Abstract
Bivalve molluscs are newly discovered models of successful aging. Here, we test the hypothesis that extremely long-lived bivalves are not uniquely resistant to oxidative stressors (eg, tert-butyl hydroperoxide, as demonstrated in previous studies) but exhibit a multistress resistance phenotype. We contrasted resistance (in terms of organismal mortality) to genotoxic stresses (including topoisomerase inhibitors, agents that cross-link DNA or impair genomic integrity through DNA alkylation or methylation) and to mitochondrial oxidative stressors in three bivalve mollusc species with dramatically differing life spans: Arctica islandica (ocean quahog), Mercenaria mercenaria (northern quahog), and the Atlantic bay scallop, Argopecten irradians irradians (maximum species life spans: >500, >100, and ~2 years, respectively). With all stressors, the short-lived A i irradians were significantly less resistant than the two longer lived species. Arctica islandica were consistently more resistant than M mercenaria to mortality induced by oxidative stressors as well as DNA methylating agent nitrogen mustard and the DNA alkylating agent methyl methanesulfonate. The same trend was not observed for genotoxic agents that act through cross-linking DNA. In contrast, M mercenaria tended to be more resistant to epirubicin and genotoxic stressors, which cause DNA damage by inhibiting topoisomerases. To our knowledge, this is the first study comparing resistance to genotoxic stressors in bivalve mollusc species with disparate longevities. In line with previous studies of comparative stress resistance and longevity, our data extends, at least in part, the evidence for the hypothesis that an association exists between longevity and a general resistance to multiplex stressors, not solely oxidative stress. This work also provides justification for further investigation into the interspecies differences in stress response signatures induced by a diverse array of stressors in short-lived and long-lived bivalves, including pharmacological agents that elicit endoplasmic reticulum stress and cellular stress caused by activation of innate immunity.
Key Words: Arctica islandica, Bivalves, Comparative biology, Endoplasmic reticulum stress, Longevity, Oxidation, Stress resistance.
WITHIN the class Bivalvia, maximum life span differs more than 200-fold, more than any other noncolonial animal group (from in excess of 500 years (1) to less than 2 years) (2). This natural variation of life span makes them ideal model organisms to test predictions of major hypotheses of aging (3–15). Using the burrowing clam Arctica islandica (ocean quahog), which is the longest lived of all noncolonial animal species on earth (maximum species life span: >500 years) (1,7,8,16), we recently demonstrated that in bivalve models of extreme longevity there is an association between maximum species life-span potential and resistance to oxidative stress–induced mortality and also a marked resistance to oxidative stress–induced apoptotic cell death (17).
Originally studies in the invertebrate model organism Caenorhabditis elegans have suggested that antiaging genetic mutations extend life span by simultaneous activation of multiple cellular pathways that protect the tissues and organs from multiple forms of injury (18). Longevity evolved independently many times in various phyla and a wide range of comparative studies is needed to prove that expression of a multistress resistance phenotype is a key mechanism of successful aging, which is conserved among these various long-lived groups (19). The hypothesis that the ability to develop resistance to multiple stressors is associated with life span has been supported, at least in part, by recent studies showing that an association exists between superior cellular stress resistance and increased species longevity in insects (20–22), rodents (23–29), bats (30), and birds (31). These discoveries led to the development of unifying multiplex stress resistance models of aging (18,32). These models imply that an organism’s ability to resist a multitude of environmental and cytotoxic stressors is associated with enhanced life span (33). It is yet to be determined whether this model is valid for bivalves. It has been hypothesized that maximal life spans of benthic communities (such as species of clam) evolve in relation to long-term cycles in benthic communities (34), which exert selective pressure for longevities somewhat longer than the cycle affecting recruitment. In support of this, long periods of failed recruitment have been reported for populations of A islandica (1). Under such evolutionary pressures, somatic maintenance will be favored more than reproductive output and one would expect to see enhanced cellular defense and repair mechanisms in those species (35). Although bivalves are unlikely to be exposed to the toxicants used in this study, their comparative resistance to these stressors is indicative of their ability to resist stressors and repair damage.
This study was designed to test the hypothesis that extremely long-lived molluscan species are not uniquely resistant to oxidative stressors but exhibit a multistress resistance phenotype. To test this hypothesis, we contrasted resistance to genotoxic stresses and to mitochondrial oxidative stressors in three bivalve mollusc species with dramatically differing life spans. We tested whether resistance to genotoxic stresses is associated with exceptional longevity because recent studies raise the possibility that DNA repair efficiency associates with life span in a diverse group of mammals (including muroid rodents (28,36), bats, and primates; Podlutsky and Austad, unpublished data, 2009). Moreover, a decline in DNA repair capacity has been linked to premature aging, cancer, and short life span in multiple models of aging. In this study, we focused on A islandica and the taxonomically related burrowing clam, Mercenaria mercenaria (northern quahog), which lives in a similar environment, has similar physiology, but has a shorter life span (maximum species life span: >100 years, Table 1). Recent studies have characterized several aspects of A islandica and M mercenaria physiology, which render these species especially useful models for aging research (1,6,17,37,38). It has been documented that both clam species used in this study are exceptionally long lived (1,13,38) and that A islandica demonstrates negligible rates of aging, little age-related decline in antioxidant capacity, nor increase in macromolecular damage (7), and an increased resistance to cellular stress than its shorter lived relative (17). Although early studies investigating the exceptional longevity of bivalves implicated superior antioxidant status (7), this is now not believed to be the case (35). Here, we test the hypothesis that extremely long-lived molluscan species are not uniquely resistant to oxidative stressors but exhibit a multistress resistance phenotype with longer lived species having evolved superior defense and repair mechanisms. We contrasted multistress resistance in the aforementioned species with that in the short-lived Atlantic bay scallop (Argopecten irradians irradians; maximum species life span: 2 years, Table 1). We assessed organismal mortality in response to both mitochondrial oxidative stressors and genotoxic DNA damaging agents, including topoisomerase inhibitors and agents that cross-link DNA or impair genomic integrity through DNA alkylation or methylation.
Table 1.
Chronological Age, Maximum Reported Life Span, and Physiological Characteristics of the Marine Bivalve Species Used in This Study
| Species | Common Name | Average Chronological Age (y) | Maximum Life Span (y) | Maximum Size (mm) | Growth Rate (K; VBGF) | Mortality Rate (Z) | Age at Maturity (y) | Lifestyle | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Artica islandica | Ocean quahog, mahogany clam | ~20 | >500 | 118 | 0.02 | 0.03 | 7–14 | Infaunal burrower | 16, 83 | |||||||||
| Mercenaria mercenaria | Northern quahog, hard clam | ~8 | 106 | 150 | 0.210 | 1.32 | 2–5 | Infaunal burrower | 3, 17, 84 | |||||||||
| Argopecten irradians irradians | Atlantic bay scallop | ~1 | 2 | 60 | n/a | 1.41 | 1 | Active swimmer | 85 |
Note: n/a = not applicable; VGBF = von Bertalanffy growth function.
Methods
Clam Collection and Maintenance
The extremely long-lived ocean quahog (A islandica), the long-lived northern quahog (M mercenaria), and the short-lived bay scallop (A i irradians) were used in this study. Maximum species life span and physiological characteristics for each species are shown in Table 1. All clams used in this study were collected in July 2011 in the coastal waters of New England. In the coastal waters of New England, where these specimens were collected from A islandica, for M mercenaria and A i irradians are estimated to have maximum life spans of 220, 106, and 2 years, respectively (38–40). The clams were transported to the Marine Aquatic Resources Center of the Marine Biological Laboratory (Woods Hole, MA), where they were kept at constant temperature (12°C for A islandica, 20°C for M mercenaria and A i irradians, which typically live in warmer water than A islandica) in 500-L tanks for more than 1 week prior to the studies.
Studies on Stress Resistance
For each experimental condition, 10–15 individuals of each species (A islandica, M mercenaria, and A i irradians) were placed in 10-L plastic aquaria containing fresh sea water. Periodically, aquaria were checked to observe siphons, which indicated that the stressors were inhaled. Additionally to ensure infiltration of the stressors, a section of the ventral margin of the shell of both clam species (A islandica and M mercenaria) was carefully removed, minimizing damage to tissues, using a handheld rotary saw. This prevented the animals sealing themselves off from the external environment, which they can do for prolonged periods of time (1). To induce mitochondrial oxidative stress, animals were treated with paraquat (1,1′-dimethyl-4,4′-bipyridinium dichloride; 1 mmol/L), which induces reactive oxygen species (ROS) production in mitochondria, or rotenone (25 µmol/L), which inhibits complex I and generates ROS in mitochondria. A range of genotoxic DNA damaging agents were used during the investigation. Topoisomerases participate in cellular processes associated with separation of DNA strands such as replication, transcription, recombination, and repair, and inhibitors of topoisomerases ultimately induce DNA breaks. To induce genotoxic stress by inhibition of the topoisomerase, we used the topoisomerase II poisons etoposide (0.1mg/L) and camptothecin (50 nmol/L), a cytotoxic quinoline alkaloid, which inhibits topoisomerase I preventing DNA religation and therefore causes DNA damage and apoptosis. We also used epirubicin (1mg/L), which acts by intercalating into DNA strands, which results in formation of complexes inhibiting DNA and RNA synthesis. It also triggers DNA cleavage and promotes the generation of ROS that cause cell and DNA damage. Mechlorethamine hydrochloride (nitrogen mustard, 100 µmol/L) was used as an alkylating agent that acts by binding to DNA, cross-linking two strands, and preventing cell duplication. We also tested the affects on organismal survival of methyl methanesulfonate (MMS; 200 µmol/L), which methylates DNA, stalling replication forks, and mitomycin C (100 nmol/L) as well as cisplatin (1mg/L), which are potent DNA cross-linkers. To study organismal resistance to mitochondrial oxidative stress and genotoxic stresses, the survival of A islandica, M mercenaria, and A i irradians exposed to the aforementioned drugs was recorded for up to 15 days. During the stressor exposure, M mercenaria and A i irradians were held at 20°C, whereas A islandica were held at 10°C. The optimum concentrations of each chemical used were selected on the basis of a review of the literature and lethal doses used in previous in vivo and in vitro investigations with other species. Tanks were checked regularly and death was recorded when the bivalve shell “gaped” (opened because their adductor muscles no longer functioned) and did not respond to external stimuli.
Data Analysis
Survival curves were compared using the logrank test in GraphPad Prism 4.0 software. p < .05 was considered statistically significant. Data are expressed as means ± SEM, unless otherwise indicated (41–44).
Results
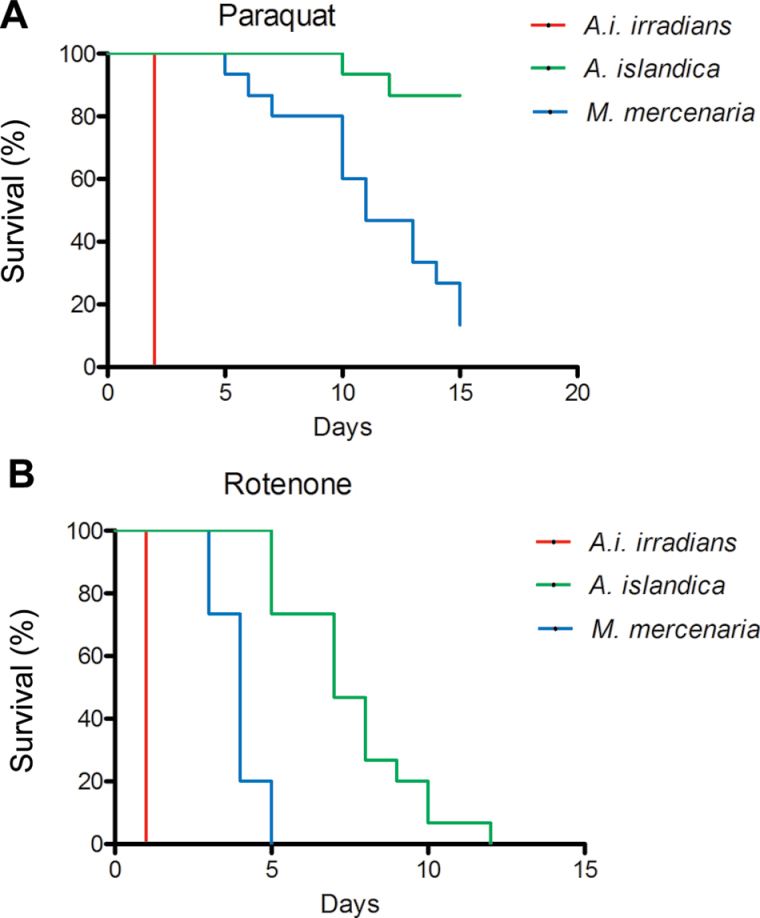
To assess resistance to mitochondrial oxidative stress, we obtained survival curves of the clams in the presence of rotenone and paraquat. In the case of both paraquat (Figure 1A) and rotenone (Figure 1B), A islandica were significantly the most resistant to mortality, as compared with both M mercenaria (p < .0001) and A. i. irradians (p < .0001). Mercenaria mercenaria were significantly more resistant to mortality than A i irradians (p < .0001). Argopecten irradians irradians were particularly susceptible to both, paraquat and rotenone, with all specimens dead after 1 and 2 days, respectively.
Figure 1.
Survival analysis of Argopecten irradians irradians, Mercenaria mercenaria, and Artica islandica underexposure to the mitochondrial oxidative stressors paraquat (1 mmol/L; A) and rotenone (25 µmol/L; B).
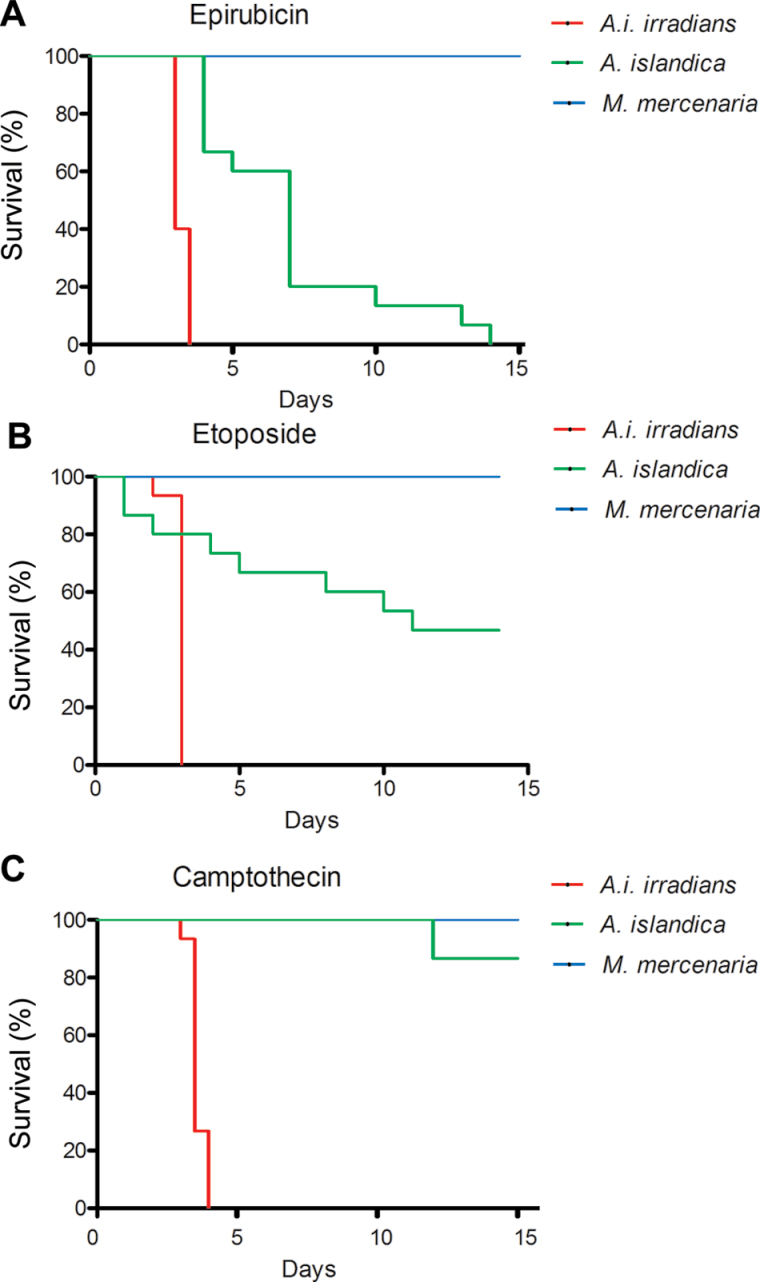
Next, we assessed organismal resistance to the genotoxic stressors epirubicin (Figure 2A), etoposide (Figure 2B), and camptothecin (Figure 2C). No mortality was observed in M mercenaria after 15 days exposure to any of the three stressors and again A i irradians was significantly least resistant to all three stressors (p < .0001 with all comparisons and stressors). In the case of epirubicin and etoposide, A islandica was less resistant than M mercenaria (p < .0001 with both stressors), but with camptothecin, no significant difference in survival was observed between the two species after 15 days of exposure (p = .15).
Figure 2.
Survival analysis of Argopecten irradians irradians, Mercenaria mercenaria, and Artica islandica underexposure to the DNA intercalating agent, epirubicin, the topoisomerase II inhibitor etoposide, and the topoisomerase I inhibitor, camptothecin: (A) 1mg/L epirubicin, (B) 0.1mg/L etoposide, and (C) 50 nmol/L camptothecin.
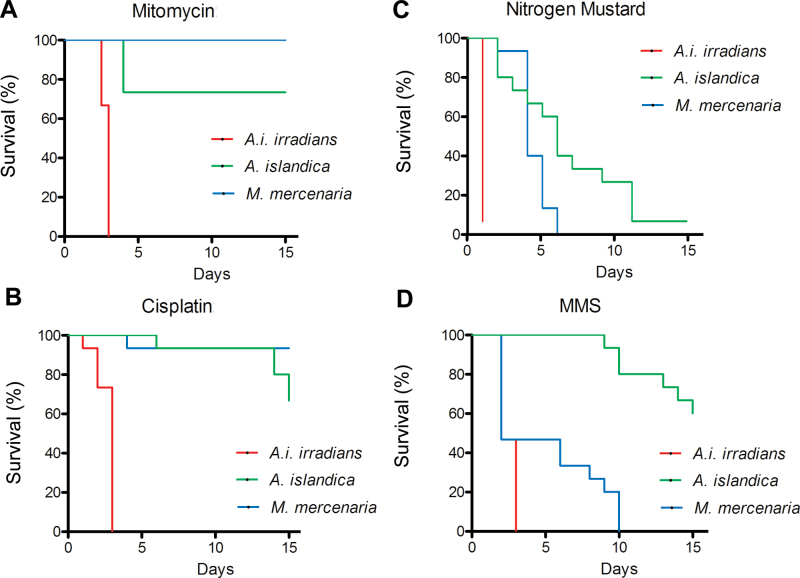
We also assessed organismal resistance to a second set of genotoxic drugs, including mitomycin C (Figure 3A), cisplatin (Figure 3B), nitrogen mustard (Figure 3C), and MMS (Figure 3D). For any of these DNA damaging agents, A i irradians was again significantly less resistant to mortality than the two longer lived species (p < .0001, in all cases). There was no significant difference in survival rates of A islandica and M mercenaria when exposed to cisplatin (p = .0881), whereas M mercenaria was significantly more resistant to mortality induced by mitomycin C than the longer lived A. islandica (p < .05). However, in the case of the other two genotoxic agents, M mercenaria was significantly less resistant to mortality than the longer lived A islandica (MMS, p < .0001; nitrogen mustard, p < .05).
Figure 3.
Survival analysis of Argopecten irradians irradians, Mercenaria mercenaria, and Artica islandica underexposure to the DNA cross-linking agents, mitomycin and cisplatin, the DNA alkylating agent, nitrogen mustard, and the DNA methylating agent, methyl methanesulfonate (MMS): (A) 100 nmol/L mitomycin, (B) 1mg/L cisplatin, (C) 100 µmol/L nitrogen mustard, and (D) 200 µmol/L MMS.
To summarize, we exposed the three species of bivalves to nine different stressors. With all stressors, the short-lived A i irradians were significantly less resistant to mortality than the two longer lived species, M mercenaria and A islandica. Arctica islandica were consistently more resistant than M mercenaria to mortality induced by mitochondrial oxidative stressors as well as to mortality induced by the DNA methylating agent nitrogen mustard and the DNA alkylating agent MMS. In case of the genotoxic DNA cross-linking agents mitomycin and cisplatin, M mercenaria were the most resistant species. In contrast, M mercenaria tended to be more resistant to the DNA intercalating agent epirubicin and the genotoxic stressors etoposide and campthotecin, which cause DNA damage by inhibiting topoisomerases.
Discussion
Accumulating empirical data obtained in diverse vertebrate species and invertebrate model organisms suggest that resistance to the aging process is often reflected in resistance to oxidative stressors both at the cellular and organismal level (17,45,46). The first interesting finding in this study is that in bivalve models of extreme longevity, we documented an association between species life span and organismal resistance to the mitochondrial oxidative stressors paraquat and rotenone (Figure 1A,B). These findings are in accordance with the conclusions of our recent studies documenting an association between longevity and resistance to mortality induced by the oxidative stressor tert-butyl hydroperoxide and also marked cellular resistance to tert-butyl hydroperoxide stress–induced apoptotic cell death in long-lived A islandica, as compared with the shorter lived M mercenaria (17) and A i irradians (Ungvari, Csiszar, and Ridgway, unpublished data, 2011). These findings also extend previous observations in a wide variety of experimental settings, ranging from invertebrate model organisms to laboratory rodents and primate fibroblasts (23,45–47). Recent findings in genetically manipulated laboratory mice (48–58) question the classical interpretation of the oxidative stress theory that increased levels of ROS per se play a key role in the aging process (59,60). Yet, the remarkable correlation between longevity and the increased oxidative stress resistance phenotype of longer lived animals in evolutionarily distant phyla is consistent with the existence of evolutionarily highly conserved pathways involved in both cellular oxidative stress resistance and life-span regulation, providing support for the oxidative stress hypothesis of aging.
The mechanisms underlying the increased cellular resistance to mitochondrial oxidative stress in long-lived bivalves are likely multifaceted. Previous studies demonstrate that differences in the efficiency of cellular antioxidant systems likely do not explain the superior oxidative stress resistance of A islandica (6,17). Recent studies also suggest that A islandica also does not exhibit a more pronounced homeostatic antioxidant response than shorter lived clams (17). In that context, it is interesting to note that in mice with overexpression or genetic knockout of major mitochondrial antioxidant enzymes (including MnSOD, catalase, and glutathione peroxidase), there is also no correlation between alterations of cellular antioxidant capacity and life span (48,50). Maintenance of protein homeostasis is also thought to be a critical determinant of both cellular stress resistance and life span (30,61), thus, further studies are warranted to determine whether in bivalves extreme longevity and resistance to oxidative stressors is, at least in part, due to enhanced protein recycling activities (17). Interestingly, resistance to mitochondrial oxidative stress in A islandica appears to associate with a low metabolic rate (at the temperatures used in these experiments respiration rates of 0.1, 0.7, and 1.0mL O2/g/h for A islandica (62), A i irradians (40), and M mercenaria (63), respectively, have been observed). Thus, further studies are warranted to test whether an association between basal metabolic rate and resistance to mitochondrial oxidative stressors exists in a range of bivalve species (eg, in long-lived clam species living in tropical waters, such as the giant clam Tridacna derasa, which was reported to exhibit resistance to organic peroxide) (64).
We tested the hypothesis that in extremely long-lived molluscan species, resistance to mitochondrial oxidative stressors is associated with a multistress resistance phenotype. We focused on genotoxic stress resistance, as there is strong evidence that DNA mutations, DNA damage, and chromosomal abnormalities increase with age in mammals (65–68). Moreover, the existing data demonstrate an inverse relationship between DNA repair capacity and age in a number of experimental settings. Importantly, there is evidence supporting the view that in mammalian species a correlation exists between efficiency of DNA repair mechanisms (nucleotide excision repair, poly [ADP-ribose] polymerase [PARP] activity) and species life-span potential (69–72). Recent studies also demonstrate that long-lived C elegans mutants are resistant to DNA damaging agents and ultraviolet irradiation, likely due to genetically determined increases in nucleotide excision repair capacity (73). As we predicted, with all genotoxic stressors tested, the short-lived A i irradians were significantly less resistant to mortality than the two longer lived species, M mercenaria and A islandica (Figure 2 and Figure 3). The high susceptibility of A i irradians to genotoxic stressors could relate to intense proliferation rates in the animals compared with the clams such as A islandica (9). In vitro treatment of cultured mammalian cells with various genotoxic agents induces apoptosis within days by activating signaling events that couple DNA damage to the mitochondrial pathway of apoptosis (74). Because the greatest portion of mortality occurred in A i irradians over a relatively short period, future studies should also investigate interspecies differences in activation of the mitochondrial apoptotic pathway in response to DNA damage.
The data presented here suggests that both species of bivalves with a maximum life-span potential over a century have evolved regulated cellular processes to minimize the accumulated DNA damage or mutation. Further work is now required to investigate how DNA repair mechanisms differ between short-lived and long-lived species and how these are associated with incidences of neoplasias in species of bivalve. To date, only a few primary proliferative diseases (eg, sarcoma) have been reported in bivalves (75–77). The Registry of Tumours in Lower Animals contains a single report of gonadal neoplasia in A islandica (75), however, due to the lack of research effort, few reliable incidence statistics are available. Future work should also seek to define the relationship between cellular resistance to genotoxic stresses and expression and/or activity of proteins (eg, p53, PARP-1) that participate in DNA damage response pathways and to proteins that control DNA damage–induced changes in cell cycle regulation.
Contrary to our predictions, comparison of resistance to genotoxic stresses in A islandica and M mercenaria provides contrasting results. Although the longest living species, A islandica, was more resistant to mortality induced by the DNA methylating agent, nitrogen mustard, and the DNA alkylating agent, MMS, than the relatively shorter living M mercenaria (Figure 3C,D), the same trend was not observed for genotoxic agents that act through cross-linking DNA (Figure 3A,B). Furthermore, we found that M mercenaria tended to be more resistant to epirubicin and to genotoxic stressors, which cause DNA damage by inhibiting topoisomerases (Figure 2). Taken together, the aforementioned data do not fully support the validity of the hypothesis that in relatively long-lived burrowing clams a close association exists between longevity and a general resistance to multiplex stressors. Interestingly, although previous studies in mammals observed a general association between increased cellular resistance to multiplex stresses and longevity, they also observed incidences where the longer lived species did not exhibit greater resistance to all stressors (78). For example, cells from the long-lived naked mole rat are more sensitive than mouse cells to H2O2, ultraviolet light, rotenone, and to pharmacological agents that elicit endoplasmic reticulum stress (including tunicamycin and thapsigargin) (24). Further, cells of long-lived bat species are resistant to gamma-irradiation but are more sensitive to ultraviolet irradiation than cells of the shorter lived M musculus (Dr. Andrej Podlutsky, personal communication 2009). It is a likely explanation for the inconsistent pattern of differential stress resistance in the aforementioned cases that the different stress responsive and DNA repair pathways are not closely coordinated. We predict that the relative role of cellular DNA repair mechanisms and other stress resistance pathways in the evolution of longevity in the different animal groups depends on the relative importance of the different types of stress-induced macromolecular damages in the aging process and pathophysiology of these species.
Limitations of the Study
Although we have knowledge of the relationship between age and size in the species studied, we did not ascribe age to each of the animals used. Yet, all animals in the study could be classified as young sexually mature adults below the maximum asymptotic size attained for each of the species. Although all animals were deemed sexually mature, we did not control for sex or reproductive status, however, a review of the literature provides little evidence of any indication of gender effects on susceptibility to stress in bivalves (79,80). Additionally at Ocean Sciences, Bangor University, we have analyzed the ages of more than 1,000 individuals from a specific population of A islandica and found no significant difference in longevity between the sexes (unpublished data).
Future studies should also seek to use a greater range of species and investigate cellular pathways involved in resistance to genotoxic stresses, including DNA repair pathways. Although no continuous cell line has been developed from marine bivalves and efforts to establish stable primary tissue cell cultures have been problematic, recent work demonstrates stable hemocyte cell cultures could be maintained for up to 1 week. Using such cell cultures, assessment of cellular DNA repair efficiency using the comet assay is technically feasible. Additionally, future studies should monitor toxicant concentrations in the seawater during the exposure periods to understand the role of differential uptake of the toxicants attributed to interspecies differences in respiratory anatomy and ventilation rate on the observed mortalities.
We have used the quinone-binding site inhibitor rotenone to inhibit complex I, which is known to significantly increase superoxide production rates during forward electron transport (81). Although rotenone is a useful tool in laboratory studies to test resistance to mitochondrial oxidative stress, it also interferes with ATP production, which may importantly contribute to its toxic effect in vivo. Because the lipophilic rotenone is easily taken up through the gills, it is highly toxic to aquatic life. Because no direct measurements of ROS production in vivo were recorded in this study, we cannot exclude that the different mortality curves observed were, in part, due to differential sensitivity of respiratory inhibition. To substantiate our findings, we have also used paraquat to induce mitochondrial oxidative stress. Paraquat, upon its carrier-mediated uptake to the mitochondrial matrix, is reduced by NADPH dehydrogenases to form the paraquat radical cation that then reacts with oxygen to form superoxide (82), which is responsible for mitochondrial injury. Importantly, both long-lived species exhibited similar resistance to the two different mitochondrial stressors.
Conclusions
To our knowledge, this is the first study comparing resistance to genotoxic stressors in bivalve mollusc species with disparate longevities. In line with previous studies of comparative stress resistance and longevity, our data extends, at least in part, the evidence for the hypothesis that an association exists between longevity and a general resistance to multiplex stressors, not solely oxidative stress. This work also provides justification for further investigation into the interspecies differences in stress response signatures induced by a diverse array of stressors in short-lived and long-lived bivalves, including pharmacological agents that elicit endoplasmic reticulum stress (24,31) and cellular stress caused by activation of innate immunity.
Funding
This work was supported by grants from the American Federation for Aging Research (to A.C. and W.E.S.), the Oklahoma Center for the Advancement of Science and Technology (to A.C., Z.U., and W.E.S.), the University of Oklahoma College of Medicine Alumni Association (to A.C.), the American Heart Association (A.C. and Z.U.) and the National Institutes of Health (NIH) (AG031085 to A.C.; AT006526 to Z.U.; AG038747, NS056218, and P01 AG11370 to W.E.S.; and AG022873 and AG025063 to S.N.A.).
Acknowledgments
The experiments took place during the 2011 Biology of Aging Course at the Marine Biological Laboratory (Woods Hole, MA) organized by S.N. Austad, for which we thank The Ellison Medical Foundation. We would like to thank Mr. Ed Enos (Superintendent, Aquatic Resources Division, Marine Biological Laboratory, Woods Hole MA) for his invaluable help with the maintenance of the bivalves used in this study. The authors would like to express their gratitude for the support of the Donald W. Reynolds Foundation, which funds aging research at the University of Oklahoma Health Sciences Center under its Aging and Quality of Life Program.
References
- 1. Ridgway ID, Richardson CA. Arctica islandica: the longest lived non colonial animal known to science. Rev Fish Biol Fisher. 2010. doi:10.1007/s11160-11010-19171-11169 [Google Scholar]
- 2. Broom MJ. Synopsis of biological data on scallops (Chlamys (Aequipecten) opercularis (Linnaeus), Argopecten Irradians (Lamarck), Argopecten gibbus (Linnaeus)).. FAO Fisheries Synopsis No 114 FIRS/S114 SAST. Rome: FAO; 1976: 44 [Google Scholar]
- 3. Plisetskaya E, Kazakov VK, Soltitskaya L, Leibson LG. Insulin-producing cells in the gut of freshwater bivalve molluscs Anodonta cygnea and Unio pictorum and the role of insulin in the regulation of their carbohydrate metabolism. Gen Comp Endocrinol. 1978; 35: 133–145 [DOI] [PubMed] [Google Scholar]
- 4. Krug AW, Allenhöfer L, Monticone R, et al. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase ½ mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010; 55: 1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Philipp E, Brey T, Pörtner HO, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev. 2005; 126: 598–609 [DOI] [PubMed] [Google Scholar]
- 6. Abele D, Brey T, Philipp E. Bivalve models of aging and the determination of molluscan lifespans. Exp Gerontol. 2009; 44: 307–315 [DOI] [PubMed] [Google Scholar]
- 7. Abele D, Strahl J, Brey T, Philipp EE. Imperceptible senescence: ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008; 42: 474–480 [DOI] [PubMed] [Google Scholar]
- 8. Strahl J, Philipp EE, Brey T, Broeg K, Abele D. Physiological aging in the Icelandic population of the ocean quahog Arctica islandica . Aqua Biol. 2007; 1: 77–84 [Google Scholar]
- 9. Strahl J, Abele D. Cell turnover in tissues of the long-lived ocean quahog Arctica islandica and the short-lived scallop Aequipecten opercularis . Marine Biol. 2010; 157: 1283–1290 [Google Scholar]
- 10. Philipp E, Heilmayer O, Brey T, Abele D, Portner HO. Physiological ageing in a polar and a temperate swimming scallop. Mar Ecol Prog Ser. 2006; 307: 187–198 [Google Scholar]
- 11. Philipp E, Pörtner HO, Abele D. Mitochondrial ageing of a polar and a temperate mud clam. Mech Ageing Dev. 2005; 126: 610–619 [DOI] [PubMed] [Google Scholar]
- 12. Ungvari Z, Philipp EE. Comparative gerontology—from mussels to man. J Gerontol A Biol Sci Med Sci. 2011; 66: 295–297 [DOI] [PubMed] [Google Scholar]
- 13. Ridgway ID, Richardson CA, Austad SN. Maximum shell size, growth rate, and maturation age correlate with longevity in bivalve molluscs. J Gerontol A Biol Sci Med Sci. 2011; 66: 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austad SN. Comparative biology of aging. J Gerontol A Biol Sci Med Sci. 2009; 64: 199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austad SN. Is there a role for new invertebrate models for aging research? J Gerontol A Biol Sci Med Sci. 2009; 64: 192–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wanamaker AD, Heinemeier J, Scourse JD, Richardson CA, Butler PG, Eiriksson J, Knudsen KL. Very long-lived molluscs confirm 17th century AD tephra-based radiocarbon reservoir ages for north Icelandic shelf waters. Radiocarbon. 2008; 50: 1–14 [Google Scholar]
- 17. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011; 66: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009; 64: 179–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Austad SN. Cats, “rats,” and bats: the comparative biology of aging in the 21st century. Integr Comp Biol. 2010; 50: 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vermeulen CJ, Loeschcke V. Longevity and the stress response in Drosophila. Exp Gerontol. 2007; 42: 153–159 [DOI] [PubMed] [Google Scholar]
- 21. Vermeulen CJ, Van De Zande L, Bijlsma R. Resistance to oxidative stress induced by paraquat correlates well with both decreased and increased lifespan in Drosophila melanogaster. Biogerontology. 2005; 6: 387–395 [DOI] [PubMed] [Google Scholar]
- 22. Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998; 282: 943–946 [DOI] [PubMed] [Google Scholar]
- 23. Harper JM, Salmon AB, Leiser SF, Galecki AT, Miller RA. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell. 2007; 6: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salmon AB, Sadighi Akha AA, Buffenstein R, Miller RA. Fibroblasts from naked mole-rats are resistant to multiple forms of cell injury, but sensitive to peroxide, ultraviolet light, and endoplasmic reticulum stress. J Gerontol A Biol Sci Med Sci. 2008; 63: 232–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Csiszar A, Labinskyy N, Zhao X, et al. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell. 2007; 6: 783–797 [DOI] [PubMed] [Google Scholar]
- 26. Labinskyy N, Mukhopadhyay P, Toth J, et al. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol. 2009; 296: H946–H956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008; 13: 5056–5070 [DOI] [PubMed] [Google Scholar]
- 28. Ungvari Z, Krasnikov BF, Csiszar A, et al. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways, and DNA repair efficiency. Age (Dordr). 2008; 30: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009; 64: 819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salmon AB, Leonard S, Masamsetti V, et al. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009; 23: 2317–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harper JM, Wang M, Galecki AT, Ro J, Williams JB, Miller RA. Fibroblasts from long-lived bird species are resistant to multiple forms of stress. J Exp Biol. 2011; 214(Pt 11):1902–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lithgow GJ, Miller RA. The determination of aging rate by coordinated resistance to multiple forms of stress. In: Guarente L, Partridge L, Wallace D, eds. The Molecular Biology of Aging. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2008. [Google Scholar]
- 33. Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005; 289: E23–E29 [DOI] [PubMed] [Google Scholar]
- 34. Powell EN, Cummins H. Are molluscan maximum life spans determined by long-term cycles in benthic communities? Oecologia. 1985; 67: 177–182 [DOI] [PubMed] [Google Scholar]
- 35. Ridgway ID. Comments on the biology and functional morphology of Arctica islandica by Brian Morton, Marine Biology Research, 2011. Mar Biol Res. 2012; 8: 95–97 [Google Scholar]
- 36. Salmon AB, Ljungman M, Miller RA. Cells from long-lived mutant mice exhibit enhanced repair of ultraviolet lesions. J Gerontol A Biol Sci Med Sci. 2008; 63: 219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Philipp EE, Abele D. Masters of longevity: lessons from long-lived bivalves—a mini-review. Gerontology. 2010; 56: 55–65 [DOI] [PubMed] [Google Scholar]
- 38. Ridgway ID, Richardson AC, Enos E, Ungvari Z, Austad SN, Philipp EER, Csiszar A. New species longevity record for the northern quahog (=hard clam), Mercenaria mercenaria . J Shellfish Res. 2011; 30: 35–38 [Google Scholar]
- 39. Jones DS. Sclerochronology: reading the record of the molluscan shell. Am Sci. 1983; 71: 384–391 [Google Scholar]
- 40. Bricelj VM, Epp J. Malouf RE comparative physiology of young and old cohorts of bay scallop Argopecten irradians irradians (Lamarck): mortality, growth, and oxygen consumption. J Exp Mar Biol Ecol. 1987; 112: 73–91 [Google Scholar]
- 41. Bailey-Downs LC, Mitschelen M, Sosnowska D, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 313–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ungvari Z, Bailey-Downs L, Gautam T, et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011; 66: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bailey-Downs LC, Sosnowska D, Toth P, et al. Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging. J Gerontol A Biol Sci Med Sci. 2012; 67: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ungvari Z, Gautam T, Koncz P, et al. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci. 2010; 65: 1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Austad SN. An experimental paradigm for the study of slowly aging organisms. Exp Gerontol. 2001; 36: 599–605 [DOI] [PubMed] [Google Scholar]
- 46. Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999; 26: 495–500 [DOI] [PubMed] [Google Scholar]
- 47. Lithgow GJ, Walker GA. Stress resistance as a determinate of C. elegans lifespan. Mech Ageing Dev. 2002; 123: 765–771 [DOI] [PubMed] [Google Scholar]
- 48. Pérez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009; 8: 73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y, Ikeno Y, Qi W, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009; 64: 1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jang YC, Lustgarten MS, Liu Y, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010; 24: 1376–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ran Q, Liang H, Ikeno Y, et al. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. J Gerontol A Biol Sci Med Sci. 2007; 62: 932–942 [DOI] [PubMed] [Google Scholar]
- 52. Van Remmen H, Jones DP. Current thoughts on the role of mitochondria and free radicals in the biology of aging. J Gerontol A Biol Sci Med Sci. 2009; 64: 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pérez VI, Cortez LA, Lew CM, et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci. 2011; 66: 1286–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sentman ML, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006; 281: 6904–6909 [DOI] [PubMed] [Google Scholar]
- 55. Mansouri A, Muller FL, Liu Y, et al. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006; 127: 298–306 [DOI] [PubMed] [Google Scholar]
- 56. Van Remmen H, Ikeno Y, Hamilton M, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003; 16: 29–37 [DOI] [PubMed] [Google Scholar]
- 57. Wu S, Li Q, Du M, Li SY, Ren J. Cardiac-specific overexpression of catalase prolongs lifespan and attenuates ageing-induced cardiomyocyte contractile dysfunction and protein damage. Clin Exp Pharmacol Physiol. 2007; 34: 81–87 [DOI] [PubMed] [Google Scholar]
- 58. Mele J, Van Remmen H, Vijg J, Richardson A. Characterization of transgenic mice that overexpress both copper zinc superoxide dismutase and catalase. Antioxid Redox Signal. 2006; 8: 628–638 [DOI] [PubMed] [Google Scholar]
- 59. Pérez VI, Bokov A, Van Remmen H, et al. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009; 1790: 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salmon AB, Richardson A, Pérez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010; 48: 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pérez VI, Buffenstein R, Masamsetti V, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009; 106: 3059–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taylor AC, Brand AR. Effects of hypoxia and body size on the oxygen consumption of the bivalve Arctica islandica (L.). J Exp Mar Biol Ecol. 1975; 19: 187–196 [Google Scholar]
- 63. Ansell AD, Lander KF. Studies on the hard-shell clam Venus mercenaria in British Waters. III. Further observations on the seasonal biochemical cycle and on spawning. J Appl Ecol. 1967; 4: 425–435 [Google Scholar]
- 64. Ungvari Z, Csiszar A, Sosnowska D, et al. Testing predictions of the oxidative stress hypothesis of aging using a novel invertebrate model of longevity: the giant clam (Tridacna derasa). J Gerontol A Biol Sci Med Sci. 2012. doi:10.1093/gerona/gls159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vijg J. Somatic mutations and aging: a re-evaluation. Mutat Res. 2000; 447: 117–135 [DOI] [PubMed] [Google Scholar]
- 66. Dollé ME, Vijg J. Genome dynamics in aging mice. Genome Res. 2002; 12: 1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dollé ME, Giese H, Hopkins CL, Martus HJ, Hausdorff JM, Vijg J. Rapid accumulation of genome rearrangements in liver but not in brain of old mice. Nat Genet. 1997; 17: 431–434 [DOI] [PubMed] [Google Scholar]
- 68. Esposito D, Fassina G, Szabo P, et al. Chromosomes of older humans are more prone to aminopterine-induced breakage. Proc Natl Acad Sci USA. 1989; 86: 1302–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cortopassi GA, Wang E. There is substantial agreement among interspecies estimates of DNA repair activity. Mech Ageing Dev. 1996; 91: 211–218 [DOI] [PubMed] [Google Scholar]
- 70. Hart RW, Setlow RB. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci USA. 1974; 71: 2169–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hart RW, Sacher GA, Hoskins TL. DNA repair in a short- and a long-lived rodent species. J Gerontol. 1979; 34: 808–817 [DOI] [PubMed] [Google Scholar]
- 72. Grube K, Bürkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci USA. 1992; 89: 11759–11763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hyun M, Lee J, Lee K, May A, Bohr VA, Ahn B. Longevity and resistance to stress correlate with DNA repair capacity in Caenorhabditis elegans. Nucleic Acids Res. 2008; 36: 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Karpinich NO, Tafani M, Rothman RJ, Russo MA, Farber JL. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J Biol Chem. 2002; 277: 16547–16552 [DOI] [PubMed] [Google Scholar]
- 75. Peters EC, Yevich PP, Harshbarger JC, Zaroogian GE. Comparative histology of gonadal neoplasms in marine bivalve molluscs. Dis Aquat Org. 1994; 20: 59–76 [Google Scholar]
- 76. Barber BJ. Neoplastic diseases of commercially important marine bivalves. Aquat Living Resour. 2004; 17: 449–466 [Google Scholar]
- 77. Poder M, Auffret M. Sarcomatous lesion in the cockle Cerastoderma edule I. Morphology and population survey in Brittany, France. Aquaculture. 1986; 58: 1–8 [Google Scholar]
- 78. Csiszar A, Podlutsky A, Podlutskaya N, et al. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012; 67: 841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McClellan-Green P, Romano J, Oberdörster E. Does gender really matter in contaminant exposure? A case study using invertebrate models. Environ Res. 2007; 104: 183–191 [DOI] [PubMed] [Google Scholar]
- 80. Zilberberg C, Sereno D, Lima G, Custódio MR, Lôbo-Hajdu G. Effect of mussel’s gender and size on a stress response biomarker. Water Air Soil Pollut. 2011; 217: 317–320 [Google Scholar]
- 81. Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J Biol Chem. 2004; 279: 39414–39420 [DOI] [PubMed] [Google Scholar]
- 82. Cochemé HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008; 283: 1786–1798 [DOI] [PubMed] [Google Scholar]
- 83. Thórarinsdóttir GG, Einarsson ST. Distribution, abundance, population structure and meat yield of the ocean quahog, Arctica islandica, in Icelandic waters. J Mar Biol Assoc UK. 1996; 76: 1107–1114 [Google Scholar]
- 84. Peterson CH. Quantitative allometry of gamete production by Mercenaria mercenaria into old age. Mar Ecol Prog Ser. 1986; 29: 93–97 [Google Scholar]
- 85. Bricelj VM, Eppa J, Maloufa RE. Comparative physiology of young and old cohorts of bay scallop Argopecten irradians irradians (Lamarck): mortality, growth, and oxygen consumption. J Exp Mar Biol Ecol. 1987; 112: 73–91 [Google Scholar]