Abstract
Polysaccharide-encapsulated fungi are the chief source of diseases in immunocompromised hosts such as those infected with human immunodeficiency virus or neutropenia patients. Currently available polysaccharide-protein conjugate vaccines are mainly T cell dependent and are usually ineffective in weakened immune systems. In this study, laminarin, a well-characterized β-1,3-glucan, was conjugated with a prokaryotically expressed recombinant fragment (amino acids [aa] 39 to 272) of calreticulin (rCRT/39–272), which exhibits extraordinarily potent immunogenicity and adjuvanticity in experimental animals. The resultant conjugate reserves the immunostimulatory effect of rCRT/39–272 on naïve murine B cells and is capable of eliciting anti-β-glucan IgG (mostly IgG1) responses in not only BALB/c mice but also athymic nude mice. Laminarin-CRT-induced mouse antibodies (Abs) are able to bind with Candida albicans and inhibit its growth in vitro. In addition, vaccination with laminarin-CRT partially protects mice from lethal C. albicans challenge. These results imply that rCRT/39–272 could be used as an ideal carrier or adjuvant for carbohydrate vaccines aimed at inducing or boosting IgG responses to fungal infections in immunodeficient hosts.
INTRODUCTION
Candida albicans is a commensal fungus that colonizes different areas of the human body such as the gastrointestinal tract, pharynx, and vaginal mucosa. It is usually not harmful to the hosts but can become pathogenic and cause local or systemic infections in immunocompromised subjects. For example, neutropenia is a hallmark risk factor for development of disseminated candidasis, while human immunodeficiency virus (HIV)-infected patients are known to be more susceptible to mucosal candidiasis (1, 2). Despite the fact that cell-mediated immunity plays an important role in antifungal defense, accumulating evidence suggests that antibodies (Abs), particularly IgG, specific for cell surface glycan epitopes of fungi are also indispensable (3–5). As one of the major components of the cell wall of C. albicans, β-glucans (β-1,3-glucan backbone with β-1,6-linked branches) have been targeted in antifungal vaccine designs by many laboratories (6–9). Like other polysaccharides, β-glucans are T-cell-independent type 2 antigens (Ags) that stimulate only short-lived B cells and drive the production of IgM Abs in vivo (10). Chemical conjugation of polysaccharides to protein carriers such as tetanus toxoid (TT) and diphtheria toxoid (DT) enhances their immunogenicity and enables them to elicit production of anti-polysaccharide IgG Abs in vivo (11, 12). Unfortunately, traditional protein-polysaccharide conjugate vaccines are unable to elicit carbohydrate antigen-specific IgG responses in T-cell-immunodeficient hosts (13, 14). Furthermore, most of the experimental glycoprotein vaccines require strong adjuvants (e.g., complete Freund's adjuvant) to achieve sufficient immunogenicity, while these adjuvants are usually incompatible with human use (15, 16). Xin et al. have recently reported a TT-coupled self-adjuvanting glycopeptide vaccine (17); whether such a vaccine could also overcome the requirement for T-cell help remains to be tested. Thus, better adjuvant and/or carrier proteins are needed as a necessary component(s) in vaccines for the control of fungal infections in weakened immune systems.
Calreticulin (CRT) is a major Ca2+-binding protein in the endoplasmic reticulum (ER); it is also a molecular chaperone capable of facilitating peptide presentation and correctly folding of major histocompatibility complex (MHC) class I molecules in the cell (18–20). Although structurally unique, CRT exhibits certain functions similar to those of heat shock proteins (HSPs) (21, 22). Consequently, CRT is considered a member of the HSP family that possesses potent immunostimulatory activities (23). We have recently reported that a recombinant fragment (amino acid residues 39 to 272) of mouse CRT (rCRT/39–272) exhibits a robust B-cell-stimulating effect and also that a protein resulting from fusion between rCRT/39–272 and recombinant enhanced green fluorescent protein (rEGFP) is able to induce anti-EGFP IgG Abs in not only BALB/c mice but also athymus nude mice (24). Furthermore, CRT has also been tested as an adjuvant for various vaccines against different viruses with promising results, as reported by previous investigators (25, 26). Our group also demonstrated that a protein resulting from fusion between CRT/39–272 and a fragment consisting of residues 450 to 650 of the spike protein (S450–650) of severe acute respiratory syndrome (SARS) coronavirus was also able to induce S450–650-specific IgG in mice (27). On the basis of these data, we wondered if CRT/39–272 could be used as an effective carrier or adjuvant for carbohydrate antigens and assist the production of glycotope-specific IgG Abs in healthy as well as immunocompromised hosts.
In this study, laminarin (LAM), a well-characterized β-1,3-glucan preparation from the brown alga Laminaria digitata (28), was chosen as a representative polysaccharide and conjugated with rCRT/39–272, and the resultant conjugate was tested for its ability to elicit β-glucan-specific IgG responses in not only BALB/c mice but also athymic (T-cell-deficient) nude mice. Furthermore, the antifungal effect of vaccination with this conjugate was tested using a mouse model of disseminated candidiasis according to the method of Bromuro et al. (29).
MATERIALS AND METHODS
Mice and their immunization.
Female BALB/c and BALB/c nu/nu (nude) mice between 6 and 8 weeks of age were purchased from the Chinese Academy of Military Medical Science (Beijing, China). All animals were maintained under specific-pathogen-free conditions in the Department of Immunology, Beijing University, and animal usage was conducted according to protocols approved by the Beijing University Institutional Animal Care and Use Committee. Mice were immunized subcutaneously (s.c.) at the base of the tail with 100 μg of antigen in a total of 50 μl of phosphate-buffered saline (PBS). For booster immunization, 50 μg of antigen–100 μl of PBS was injected s.c. Serum samples were collected by tail bleeding at different time points, divided into aliquots, and kept at −20°C until further use.
Conjugation of laminarin with rCRT/39–272 or EGFP.
rCRT/39–272 and rEGFP were expressed as previously described (24). The conjugation procedure was done essentially as previously described by Wessels et al. (30) with some modifications. Laminarin (Sigma-Aldrich) (15 mg) was incubated with 4 mM sodium periodate–1.5 ml PBS (pH = 7.0) in the dark for 1.5 h at room temperature. Glycerol was added to consume any residual periodate. The mixture was passed through a Sephadex G-25 column (Pharmacia) and lyophilized. The oxidized laminarin (10 mg) was dissolved in 0.1 M sodium bicarbonate (pH 8.5) and mixed with rCRT/39–272 or rEGFP (5 mg). Sodium cyanoborohydride was added to achieve a final concentration of 20 mg/ml, and the mixture was incubated at 37°C for 5 days. When the conjugation was complete, sodium borohydride (10 mg/ml) was added to reduce any remaining free aldehyde groups. The conjugate was purified using nickel-nitrilotriacetic acid resin (Novagen) and judged by the use of Coomassie blue-stained SDS-PAGE gels. The carbohydrate content of the conjugate was determined by the phenol-sulfuric acid method, and the protein was verified with a Micro bicinchoninic acid (BCA) protein assay (Bio-Rad). All antigen preparations were filtered through a 0.2-μm-pore-size sterile syringe filter (Corning) and passed twice through a polymyxin B column (Sigma-Aldrich), and the endotoxin levels were less than 1 ng/mg, as determined by the use of Endospecy (Seikagaku Kogyo Ltd.).
Proliferation and IgM production assays.
Splenocytes were freshly collected from naïve BALB/c mice or nude mice and then immediately used for fractionation of B and T cells by negative selection with B- and T-cell isolation kits (Miltenyi Biotec) following the manufacturer's instructions. The resultant cells were over 95% positive for surface CD19/B220 (double staining) and CD3, respectively, as determined by fluorescence-activated cell sorter (FACS) analysis using a BD FACSCalibur flow cytometer (BD Biosciences).
For proliferation assays, purified B and T cells (2 × 105 cells/well) from BALB/c mice or splenocytes from nude mice (3 × 105 cells/well) were stimulated, in triplicate wells of 96-well plates, with appropriate antigens in 200 μl of RPMI 1640 medium supplemented with 10% (vol/vol) fetal bovine serum (FBS) (HyClone Laboratories), penicillin and streptomycin (100 U/ml), l-glutamine (2 mM), and 2-mercaptoethanol (2-ME) (5 × 10−5 M) for 48 h in a 5% CO2 incubator at 37°C. A combination of phorbol myristate acetate (PMA) (20 ng/ml) and ionomycin (1 μg/ml) (Sigma-Aldrich) was used as a positive control. For the last 8 h of incubation, the cells were pulsed with 0.2 μCi/well of [3H]thymidine (Atom HighTech); the cultures were subsequently harvested on an automatic cell harvester (Tomtec), and the radioactivity was counted in a β-counter (EG&G Wallac). The concentration of IgM in cell culture supernatant after 48 h was determined using a mouse IgM enzyme-linked immunosorbent assay (ELISA) quantitation set (Bethyl Laboratories) according to the manufacturer's instructions.
ELISAs.
For polysaccharide-based ELISA, polyvinyl ELISA plates were coated overnight at 4°C with different polysaccharides, namely, laminarin (primarily β-1,3-glucan with some 1-6-linked branches; Sigma L9634), linear β-1,3-glucan (with no branching; Sigma 89862), dextran (linear α-1,6-glucan with α-1,3-linked branches; Sigma D9260), mannan (Sigma M7504), alginic acid (polyuronic acid composed primarily of anhydro-β-d-mannuronic acid residues with 1-4 linkage; Sigma A7003), heparin (polymer of d-glucuronic and l-iduronic acids and N-acetyl-d-glucosamine; Sigma H3149), and pustulan (linear β-1,6-glucan; Calbiochem 540501), at 50 μg/ml in carbonate buffer (pH 9.6). β-1,3-Glucan was first dissolved in dimethyl sulfoxide (DMSO) and then diluted in carbonate buffer at 50 μg/ml. Coated plates were subsequently incubated with blocking solution (2% bovine serum albumin [BSA]–PBS) for 2 h at 37°C. The wells were washed five times with PBS containing 0.05% Tween 20 (PBS-T), and then 100 μl of diluted mouse sera was added into the well and incubated for 2 h at 37°C. After five washes with PBS-T, the plates were incubated with horseradish peroxidase (HRP)-labeled goat anti-mouse IgM, IgG, IgG1, IgG2a, IgG2b, or IgG3 antibody (SouthernBiotech) for 1 h at 37°C. The reaction was developed with 100 μl of O-phenylenediamine (OPD) (Sigma-Aldrich) for 5 min and stopped with 100 μl of 2 M H2SO4. The optical density at 492 nm (OD492) was measured in an ELISA spectrophotometer (Titertek Multiscan Plus MK II).
Purification of anti-LAM IgG antibody.
On the 28th day after the first immunization with LAM-CRT, BALB/c mice or BALB/c nu/nu mice were sacrificed for sera that were used for purification of LAM-specific IgG Abs using laminarin-conjugated Sepharose 6B beads (GE Healthcare). Protein A/G-agarose (Vigorous, China) was then used to purify IgG from the affinity-purified anti-laminarin Abs. The concentration and purity of Abs were examined by Coomassie blue staining and SDS-PAGE.
Preparation of C. albicans cells and C. albicans growth inhibition assay.
C. albicans strain BP was routinely maintained on Sabouraud agar slants. For experimental purposes, the fungus was cultured in yeast form in liquid Winge medium at 28°C, washed twice in saline solution, counted in a hemocytometer, and resuspended to the desired concentration in sterile saline solution. For preparation of inactivated C. albicans, yeast cell suspensions (1 × 108 cells/ml) were inactivated at 80°C for 30 min, washed, and stored at 4°C for no more than 1 week.
The C. albicans growth inhibition assay was done as described by Torosantucci et al. (8). A total of 150 C. albicans germ tubes were incubated in 200 μl of RPMI-fetal calf serum (FCS) medium in the presence of sera (undiluted or diluted 1:10) from LAM-CRT-immunized mice (collected 28 days after the first immunization). Control cultures were prepared with normal mouse sera (NMS) or rCRT/39–272-induced mouse antisera at the same protein concentration. Cultures were incubated overnight at 37°C, and C. albicans growth was evaluated by CFU counts.
Systemic infection with C. albicans and assessment of protection.
An acute mouse candidiasis model was implemented as described by Bromuro et al. (29). Mice that had been immunized with LAM-CRT or rCRT/39–272 28 days earlier were infected by the intravenous (i.v.) route with a lethal dose of C. albicans cells (2 × 106 cells/ml stock [0.1 ml/mouse]). Protection was evaluated by monitoring animal survival for 40 days and by quantifying the extent of C. albicans outgrowth in the kidneys of the infected animals. For the latter purpose, the left kidneys of sacrificed animals were aseptically removed on day 2 postchallenge and homogenized in sterile saline solution containing 0.1% Triton X-100 (Sigma-Aldrich). The number of CFU per organ was determined by a plate dilution method on Sabouraud dextrose agar. Each kidney was examined separately, and at least three distinct dilutions from each sample were assayed in triplicate.
Statistical analysis.
All experiments were repeated at least three times, and the results are expressed as means ± standard deviations (SD). Statistical analysis was performed using the independent samples and a t test or one-way analysis of variance (ANOVA) (Tukey's multiple-comparison test) for comparisons between groups using Graphpad Prism 4.03. Differences were considered statistically significant at a P of <0.05.
RESULTS
Characterization of protein-conjugated laminarin.
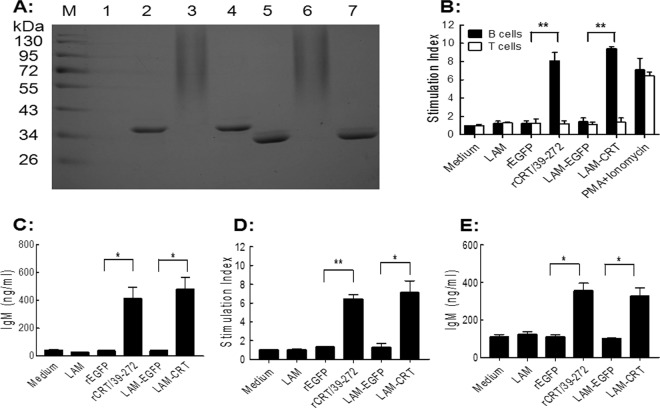
Laminarin was conjugated with rCRT/39–272 or rEGFP using a three-step reaction as described in Materials and Methods, and the resultant conjugate, namely, LAM-CRT or LAM-EGFP, respectively, was visualized in Coomassie blue-stained SDS-PAGE 12% gels (Fig. 1A). Free rCRT/39–272 and rEGFP appeared as single protein bands (36 kDa and 31 kDa, respectively), while LAM-CRT and LAM-EGFP were smears (due to heterogeneity in the length of laminarin and different numbers of laminarin molecules conjugated with the recombinant proteins) of much higher molecular mass. Free recombinant proteins were undetectable in the laminarin-protein conjugation products (Fig. 1A, lanes 3 and 6), indicating that virtually all were conjugated with laminarin. Both LAM-CRT and LAM-EGFP remained soluble at 10 mg/ml in PBS. The saccharide/protein ratios of LAM-CRT and LAM-EGFP were estimated, by using the phenol-sulfuric acid and BCA method, to be 0.75 and 0.7, respectively, reflecting high conjugation efficiency.
Fig 1.
Characterization of LAM-CRT and LAM-EGFP. (A) Samples of laminarin, rCRT/39–272, LAM-CRT, a mixture of laminarin and rCRT/39–272, rEGFP, LAM-EGFP, and a mixture of laminarin and rEGFP were run in 12% SDS-PAGE followed by Coomassie blue staining (lanes 1 to 7, respectively). Protein molecular mass markers (lane M) were loaded in the left-hand lane. (B) Freshly fractionated B and T cells from naïve BALB/c mouse splenocytes (3 mice pooled) were stimulated, in triplicate, with rCRT/39–272 or rEGFP (10 μg/ml) or with LAM-CRT, LAM-EGFP, or laminarin (50 μg/ml) for 48 h. A combination of PMA (20 ng/ml) and ionomycin (1 μg/ml) was included as a positive control. [3H]thymidine was added to the cultures (0.2 μCi/well) for the last 8 h of incubation, and then [3H]thymidine incorporation of each well was counted (cpm). The results were calculated using the “cell and medium only” wells as a reference and are expressed as mean stimulation index ± SD of the results determined with triplicate wells. (C) The IgM concentration in the B-cell culture supernatant was quantitated using ELISA, and the results are expressed as mean concentration (ng/ml) ± SD. (D and E) Freshly prepared naïve BALB/c nu/nu mouse splenocytes were used as responder cells in parallel experiments. *, P < 0.05; **, P < 0.01. Data represent results from 3 independent experiments.
We have previously shown that rCRT/39–272 was able to drive B-cell activation and proliferation and IgM production (24). Consistently, LAM-CRT, but not LAM-EGFP, rEGFP, or laminarin alone, induced vigorous proliferation of splenic B cells freshly fractionated from naïve BALB/c mice, which was accompanied by IgM secretion detectable in the culture supernatant by ELISAs (Fig. 1B and C). Similarly, splenocytes from naïve athymic nude mice (∼85% B cells and almost no detectable T cells as judged by flow cytometric analysis) were also effectively activated by LAM-CRT in vitro, as evidenced by their proliferation and IgM secretion (Fig. 1D and E). Consistent with our previous reports (24, 27), LAM-CRT and rCRT/39–272 were unable to induce proliferative response of freshly fractionated T cells from naïve BALB/c mice (Fig. 1B). It is thus clear that the CRT fragment in LAM-CRT well reserved its immunobiological activities after the conjugation procedures.
Enhanced immunogenicity of LAM-CRT and LAM-EGFP over laminarin.
The laminarin-protein conjugates were tested in BALB/c mice for the ability to elicit carbohydrate Ag-specific Ab responses. LAM-EGFP induced reasonable levels of anti-laminarin serum IgM and IgG Abs in BALB/c mice when s.c. injected twice in the absence of adjuvant, while laminarin alone was hardly able to do so (Fig. 2A and B). LAM-CRT was a better immunogen than LAM-EGFP in eliciting laminarin-specific IgG responses in vivo (Fig. 2C). Analysis of Ab subtypes revealed that anti-laminarin IgG Abs elicited by LAM-CRT were mainly of the IgG1 subtype (Fig. 2D). By comparing titration curves of the two groups (Fig. 2E), it can be estimated that the titers of laminarin-specific IgG1 Abs in mice immunized with LAM-CRT were approximately 16-fold higher than that of the LAM-EGFP-immunized group. It should also be emphasized that antisera from mice immunized with rCRT/39–272 showed strong binding only with the immunizing Ag (24) but not with any of the carbohydrate Ags such as laminarin, lipopolysaccharide (LPS), mannan, and pustulan (Fig. 2F).
Fig 2.
Enhanced immunogenicity of LAM-CRT and LAM-EGFP over unconjugated laminarin. Groups of BALB/c mice (6 per group) were s.c. injected with laminarin (A), LAM-EGFP (B), or LAM-CRT (C) or with rCRT/39–272 (F) (in PBS; 100 μg/mouse) and boosted with 50 μg of the same Ag preparations 2 weeks later. The mice were bled at different time points thereafter, and the combined sera (1/200 dilution) were assayed, in triplicate wells, for IgM and IgG using laminarin-based ELISAs. The detection antibody was HRP-conjugated goat anti-mouse IgM or IgG with OPD as the substrate. (D) Sera of the LAM-CRT group (mixture of equal proportions, 1/200 dilution), collected on day 28, were assayed in laminarin-based ELISAs for IgG subclasses using HRP-conjugated goat anti-mouse Abs against IgG1, IgG2a, IgG2b, or IgG3 for detection. (E) Antisera from all 3 groups (mixture of equal proportions for each group) were also titrated against laminarin using HRP-labeled IgG1-specific Abs for detection. (F) ELISAs were also carried out to test cross reactivity of antisera from the rCRT/39–272 group using microtiter plates precoated, in triplicate, with rCRT/39–272, rEGFP, pustulan, laminarin, mannan, alginic acid, dextran, and LPS. The results are expressed as mean OD492 ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant. Results are representative of three independent experiments.
LAM-CRT-induced IgG responses in nude mice.
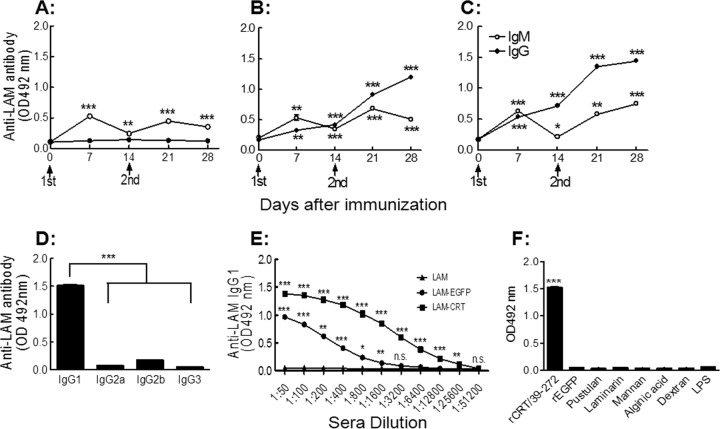
T-cell-deficient athymic nude mice are unable to mount IgG responses to carbohydrate or protein antigens, as T-helper cells are normally indispensable for class switching of the responding B lymphocytes (31). We have previously shown, however, that rCRT/39–272 was able to assist IgG production against covalently linked Ags in nude mice (24). Groups of nude mice were s.c. immunized twice with laminarin, LAM-EGFP, or LAM-CRT and monitored 2 weeks thereafter for laminarin-specific serum IgG by ELISAs. Only LAM-CRT, but not laminarin or LAM-EGFP, was able to induce laminarin-specific IgG responses (Fig. 3A to C). Laminarin-specific memory B cells seemed to be generated by LAM-CRT immunization in nude mice, since an elevated response to a booster dose was seen (Fig. 3C), although additional analyses of the phenotype of the responding B cells are needed for a concrete conclusion. These data suggest that the LAM-CRT conjugate could elicit polysaccharide-specific IgG responses in a manner independent of T-cell help in vivo.
Fig 3.
IgG production in LAM-CRT-immunized nude mice. Groups of BALB/c nu/nu mice (6 per group) were immunized with laminarin (A), LAM-EGFP (B), or LAM-CRT (C) and then bled at different time points. The antisera (mixture of equal proportions; 1/200 dilution) of each group were assayed, in triplicate wells, for IgM and IgG using laminarin-based ELISAs. Sera from day 28 were titrated against laminarin for IgG1 (D). The results are expressed as mean OD492 ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant. Results are representative of 3 independent experiments.
Specific recognition of yeast particles by LAM-CRT-induced Abs.
The fine specificity of the LAM-CRT-induced antisera from wild-type BALB/c mice (Fig. 4A) and nude mice (Fig. 4B) was analyzed by using ELISAs based on carbohydrate antigens, including dextran, pustulan, linear β-1,3-glucan, laminarin, mannan, alginic acid, and heparin. Laminarin and unbranched linear β-1,3-glucan, but not other carbohydrate Ags tested here, were recognized by IgG Abs in the LAM-CRT-induced antisera. β-Glucan-specific IgG Abs were then affinity purified from the antisera using a laminarin column. The resultant Abs were able to bind with yeast particles, as evidenced in ELISAs as well as in flow cytometric analysis (Fig. 5). Specificity of the binding was further confirmed by nearly complete blocking by soluble laminarin, but not dextran, in ELISAs (Fig. 5A and B).
Fig 4.
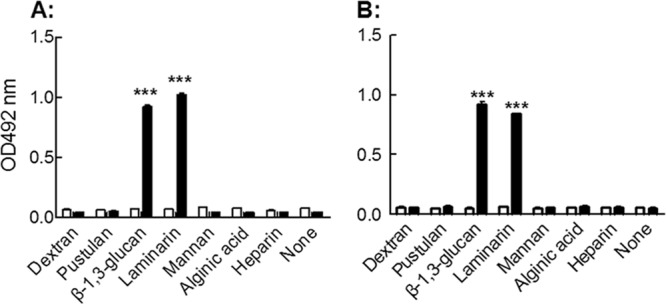
Carbohydrate antigen specificity of LAM-CRT-induced Abs. Groups of BALB/c mice (A) and nude mice (B) were s.c. injected with LAM-CRT (in PBS; 100 μg/mouse, 6 per group) and boosted with 50 μg of the same Ag preparations 2 weeks later. Antisera collected on day 28 from each group were pooled, diluted 1/200, and dispensed in triplicate wells that had been precoated with dextran, pustulan, linear β-1,3-glucan, laminarin, mannan, alginic acid, and heparin for ELISAs. HRP-conjugated goat anti-mouse IgG was employed as a detection Ab followed by development with OPD. Wells not precoated with any Ags (None) were included as a control. The results are expressed as OD492 with SD. ***, P < 0.001.
Fig 5.
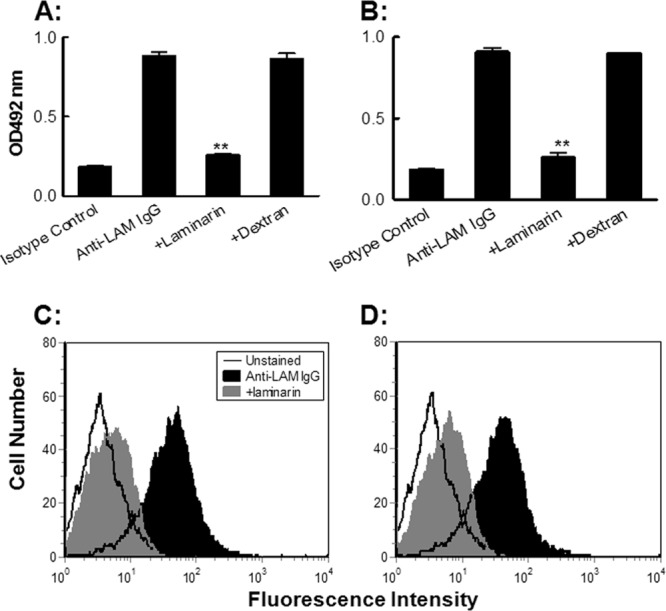
Recognition of yeast particles by IgG Abs induced by LAM-CRT vaccination. (A and B) Laminarin-specific IgG Abs (20 μg/ml), affinity purified from LAM-CRT-induced antisera of BALB/c (A) or nude (B) mice by using a laminarin-conjugated Sepharose 6B column and then a protein A/G-agarose column, were tested for the ability to bind viable C. albicans particles precoated onto ELISA plates, and HRP-conjugated goat anti-mouse IgG was employed as a detection Ab; the results are expressed as the mean OD492 ± SD of triplicate wells. For competition inhibition, the affinity-purified IgG Abs were incubated with either laminarin or dextran (100 μg/ml) at 37°C for 1 h and then dispensed in ELISAs. **, P < 0.01. The affinity-purified IgG Abs from BALB/c (C) and nude (D) mice were further analyzed for the ability to bind C. albicans by flow cytometry. Heat-killed C. albicans cells (2 × 106 particles) were sequentially treated with affinity-purified Abs (filled dark histograms, 20 μg/ml) or left untreated (open histogram) and then treated with fluorescein isothiocyanate (FITC)-labeled anti-mouse IgG followed by analysis using a BD FACSCalibur flow cytometer. The yeast cells were also stained with affinity-purified Abs preincubated with laminarin (100 μg/ml) at 37°C for 1 h (filled gray histogram) as an additional specificity control. Data represent the results from at least three independent experiments.
Vaccination with LAM-CRT protects against C. albicans challenge in mice.
To address the issue of whether LAM-CRT vaccination could induce protective immunity against fungal infection in vivo, BALB/c mice were s.c. immunized twice with LAM-CRT, or rCRT/39–272 alone as a control, followed by a lethal-dose i.v. challenge with viable C. albicans (2 × 105 cells/mouse) 28 days later. As illustrated in Fig. 6A, LAM-CRT-immunized mice survived longer than the mice in the control group, which is correlated to a much lower number of viable C. albicans cells recoverable from the kidney tissues of the LAM-CRT group 2 days after the challenge (Fig. 6B). Moreover, antisera from the LAM-CRT-immunized mice, but not control sera, showed effective inhibition of the growth of C. albicans cells in vitro (Fig. 6C), supporting a protective role for anti-β-glucan Abs induced by LAM-CRT vaccination in vivo.
Fig 6.
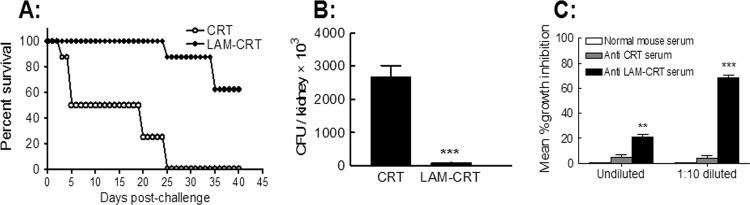
Vaccination with LAM-CRT confers protective immunity against C. albicans infection. BALB/c mice (8 per group) were immunized twice with LAM-CRT or rCRT/39–272 followed by a lethal i.v. challenge with C. albicans 28 days later. (A) The mice were observed daily for up to 40 days, and percent survival was calculated. (B) Groups of BALB/c mice (4/group) that had been similarly immunized were sacrificed for kidney analyses 2 days after a lethal challenge with C. albicans. Viable yeast cells in the kidneys were individually quantitated using the plate dilution method, and the results are expressed as mean CFU/kidney (of 4 kidneys) ± SD. (C) C. albicans cells were cultured overnight in the presence of NMS (open bars)- or LAM-CRT (filled black bars)- or rCRT/39–272 (filled gray bars)-induced antisera. Viable C. albicans cells in the cultures were then quantitated, and the results were calculated using the “NMS” wells as a reference and are expressed as mean percent growth inhibition ± SD of the results determined with triplicate wells. Data are from three independent immunization experiments. **, P < 0.01; ***, P < 0.001.
DISCUSSION
In this study, we have shown that s.c. immunization with LAM-CRT induced high-titer anti-β-glucan IgG1 Abs in not only BALB/c mice but also T-cell-deficient nude mice. The Abs thus induced could inhibit the growth of C. albicans in vitro; vaccination with LAM-CRT conferred partial protective immunity against lethal challenge with C. albicans in mice. We have also conjugated rCRT/39–272 with dextran, pustulan, or alginic acid, and the resultant conjugates were as effective in eliciting carbohydrate Ag-specific IgG responses in mice (data not shown). Traditional carrier proteins such as keyhole limpet hemocyanin (KLH) and TT are also able to enhance humoral responses to carbohydrate antigens (11, 12). However, there is no evidence suggesting that any of them could assist Ig class switching in T-cell-deficient hosts.
Since CRT is constitutively expressed in mammalian cells, possible induction of pathological autoimmune damage in the hosts by vaccination with rCRT/39–272-containing products is always of concern. However, results from animal experiments completed thus far suggest that this is unlikely to occur. First, despite the facts that CRT/39–272 was cloned from a mouse source and can be considered a true self-antigen to mice and also that CRT-specific IgG Abs are relatively easily induced in rodents (including BALB/c, C57/BL6N, and C3H mice and Sprague Dawley rats) and rabbits by immunization with rCRT/39–272 or fusion protein containing rCRT/39–272, no signs of autoimmune damage were observed throughout the 6-month period after the antigen inoculation (W. J. Li, H.-L. Dong, and C. Hong, unpublished observation). Second, we have recently found that intraperitoneal administration of rCRT/39–272 effectively modulated cell-mediated immunity (delayed type hypersensitivity [DTH]) and suppressed experimental autoimmune encephalomyelitis (EAE) in mice, which is attributable to (i) the production of anti-CRT IgG Abs that are able to downregulate Th1 and Th17 cell differentiation and activation (32) and (ii) activation and expansion of CD1dhigh CD5+ interleukin-10 (IL-10)-producing B cells (regulatory B cells) that are able to suppress autoimmune responses in vivo (C. Hong, T. T. Zhang, and X.-M. Gao, manuscript in preparation).
rEGFP rather than traditional carrier proteins such as KLH or TT was employed as a control in our study for the following reasons: (i) both rEGFP and rCRT/39–272 were expressed in Escherichia coli with a histidine tag; (ii) rEGFP and rCRT/39–272 have similar molecular masses (31 kDa and 36 kDa, respectively); (iii) rEGFP and rCRT/39–272 are highly soluble; and (iv) our study mainly focused on the ability of rCRT/39–272 to assist humoral responses to conjugated carbohydrate Ags (e.g., laminarin) in vivo. Unlike prokaryotically expressed recombinant proteins, traditional carrier proteins such as KLH, TT, and DT are glycoproteins, and their carbohydrate moieties might be able to induce Abs that are cross reactive with laminarin. Moreover, there is no evidence suggesting that KLH and TT could assist Ig class switching in T-cell-deficient hosts; they therefore represented no advantage over rEGFP as a control in our study.
CRT and several members of the HSP families (e.g., HSP70 and HSP110) have been exploited as adjuvants in enhancing vaccine potency with promising results, which is ascribed to their chaperoning properties (25, 26, 33, 34). The ability of rCRT/39–272 to trigger IgG class switching by B cells (24), an ability that has so far not been reported in HSPs, is likely the key point in eliciting IgG responses by the conjugates in vivo.
Covalent linkage of rCRT/39–272 to laminarin is essential for its adjuvant effect, because a mixture of rCRT/39–272 and laminarin was not different from laminarin alone in the ability to elicit humoral responses in mice (data not shown). Presumably, LAM-CRT can activate B cells via both B-cell receptors (BCR) and CRT-binding proteins in a synergistic manner, thereby inducing IgG production in the absence of T-cell help. Its binding with cognate BCR selects target Ag-specific B cells, while its contact with CRT-binding proteins on the cell surface drives the activation and class switching of the responding B lymphocytes. CD91 (35), CD59 (36), and scavenger receptors (37) have been shown to bind CRT and to facilitate CRT recognition and endocytosis by antigen-presenting cells (APCs).
Unlike B cells, purified murine T cells were unresponsive to stimulation with rCRT/39–272 or LAM-CRT (Fig. 1B). Given that APCs play pivotal roles in T-cell activation, we also tested B-cell-depleted mouse splenocytes (containing T cells, macrophages, and dendritic cells as APCs) for responsiveness to stimulation with LAM-CRT and rCRT/39–272, but no positive response was observed (not shown). It should also be emphasized that, apart from its potent adjuvanticity, rCRT/39–272 can also function like ordinary carrier proteins in assisting IgG responses to target Ags in a T-cell-dependent fashion. This finding is supported by the fact that the titer of anti-laminarin IgG in LAM-CRT-immunized BALB/c mice was approximately 4-fold higher than that seen with nude mice (compare Fig. 2E and 3D). In a previous study, we were able to establish CD4+ helper T-cell lines using draining lymph node cells from mice s.c. immunized with rCRT/39–272 (27), suggesting that rCRT/39–272 could be presented via the class II antigen-processing pathway.
β-Glucan carries a substantial advantage as a candidate vaccine antigen over other fungus-encapsulating polysaccharides such as mannan, as its expression is highly conserved and stable across most species of pathogenic fungi (38). Several β-glucan–protein conjugates have been generated and shown to be able to successfully induce anti-β-glucan Ab-based protection against experimental candidiasis (6–9). Consistent with the in vivo data, Abs generated by LAM-CRT vaccination could efficiently inhibit fungal growth in vitro (Fig. 6). It is noteworthy that several previous groups have also reported direct candicidal activity of Abs specific for glycan antigens of pathogenic fungi (8, 39–41).
The breakthrough represented by LAM-CRT is its ability to bypass T-cell help in eliciting target antigen-specific IgG production, implying effectiveness in protecting hosts whose acquired immune system is impaired. Additionally, rCRT/39–272 could greatly improve the immunogenicity of β-glucans in healthy mice such that additional adjuvants are not needed for vaccination protocol (Fig. 2 and 3). Adjuvants such as colonization factor antigen (CFA) are commonly needed in experimental vaccination with glycoprotein conjugates, but there is always a concern in translating preclinical results into clinical trials (15). Although replacing CFA with other human-compatible adjuvants is one possible solution (6), it is still worth expecting that some glycoprotein conjugates could dismiss adjuvants. Interestingly, Xin et al. have recently reported that addition of TT to glycopeptide β-(Man)3-Fba (β-1,2-mannotriose conjugated with a 14-mer Fba peptide derived from the N-terminal portion of fructose-bisphosphate aldolase) resulted in a self-adjuvanting vaccine that promotes robust antibody responses to disseminated candidiasis without the need for an additional adjuvant (17).
Taken together, the results presented here highlight the advantage of glyco-CRT conjugates as potential vaccines aimed at inducing carbohydrate Ag-specific IgG responses in immunologically incompetent hosts. CRT deserves further investigation for its application as a carrier protein in glycoconjugate vaccine formulation.
ACKNOWLEDGMENTS
This study was supported by grants from PCSIRT (IRT1075), National Foundation of Natural Science of China (30890142), National Key Basic Research Programs (2010CB529102), and also Priority Academic Program Development of Jiangsu Province Higher Education Institutions.
Footnotes
Published ahead of print 13 February 2013
REFERENCES
- 1. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48: 503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moyes DL, Naglik JR. 2011. Mucosal immunity and Candida albicans infection. Clin. Dev. Immunol. 2011: 346307 doi:10.1155/2011/346307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xin H, Dziadek S, Bundle DR, Cutler JE. 2008. Synthetic glycopeptide vaccines combining beta-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. U. S. A. 105: 13526–13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han Y, Cutler JE. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63: 2714–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cutler JE. 2005. Defining criteria for anti-mannan antibodies to protect against candidiasis. Curr. Mol. Med. 5: 383–392 [DOI] [PubMed] [Google Scholar]
- 6. Bromuro C, Romano M, Chiani P, Berti F, Tontini M, Proietti D, Mori E, Torosantucci A, Costantino P, Rappuoli R, Cassone A. 2010. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 28: 2615–2623 [DOI] [PubMed] [Google Scholar]
- 7. Pietrella D, Rachini A, Torosantucci A, Chiani P, Brown AJ, Bistoni F, Costantino P, Mosci P, d'Enfert C, Rappuoli R, Cassone A, Vecchiarelli A. 2010. A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 28: 1717–1725 [DOI] [PubMed] [Google Scholar]
- 8. Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202: 597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Torosantucci A, Chiani P, Bromuro C, De Bernardis F, Palma AS, Liu Y, Mignogna G, Maras B, Colone M, Stringaro A, Zamboni S, Feizi T, Cassone A. 2009. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One 4: e5392 doi:10.1371/journal.pone.0005392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coutinho A, Moller G. 1973. Mitogenic properties of the thymus-independent antigen pneumococcal polysaccharide S3. Eur. J. Immunol. 3: 608–613 [DOI] [PubMed] [Google Scholar]
- 11. Beuvery EC, van Rossum F, Nagel J. 1982. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect. Immun. 37: 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ragupathi G, Koganty RR, Qiu D, Lloyd KO, Livingston PO. 1998. A novel and efficient method for synthetic carbohydrate conjugate vaccine preparation: synthesis of sialyl Tn-KLH conjugate using a 4-(4-N-maleimidomethyl) cyclohexane-1-carboxyl hydrazide (MMCCH) linker arm. Glycoconj. J. 15: 217–221 [DOI] [PubMed] [Google Scholar]
- 13. Mochon AB, Cutler JE. 2005. Is a vaccine needed against Candida albicans? Med. Mycol. 43: 97–115 [DOI] [PubMed] [Google Scholar]
- 14. Deepe GS., Jr 2004. Preventative and therapeutic vaccines for fungal infections: from concept to implementation. Expert Rev. Vaccines 3: 701–709 [DOI] [PubMed] [Google Scholar]
- 15. Pollard AJ, Perrett KP, Beverley PC. 2009. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat. Rev. Immunol. 9: 213–220 [DOI] [PubMed] [Google Scholar]
- 16. Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. 1995. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm. Biotechnol. 6: 277–296 [DOI] [PubMed] [Google Scholar]
- 17. Xin H, Cartmell J, Bailey JJ, Dziadek S, Bundle DR, Cutler JE. 2012. Self-adjuvanting glycopeptide conjugate vaccine against disseminated candidiasis. PLoS One 7: e35106 doi:10.1371/journal.pone.0035106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. 1999. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem. 274: 16917–16922 [DOI] [PubMed] [Google Scholar]
- 19. Michalak M, Corbett EF, Mesaeli N, Nakamura K, Opas M. 1999. Calreticulin: one protein, one gene, many functions. Biochem. J. 344(Pt 2): 281–292 [PMC free article] [PubMed] [Google Scholar]
- 20. Vassilakos A, Michalak M, Lehrman MA, Williams DB. 1998. Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry 37: 3480–3490 [DOI] [PubMed] [Google Scholar]
- 21. Pockley AG. 2003. Heat shock proteins as regulators of the immune response. Lancet 362: 469–476 [DOI] [PubMed] [Google Scholar]
- 22. Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. 2002. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 23: 130–135 [DOI] [PubMed] [Google Scholar]
- 23. Gold LI, Eggleton P, Sweetwyne MT, Van Duyn LB, Greives MR, Naylor SM, Michalak M, Murphy-Ullrich JE. 2010. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J. 24: 665–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hong C, Qiu X, Li Y, Huang Q, Zhong Z, Zhang Y, Liu X, Sun L, Lv P, Gao XM. 2010. Functional analysis of recombinant calreticulin fragment 39-272: implications for immunobiological activities of calreticulin in health and disease. J. Immunol. 185: 4561–4569 [DOI] [PubMed] [Google Scholar]
- 25. Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, Wu TC. 2001. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J. Clin. Invest. 108: 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park YS, Lee JH, Hung CF, Wu TC, Kim TW. 2008. Enhancement of antibody responses to Bacillus anthracis protective antigen domain IV by use of calreticulin as a chimeric molecular adjuvant. Infect. Immun. 76: 1952–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu X, Hong C, Li Y, Bao W, Gao XM. 2012. Calreticulin as a hydrophilic chimeric molecular adjuvant enhances IgG responses to the spike protein of severe acute respiratory syndrome coronavirus. Microbiol. Immunol. 56: 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Read SM, Currie G, Bacic A. 1996. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 281: 187–201 [DOI] [PubMed] [Google Scholar]
- 29. Bromuro C, Torosantucci A, Chiani P, Conti S, Polonelli L, Cassone A. 2002. Interplay between protective and inhibitory antibodies dictates the outcome of experimentally disseminated Candidiasis in recipients of a Candida albicans vaccine. Infect. Immun. 70: 5462–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wessels MR, Paoletti LC, Kasper DL, DiFabio JL, Michon F, Holme K, Jennings HJ. 1990. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J. Clin. Invest. 86: 1428–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wortis HH. 1974. Immunological studies of nude mice. Contemp. Top. Immunobiol. 3: 243–263 [DOI] [PubMed] [Google Scholar]
- 32. Qiu X, Hong C, Zhong Z, Li Y, Zhang T, Bao W, Xiong S, Gao XM. 2012. Modulation of cellular immunity by antibodies against calreticulin. Eur. J. Immunol. 42: 2419–2430 [DOI] [PubMed] [Google Scholar]
- 33. Ge FF, Qiu YF, Yang YW, Chen PY. 2007. An hsp70 fusion protein vaccine potentiates the immune response against Japanese encephalitis virus. Arch. Virol. 152: 125–135 [DOI] [PubMed] [Google Scholar]
- 34. Hu T, Li D, Zhao Y. 2009. Development of the hsp110-heparanase vaccine to enhance antitumor immunity using the chaperoning properties of hsp110. Mol. Immunol. 47: 298–301 [DOI] [PubMed] [Google Scholar]
- 35. Basu S, Binder RJ, Ramalingam T, Srivastava PK. 2001. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14: 303–313 [DOI] [PubMed] [Google Scholar]
- 36. Ghiran I, Klickstein LB, Nicholson-Weller A. 2003. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecule. J. Biol. Chem. 278: 21024–21031 [DOI] [PubMed] [Google Scholar]
- 37. Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. 2004. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J. Biol. Chem. 279: 51250–51257 [DOI] [PubMed] [Google Scholar]
- 38. Brown GD, Gordon S. 2003. Fungal beta-glucans and mammalian immunity. Immunity 19: 311–315 [DOI] [PubMed] [Google Scholar]
- 39. Kavishwar A, Shukla PK. 2006. Candidacidal activity of a monoclonal antibody that binds with glycosyl moieties of proteins of Candida albicans. Med. Mycol. 44: 159–167 [DOI] [PubMed] [Google Scholar]
- 40. Moragues MD, Omaetxebarria MJ, Elguezabal N, Sevilla MJ, Conti S, Polonelli L, Ponton J. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71: 5273–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cassone A, Torosantucci A. 2006. Opportunistic fungi and fungal infections: the challenge of a single, general antifungal vaccine. Expert Rev. Vaccines 5: 859–867 [DOI] [PubMed] [Google Scholar]