Abstract
The implementation of bio-orthogonal click chemistries is a topic of growing importance in the field of biomaterials, as it is enabling the development of increasingly complex hydrogel materials capable of providing dynamic, cell-instructive microenvironments. Here, we introduce the tetrazine–norbornene inverse electron demand Diels–Alder reaction as a new cross-linking chemistry for the formation of cell laden hydrogels. The fast reaction rate and irreversible nature of this click reaction allowed for hydrogel formation within minutes when a multifunctional PEG-tetrazine macromer was reacted with a dinorbornene peptide. In addition, the cytocompatibility of the polymerization led to high postencapsulation viability of human mesenchymal stem cells, and the specificity of the tetrazine–norbornene reaction was exploited for sequential modification of the network via thiol–ene photochemistry. These advantages, combined with the synthetic accessibility of the tetrazine molecule compared to other bio-orthogonal click reagents, make this cross-linking chemistry an interesting and powerful new tool for the development of cell-instructive hydrogels for tissue engineering applications.
Introduction
Due to their high water content, excellent mass transport properties, and soft tissue like elasticity, hydrogels have emerged as diverse materials for cell encapsulation and tissue engineering. Two key applications of hydrogels are their use as carrier materials for cell delivery in vivo and their use as matrices for 3D cell culture in vitro. Synthetic poly(ethylene glycol) (PEG) hydrogels in particular have found exceptional utility, especially for 3D cell culture, because they interact minimally with proteins in cell culture media. Instead, PEG gels can be engineered from the bottom up to incorporate desired or key aspects of the native extracellular matrix (ECM) microenvironment in which cells naturally reside (e.g., integrin binding adhesive sites, enzymatically degradable cross-links).1,2 Importantly, the ability to engineer synthetic microenvironments with fully defined biochemical and biomechanical properties enables researchers to investigate the effects of specific cues on complex cell functions such as cell migration and stem cell differentiation, thereby furthering our collective fundamental understanding of these biological processes.
Several strategies have been described in the literature for encapsulating cells within covalently cross-linked, ECM-mimetic PEG hydrogels; these include chain polymerization of (meth)acrylated PEGs,3,4 radical mediated thiol–ene polymerizations,5,6 Michael additions,7,8 oxime chemistry,9 and strain promoted azide–alkyne cycloadditions (SPAAC).10,11 PEG hydrogels prepared with SPAAC as a cross-linking chemistry have been particularly interesting, as this approach has been used in concert with other bio-orthogonal chemistries to create complex microenvironments with dynamic properties. For example, DeForest et al. demonstrated that the biochemical properties of cell-laden hydrogels formed from azide functionalized peptides and difluorocyclooctyne (DIFO) functionalized PEGs could be manipulated with precise spatiotemporal control using photoinitiated thiol–ene reactions to introduce new biomolecules into the hydrogel network.10 Further increasing the possibilities for user-directed manipulation of the cellular microenvironment, subsequent studies have demonstrated that, by employing photolabile chemistries in conjunction with SPAAC and thiol–ene click reactions, it is possible to sequentially conjugate a biomolecule to a network and then either photochemically release it to remove the biochemical signal12 or degrade the network to create physical features such as channels in the hydrogel.13 While these elegant approaches to creating cell-laden hydrogels with bio-orthogonal chemistries give researchers powerful tools with which to interrogate the roles of biological signals in the cellular microenvironment, their impact on the biomaterials field has not yet been fully realized because of the synthetic difficulty associated with strained cyclooctyne molecules required for SPAAC. Moreover, this problem has not been alleviated by the commercial availability of SPAAC reagents, as the high costs and limited selection of highly reactive cyclooctyne moieties such as DIFO remain prohibitive for many applications.
As an alternative synthetic approach, we hypothesized that the inverse electron demand Diels–Alder click reaction between tetrazine and an appropriate dienophile (e.g., norbornene, trans-cyclooctene) would have many of the same benefits as SPAAC, but with simpler synthetic routes, if the kinetics of the cross-linking chemistry were fast and compatible with cellular encapsulation. Notably, numerous studies have demonstrated tetrazine click reactions as powerful bio-orthogonal chemistries for in vitro and in vivo cell labeling and imaging.14−16 Tetrazine chemistry has also been leveraged for the creation of block copolymers without requiring any additional additives, initiators, or catalysts,17 suggesting that it could also be useful for forming covalently cross-linked polymer networks. Another attractive property of tetrazines is their synthetic tractability. Karver et al. described the synthesis of 12 different tetrazine molecules, all of which were obtained in fewer than three steps and in yields greater than 15%.18 In contrast, the second-generation DIFO molecule that we previously used (i.e., DIFO3) was synthesized in 12–13 steps and obtained in approximately 8% yield.19,20
Here, we report the tetrazine–norbornene click reaction as a new cross-linking chemistry suitable for the formation of cell-laden hydrogels for 3D cell culture. We specifically used a PEG functionalized with a benzylamino tetrazine moiety that was previously shown to have high reactivity toward norbornene.14 Hydrogels were cross-linked with an ECM mimetic cell degradable dinorbornene synthetic peptide. The kinetics of hydrogel formation were evaluated by rheological characterization during in situ polymerization. The equilibrium modulus and swelling ratio of hydrogels were also characterized. To evaluate the suitability of this chemistry for 3D cell culture applications, the postencapsulation viability of human mesenchymal stem cells (hMSCs) was evaluated. Finally, the potential for photochemical modification of hydrogel networks was explored.
Experimental Section
Hydrogel Precursors
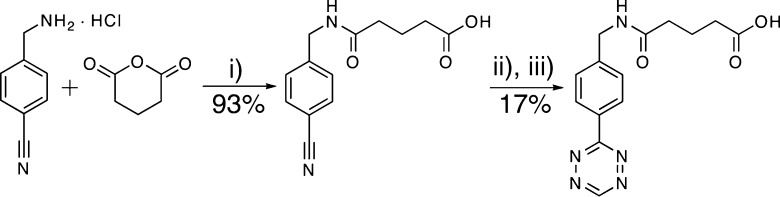
A clickable PEG-tetrazine (PEG-Tz) macromer was synthesized by coupling 5-(4-(1,2,4,5-tetrazin-3-yl)benzylamino)-5-oxopentanoic acid (Tz-COOH; see Scheme 1 for summary of synthetic route) to a multifunctional PEG-NH2 (four-arm, Mn ≈ 20 000 Da; JenKem Technologies USA) using standard acid-amine conjugation techniques. The PEG-Tz was estimated to be 75% functionalized, which should lead to a statistical distribution of polymer functionalization with an average functionality of 3. A dinorbornene cell degradable cross-linker peptide norb-KGPQGIWGQKK-norb and mononorbornene pendant peptides norb-AhxRGDS and norb-GGKGGC were synthesized using standard Fmoc solid phase peptide synthesis protocols and purified by reverse phase HPLC. Chemical structures of the hydrogel precursors and schematics of the cross-linking reaction and cellular encapsulation are presented in Figure 1. Complete synthetic details are presented in the Supporting Information.
Scheme 1. Synthesis of Tz-COOH.
(i) 1.1 equiv Et3N, CH3CN, reflux, 15 h. (ii) 18 equiv NH2–NH2, 4 equiv HN=CHNH2·CH3COOH, 1 equiv S, room temp, 20 h. (iii) CH3COOH, 5 equiv NaNO2, 0 °C, ∼ 1 h.
Figure 1.
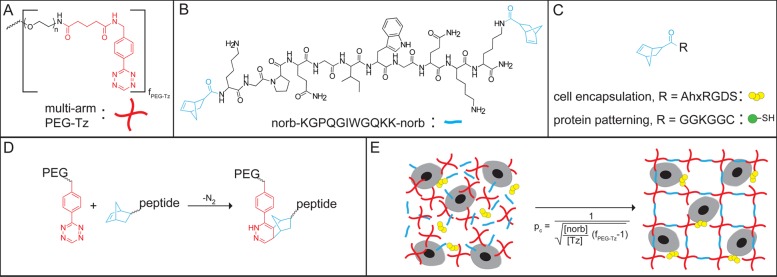
Overview of hydrogel formation by tetrazine-norbornene click chemistry. Chemical structures of (A) mutli-functional PEG-Tz, where fPEG-Tz is the average functionality of the polymer, (B) cell degradable dinorbornene cross-linker peptide, and (C) mononorbornene functionalized peptides used for cell encapsulation and protein patterning experiments. (D) Reaction between PEG-Tz and norbornene-functionalized peptide showing the cycloaddition product. (E) Idealized schematic for cell encapsulation (image not to scale).
Characterization of Hydrogel Properties
To evaluate the kinetics of gel formation, hydrogels were prepared using 7.5 wt % PEG-Tz (10.5 mM Tz) and 5.25 mM norb-KGPQGIWGQKK-norb in phosphate buffered saline (PBS) and polymerized in situ on a TA Instruments DHR-3 rheometer equipped with a 20 mm, 2° cone. A peltier plate was used to maintain the temperature at 22 °C, and a solvent trap was put in place to prevent the hydrogel from drying out during testing. During polymerization, the hydrogels were subjected to oscillatory shear at 10 rad/s and 10% strain and the evolution of the storage and loss moduli (i.e., G′ and G″) was monitored for 30 min.
In addition, hydrogels were prepared with the following stoichiometrically balanced formulations (i.e., 1:1 total Tz:norb) and stored in PBS at 37 °C for 2 days: (1) 10 wt % PEG-Tz (14 mM Tz), 6.5 mM norb-KGPQGIWGQKK-norb, and 1 mM norb-AhxRGDS; (2) 7.5 wt % PEG-Tz (10.5 mM Tz), 4.75 mM norb-KGPQGIWGQKK-norb, and 1 mM norb-AhxRGDS; (3) 5 wt % PEG-Tz (7 mM Tz), 3 mM norb-KGPQGIWGQKK-norb, and 1 mM norb-AhxRGDS. Equilibrium modulus measurements were made by subjecting these hydrogels to oscillatory shear, as described above, using an 8 mm parallel plate geometry. The gels were tested while immersed in a 37 °C water bath. The 10 wt % and 7.5 wt % gels were tested at 10 rad/s and 10% strain. The 5 wt % gels were tested at 1 rad/s and 1% strain in order to be within the linear viscoelastic regime for this formulation. Finally, wet and dry weights of the gels were recorded to calculate the mass swelling ratio for each formulation. Calculated values for the critical cross-link density required to achieve gelation, ρc, for each formulation are provided in the Supporting Information.
Cell Encapsulation
To evaluate the cytocompatibility of the tetrazine–norbornene cross-linking reaction, hMSCs isolated from human bone marrow were encapsulated in PEG-peptide hydrogels, and their viability was assessed via a membrane integrity assay at 24 and 72 h postencapsulation. Briefly, hMSCs were resuspended at a final cell density of 5 × 106 cells/ml in a solution of 7.5 wt % PEG-Tz, 4.75 mM norb-KGPQGIWGQKK-norb, and 1 mM norb-RGDS. The cell suspension was pipet mixed and then quickly transferred to sterile syringe tip molds (i.e., 1 mL syringes that had been cut to remove the tips and then inverted) in 30 μL aliquots. After allowing 15 min for gelation, the cell-laden hydrogels were transferred to a 24-well plate and cultured under standard conditions. hMSC viability was assessed using the commercially available Live/Dead staining kit (Invitrogen), which differentiates viable cells from dead cells based on membrane integrity. Stained hydrogels were imaged on a Zeiss LSM NLO laser scanning confocal microscope, and percent viability was determined by analysis in Image J.
Protein Photopatterning
To demonstrate orthogonality with photochemical patterning techniques, hydrogels were prepared using 7.5 wt % PEG-Tz, 4.75 mM norb-KGPQGIWGQKK-norb, and 1 mM norb-GGKGGC. After gelation, the gels were immersed in a solution of 0.1 mg/mL norbornene-functionalized fluorescein–bovine serum albumin (norb/FL-BSA; see SI for functionalization protocol) and 2.2 mM 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1- propanone (trade name I2959; Ciba) photoinitiator in PBS, incubated at room temperature on an orbital shaker for 2 h, and then irradiated with collimated UV light (365 nm, 10 mW/cm2, Omnicure lamp) through a chrome on a quartz photomask (100 μm lines with 100 μm spacing) for 10 min. The patterned gel was transferred to fresh PBS, incubated for 1 h at room temperature on an orbital shaker, and the patterning process was repeated with norbornene-functionalized tetrametheylrhodamine-BSA (norb/TAMRA-BSA). In the second patterning step, the chrome photomask was rotated approximately 90° to generate a grid pattern. Single and dual protein patterned hydrogels were imaged at 10X magnification through a water immersion lense on a Ziess widefield fluorescence microscope. Quantitative measurements of the patterned lines were made to confirm pattern fidelity.
Results and Discussion
To obtain hydrogels cross-linkable by tetrazine–norbornene click chemistry, we synthesized 5-(4-(1,2,4,5-tetrazin-3-yl)benzylamino)-5-oxopentanoic acid (i.e., Tz-COOH). Notably, this product was obtained in three steps over approximately 3–4 days in 16% total synthetic yield (Scheme 1), which is a significant improvement when compared to the synthetic routes for cyclooctynes required for SPAAC. Subsequent coupling to a multifunctional PEG-NH2 yielded a clickable PEG-Tz macromer with excellent water solubility (>20 wt %) and with high reactivity toward norbornene-functionalized macromolecules.
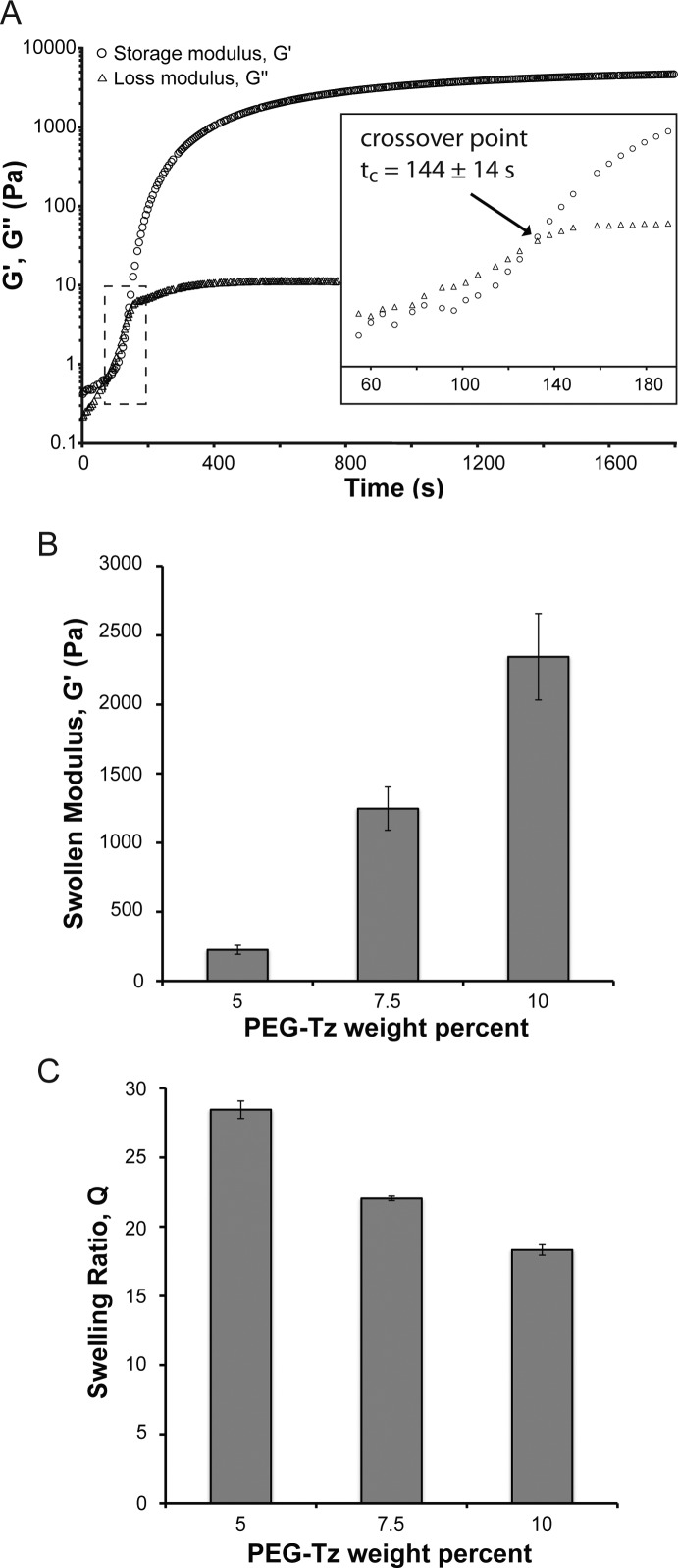
Importantly, although the average functionality of the PEG-Tz was ∼3, hydrogels formed readily at both ambient and near physiologic conditions when the PEG-Tz was mixed with a stoichiometrically balanced amount of a dinorbornene peptide cross-linker (ρc = 0.71; see Supporting Information). In general, all gels formed in <5 min, even at the lowest reactive group concentration (i.e., [norb] = [Tz] = 7 mM). Gaseous nitrogen was released as a byproduct during polymerization. Monitoring of the shear modulus evolution during in situ polymerization of 7.5 wt % PEG-Tz hydrogels (10.5 mM Tz, 5.25 mM norb-KGPQGIWGQKK-norb) showed that the crossover point, which marks the transition from liquid-like to solid-like behavior and is often used as an estimate of the gel point, was reached after just 144 ± 14 s (Figure 2A). Interestingly, these gelation kinetics are faster than what has been previously observed for DIFO3-based SPAAC cross-linked gels,10 which is consistent with the rate constants published in the literature (DIFO3-benzyl azide: k = 0.086 M–1 s–1; Tz-norbornene: k = 1.9 M–1 s–1).14,20 While the kinetics of tetrazine Diels–Alder reactions can be tuned by changing the dienophile (e.g., reactivity of transcyclooctene ≫ norbornene), norbornene, which is commercially available at low cost, appears to be ideal, allowing sufficient time for the user to prepare solutions without encountering premature gelation, but also reacting quickly enough to achieve gelation on a reasonable time scale. In fact, the reaction kinetics allowed facile synthesis of hydrogels with a wide range of material properties, simply by varying the weight percent of PEG-Tz in the initial monomer formulation. The equilibrium shear moduli of hydrogels prepared with 5, 7.5, and 10 wt % PEG-Tz and 1 mM norb-AhxRGDS were 225 ± 32, 1246 ± 156, and 2345 ± 312 Pa, respectively (Figure 2B; n = 3). The respective swelling ratios for these formulations were 28.4 ± 0.6, 22.0 ± 0.2, and 18.3 ± 0.4 (Figure 2C; n = 3). In interpreting this data, it should be noted that all three formulations contained 1 mM norb-AhxRGDS. While the incorporation of monofunctional peptides changes ρc in each formulation (see Supporting Information), this characterization provides useful information for cell encapsulation, as concentrations of adhesive peptides such as RGD are typically on the order of 1 mM.
Figure 2.
Characterization of hydrogel properties. (A) Representative plot of modulus evolution with an inset showing crossover at tc = 144 ± 14 s (n = 3). Hydrogels were prepared with 7.5 wt % PEG-Tz (10.5 mM Tz) and 5.25 mM dinorbornene cross-linker. (B) Swollen modulus and (C) swelling ratio of hydrogels prepared with the following formulations: (1) 5 wt % PEG-Tz (7 mM Tz), 1 mM norb-AhxRGDS, 3 mM dinorbornene cross-linker; (2) 7.5 wt % PEG-Tz (10.5 mM Tz), 1 mM norb-AhxRGDS, 4.75 mM dinorbornene cross-linker; (3) 10 wt % PEG-Tz (14 mM Tz), 1 mM norb-AhxRGDS, 6.5 mM dinorbornene cross-linker (n = 3).
To demonstrate the tetrazine–norbornene click reaction as a cytocompatible cross-linking chemistry for primary cell encapsulation, hMSCs were encapsulated in ECM mimetic hydrogels using the 7.5 wt % formulation with 1 mM norb-AhxRGDS. Notably, the dinorbornene peptide cross-linker shown in Figure 1B was designed to be susceptible to degradation by cell secreted matrix metalloprotease (MMP) enzymes, as it contains the MMP cleavable sequence GPQGIWGQ derived from type I collagen.21 MMP degradability is desirable for three-dimensional (3D) cell culture because it allows for cell spreading and migration within PEG hydrogels.22,23 In addition, to be consistent with the prior studies on hMSC encapuslation in synthetic hydrogels,5,24−26 the fibronectin mimetic monofunctional norb-AhxRGDS peptide was incorporated at 1 mM to promote integrin-mediated cell attachment, which can be critical for maintaining the viability of anchorage-dependent cells such as hMSCs.26 Following gel polymerization, the hMSC laden hydrogels were cultured under standard conditions, and cell viability was assessed at 24 and 72 h via Live/Dead staining, which discriminates dead cells from viable cells based on membrane integrity (Figure 3). Quantitative analysis of images obtained by laser scanning confocal microscopy showed that the viability of hMSCs was excellent at 24 h postencapsulation (92 ± 3%; n = 10 images taken from two gels), clearly demonstrating the cytocompatibility of the cross-linking reaction. Furthermore, cell viability remained high after 72 h (79 ± 6%; n = 10 images taken from two gels), although the low degree of cell spreading seen at high magnification suggests that future optimization of the hydrogels may be needed. In a synthetic PEG hydrogel system with a lower cross-linking density and shear modulus (∼700 Pa), Fairbanks et al. noted a significant effect of RGD concentration and gel degradability on hMSC spreading at 6 days.5 Thus, future investigation will focus on tuning the stiffness, degradability, and RGD concentration to promote hMSC spreading.
Figure 3.
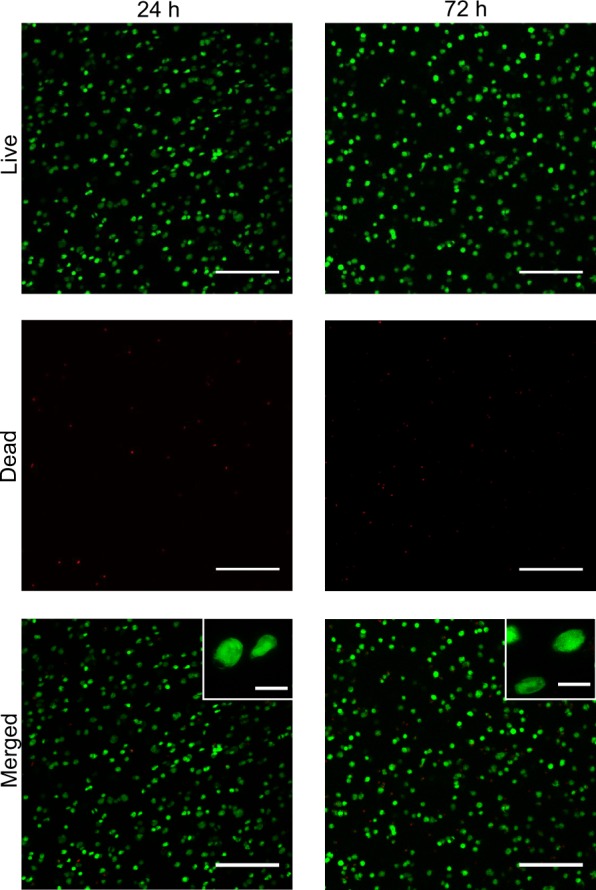
Representative Live/Dead images showing high viability of hMSCs at 24 and 72 h postencapsulation. Cells were encapsulated at 5 × 106 cells/ml in 7.5 wt % PEG-Tz hydrogels containing 1 mM norb-AhxRGDS peptide. Low magnification images are projections of 300 μm z-stacks. High-magnification insets show cell morphology. % Live at 24 h = 92 ± 3%. % Live at 72 h = 79 ± 6% (note: green cells = live, red cells = dead, scalebars = 200 and 20 μm for low- and high-magnification images, respectively; n = 10 images from two gels).
The final aspect of this study was to explore the potential for photochemical modification of the hydrogel network by exploiting the specificity of the initial tetrazine click reaction. Importantly, photochemical reactions provide a versatile means for user-controlled manipulation of cellular microenvironments. To demonstrate that tetrazine–norbornene click hydrogels can be used in combination with photochemical patterning to create dynamic hydrogel microenvironments, PEG-peptide hydrogels were prepared using the same 7.5 wt % PEG-Tz formulation as for cell encapsulation, except that the hetero-bifunctional peptide with the sequence norb-GGKGGC (Figure 1C) was incorporated into the hydrogels at 1 mM rather than the norb-AhxRGDS peptide. This formulation introduced pendant thiols, which were subsequently used to modify the chemical microenvironment with precise spatiotemporal control using a photoinitiated thiol–ene click reaction. Here, we photopatterned norbornene-functionalized fluorescent BSA proteins. Although protein coupling to unreacted tetrazine within the hydrogel could potentially occur when using this approach, the initial tetrazine–norbornene cross-linking reaction was stoichiometrically balanced to minimize this possibility. Photocoupling was achieved using a collimated UV light source (365 nm) and I2959 as the photoinitiator. In a first patterning step, a stripped pattern (100 μm lines spaced 100 μm apart) of fluorescein-labeled BSA was generated. Minimal background fluorescence was observed. The process was then repeated with tetramethylrhodamine labeled BSA. During the second patterning step, the photomask was rotated approximately 90°, resulting in four distinct biochemical environments (i.e., nonpatterned, FL-BSA, TAMRA-BSA, and double-patterned; Figure 4). Excellent pattern fidelity was achieved for both steps, as the measured line widths were 101 ± 1 μm (n = 12). These results demonstrating sequential photopatterning are comparable to what has been previously reported using SPAAC and copper click cross-linked PEG hydrogels.10,27
Figure 4.
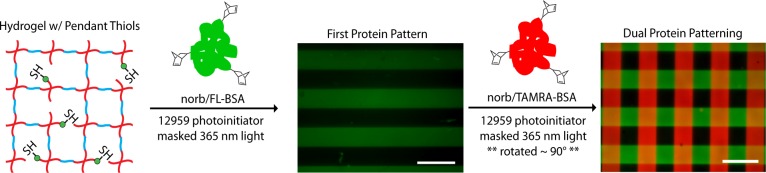
Sequential biochemical patterning in tetrazine click gels. To demonstrate the potential for biochemical patterning, 7.5 wt % tetrazine click hydrogels were formed with 1 mM norb-GGKGGC, leaving pendant thiols in the network. Aqueous solutions of norbornene/fluorophore labeled bovine serum albumin (norb/FL-BSA and norb/TAMRA-BSA, 0.1 mg/mL) and the photoinitiator 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1- propanone (I2959, 2.2 mM) were sequentially swollen into the network and patterned through a photomask using collimated UV light (365 nm, 10 mW/cm2, 10 min) to create a grid pattern (100 μm lines, 100 μm spacing). High patterning fidelity was observed, as measured line widths were 101 ± 1 μm (note: epifluorescent images of the pattern at the gel surface; scalebars =200 μm; n = 12 for line width measurements).
Conclusion
In summary, this communication presents the tetrazine-norbornene click reaction as a powerful new cross-linking chemistry to synthesize hydrogels for cellular encapsulation and 3D culture. The bio-orthogonality, ideal reaction kinetics, and amenability to photochemical patterning render this hydrogel platform useful for a variety of fundamental as well as translational tissue engineering applications. The key advantage, however, is the synthetic accessibility of the tetrazine moiety, which should make this hydrogel platform more broadly useful to researchers in materials development than previously described click hydrogel platforms and enable the design of highly defined cell-instructive matrices for applications ranging from stem cell biology to tissue regeneration.
Acknowledgments
The authors thank Dr. Kelly Schultz for assistance in the rheological characterization and Prof. Matthew Liberatore of the Department of Chemical and Biological Engineering at Colorado School of Mines for access to rheological equipment. This work was funded in part by grants from the National Institutes of Health (5R01DE016523), the National Science Foundation (DMR 1006711), and the Howard Hughes Medical Institute.
Supporting Information Available
Synthetic procedures, NMR, mass spectroscopy, experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Tibbitt M. W.; Anseth K. S. Biotechnol. Bioeng. 2009, 103, 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M. P.; Hubbell J. A. Nat. Biotechnol. 2005, 23, 47. [DOI] [PubMed] [Google Scholar]
- Cruise G. M.; Hegre O. D.; Lamberti F. V.; Hager S. R.; Hill R.; Scharp D. S.; Hubbell J. A. Cell Transplant 1999, 8, 293. [DOI] [PubMed] [Google Scholar]
- Elisseeff J.; McIntosh W.; Anseth K.; Riley S.; Ragan P.; Langer R. J. Biomed. Mater. Res. 2000, 51, 164. [DOI] [PubMed] [Google Scholar]
- Fairbanks B. D.; Schwartz M. P.; Halevi A. E.; Nuttelman C. R.; Bowman C. N.; Anseth K. S. Adv. Mater. 2009, 21, 5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H.; Lin C. C. Biomacromolecules 2012, 13, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf M. P.; Lauer-Fields J. L.; Schmoekel H. G.; Metters A. T.; Weber F. E.; Fields G. B.; Hubbel J. A. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A.; Enemchukwu N. O.; Fiore V. F.; Sy J. C.; Murthy N.; Sulchek T .A.; Barker T. H.; Garcia A. J. Adv. Mater. 2012, 24, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover G. N.; Lam J.; Nguyen T. H.; Segura T.; Maynard H. D. Biomacromolecules 2012, 13, 3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForest C. A.; Polizzotti B. D.; Anseth K. S. Nat. Mater. 2009, 8, 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J.; Smith Callahan L. A.; Hao J.; Guo K.; Wesdemiotis C.; Weiss R. A.; Becker M. L. ACS Macro Lett. 2012, 1, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForest C. A.; Anseth K. S. Angew. Chem., Int. Ed. Engl. 2012, 51, 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForest C. A.; Anseth K. S. Nat. Chem. 2011, 3, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj N. K.; Weissleder R.; Hilderbrand S. A. Bioconjug. Chem. 2008, 19, 2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj N. K.; Thurber G. M.; Keliher E. J.; Marinelli B.; Weissleder R. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitchik J. L.; Peeler J. C.; Taylor M. T.; Blackman M. L.; Rhoads T. W.; Cooley R. B.; Refakis C.; Fox J. M.; Mehl R. A. J. Am. Chem. Soc. 2012, 134, 2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell C. F.; Espeel P.; Stamenovic M. M.; Barker I. A.; Dove A. P.; Du Prez F. E.; O’Rielly R. K. J. Am. Chem. Soc. 2011, 133, 13828. [DOI] [PubMed] [Google Scholar]
- Karver M. R.; Weissleder R.; Hilderbrand S. A. Angew. Chem., Int. Ed. Engl. 2012, 51, 920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims E. A.; Deforest C. A.; Anseth K. S. Tetrahedron Lett. 2011, 52, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codelli J. A.; Baskin J. M.; Agard N. J.; Bertozzi C. R. J. Am. Chem. Soc. 2008, 130, 11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.; Hubbell J. A. Biomaterials 2010, 31, 7836. [DOI] [PubMed] [Google Scholar]
- Raeber G. P.; Lutolf M. P.; Hubbell J. A. Biophys. J. 2005, 89, 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin A. S.; West J. L. FASEB J. 2002, 16, 751. [DOI] [PubMed] [Google Scholar]
- Jongpaiboonkit L.; King W. J.; Murphy W. L. Tissue Eng., Part A 2009, 15, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas C. N.; Anseth K. S. J. Tissue Eng. Regener. Med. 2008, 2, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttelman C. R.; Tripodi M. C.; Anseth K. S. Matrix Biol. 2005, 24, 208. [DOI] [PubMed] [Google Scholar]
- Polizzotti B. D.; Fairbanks B. D.; Anseth K. S. Biomacromolecules 2008, 9, 1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.