Abstract
Group 8 mite allergens exhibit sequence homology to glutathione S-transferases (GSTs), such as that from Dermatophagoides pteronyssinus (Der p 8). GSTs have been identified as important allergens in studies of allergens from house dust mites, cockroaches, and fungi. Our objective was to purify the native group 8 allergen from Tyrophagus putrescentiae (nTyr p 8) and generate recombinant Tyr p 8 (rTyr p 8) for immunological characterization. The allergenicity was determined by antibody recognition, IgE inhibition, and triggering of the basophil-sensitized release of histamine, using T. putrescentiae hypersensitivity sera. The results showed that the mRNA transcript of nTyr p 8 is 657 bp long, contains 218 amino acids with a molecular mass of 26 kDa, and exhibits 83% sequence homology to Der p 8. Serum samples from the allergic patients with an IgE-positive response to T. putrescentiae were analyzed to determine their IgE response to rTyr p 8. The results showed that the sera of 48 subjects (45.3%) had specific IgE against rTyr p 8. However, sera of only 19 subjects (17.9%) had specific IgE against rTyr p 8 after D. pteronyssinus absorption. Histamine release was observed from T. putrescentiae-allergic subjects in the presence of rTyr p 8. Both the nTyr p 8 and T. putrescentiae crude extract had been demonstrated to possess GST enzymatic activity. Although the specific binding of serum IgE to rTyr p 8 was only 17.9%, which indicates that rTyr p 8 was not a major allergen, the positive response to rTyr p 8 was due to the cross-reactivity with Der p 8. The group 8 mite allergen might be of use in the design of a suitable allergen for diagnosis and for the development of novel immunotherapies.
INTRODUCTION
Storage mites have been increasingly recognized as a risk factor for allergen sensitization and the development of allergic diseases (1). They can cause allergic respiratory symptoms after occupational exposure on farms and in grain elevator stores, and researchers recently investigated the allergenicity of storage mites in domestic environments. The storage mite, Tyrophagus putrescentiae, has been described as infesting various stored foodstuffs that have high lipid and protein content, such as ham, cheese, kippers, dried fruits, and grain. T. putrescentiae might cause allergic respiratory diseases in urban areas and can pose an occupational hazard in rural areas (2, 3). Our previous study demonstrated, with an enzyme-linked immunosorbent assay (ELISA), that around 38% of patients suffering from allergic symptoms had sera containing IgE against T. putrescentiae crude extract. Characterization of storage mite allergens is important for the development of diagnostic and therapeutic agents against mite-associated allergic disorders. At least 20 IgE-binding allergenic components of T. putrescentiae have been discovered (4). So far, only six allergens have been cloned and characterized: Tyr p 2, Tyr p 3, Tyr p 10, Tyr p 13, α-tubulin, and troponin C. Among these allergens, the major ones have been identified as Tyr p 2 and Tyr p 3 (5, 6). However, many allergenic components of T. putrescentiae have not been clarified to date. Identification of more allergens and characterization of their biochemical functions or IgE-binding activities will allow us to better understand the allergenicity of T. putrescentiae. Furthermore, evaluation of the cross-reactivity between T. putrescentiae and the house dust mite Dermatophagoides pteronyssinus might shed light on the effects of certain components of cross-reacting or species-specific allergens.
Glutathione S-transferases (GSTs) comprise a ubiquitous superfamily of enzymes which catalyze nucleophilic attack by reducing glutathione (GSH) in nonpolar compounds that contain an electrophilic carbon, nitrogen, or sulfur atom. They play a physiological role in detoxification of xenobiotics (drugs, insecticides, and herbicides) and endogenous compounds in almost all living organisms (7). GST from the house dust mite (D. pteronyssinus) has been cloned, sequenced, and identified as a major allergen (8). Native and recombinant GSTs have been purified from D. pteronyssinus and are known as Der p 8. These allergens have been found at a high frequency among allergic subjects, but their titers of IgE reactivity were low (9). The mite GST has been demonstrated to be a cross-reactive allergen in D. pteronyssinus and cockroach GST, which suggests that it is a panallergen (9). GST has also been shown as an allergen in Blatella germanica (Bla g 5), arthropods, and fungi (10, 11, 12). However, the presence of GST in T. putrescentiae has not been identified. Thus, in the present study, we attempted to subclone, generate, and purify the group 8 allergen from T. putrescentiae to determine whether its sensitization is due to cosensitization or cross-reactivity and to distinguish specific IgE responses between D. pteronyssinus and T. putrescentiae.
Recombinant allergens have shown a potential for diagnosis, immunotherapy, synthesis of hypoallergenic variants, and epitope mapping (13, 14). However, recombinant proteins for use in clinical practice should have physiochemical, biological, and immunological properties similar to those of native proteins. Therefore, in the present study we purified, cloned, and sequenced an allergenic protein homologous to GST from T. putrescentiae. Furthermore, recombinant Tyr p 8 (rTyr p 8) was generated for biochemical and immunological characterization.
MATERIALS AND METHODS
Preparation of mite extracts.
Two kinds of mite (D. pteronyssinus and T. putrescentiae) crude extracts were used in this study. The crude extracts of D. pteronyssinus were prepared from lyophilized whole-mite bodies purchased from Allergon (Angelholm, Sweden). The T. putrescentiae mites were cultured in our laboratory in medium consisting of yeast extract and mouse chow, and then the mite bodies were separated from the medium. The mites were separated from the medium by gently stirring the medium with a glass rod following overnight culture. The mites that migrated to the cover were collected. These collections included a large proportion of mite bodies and were almost free of medium. Frozen T. putrescentiae mites were homogenized and extracted with phosphate-buffered saline (PBS) (pH 7.2). The protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA) as a standard.
Patient sera.
Serum samples were obtained from the patients who visited the clinic in the Division of Allergy, Immunology and Rheumatology in Taichung Veterans General Hospital (TCVGH), Taichung, Taiwan. These patients were diagnosed with allergy if they had a history of asthma, rhinitis, atopic dermatitis, and/or eczema and a positive test for T. putrescentiae with ELISA screening. A total of 106 allergic patients were enrolled, and 10 nonallergic individual serum samples were obtained. The collection of blood samples from patients and volunteers was approved by the institutional review board of TCVGH (TCVGH IRB no. C07126).
IgE ELISA reactivity of T. putrescentiae.
IgE antibodies in the serum samples from the allergic patients were detected by ELISA as described previously (6, 15). Our preliminary test showed that the optimal level of absorption can be obtained by coating a polyvinyl microtiter plate (Costar, Cambridge, MA) with 10 μg/ml T. putrescentiae crude extract in 0.1 M NaHCO3 (pH 8.4) for 4 h at room temperature (RT). After being blocked with 1% skim milk, the extract was then incubated for 1 h at RT. The wells were washed with PBS containing 0.05% Tween 20 (PBST) (Southern Biotech Association, Birmingham, AL). One hundred microliters of diluted human sera was added for overnight incubation at 4°C. The plates were washed and incubated with horseradish peroxidase (HRP)-conjugated goat anti-human IgE (1:1,000) for 1 h at RT. After being washed with PBST three times, the bound enzyme substrates were detected with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Invitrogen, Carlsbad, CA). The reactions were stopped with 50 μl 1 N H2SO4 after 15 min, and the optical density (OD) was measured at 450 nm in a multiscan spectrophotometer (Sunrise; Tecan, Switzerland).
Immunoblot and inhibition analyses.
For analysis of the components recognized by IgE antibodies, protein components of mite extracts were separated by SDS-PAGE and analyzed by immunoblotting T. putrescentiae allergenic components as described previously. T. putrescentiae crude extract (300 μg) was separated by SDS-PAGE (12.5%) and transferred onto a polyvinylidene difluoride (PVDF) membrane. After being blocked with 3% skim milk in PBST, the blots were incubated with diluted serum samples (5× dilution in PBST) and 1% skim milk for 16 h at 4°C. After being washed with PBST, the blots were incubated with alkaline phosphatase-conjugated monoclonal anti-human IgE antibodies (Pharmingen, CA) for 2 h at room temperature. The blots were developed in a substrate solution of carbonate buffer (0.1 mol/liter NaHCO3, 1 mol/liter MgCl2 [pH 9.8]) containing 0.35 mol/liter 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt (BCIP) and 0.37 mol/liter p-nitroblue tetrazolium chloride (NBT) for about 30 min. After washing the membrane with H2O, the deposition of antigen-antibody binding was recorded photographically. In the immunoblot inhibition, each serum sample (100 μl) was preincubated with 50 μl of D. pteronyssinus crude extracts (50 μg/ml) and left overnight at 4°C. After the preabsorption, the subsequent procedures for determining IgE by immunoblotting were the same as those described previously.
Basophil histamine release assay.
Washed polymorphonuclear cells were obtained from the venous blood of nonatopic donors using Polymorphprep solution (Axis-Shield PoC AS, Oslo, Norway). The cells were resuspended in RPMI 1640 (Invitrogen, Carlsbad, CA) by adjusting to 2 × 106 cells/ml using trypan blue assessment (0.4%) (Invitrogen). Passive sensitization of basophils with specific IgE from sera from allergic patients (P1 to P10) and healthy individuals (N1 to N4) was performed. Following sensitization, cells were incubated with serial concentrations (0.01 to 100 μg/ml) of GST-T. putrescentiae for 30 min at 37°C, and the supernatant was collected and reacted with o-phthalaldehyde (5 mM) for 7 min. The reaction was stopped by adding H2SO4 (0.04 M). The histamine released into the supernatant was measured by a fluorescent spectrophotometer (F4500; Hitachi, Tokyo, Japan).
Purification of native Tyr p 8 and measurement of enzymatic activity.
A total of 50 mg dry T. putrescentiae mites was ground using a homogenizer in 10 mM Tris, 300 mM NaCl buffer in the presence of 1 mM phenylmethylsulfonyl fluoride (PMSF) for 30 min on ice. The crude extract was obtained after centrifugation at 14,000 × g for 30 min. The native Tyr p 8 (nTyr p 8) was affinity purified on a glutathione (GSH)-Sepharose 4B column (GE Healthcare). The column of unbound proteins was washed with PBS until the eluate reached an absorbance (optical density at 280 nm [OD280]) of <0.01. The nTyr p 8 from T. putrescentiae was eluted with 10 mM reduced GSH (Sigma, St. Louis, MO). The fraction containing GST activity was pooled, dialyzed, and lyophilized, and the protein content was estimated by the modified Lowry's method (16), followed by analysis by SDS-PAGE and Western blotting with anti-GST peroxidase (1:2,500 [vol/vol]) (Sigma, St. Louis, MO). The enzymatic activity of GST can catalyze the conjugation of GSH with 1-chloro-2,4-dinitrobenzene (CDNB) as CDNB-GSH. Therefore, the enzymatic activity of GST was determined using CDNB as the substrate. The absorbance was measured at 340 nm, and the activity was calculated using an extinction coefficient of 9.6 mM−1 (9).
Edman degradation.
Because the sequence of T. putrescentiae GST was not known, Edman degradation was used to analyze the N-terminal amino acid of T. putrescentiae GST. The purification and Edman degradation of GSTs from other species were described previously (9–11). In brief, the amino acid was determined by Edman degradation using a pulsed-liquid protein sequencer (Procise 494A; Applied Biosystems) equipped with a special C18 column for phenylthiohydantoin (PTH) amino acid analysis (Spheri-5 PTH, 5 μm, 220 by 2.1 mm; PerkinElmer). This process was repeated iteratively for each subsequent terminal amino acid of the protein. With the known sequence of Tyr p 8, we designed the degenerate primer, and then the degenerate PCR was used to produce the cDNA of GST-T. putrescentiae. The cDNA was sequenced and used to express GST-T. putrescentiae.
Determination of GST allergen from T. putrescentiae.
Because of the cross-reactivity of D. pteronyssinus and T. putrescentiae, the species-specific components of T. putrescentiae were identified. In order to clarify the components, two-dimensional gel electrophoresis (2DGE) was used, coupled with liquid chromatography-mass spectrometry (LC-MS). The isoelectric focusing (IEF) was performed with a ReadyStrip IPG strip (pH 3 to 10; linear, 7 cm) using a Protean IEF cell (Bio-Rad) according to the manufacturer's protocol. T. putrescentiae crude extract was mixed in 125 liters rehydration buffer (7 M urea, 2 M thiourea, 2% CHAPS [3-([3-cholamidopropyl]-dimethylammonio)-1-propanesulfonate], 65 mM dithiothreitol [DTT], 8% ampholytes, 1% Zwittergent 3-10, 0.01% bromophenol blue) and rehydrated at 50 V for 15 min in buffer (50 mM Tris-HCl [pH 6.8], 6 M urea, 2% SDS, 30% glycerol, 0.0001% bromophenol blue) containing 2% DTT at room temperature for 15 min, followed by the addition of equilibration buffer containing 2.5% iodoacetamide for 15 min. The strips were transferred to a 12% SDS-PAGE gel for two-dimensional electrophoresis using a PROTEAN II xi 2-D cell (Bio-Rad). After electrophoresis, the gel was stained with silver nitrate or transferred to a nitrocellulose (NC) or PVDF membrane for Western blotting. After Western blotting, the silver-stained spots were separated from the gel and stored at −20°C. These spots were sent for analysis at the Proteomics Research Core Laboratory of National Cheng Kung University (NCKU) to identify the unknown protein, followed by further analysis by LC-MS.
Expression of the recombinant protein.
The recombinant proteins of T. putrescentiae allergen components were cloned. Total RNA of mites was isolated with TRIzol reagent, and cDNA was synthesized with primer dT and Moloney murine leukemia virus (MMLV) reverse transcriptase (Gibco-BRL, New York, NY). The specific primers were designed based on sequence data deposited in the National Center for Biotechnology Information under the relevant accession numbers. PCR was carried out at 94°C for 10 s, at 55°C for 20 s, and at 72°C for 3 min for 30 cycles. The PCR products were purified and ligated into a pQE30 vector and transformed in Escherichia coli M15. Transformants were selected by kanamycin (25 g/ml) and ampicillin (100 g/ml). Expressions of recombinant allergens were determined according to the methods described in the directions for the QIAexpressionist kit (Qiagen, Hilden, Germany). The recombinant proteins were expressed as a 6×His-tagged protein using 1 mM isopropyl-β-d-thiogalactopyranoside (Promega, Madison, WI) induction. The proteins were purified by nickel-nitrilotriacetic acid (Ni-NTA) agarose metal affinity column chromatography under native conditions.
RESULTS
Purification of nTyr p 8 from T. putrescentiae and identification by Western blotting.
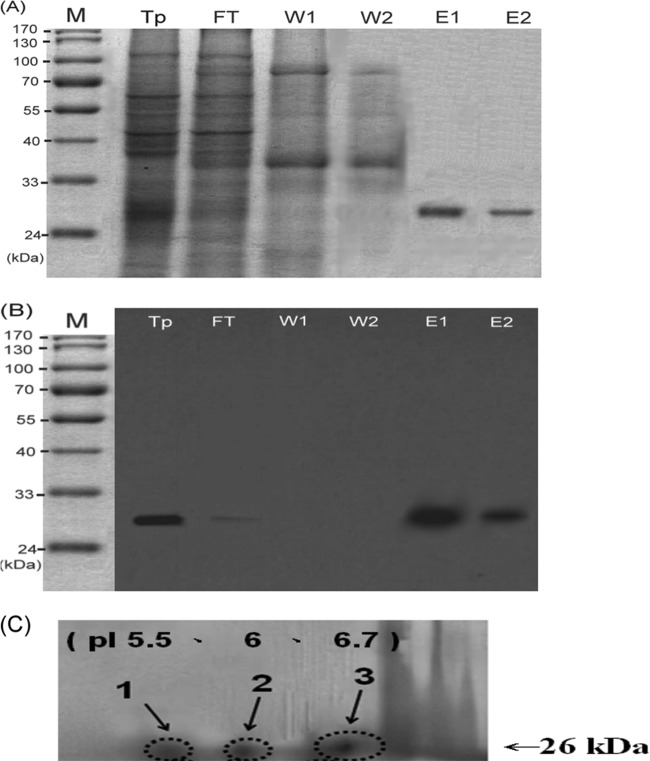
Chromatography with a GSH-Sepharose 4B affinity column was used to purify nTyr p 8 from T. putrescentiae crude extract. The protein purification profiles on SDS-PAGE showed several protein bands in T. putrescentiae crude extract (Fig. 1A). After the binding with the GSH-Sepharose 4B affinity column, the flow-through data in lanes E1 and E2 of Fig. 1 shows a band at approximately 26 kDa. When the column was washed and eluted, a protein band of 26 kDa remained. The cell lysates in the purification process were identified by Western blotting, and the results showed that the purified nTyr p 8 from T. putrescentiae crude extract can be recognized by anti-GST antibodies (Fig. 1B). The two-dimensional gel electrophoresis of native Der p 8 (nDer p 8) showed that there were three isoforms with a range of isoelectric points (pIs) from 5.5 to 6.7 (Fig. 1C).
Fig 1.
(A) SDS-PAGE analysis of T. putrescentiae GSTs. Electrophoresis was performed on a 12% gel. Lane M, molecular markers (kDa values are indicated by the scale on the left); T. putrescentiae (Tp), 20-μg crude extract of T. putrescentiae; FT, flow through of crude extract; W1, wash with 100 ml PBS; W2, wash with the second 100 ml PBS; E1 and E2, eluates with 10 mM reduced glutathione. (B) Western blot analysis of T. putrescentiae GSTs. The proteins were transferred to a membrane and blotted with mouse anti-Schistosoma japonicum GST serum. (C) Two-dimensional electrophoresis of T. putrescentiae GSTs. The first dimension is isoelectric focusing, which separates proteins according to their isoelectric point (pI); the second dimension is SDS-PAGE, according to the molecular masses. The isoelectric focusing was performed with linear pHs ranging from 3 to 10.
Purification, sequence analyses, and molecular cloning of Tyr p 8 derived from T. putrescentiae.
The N-terminal amino acid sequence of the T. putrescentiae GST was determined to be SSKPVLGYWDIRGLAQ by Edman degradation. The nTyr p 8 obtained with the GSH-Sepharose 4B affinity column was applied to perform identification with liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses. Additional sequence information was obtained from LC-MS/MS analyses of peptide generated from digestions using trypsin protease. The result showed that nTyr p 8 has a sequence of AYDPNADKLKPD (data not shown), which was homologous with glutathione S-transferase analyzed by using BLAST (National Center for Biotechnology Information).
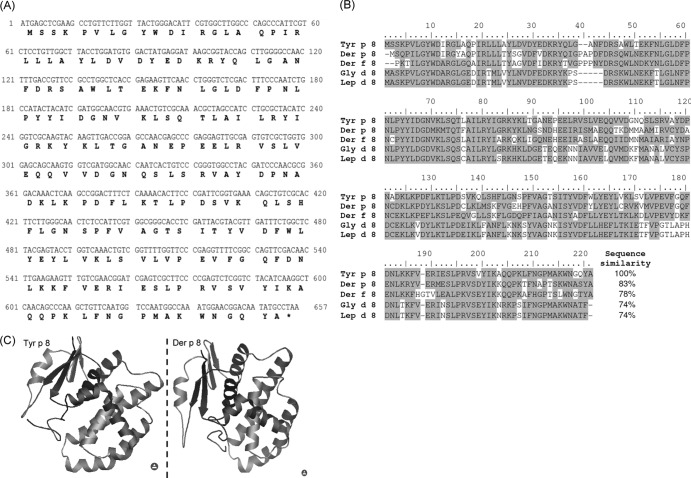
cDNA encoding the Tyr p 8 from T. putrescentiae was cloned using degenerate primers and the modified rapid amplification of cDNA ends (RACE) technique. The degenerate primers were designed by data obtained from LC-MS/MS analyses and mite GST-homologous sequences. Total RNA was isolated from live T. putrescentiae, and the amplicon of nTyr p 8 fragments was amplified by RACE PCR of cDNA which had been obtained from reverse transcription. The recovered PCR product was cloned into the plasmid vector yT&A and transformed into competent cells of Escherichia coli strain JM109. The full-length PCR product of nTyr p 8 from T. putrescentiae was nearly 650 bp, as shown in Fig. 2. The clones were selected by blue/white screening determined by restriction enzyme digestion and further sequencing of its GST-homologous sequence. The sequence of nTyr p 8 had a start codon (ATG), a stop codon (TAA), and a 3′ termination codon (AATTAAAA). The full-length sequence of nTyr p 8 was 657 nucleotides long, and the open reading frame encoded a protein of 218 amino acids (Fig. 3A). In comparison with other group 8 allergen sequences, rTyr p 8 had 83%, 78%, 74%, and 74% homology with the group 8 mite allergens Der p 8, Der f 8, Gly d 8, and Lep d 8, respectively, as shown by protein alignment analysis (Fig. 3B). When the amino acid sequence was aligned with those of other members of the group 8 allergens, Tyr p 8 appeared to be highly conserved based on findings reported in other species of mites, which included house dust mites and storage mites. The three-dimensional (3D) tertiary structure of Tyr p 8 was predicted using the (PS)2: Protein Structure Prediction Server (http://ps2.life.nctu.edu.tw/), which is maintained by the Molecular Bioinformatics Center, National Chiao Tung University, Taiwan, Republic of China. The ribbon representation of the Tyr p 8 is shown in Fig. 3C. The 3D tertiary structure predictions of Tyr p 8 and Der p 8 were compared, and the findings revealed a high degree of similarity between them.
Fig 2.
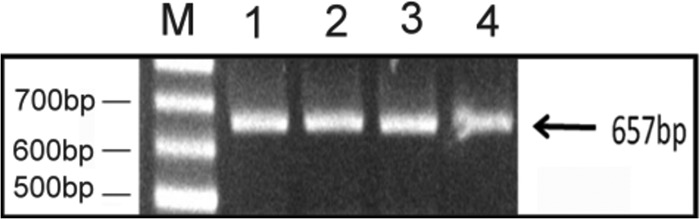
Full-length cDNA of Tyr p 8. The reverse transcription (RT)-PCR products were amplified from a different batch of cDNA with primers p8-EF and p8-ER and analyzed with 1% agarose gel electrophoresis in lanes 1 to 4. Lane M, DNA marker.
Fig 3.
(A) Nucleotides and deduced amino acid sequences of Tyr p 8. The accession number for Tyr p 8 in the GenBank database is AN-JX255733. (B) Alignments of the amino acid sequences of Tyr p 8 and other group 8 mite allergens. The conserved residues are shaded, and the gaps are indicated by dashes. (C) 3D tertiary structure predictions of Tyr p 8 and Der p 8.
Subcloning, expression, and purification.
After the recombinant plasmid vector yT&A-Tyr p 8 was digested by both BamHI and XhoI, the recovered cDNA encoding rTyr p 8 was subcloned into the expression vector of pQE30 and identified by restriction analysis. The plasmid pQE30-GST was then transformed into E. coli M15, and the protein was expressed in the presence of the isopropyl-β-d-thiogalactopyranoside (IPTG) inducer. The rTyr p 8 was purified from E. coli induction lysate using His-tagged affinity chromatography with Ni-NTA–Sepharose. The protein gave a single homogeneous band by SDS-PAGE of 26 kDa (Fig. 4).
Fig 4.
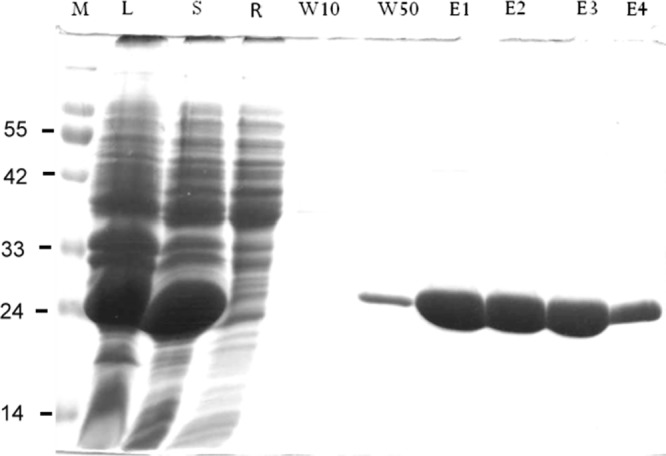
SDS-PAGE analysis of recombinant T. putrescentiae GST. The T. putrescentiae recombinant GST was purified from E. coli induction lysate using His-tagged affinity chromatography with Ni-NTA–Sepharose. L, cell lysate; S, supernatant collected from the lysate after sonication and centrifugation; R, flowthrough; W10, wash with 10 mM imidazole; W50, wash with 50 mM imidazole; E1 to E4, 250 mM imidazole elution.
Identification of T. putrescentiae-specific allergenic components by IgE immunoblot inhibition with rTyr p 8.
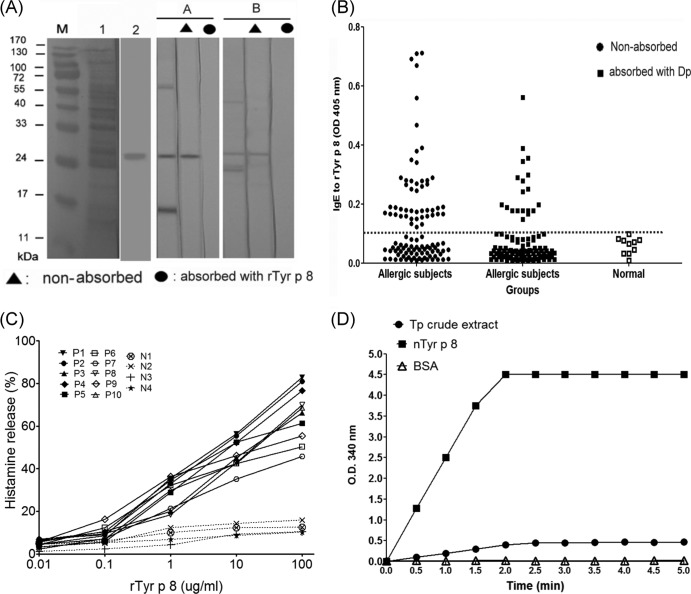
The two serum samples from allergic patients were selected for IgE immunoblot inhibition, and a PVDF membrane with the T. putrescentiae crude extract and rTyr p 8 was used to identify GST-specific allergens reacting with the sera after the absorption of rTyr p 8. The results of immunoblot analyses with T. putrescentiae crude extract and immunoblot inhibition with rTyr p 8 are shown in Fig. 5A. There were several IgE-binding proteins in the T. putrescentiae crude extract as determined by the T. putrescentiae-allergic sera, including one with a molecular mass of 26 kDa. This 26-kDa IgE-binding component was totally absorbed by rTyr p 8, which indicated that this allergenic component might be a T. putrescentiae-specific allergen and homologous to a GST protein.
Fig 5.
Allergenicity of T. putrescentiae GST. (A) T. putrescentiae allergenic components were identified by immunoblot inhibition analysis of IgE reactivity with sera from allergic subjects. Lane M, protein marker; lane 1, T. putrescentiae crude extract; lane 2, recombinant Tyr p 8; A and B, allergic patients. (B) IgE-binding ability of rTyr p 8 in patients allergic to T. putrescentiae. ELISA was performed using sera from T. putrescentiae-allergic subjects (n = 106) and healthy subjects (n = 10) before or after D. pteronyssinus absorption. The dashed line indicates the cutoff value for a positive response. The IgE-binding activity was determined by ELISA at an optical density of 405 nm. (C) Percentage of histamine release from basophils. The basophil histamine release assay was triggered with rTyr p 8 (0.01 to 100 μg/ml) using basophils presensitized with sera from the allergic patients (P1 to P10) or healthy individuals (N1 to N4). (D) Enzymatic activity assay for nTyr p 8. T. putrescentiae crude extract, nTyr p 8, and negative control BSA were added to the reaction solution, and the absorbance was measured at 340 nm for the GST enzymatic activity assay.
Frequency of IgE binding of rTyr p 8 in T. putrescentiae-allergic subjects.
Sensitization of the 106 T. putrescentiae-allergic subjects to GST was determined by measuring serum IgE antibodies against rTyr p 8. The results showed that 45.3% (48/106) of the T. putrescentiae-allergic subjects possessed the IgE antibodies against rTyr p 8 (Fig. 5B). The ELISA IgE inhibition assay was performed with D. pteronyssinus absorption to identify the cross-reactivity of group 8 allergens between T. putrescentiae and D. pteronyssinus, and the results showed that 17.9% (19/106) of the T. putrescentiae-allergic subjects had a positive response to rTyr p 8. Although some patients showed a positive reaction to rTyr p 8, most showed a weak response to rTyr p 8 after D. pteronyssinus absorption. These results indicate that this allergic component (Tyr p 8) showed a high level of cross-reactivity between T. putrescentiae and D. pteronyssinus, which suggests that the group 8 allergen in T. putrescentiae might act as an important allergen triggering IgE-mediated hypersensitivity and that it is highly cross-reactive with D. pteronyssinus.
Role of rTyr p 8 in triggering histamine release in IgE-mediated hypersensitivity.
A total of 14 samples, 10 from allergic patients (P1 through P10) with positive IgE responses against rTyr p 8 and four from nonallergic healthy volunteers (N1 through N4), were used for the basophil histamine release assay. In the presence of rTyr p 8, the histamine release from basophils presensitized with sera from T. putrescentiae-sensitive subjects increased in a dose-dependent manner. In the presence of rTyr p 8, there were no significant differences in the histamine release from basophils presensitized with normal serum. The percentage of histamine release resulting from basophil degranulation was higher in allergic patients, ranging from 40 to 80%, especially in the sera from P1 and P2 (Fig. 5C).
Enzymatic activity of nTyr p 8.
The enzymatic activity of GST catalyzed the conjugation of GSH with CDNB to form CDNB-GSH, which was measured by the absorbance at 340 nm. Enzymatic activity assays showed that T. putrescentiae crude extract catalyzed the CDNB to generate CDNB-GSH with enzymatic activity of GST up to an OD340 of 0.47/μg measured in 5 min. Through the purification, the enzymatic activity of nTyr p 8 was increased to an OD340 of 4.5/μg measured in 5 min (Fig. 5D). The nTyr p 8 exhibited high enzymatic activity of GST, but the BSA showed no enzymatic activity.
DISCUSSION
The airway allergies are important factors in the genesis of allergic asthma and rhinitis. There is increasing evidence that exposure to airborne allergens, including mites, cockroaches, fungi, and pollens, leads to airway allergy. In general, sensitivity to inhaled allergens from domestic mites is the most consistent risk factor for the development of allergic diseases. Domestic mites can be divided into two categories, pyroglyphid and nonpyroglyphid, which are commonly referred to as house dust mites and storage mites, respectively. Due to their abundance and high allergenicity, the house dust mite (D. pteronyssinus) and the storage mite (T. putrescentiae) are common causes of allergy and at high levels of infestation are associated with airway hypersensitivity. The notable appearance and abundance of mites might be influenced by the warm and humid climate in Taiwan. D. pteronyssinus is the major cause of allergy in more than 80% of allergic patients with bronchial asthma in Taiwan. In most cases, T. putrescentiae can cause allergic respiratory symptoms after occupational exposure on farms and in grain stores; currently, more attention is being paid to its allergenicity in nonoccupational environments.
Although D. pteronyssinus and Dermatophagoides farinae mites are the predominant species and coexist in most geographical regions, there are many microenvironmental variations (17). D. pteronyssinus prefers temperate/tropical coastal regions, whereas D. farinae is more abundant in continental climates. The allergens of D. pteronyssinus and D. farinae typically have 15 to 20% amino acid sequence disparity, and although they are immunologically cross-reactive, they also have unique epitopes (18). The importance of using extracts tailored to the species prevalent in the local environment is not known and is difficult to ascertain because of the variation in extracts obtained from the same species. Mites from storage mite families can also be important house dust mites; however, because their allergens have 70% amino acid sequence disparity with Dermatophagoides spp., there is minimal IgE cross-reactivity. The T. putrescentiae mites found in Korea and Spain and the Blomia tropicalis mites found in South America, the Caribbean, and Singapore are two important types of storage mites (19, 20) which usually coexist with D. pteronyssinus.
GSTs comprise a ubiquitous superfamily of enzymes that play a significant role in the detoxification of xenobiotics such as insecticides (21). Upregulation of GST production in insects is associated with resistance to insecticides (22). The majority of GSTs are in the mu, delta, and epsilon classes, and the additional enzymes are in the omega, sigma, theta, and zeta classes. Cytosolic GST from the house dust mite D. pteronyssinus (Der p 8), classified as being in the mu class (8), and that from the cockroach Blattella germanica (Bla g 5), classified as being in the sigma class (10), have been shown to act as allergens. In this study, protein alignment analysis showed that rTyr p 8 had a high homology with the other group 8 mite allergens. In particular, there was 83% protein homology with the Der p 8 mu class. Therefore, we presumed that the Tyr p 8 we purified belonged to the mu class. The GST mu class appeared to be highly conserved in mite species, which included house dust mites and storage mites. However, a number of GST isoforms from the crude mite extract had been isolated by glutathione affinity column purification. At least eight isoforms in the nDer p 8 with a pI range from 6.3 to 8.5 had been identified by two-dimensional gel electrophoresis and immunoblotting analyses (9). In this study, we report the detection of at least three isoforms. GST is widely distributed in nature and often exists as multiple isoenzymes. It will be interesting to perform further studies to investigate the allergenicities and family classes of nTyr p 8 isoforms.
Allergenicity can be measured by the specific binding of IgE and its ability to induce an immune response, leading to hypersensitivity caused by IgE. An in vitro test can measure allergen-induced histamine release from IgE antibody-armed basophils in tissue culture. In this study, nTyr p 8 showed the ability to bind IgE in the sera of T. putrescentiae-sensitive patients and stimulate the release of histamine from basophils. These findings demonstrate that nTyr p 8 exhibits allergenic properties.
In a large series of allergic subjects, approximately 50% of the IgE binding in sera was accounted for by Der p 1 and 2, and in another 30%, Der p 4, 5, and 7 contributed equally. In this study, the IgE binding levels of rTyr p 8 before and after D. pteronyssinus absorption were 45.3% and 14.3%, indicating that rTyr p 8 is a minor allergen in T. putrescentiae-allergic subjects. In the analysis of the sequence homology of Der p 8 and Tyr p 8, a sequence similarity of 83% was found between these two allergens, and the frequency of IgE binding to rTyr p 8 was decreased after D. pteronyssinus absorption, indicating that there were high levels of cross-reactivity between Tyr p 8 and Der p 8. It has been reported that Der p 8 reacts with IgE antibodies (Abs) in sera of 40% mite-allergic patients (8). Our data revealed that 45.3% of T. putrescentiae-allergic patients had IgE antibodies against rTyr p 8.
Although there was a high frequency of IgE binding to rTyr p 8 and a high sequence homology between Der p 8 and Tyr p 8, the specific binding of serum IgE to GST-T. putrescentiae was less than 20%, indicating that Tyr p 8 was not a major allergen, and T. putrescentiae-allergic subjects might have had a positive response to rTyr p 8 due to the cross-reactivity with Der p 8. To the best of our knowledge, the present study is the first to report that rTyr p 8 is an allergen. It is important to note that this antigen exhibits cross-reactions between T. putrescentiae and D. pteronyssinus, but the precise mechanisms of interaction have yet to be fully elucidated. Further investigation is required to confirm the cross-reactivity of Der p 8 and Tyr p 8.
ACKNOWLEDGMENT
This study was supported by a grant (TCVGH-1017310C) from Taichung Veterans General Hospital.
Footnotes
Published ahead of print 30 January 2013
REFERENCES
- 1. Kondreddi PK, Elder BL, Morgan MS, Vyszenski-Moher DL, Arlian LG. 2006. Importance of sensitization to Tyrophagus putrescentiae in the United States. Ann. Allergy Asthma Immunol. 96: 124. [DOI] [PubMed] [Google Scholar]
- 2. Park JW, Ko SH, Yong TS, Ree HI, Jeoung BJ, Hong CS. 1999. Cross-reactivity of Tyrophagus putrescentiae with Dermatophagoides farinae and Dermatophagoides pteronyssinus in urban areas. Ann. Allergy Asthma Immunol. 83: 533–539 [DOI] [PubMed] [Google Scholar]
- 3. Müsken H, Fernandez-Caldas E, Maranon F, Franz JT, Masuch G, Bergmann KC. 2002. In vivo and in vitro sensitization to domestic mites in German urban and rural allergic patients. J. Investig. Allergol. Clin. Immunol. 12: 177–181 [PubMed] [Google Scholar]
- 4. Arlian LG, Geis DP, Vyszenski-Moher DL, Bernstein IL, Gallagher JS. 1984. Antigenic and allergenic properties of the storage mite Tyrophagus putrescentiae. J. Allergy Clin. Immunol. 74: 166–171 [DOI] [PubMed] [Google Scholar]
- 5. Eriksson TL, Johansson E, Whitley P, Schmidt M, Elsayed S, van Hage-Hamsten M. 1998. Cloning and characterisation of a group II allergen from the dust mite Tyrophagus putrescentiae. Eur. J. Biochem. 251: 443–447 [DOI] [PubMed] [Google Scholar]
- 6. Liao EC, Hsu EL, Tsai JJ, Ho CM. 2009. Immunologic characterization and allergenicity of recombinant Tyr p 3 allergen from the storage mite tyrophagus putrescentiae. Int. Arch. Allergy Immunol. 150: 15–24 [DOI] [PubMed] [Google Scholar]
- 7. Ketterman AJ, Saisawang C, Wongsantichon J. 2011. Insect glutathione transferases. Drug Metab. Rev. 43: 253–265 [DOI] [PubMed] [Google Scholar]
- 8. O'Neill GM, Donovan GR, Baldo BA. 1994. Cloning and characterization of a major allergen of the house dust mite, Dermatophagoides pteronyssinus, homologous with glutathione S-transferase. Biochim. Biophys. Acta 1219: 521–528 [DOI] [PubMed] [Google Scholar]
- 9. Huang CH, Liew LM, Mah KW, Kuo IC, Lee BW, Chua KY. 2006. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin. Exp. Allergy 36: 369–376 [DOI] [PubMed] [Google Scholar]
- 10. Arruda LK, Vailes LD, Platts-Mills TA, Hayden ML, Chapman MD. 1997. Induction of IgE antibody responses by glutathione S-transferase from the German cockroach (Blattella germanica). J. Biol. Chem. 272: 20907–20912 [DOI] [PubMed] [Google Scholar]
- 11. Shankar J, Gupta PD, Sridhara S, Singh BP, Gaur SN, Arora N. 2005. Immunobiochemical analysis of cross-reactive glutathione-S-transferase allergen from different fungal sources. Immunol. Invest. 34: 37–51 [PubMed] [Google Scholar]
- 12. Galindo PA, Lombardero M, Borja J, Gomez E, Feo F, Barber D, Garcia R. 2001. A new arthropod panallergen? Allergy 56: 195–197 [DOI] [PubMed] [Google Scholar]
- 13. Lorenz AR, Scheurer S, Haustein D, Vieths S. 2001. Recombinant food allergens. J. Chromatogr. B Biomed. Sci. Appl. 756: 255–279 [DOI] [PubMed] [Google Scholar]
- 14. Hiller R, Laffer S, Harwanegg C, Huber M, Schmidt WM, Twardosz A, Barletta B, Becker WM, Blaser K, Breiteneder H, Chapman M, Crameri R, Duchene M, Ferreira F, Fiebig H, Hoffmann-Sommergruber K, King TP, Kleber-Janke T, Kurup VP, Lehrer SB, Lidholm J, Muller U, Pini C, Reese G, Scheiner O, Scheynius A, Shen HD, Spitzauer S, Suck R, Swoboda I, Thomas W, Tinghino R, Van Hage-Hamsten M, Virtanen T, Kraft D, Muller MW, Valenta R. 2002. Microarrayed allergen molecules: diagnostic gatekeepers for allergy treatment. FASEB J. 16: 414–416 [DOI] [PubMed] [Google Scholar]
- 15. Shen HD, Chua KY, Lin WL, Chen HL, Hsieh KH, Thomas WR. 1996. IgE and monoclonal antibody binding by the mite allergen Der p 7. Clin. Exp. Allergy 26: 308–315 [PubMed] [Google Scholar]
- 16. Singh BP, Sridhara S, Arora N, Gangal SV. 1992. Evaluation of protein assay methods for pollen and fungal spore extracts. Biochem. Int. 27: 477–484 [PubMed] [Google Scholar]
- 17. Arlian LG, Morgan MS, Neal JS. 2002. Dust mite allergens: ecology and distribution. Curr. Allergy Asthma Rep. 2: 401–411 [DOI] [PubMed] [Google Scholar]
- 18. Thomas WR, Heinrich TK, Smith WA. 2007. Pyroglyphid house dust mite allergens. Protein Pept. Lett. 14: 943–953 [DOI] [PubMed] [Google Scholar]
- 19. Fernández-Caldas E, Lockey RF. 2004. Blomia tropicalis, a mite whose time has come. Allergy 59: 1161–1164 [DOI] [PubMed] [Google Scholar]
- 20. Chua KY, Cheong N, Kuo IC. 2007. The Blomia tropicalis allergens. Protein Pept. Lett. 14: 325–333 [DOI] [PubMed] [Google Scholar]
- 21. Sheehan D, Meade G, Foley V, Dowd CA. 2001. Structure, function and evolution of glutathione S-transferases: implications for classification of nonmammalian members of an ancient enzyme superfamily. Biochem. J. 360: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fournier D, Bride JM, Poirie M, Bergé JB, Plapp FW., Jr 1992. Insect glutathione S-transferases. Biochemical characteristics of the major forms from houseflies susceptible and resistant to insecticides. J. Biol. Chem. 267: 1840–1845 [PubMed] [Google Scholar]