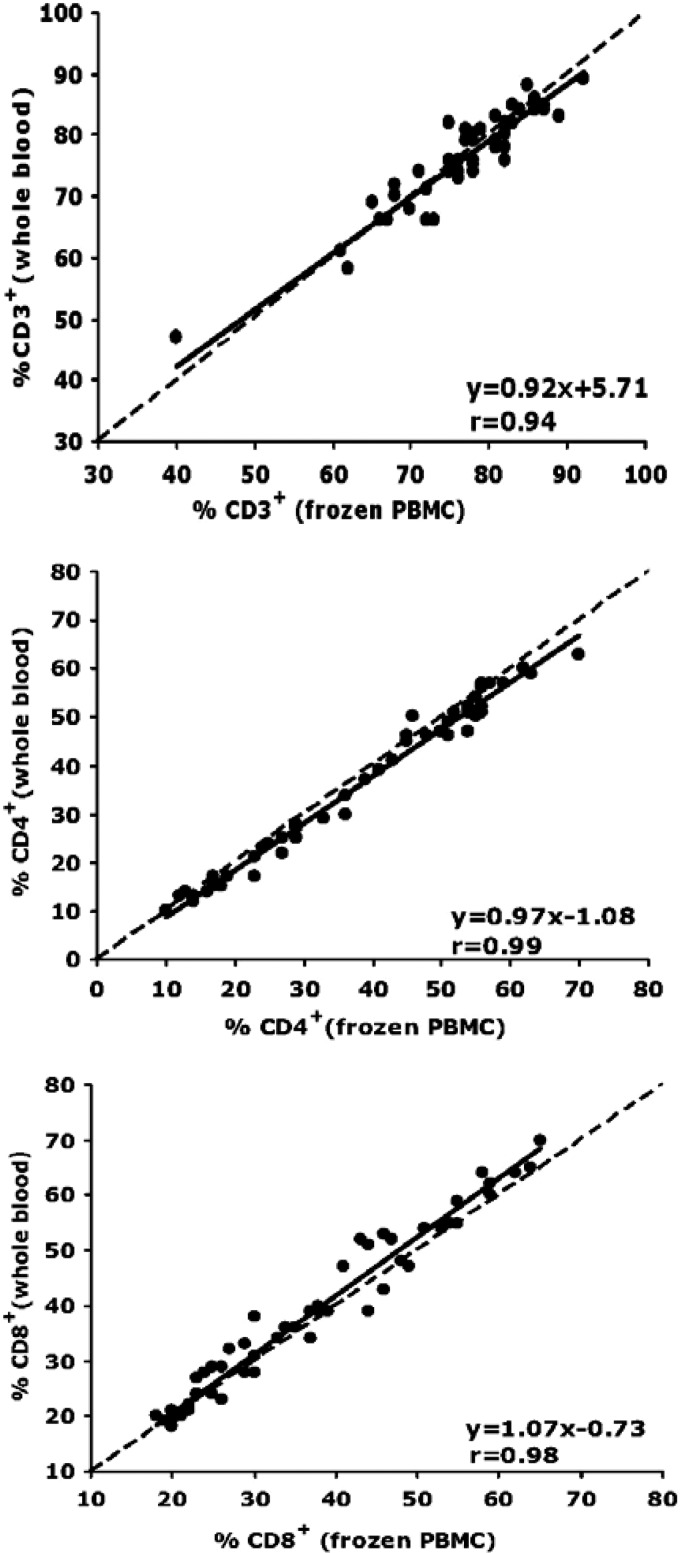
Fifty-four paired samples of fresh whole blood and cryopreserved PBMC were examined. Our a priori criterion for T cell subset analyses was >85% viability for thawed cells, and all cryopreserved samples met this criterion. As shown in Table 1, the mean and median percentages of CD3+, CD4+, and CD8+ T cells were very similar for fresh and cryopreserved T cells and did not differ significantly between the two sample types. In addition, regression analysis between results from fresh and cryopreserved PBMC for percentages of CD3+, CD4+, and CD8+ T cells showed that for all three phenotypes the regression slopes and correlation coefficients were very close to 1, indicating excellent agreement between measurements (Fig. 5).
Table 1.
T lymphocyte phenotypes in fresh whole-blood and cryopreserved PBMC
| Percentage type | CD3+ (%) |
CD3+ CD4+ (%) |
CD3+ CD8+ (%) |
|||
|---|---|---|---|---|---|---|
| Fresh | Cryopreserved | Fresh | Cryopreserved | Fresh | Cryopreserved | |
| Mean | 76 | 76 | 35 | 37 | 40 | 38 |
| Minimum | 47 | 40 | 10 | 10 | 18 | 18 |
| Maximum | 89 | 92 | 63 | 70 | 76 | 77 |
| Median | 76 | 78 | 36 | 38 | 39 | 36 |
| IQRa | 10.1 | 10.1 | 31.3 | 31.3 | 25.1 | 23.2 |
IQR is the interquartile range, the difference between the 25th and 75th percentiles.
Fig 5.
Deming regression analyses of expression of cell surface markers CD3 (top panel), CD4 (middle panel), and CD8 (bottom panel) on paired samples of fresh whole-blood and cryopreserved PBMC. A total of 54 paired blood samples (27 from HIV-uninfected and 27 from HIV-infected participants) from the 4 MACS sites were evaluated. The dashed lines represent the line of identity (y = x), and the solid lines represent the least-squares regression lines of the data.
DISCUSSION
In this study, we examined the recovery, proliferative capacity, and T cell surface phenotype of PBMC that had been cryopreserved during a 6-year period at the 4 MACS laboratories. We found excellent cell viabilities and recovery of viable cells for both HIV-uninfected and -infected participants. Proliferative responses of cryopreserved PBMC to the T cell mitogen PHA were also excellent, with all samples having stimulation indices >30 except those from two HIV-infected donors who had very low percentages of CD4+ T cells. This response was selected for analysis because cryopreservation can induce apoptosis and cell death in the first 24 to 48 h after thawing of cells (9), which might reduce net proliferation over a 4-day culture. However, we saw no evidence of this after evaluating thawed cells for viability after a 24-h culture period (data not shown), as previously reported (10). Other studies have used a stimulation index >5 to indicate T cell responsiveness to mitogens (11, 12). Given the high viability of thawed PBMC in the present study, our results are consistent with a previous report that cryopreserved PBMC with a viability of >70% proliferated well in response to mitogen (13) and that cells with >75% viability were active in functional assays including proliferation to various stimuli (14). We also found that T cell proliferation to PHA correlated positively (r = 0.36) and negatively (r = −0.35) with the percentage of CD4- and CD8-positive cells, respectively, which is in agreement with a previous study of HIV-infected children on highly active antiretroviral therapy (8). Taken together, these studies indicate that cell viability and proliferative capacity can be used to assess the quality of cryopreserved PBMC and that the MACS cryopreservation protocol provides PBMC suitable for future studies of immunological function.
Further support for the quality of the MACS cryopreservation program comes from the near identity for expression of the T cell markers CD3+, CD4+, and CD8+ in paired fresh whole-blood and cryopreserved PBMC samples. These markers have been reported not to be affected by cryopreservation (11, 14), so this result was very reassuring. In contrast, other cell surface markers such as CD45RA, CD25, CD62L, and CCR5 are altered by the process of cryopreservation (11, 15, 16, 17). Furthermore, cryopreservation of HIV-infected PBMC results in decreased gamma interferon (IFN-γ) production by T cells in response to HIV peptides, whole proteins (Gag), and cytomegalovirus (CMV) cell lysates (16). The reported decreases in IFN-γ production are more pronounced when PBMC are stored cryopreserved for more than 6 months. The proliferative responses of HIV-infected PBMC to HIV (p24), CMV, and influenza antigens have also been shown to be diminished following cryopreservation (15).
The median viable cell recovery in this study was higher than the 6.9 × 106 cells per vial obtained in a previous MACS study that examined PBMC cryopreserved for up to 12 years (5). This discrepancy may be due to the fact that in the earlier study the cells were thawed in two different laboratories, while in the present study this was done by a single experienced technologist in one laboratory. Differences in the processing of blood and cryopreservation of PBMC can ultimately influence viable cell recovery after thawing and must be carefully controlled and monitored in multicenter studies by using standardized protocols that are rigidly followed by staff working in different laboratories.
Despite the overall good quality of the cryopreserved PBMC in this study, there were problems identified by the QA program that resulted in better performance of the cryopreservation program toward the end of the study period. In one case, a specific intervention was carried out, namely, repair of a particle counter, which likely accounted for the improved cell recoveries from one of the MACS sites. In another case where cell counts were probably underestimated, resulting in greater than 107 cells being placed in each vial, no specific intervention was carried out other than conveying this information to the sites. However, improved performance by the Chicago site was observed with only 2 vials containing greater than 100% viable cell recovery toward the end of the study. Likewise, after discussions with 2 of the sites regarding low viable cell recoveries, there was noticeable improvement with only 2 samples from one of the sites yielding viable cell recoveries lower than 74% after QA round 13. These improvements were probably due to more careful and attentive adherence to established protocols by better-trained and better-supervised laboratory staff. This is another benefit of having a QA program in place.
In this study, we monitored mitogen responsiveness as an indicator of cryopreserved cell function. We chose not to monitor antigen-specific proliferative responses because cryopreservation of HIV-infected PBMC is known to reduce proliferative responses to the HIV-p24 antigen and recall antigens such as CMV and influenza when cryopreserved under favorable conditions (15). There are certainly other measures of cellular function that could be used to monitor the cryopreservation and thawing process, such as cytokine production, in response to specific antigens and/or mitogens. However, cryopreservation has been reported to have a detrimental effect on IFN-γ production by T cells (based on intracellular cytokine staining) in response to HIV peptides, HIV p55 protein, and CMV lysates (16). Thus, it is important to select a marker(s) of cellular function that remains intact when PBMC are optimally cryopreserved and evaluate the costs and cell numbers required to perform the assays and balance this against the benefits received from an expanded QA program. Mitogen responsiveness appears to represent a good balance between costs and benefits, in that it is relatively inexpensive and provides an indicator of cellular function that has been shown to correlate with other markers of cellular integrity such as viable cell recovery (14, 18).
This study has shown the value of a QA program for monitoring the quality of PBMC that are cryopreserved for long periods of time by multiple laboratories in support of multicenter studies. In addition, we have illustrated how the frequent monitoring of cryopreserved PBMC and the dissemination of monitoring results can result in improvements in the overall performance of a cryopreservation program. In addition to a rigorous monitoring program, it is also important that laboratories cryopreserving PBMC validate their processing, cell counting, and freezing procedures in order to guarantee the quality of PBMC for future studies. Blood samples should be processed shortly after collection, preferably within 8 h (6). Automated cell counters should be used whenever possible, and they should be evaluated for accuracy and precision on a regular basis. In addition, laboratory staff should be trained to meticulously follow detailed protocols and should be assessed for competency on a regular basis. When multiple laboratories are participating in multicenter studies it would be beneficial to distribute aliquots of cryopreserved PBMC from a single donor (processed and frozen at the same time) to all of the sites for evaluation. The viable cell recovery across sites could then be used to monitor the competency of testing personnel and the accuracy of the cell counting procedures. A viable cell recovery that differs significantly from the group mean would indicate a lab-specific problem that needs to be resolved. The MACS is currently considering implementing this additional quality control check as part of its assessment program.
Although other investigators have monitored viable cell recovery and functional activity of cryopreserved PBMC for short periods of time (13, 19), our QA monitoring program extended over a 6-year period. When PBMC are properly cryopreserved and routinely monitored for quality they become an attractive alternative to freshly isolated PBMC for studies of cellular activities that are not inherently altered by cryopreservation.
ACKNOWLEDGMENTS
We express our appreciation to all participants and staff of the MACS. We are also grateful for the technical assistance of Pat Otto, Stacey M. Cayetano, Alycia Knauer, Lance Hultin, Patricia Hultin, Shaun Hsueh, and Yegermal Asnake.
The data presented in this study were collected by the MACS with centers (principal investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick and Lisa P. Jacobson), Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair and Steven M. Wolinsky), University of California, Los Angeles (Roger Detels and Otoniel Martinez-Maza), and University of Pittsburgh (Charles R. Rinaldo and Lawrence Kingsley).
The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute (grants UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041).
The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Chattopadhyay PK, Douek DC, Gange SJ, Chadwick KR, Hellerstein M, Margolick JB. 2006. Longitudinal assessment of de novo T cell production in relation to HIV-associated T cell homeostasis failure. AIDS Res. Hum. Retroviruses 6: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rinaldo CR, Jr, Beltz LA, Huang XL, Gupta P, Fan Z, Torpey DJ., III 1995. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res. Hum. Retroviruses 11: 481–489 [DOI] [PubMed] [Google Scholar]
- 3. Chmiel JS, Detels R, Kaslow RA, Van Raden M, Kingsley LA, Brookmeyer R. 1987. Factors associated with prevalent human immunodeficiency virus (HIV) infection in the Multicenter AIDS Cohort Study. Am. J. Epidemiol. 126: 568–577 [DOI] [PubMed] [Google Scholar]
- 4. Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr 1987. The Multicenter AIDS Cohort Study: rationale, organization and selected characteristics of the participants. Am. J. Epidemiol. 126: 310–318 [DOI] [PubMed] [Google Scholar]
- 5. Kleeberger CA, Lyles RH, Margolick JB, Rinaldo CR, Phair JP, Giorgi JV. 1999. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin. Diagn. Lab. Immunol. 6: 14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, McElrath MJ. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hultin LE, Chow M, Jamieson BD, O'Gorman MR, Menendez FA, Borowski L, Denny TN, Margolick JB. 2010. Comparison of interlaboratory variation in absolute T-cell counts by single-platform and optimized dual-platform methods. Cytometry B Clin. Cytom. 78: 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Resino S, Abad ML, Navarro J, Bellón JM, Sánchez-Ramón S, Angeles Muñoz-Fernández M. 2003. Stimulated proliferative responses in vertically HIV-infected children on HAART correlate with clinical and immunological markers. Clin. Exp. Immunol. 131: 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baust JM, Van Buskirk R, Baust JG. 1998. Cryopreservation outcome is enhanced by intracellular-type medium and inhibition of apoptosis. Cryobiology 37: 410–411 [Google Scholar]
- 10. Riccio EKP, Neves I, Jr, Banic DM, Corte-Real S, das Gracas Alcerim M, Morgado M, Daniel-Ribeiro CT, Ferreira-da-Cruz MDF. 2002. Cryopreservation of peripheral blood mononuclear cells does not significantly affect the levels of spontaneous apoptosis after 24-h culture. Cryobiology 45: 127–134 [DOI] [PubMed] [Google Scholar]
- 11. Reimann KA, Chernoff M, Wilkening CL, Nickerson CE, Landay AL. 2000. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. Clin. Diagn. Lab. Immunol. 7: 352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinberg A, Betensky RA, Zhang L, Graham R. 1998. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin. Diagn. Lab. Immunol. 5: 804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinberg A, Zhang L, Brown D, Erice A, Polsky B, Hirsch MS, Owens S, Lamb K. 2000. Viability and functional activity of cryopreserved mononuclear cells. Clin. Diagn. Lab. Immunol. 7: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberg A, Song LY, Wilkening C, Sevin A, Blais B, Louzao R, Stein D, Defechereux P, Durand D, Riedel E, Raftery N, Jesser R, Brown B, Keller MF, Dickover R, McFarland E, Fenton T, Pediatric ACTG Cryopreservation Working Group 2009. Optimization and limitations of use of cryopreserved peripheral blood mononuclear cells for functional and phenotypic T-cell characterization. Clin. Vaccine Immunol. 16: 1176–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, Montroni M. 2003. Effects of cryopreservation on lymphocyte immunophenotype and function. J. Immunol. Methods 278: 145–155 [DOI] [PubMed] [Google Scholar]
- 16. Owen RE, Sinclair E, Emu B, Heitman JW, Hirschkorn DF, Epling CL, Tan QX, Custer B, Harris JM, Jacobson MA, McCune JM, Martin JN, Hecht FM, Deeks SG, Norris PJ. 2007. Loss of T cell responses following long-term cryopreservation. J. Immunol. Methods 326: 93–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seale AC, de Jong BC, Zaidi I, Duvall M, Whittle W, Rowland-Jones S, Jaye A. 2008. Effects of cryopreservation on CD4+ CD25+ T cell of HIV-1 infected individuals. J. Clin. Lab. Anal. 22: 153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith JG, Joseph HR, Green T, Field JA, Wooters M, Kaufhold RM, Antonello J, Caulfield MJ. 2007. Establishing acceptance criteria for cell-medicated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin. Vaccine Immunol. 14: 527–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinberg A, Louzao R, Mussi-Pinhata MM, Cruz MLS, Pinto JA, Huff MF, de Castro AC, Sucupira MC, Denny TN. 2007. Quality assurance program for peripheral blood mononuclear cell cryopreservation. Clin. Vaccine Immunol. 14: 1242–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]