Abstract
Background:
Metabolic syndrome (MetS) and functional limitation have been linked, but whether and how specific components of MetS and associated factors, such as inflammation, drive this relationship is unknown.
Methods:
Data are from 2,822 men and women, aged 70–79 years, participating in the Health, Aging, and Body Composition (Health ABC) study and followed for 5 years. Presence of MetS at baseline was defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines. Interleukin-6, C-reactive protein, and body fat mass were measured at baseline. Measures of physical performance, including 400-m walk time, 20-m walking speed, and the Health ABC physical performance battery (PPB) were obtained at baseline and examination years 2, 4, and 6.
Results:
A total of 1,036 (37%) individuals met criteria for MetS. MetS was associated with poorer physical performance at baseline. Effect estimates between MetS and gait speed, and components of the Health ABC PPB (standing balance and repeated sit-to-stand performance) were modestly attenuated after adjustment for inflammation. All associations were attenuated to nonsignificance after adding total body fat mass to the model. Longitudinal analyses yielded similar results. Individual MetS component analysis revealed that abdominal obesity explained the largest fraction of the variation in physical performance.
Conclusions:
Although inflammatory biomarkers partially accounted for the relationship between MetS and aspects of physical performance, overall findings implicate adiposity as the primary factor explaining poorer physical performance in older adults with MetS.
Key Words: Metabolic syndrome, Physical function, Inflammation, Obesity.
FOR older adults, preservation of physical function is critically important to independent living. Indices of physical performance decline with age (1,2), and these declines predict higher rates of disability, institutionalization, and mortality (3–5). Given the growing demographic of persons aged 65 years and older (6), coupled with the economic (7) and emotional burden related to age-associated loss of physical function, prompt identification of modifiable functional decline risk factors is of considerable public health concern.
Metabolic syndrome (MetS) is a clustering of cardiovascular risk factors, including abdominal obesity, dyslipidemia, hypertension, and impaired glucose tolerance. This prevalent, yet treatable, condition affects at least 42% of adults aged 70 years and older (8). Although slightly different operational definitions of the disorder exist (9–12), essential components are similar and diagnosis requires the presence of at least three criteria. Cross-sectional studies have linked MetS and poorer physical performance (13,14), and recent longitudinal research suggests that older individuals with MetS, especially those with abdominal obesity, are at greater risk for developing self-reported functional impairments than those without MetS (15,16). However, these findings are based on self-reported measures of disability. Thus, confirmation is warranted using objective measures of physical function to determine the independent role of MetS as a risk factor for age-related functional decline.
Whether individual MetS components or underlying factors mediate the relationship between MetS and functional decline is also unknown. For instance, several observational studies demonstrate a direct relationship between well-known markers of inflammation and prevalence and severity of MetS (17–21). Likewise, inflammation has also been implicated in physical dysfunction in older adults (22–25), leading to speculation that the inflammatory pathway may be one mechanism by which MetS leads to functional decline. Additionally, evidence from several observational studies implicates obesity (as measured by body mass index [BMI]) in reduced physical performance (26–28). Abdominal obesity, measured via waist circumference, is a diagnostic component of MetS and contributor to inflammatory burden (29). Therefore, it is also plausible that associations between MetS and physical performance are mediated by excessive fat mass, particularly abdominal fat. Collectively, these data suggest involvement of MetS, inflammation, and global and regional adiposity in the progression of functional decline in older adults, although relative contributions of each are unclear.
The purpose of this study was to evaluate the role of MetS as a risk factor for decline in functional performance in late life. Secondarily, we sought to examine whether this association is independent of inflammation or fat mass in the presence of MetS. We hypothesized that the presence of MetS is predictive of lower physical performance at baseline and over time, and that inflammation and adiposity are both partial mediators of this relationship.
Methods
Study Population
Participants were from the Health, Aging, and Body Composition (Health ABC) Study, a prospective cohort study of 3,075 well-functioning white and black older adults, aged 70–79 years. Participants were recruited in 1997 and 1998 from a random sample of white and all black Medicare-eligible beneficiaries residing in the areas surrounding Pittsburgh, Pennsylvania, and Memphis, Tennessee. Participants were excluded if they (a) reported difficulty in walking for a quarter of a mile, walking up 10 steps, or performing activities of daily living; (b) had active cancer treatment in the past 3 years, or (c) planned to move from the area in the next 3 years. Of the entire Health ABC cohort, 252 participants were excluded from the primary cross- sectional analysis due to missing covariate information (62 were missing MetS status, 20 body fat mass, 5 smoking status, 11 alcohol consumption, 3 self-reported diabetes status, 9 anti-inflammatory drug use, 11 C-reactive protein [CRP] level, and 131 interleukin-6 [IL-6] level) and 1 participant was excluded due to missing performance measures at baseline, leaving a study sample size of 2,822. For longitudinal analyses, sample sizes for all outcomes indicate the number of Health ABC participants with data at baseline and at least one follow-up time point. Although longitudinal sample size varied by outcome measure, of 2,719 participants with baseline Health ABC PPB score information at baseline, 19% were lost to follow-up during the 5-year period. All participants provided written informed consent, approved by the Institutional Review Boards of the clinical sites.
Measurements
Metabolic syndrome.—The presence of MetS was defined using the National Cholesterol Education Program Adult Treatment Panel III guidelines (30). Specifically, MetS diagnosis was contingent on the presence of at least three of the following criteria: (a) abdominal obesity (waist circumference >102cm in men and >88cm in women), (b) triglyceride level ≥150mg/dL, (c) low high-density lipoprotein (HDL) cholesterol (<40mg/dL in men and <50mg/dL in women), (d) systolic blood pressure ≥130mm Hg and/or diastolic blood pressure ≥85mm Hg or use of antihypertensive medication, and (e) high fasting glucose (≥110mg/dL or use of antidiabetic medication). At the baseline visit, waist circumference and blood pressure were both averaged over two measurements. Lipid and blood glucose levels were measured using standard procedures (31) after an overnight fast. All prescription medications taken within 2 weeks of the baseline assessment were documented on a standardized medication inventory form.
Physical performance.—Physical performance measures were assessed as previously described, and include the 400-m walk (32), 20-m walk (32), and Health ABC physical performance battery (PPB) (33), comprised a 6-m usual and narrow walk, standing balance, and repeated chair stand test (34). Collectively, these objective measures provide information about physical performance and were measured in years 1 (baseline), 2 (400-m and 20-m walk only), 4, and 6 of the Health ABC study.
Inflammatory biomarkers.—Baseline inflammatory biomarkers included in these analyses are CRP and IL-6. Fasting blood samples were obtained from participants by venipuncture. Serum concentrations of the cytokines were measured in duplicate by enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN); methods were previously published (35).
Covariates.—Demographic characteristics (age, race, sex, study site, and educational level), smoking status, alcohol consumption, physical activity, presence of diabetes (DM), cardiovascular disease (CVD; including coronary heart disease, congestive heart failure, and stroke), chronic knee pain, and anti-inflammatory drug use were ascertained by an interviewer-administered questionnaire at study baseline. The time and intensity of self-reported physical activities performed in the past 7 days were summed (kcal/week) and divided into three levels defined as low (<1000 kcal/week), medium (1000–2000 kcal/week), and high (≥2000 kcal/week) activity levels. Body weight and height were measured at baseline, and BMI was calculated as weight divided by the square of height (kg/m2). Finally, body fat mass was determined by dual-energy x-ray absorptiometry (QDR 4500; Hologic Inc., Waltham, MA).
Statistical analysis.—All data were initially analyzed using descriptive statistics. Sample means and standard deviations were computed for continuous variables and counts and proportions were calculated for categorical variables. Because IL-6 and CRP levels were not normally distributed, medians and interquartile ranges are provided. Linear regression models were used to fit the association between presence of MetS (binary covariate of interest) and physical performance at baseline (dependent variable). Models were adjusted for other relevant covariates, including age, race, sex, study site, educational level, height, smoking status, alcohol use, physical activity, presence of comorbidities (DM and CVD), chronic knee pain, and anti-inflammatory drug use. CRP, IL-6, and total body fat were also included independently as potential mediators.
To model the association between presence of MetS and change in physical performance over time, mixed effects models containing the same covariates listed above, plus baseline outcome measure and time, were used. An unstructured covariance matrix was used to model the correlation of repeated measures. MetS by time and Mets by gender interactions were tested, with stratification performed when necessary. Finally, all models were re-run excluding participants with prevalent diabetes at baseline.
To explore the variation in physical performance explained by MetS or its components, the coefficient of determination (R 2) statistic for the fully adjusted covariates model and the model including covariates and MetS or its components was calculated. The partial R 2 captures the marginal contribution of MetS and/or one of its components beyond that of the covariates. Further, we tested whether the observed partial R 2 was equal to 0 using an F test. For the mixed effects model, R 2 specific to the MetS-related variables were calculated as
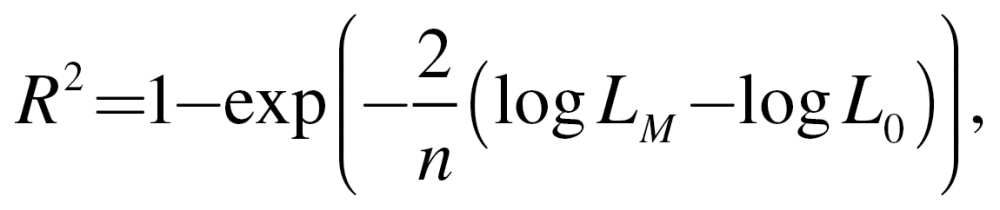 |
where n is the number of participants, log L M the log-likelihood of the full model (basic covariates plus MetS or its individual components), and log L 0 the log-likelihood of the model with basic covariates (36). All analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC) with a two-sided alpha level of 0.05 to indicate statistical significance.
Results
Participant Characteristics
The mean age of the study sample (n = 2822) at baseline was 73.6 years and 52% were women. Excluded participants (n = 253) were similar in age and sex, but were more likely to be black (51% vs 41%; p < .01). Excluded participants also had slower 6-m and 20-m walking speeds (mean difference [SE]: −0.04 [0.02] m/s and −0.07 [0.02] m/s, respectively) than the study sample, but were otherwise similar at baseline. Participant characteristics by MetS status are shown in Table 1. Of the 1,036 (37%) individuals who met the criteria for MetS, 670 (65%) had elevated triglycerides, 898 (87%) were abdominally obese, 905 (87%) had hypertension, 506 (49%) had elevated blood glucose levels, and 652 (63%) had lower HDL. Only 1% of participants with MetS had all five components of the syndrome. Nearly half (n = 500) had abdominal obesity but not glucose intolerance, whereas only 84 participants with MetS had glucose intolerance but not abdominal obesity. Overall, persons with MetS were less likely to be physically active and be a current smoker or alcohol drinker, and were more likely to be women, have high body mass, have CVD or DM, report chronic knee pain and use of anti-inflammatory medications, and have higher baseline levels of systemic inflammatory biomarkers.
Table 1.
Descriptive Baseline Characteristics According to Metabolic Syndrome Status
| Metabolic Syndrome Presence | p Value | ||
|---|---|---|---|
| No | Yes | ||
| N (%) | 1786 (63) | 1036 (37) | <.01 |
| Age (y) | 73.7±2.9 | 73.5±2.8 | .18 |
| Black race (%) | 751 (42) | 401 (39) | .09 |
| Female sex (%) | 861 (48) | 594 (57) | <.01 |
| Site (% Memphis) | 901 (50) | 516 (50) | .74 |
| Years of education (%) | |||
| Less than high school | 445 (25) | 254 (25) | .25 |
| High school graduate | 565 (32) | 358 (35) | |
| Postsecondary | 733 (43) | 422 (41) | |
| Height | 166.5±9.6 | 165.8±8.9 | .07 |
| Smoking status (%) | |||
| Former | 799 (45) | 495 (48) | <.01 |
| Current | 204 (11) | 77 (7) | |
| Never | 783 (44) | 464 (45) | |
| Drinking status (%) | |||
| None | 851 (48) | 573 (55) | <.01 |
| <1 drink/wk | 368 (21) | 217 (21) | |
| 1–7 drinks/wk | 423 (24) | 186 (18) | |
| >1 drink/d | 144 (8) | 60 (6) | |
| Prevalent disease (%) | |||
| Diabetes | 108 (6) | 309 (30) | <.01 |
| CVD | 223 (12) | 197 (19) | <.01 |
| Chronic knee pain (%) | 255 (14) | 194 (19) | <.01 |
| Anti-inflammatory drug use (%) | 911 (51) | 577 (56) | .02 |
| Physical activity (%) | |||
| <1000 kcal/wk | 891 (50) | 583 (56) | <.01 |
| 1000–2000 kcal/wk | 610 (34) | 320 (31) | |
| ≥2000 kcal/wk | 285 (16) | 133 (13) | |
| CRP (µg/mL) | 1.5 (0.9–2.7) | 2.1 (1.2–3.8) | <.01* |
| IL-6 (pg/mL) | 1.7 (1.1–2.5) | 2.1 (1.5–3.2) | <.01* |
| Fat mass (kg) | 24.6±8.1 | 30.6±8.4 | <.01 |
Note: Data are presented as means ± SD for continuous variables and n (%) for categorical variables. Differences were assessed using t tests for continuous variables and chi-squared tests for categorical variables. Due to violation of the normality assumption, CRP and IL-6 data are presented as medians (interquartile range).
Abbreviations: N = sample size; CVD = cardiovascular disease; CRP = C-reactive protein; IL-6 = interleukin-6.
*p values are presented after log transformation.
Baseline Associations Between MetS and Physical Performance
Baseline physical performance measures according to MetS status are presented in Table 2. In analyses adjusted for age, race, sex, study site, and educational level (Model 1), people with MetS had longer 400-m walk time (least squares mean difference [SE]: −14.31 [2.46] s, p < .01) and slower 20-m walking speed (least squares mean difference [SE]: −0.04 [0.01] m/s, p < .01), as well as a lower score on the Health ABC PPB (least squares mean difference [SE]: −0.11 [0.02], p < .01) compared with those without MetS. Likewise, individual components of the Health ABC PPB were also worse for those with MetS compared with those without MetS (least squares mean difference [SE]: −0.03 [0.01] m/s for 6-m walking speed, −0.02 [0.01] m/s for 6-m narrow walking speed, −3.74 [0.89] s for standing balance, and −0.02 [0.00] m/s for chair stand pace; p for all <.05). Associations between MetS and all performance measures, except chair stand pace and 6-m narrow walking speed, remained significant after additional covariate adjustment (Model 2).
Table 2.
Baseline and Follow-up Physical Performance Measures According to Metabolic Syndrome Status
| Baseline Model | Longitudinal Model | |||||
|---|---|---|---|---|---|---|
| Metabolic Syndrome Presence | p value | Metabolic Syndrome Presence | p value | |||
| No | Yes | No | Yes | |||
| 400-m walk time (s) | ||||||
| N | 1409 | 738 | 1210 | 605 | ||
| Model 1 | 333.55±1.49 | 347.86±2.04 | <.01 | 331.17±1.01 | 334.72±1.39 | .02 |
| Model 2 | 346.76±3.24 | 357.15±3.20 | <.01 | 336.29±2.25 | 339.07±2.25 | .09 |
| Model 3a | 345.76±3.02 | 354.62±2.98 | <.01 | 336.18±2.24 | 338.78±2.26 | .11 |
| Model 3b | 350.51±3.21 | 353.56±3.19 | .24 | 337.44±2.24 | 337.71±2.25 | .87 |
| 20-m walking speed (m/s) | ||||||
| N | 1585 | 880 | 1415 | 810 | ||
| Model 1 | 1.33±0.01 | 1.29±0.01 | <.01 | 1.51±0.01 | 1.47±0.01 | <.01 |
| Model 2 | 1.29±0.01 | 1.27±0.01 | .02 | 1.45±0.01 | 1.44±0.01 | .05 |
| Model 3a | 1.29±0.01 | 1.27±0.01 | .07 | 1.45±0.01 | 1.44±0.01 | .08 |
| Model 3b | 1.28±0.01 | 1.28±0.01 | .60 | 1.44±0.01 | 1.44±0.01 | .92 |
| Health ABC physical performance battery score (range, 0–4) | ||||||
| N | 1723 | 996 | 1398 | 807 | ||
| Model 1 | 2.20±0.01 | 2.08±0.02 | <.01 | 2.01±0.01 | 1.92±0.01 | <.01 |
| Model 2 | 2.07±0.02 | 2.00±0.02 | <.01 | 1.90±0.02 | 1.85±0.02 | .01 |
| Model 3a | 2.07±0.02 | 2.02±0.02 | <.01 | 1.91±0.02 | 1.86±0.02 | .02 |
| Model 3b | 2.04±0.02 | 2.05±0.02 | .69 | 1.89±0.02 | 1.86±0.02 | .18 |
| 6-m walking speed (m/s) | ||||||
| N | 1774 | 1030 | 1473 | 857 | ||
| Model 1 | 1.17±0.00 | 1.14±0.01 | <.01 | 1.08±0.01 | 1.05±0.01 | <.01 |
| Model 2 | 1.13±0.01 | 1.12±0.01 | .03 | 1.05±0.01 | 1.03±0.01 | <.01 |
| Model 3a | 1.13±0.01 | 1.12±0.01 | .09 | 1.05±0.01 | 1.03±0.01 | .02 |
| Model 3b | 1.14±0.01 | 1.14±0.01 | .82 | 1.05±0.01 | 1.03±0.01 | .21 |
| 6-m narrow walking speed (m/s) | ||||||
| N | 1546 | 843 | 1178 | 604 | ||
| Model 1 | 1.09±0.01 | 1.06±0.01 | .03 | 0.91±0.01 | 0.83±0.02 | <.01 |
| Model 2 | 1.07±0.01 | 1.06±0.01 | .21 | 0.83±0.02 | 0.77±0.02 | .02 |
| Model 3a | 1.06±0.01 | 1.06±0.01 | .42 | 0.83±0.02 | 0.78±0.02 | <.01 |
| Model 3b | 1.06±0.01 | 1.07±0.01 | .59 | 0.81±0.02 | 0.79±0.02 | .17 |
| Standing balance test (s) | ||||||
| N | 1750 | 1015 | 1434 | 840 | ||
| Model 1 | 68.23±0.54 | 64.48±0.71 | <.01 | 61.60±0.52 | 57.11±0.68 | <.01 |
| Model 2 | 64.16±1.14 | 62.04±1.12 | .03 | 57.09±1.12 | 54.42±1.08 | <.01 |
| Model 3a | 64.32±1.14 | 62.56±1.13 | .06 | 57.17±1.12 | 54.73±1.09 | <.01 |
| Model 3b | 63.16±1.14 | 63.37±1.12 | .83 | 56.52±1.12 | 55.04±1.09 | .11 |
| Chair stand pace (stands/s) | ||||||
| N | 1768 | 1022 | 1398 | 805 | ||
| Model 1 | 0.37±0.00 | 0.35±0.00 | <.01 | 0.32±0.00 | 0.30±0.00 | <.01 |
| Model 2 | 0.35±0.01 | 0.34±0.01 | .18 | 0.29±0.01 | 0.28±0.01 | .09 |
| Model 3a | 0.35±0.01 | 0.34±0.01 | .23 | 0.29±0.01 | 0.28±0.01 | .17 |
| Model 3b | 0.35±0.01 | 0.35±0.01 | .89 | 0.28±0.01 | 0.29±0.01 | .44 |
Notes: Data are presented at least squares means ± standard error. Model 1 adjusted for age, race, sex, study site, and educational level. Model 2 adjusts for all covariates specified in Model 1 plus height, smoking status, alcohol use, physical activity, presence of prevalent comorbidities (ie, DM and cardiovascular disease), chronic knee pain, and anti-inflammatory drug use. Model 3a adjusts for all covariates specified in Models 1 and 2 and accounts for the potential inflammatory mediators, CRP and IL-6. Model 3b adjusts for all covariates specified in Models 1 and 2 and accounts for the potential mediator, total body fat.
To determine whether the association between MetS and physical performance was independent of inflammation or adiposity, baseline IL-6, CRP, and total body fat mass were separately added to Model 2 (Models 3a and 3b, respectively). Inclusion of baseline IL-6 and CRP (Model 3a) modestly attenuated the strength of association between MetS and physical performance measures; however, MetS was still significantly related to 400-m walk time, Health ABC PPB score (both p < .01) and the standing balance test (p = .05). After adjustment for total body fat, the associations between MetS and all physical function measures were no longer significant (Model 3b; all p > .05). All cross-sectional associations between MetS and physical performance were similar in additional analyses in which participants with prevalent diabetes (n = 527) were excluded (data not shown).
Longitudinal Associations Between MetS and Physical Performance
Results from the longitudinal analyses are presented in Table 2. After basic adjustment (Model 1), presence of MetS was associated with lower physical performance at each time, but did not predict an accelerated rate of decline in physical performance during the 5-year follow-up period. All associations remained significant, except for 400-m walk time, after further covariate adjustment (Model 2). After adjustment for baseline IL-6 and CRP, only associations between MetS and 6-m usual and narrow walking speeds, the Health ABC PPB score, and standing balance remained statistically significant (all p < .01). No significant associations were found between MetS and physical performance measures over time after adjustment for total body fat (Model 3b). In longitudinal analyses excluding participants with prevalent diabetes, MetS status was only statistically associated with worse Health ABC PPB score (p = .01) and standing balance (p < .01) at follow-up.
Gender by MetS interactions was tested in all baseline and longitudinal models, with only the longitudinal models of 400-m walk time yielding significant interactions (all p < .05). Stratification by gender in these models revealed that men with MetS had longer follow-up 400-m walk times than those without MetS (318.41±1.36 s vs 325.03±1.95 s; p < .01; Model 1), but this relationship was attenuated after adjustment for body fat mass (322.97±2.48 s vs 326.83±2.56 s; p = .10; Model 3B). Conversely, MetS did not have an effect on follow-up 400-m walk time in women.
Partial Correlation Analyses Between MetS Components and Physical Performance Measures
To investigate which MetS component explained the largest fraction of the variation in physical performance, we analyzed associations between physical performance measures and independent MetS components, adjusting for covariates listed in Model 2. Because covariate adjustment was held constant in each model, direct comparison of the R 2 statistic to determine the relative importance of each MetS component on baseline physical function was possible (Table 3). In cross-sectional analyses, the R 2 values for abdominal obesity, impaired fasting glucose, and metabolic syndrome were statistically significant for nearly all outcome measures. Notably, abdominal obesity had the highest R 2 value across all physical performance measures, even more so than the composite MetS variable. Longitudinal analyses revealed similar findings, with the partial R 2 for abdominal obesity ranging from 0.002 to 0.009 and explaining the largest fraction of variability for five of the seven follow-up physical performance measures (400-m walk time; 20-m, 6-m, and 6-m narrow walking speed; and chair stand pace; data not shown).
Table 3.
Coefficient of Determination (R 2) Summary of Models Between Metabolic Syndrome Components and Baseline Physical Performance Measures After Adjustment for Relevant Covariates
| R 2 | 400-m Walk Time (s) | 20-m Walking Speed (m/s) | Health ABC PPB Score | 6-m Walking Speed (m/s) | 6-m Narrow Walking Speed (m/s) | Standing Balance (s) | Chair Stand Pace (stands/s) |
|---|---|---|---|---|---|---|---|
| N | 2,140 | 2,456 | 2,710 | 2,785 | 2,385 | 2,740 | 2,770 |
| Covariates only | 0.2356 | 0.2412 | 0.1937 | 0.2251 | 0.1253 | 0.0990 | 0.0624 |
| Abdominal obesity | 0.2599* | 0.2566* | 0.2245* | 0.2382* | 0.1450* | 0.1180* | 0.0732* |
| Impaired fasting glucose | 0.2386* | 0.2431* | 0.2003* | 0.2269* | 0.1308* | 0.1028* | 0.0630 |
| Elevated fasting triglycerides | 0.2361 | 0.2414 | 0.1957* | 0.2252 | 0.1260 | 0.1002 | 0.0649* |
| Low HDL | 0.2369 | 0.2415 | 0.1937 | 0.2251 | 0.1253 | 0.0990 | 0.0624 |
| High blood pressure | 0.2363 | 0.2419 | 0.1944 | 0.2264* | 0.1253 | 0.0997 | 0.0627 |
| Metabolic syndrome | 0.2470* | 0.2468* | 0.2043* | 0.2295* | 0.1312* | 0.1049* | 0.0664* |
Covariates include age, race, sex, study site, educational level, height, smoking status, alcohol use, physical activity, presence of prevalent comorbidities (ie, DM and cardiovascular disease), chronic knee pain, and anti-inflammatory drug use.
Abbreviations: R 2 = coefficient of determination; PPB = physical performance battery, N = sample size, HDL = high-density lipoprotein.
Boldface numbers indicate the largest R 2 value within a given column.
*Statistical significance (p < .05).
Discussion
These findings suggest that the presence of MetS is significantly associated with poorer physical performance in older adults, although MetS does not appear to predict an accelerated rate of functional decline. Although modest, inclusion of inflammatory biomarkers into our statistical models partially accounted for the relationship between MetS and several physical performance measures. However, inclusion of total body fat mass completely attenuated the relationship between MetS and physical performance. This finding is corroborated by a partial correlation analysis where abdominal adiposity explained the largest fraction of variability in physical performance. Overall findings from this study implicate excessive global and regional adiposity as the primary factor explaining poorer physical performance in older adults with MetS.
Our results concur with prior epidemiological literature linking MetS, and in particular the MetS component abdominal obesity, to poorer physical functioning (13–16). Several cross-sectional studies, for example, show an inverse association between objective measures of physical performance and MetS (13,14). Although not assessed in this study, impaired grip strength in the Hertfordshire cohort was associated with individual features, as well as the summary definition, of MetS (13). Likewise, a graded inverse association was found between MetS and grip strength, walking speed, and chair stand pace in the Osteoporotic Fractures in Men Study, with abdominal obesity significantly related to all performance measures (14). Finally, although evidence coming from the 1999–2002 National Health and Nutrition Examination Survey did not support an association between slower gait speed and presence of MetS, gait speed was inversely associated with abdominal obesity in women (OR = 0.48) (37).
As previously reported by Penninx and colleagues (15), a significant association exists between MetS and risk of developing self-reported mobility disability (RR = 1.46), independent of CVD and DM, in the Health ABC cohort. Risk was further elevated with increasing severity (eg, number of criteria) of MetS, with abdominal obesity conferring the highest risk among the MetS components (RR = 1.54). Similarly, in older Mexican Americans, MetS (with or without DM) was associated with progressive limitations in self-reported mobility and strength over a 3-year period (16). Our results extend these findings to objective measures of physical performance and affirm conclusions that individuals with MetS are at increased risk of developing mobility disability due to lowered physical performance. Further, our results confirm that abdominal adiposity is the MetS component most related to lower physical performance. These results suggest geriatricians should focus on targeted weight management to delay the onset of mobility disability in older adults with MetS, as our study, and others (38), highlight abdominal adiposity as a central predictor of lower physical performance.
Strengths of this study include the large study sample, long follow-up, and careful collection of standardized physical performance data, inflammatory, biomarkers and fat mass. However, our study also has limitations. First, time-varying covariates, including MetS, were not included in longitudinal models; so our results only speak to the predictive power of MetS and covariates at baseline. Second, the observational nature of this study limits our ability to draw strong causal inferences from the data; however, evidence from the longitudinal model suggests that MetS, and especially abdominal adiposity, contribute to worse physical performance at each time point.
In conclusion, we report an association between presence of MetS and poor physical performance, with excess adiposity as the driver of this relationship. Given that physical function represents a critically important and modifiable predictor of independent living in older adults, future research should test whether reductions in global and regional body fat mass in older adults with MetS results in improved physical performance and whether such improvements translate into prolonged independence.
Funding
This work was supported by National Institute on Aging (NIA) grants N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, and NIA grant R01-AG028050; National Institute on Nursing Research (NINR) grant R01-NR012459; Intramural Research Program of the NIH, National Institute on Aging, as well as an individual postdoctoral fellowship (F32-AG039186) awarded to KMB.
References
- 1. Onder G, Penninx BW, Lapuerta P, et al. Change in physical performance over time in older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2002; 57: M289–M293 [DOI] [PubMed] [Google Scholar]
- 2. Lindle RS, Metter EJ, Lynch NA, et al. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol. 1997; 83: 1581–1587 [DOI] [PubMed] [Google Scholar]
- 3. Vasunilashorn S, Coppin AK, Patel KV, et al. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2009; 64: 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000; 55: M221–M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995; 332: 556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention Public health and aging: trends in aging United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003; 52: 101–106 [PubMed] [Google Scholar]
- 7. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004; 52: 80–85 [DOI] [PubMed] [Google Scholar]
- 8. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002; 287: 356–359 [DOI] [PubMed] [Google Scholar]
- 9. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15: 539–553 [DOI] [PubMed] [Google Scholar]
- 10. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421 [PubMed] [Google Scholar]
- 11. Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004; 109: 433–438 [DOI] [PubMed] [Google Scholar]
- 12. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005; 366: 1059–1062 [DOI] [PubMed] [Google Scholar]
- 13. Sayer AA, Syddall HE, Dennison EM, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Cohort Study. QJM. 2007; 100: 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Everson-Rose SA, Paudel M, Taylor BC, et al. Metabolic syndrome and physical performance in elderly men: the osteoporotic fractures in men study. J Am Geriatr Soc. 2011; 59: 1376–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009; 64: 96–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. 2007; 62: 766–773 [DOI] [PubMed] [Google Scholar]
- 17. Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000; 102: 42–47 [DOI] [PubMed] [Google Scholar]
- 18. Ford ES, Giles WH, Myers GL, Mannino DM. Population distribution of high-sensitivity C-reactive protein among US men: findings from National Health and Nutrition Examination Survey 1999-2000. Clin Chem. 2003; 49: 686–690 [DOI] [PubMed] [Google Scholar]
- 19. Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004; 110: 380–385 [DOI] [PubMed] [Google Scholar]
- 20. Pradhan AD, Cook NR, Buring JE, Manson JE, Ridker PM. C-reactive protein is independently associated with fasting insulin in nondiabetic women. Arterioscler Thromb Vasc Biol. 2003; 23: 650–655 [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003; 107: 391–397 [DOI] [PubMed] [Google Scholar]
- 22. Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000; 55: M709–M715 [DOI] [PubMed] [Google Scholar]
- 23. Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002; 57: M326–M332 [DOI] [PubMed] [Google Scholar]
- 24. Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999; 47: 639–646 [DOI] [PubMed] [Google Scholar]
- 25. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004; 59: 242–248 [DOI] [PubMed] [Google Scholar]
- 26. Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int J Obes (Lond). 2007; 31: 1680–1687 [DOI] [PubMed] [Google Scholar]
- 27. Stenholm S, Koster A, Alley DE, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women–results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010; 65: 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koster A, Penninx BW, Newman AB, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity (Silver Spring). 2007; 15: 3122–3132 [DOI] [PubMed] [Google Scholar]
- 29. Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001; 60: 349–356 [DOI] [PubMed] [Google Scholar]
- 30. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25):3143–3421 [PubMed] [Google Scholar]
- 31. You T, Nicklas BJ, Ding J, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008; 63: 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001; 49: 1544–1548 [DOI] [PubMed] [Google Scholar]
- 33. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49: M85–M94 [DOI] [PubMed] [Google Scholar]
- 34. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001; 56: M644–M649 [DOI] [PubMed] [Google Scholar]
- 35. Hsu FC, Kritchevsky SB, Liu Y, et al. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci. 2009; 64: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Am Statist. 1990; 44(3):250–253 [Google Scholar]
- 37. Okoro CA, Zhong Y, Ford ES, Balluz LS, Strine TW, Mokdad AH. Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: a cross- sectional analysis. BMC Public Health. 2006; 6: 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouchard DR, Choquette S, Dionne IJ, Brochu M. Is fat mass distribution related to impaired mobility in older men and women? Nutrition as a determinant of successful aging: the Quebec longitudinal study. Exp Aging Res. 2011; 37: 346–357 [DOI] [PubMed] [Google Scholar]


