Abstract
Purpose
In spite of an extensive vaccination program, parvoviral infections still pose a major threat to the health of dogs.
Materials and Methods
We isolated a novel canine parvovirus (CPV) strain from a dog with enteritis. Nucleotide and amino acid sequence analysis of the isolate showed that it is a novel type 2b CPV with asparagine at the 426th position and valine at the 555th position in VP2. To develop a vaccine against CPV infection, we passaged the isolate 4 times in A72 cells.
Results
The attenuated isolate conferred complete protection against lethal homologous CPV infection in dogs such that they did not develop any clinical symptoms, and their antibody titers against CPV were significantly high at 7-11 days post infection.
Conclusion
These results suggest that the virus isolate obtained after passaging can be developed as a novel vaccine against paroviral infection.
Keywords: Canine parvoviral infection, Vaccines, Novel type 2b, Dogs, Clinical isolate
Introduction
Since its discovery in 1978, canine parvovirus (CPV) has caused widespread enteritis and myocarditis with a high mortality rate in canine pups [1,2]. Although an extensive vaccination program has been instituted for the prevention of CPV infection in pups, CPV infection remains highly prevalent in Korea. The emergence of a new type of CPV strain different from the vaccine strain was determined as the main cause of the high prevalence of CPV infections in Korea.
CPV belongs to the feline parvovirus subgroup and is closely related to the feline panleukopenia virus (FPV), suggesting that it has evolved from FPV [3,4]. CPV is a non-enveloped virus with a single-stranded DNA genome of about 5,200 nucleotides in length and contains 2 promoters that direct the expression of 2 non-structural proteins (NS1 and NS2) and 2 capsid proteins (VP1 and VP2) [5-7]. Mutations in the VP2 region have given rise to 2 new antigenic variants-CPV-2a and CPV-2b. Although the relative prevalence of CPV-2a and CPV-2b varies in different countries, these 2 variants are currently predominant in canine populations worldwide [8-16]. Some sporadic mutations have occurred since the emergence of CPV-2b, including mutations at glutamine (Glu)-426 and aspartic acid (Asp)-300, but no single significant mutation has been reported yet [14,17-19]. At least 5 conserved amino acid substitutions in the VP2 region were observed between CPV-2 and CPV-2a. CPV-2b has 2 more substitutions in VP2 than CPV-2a, where asparagine (Asn)-426 and isoleucine (Ile)-555 are replaced by Asp and valine (Val), respectively [11].
The antigenic variations in CPV can be detected by several methods, including hemagglutination, hemagglutination inhibition by monoclonal antibodies, sequence analysis, polymerase chain reaction (PCR), and real-time PCR [11,12,20,21]. Irrespective of the method used, it is critical to monitor changes in VP2, because VP2 determines the host range of the pathogen as well as other biological features. Substitution of only 1 amino acid in VP2 is known to cause dramatic changes in the antigenic properties of the virus [9,11,19,22,23].
In the present study, we isolated CPVs from the diarrheic sample of a dog with severe enteritis in order to develop a vaccine against CPV infection in dogs. We characterized the clinical isolate in terms of the nucleotide sequences of the VP2 gene and amino acid sequences of the VP2 protein. The virulence of the isolate was attenuated by passaging the isolate 4 times by repeated infection of A72 cells. Our results suggest that it is highly possible to develop a novel vaccine against CPV infection in dogs.
Materials and Methods
PCR for confirmation of CPV in fecal samples
To verify the presence of CPV in the fecal samples of a dog with clinical symptoms of severe enteritis, we performed PCR with primer sets for the conserved region in various strains of CPV (sense, 5'-AAAGAGAGCCAGGAGAGGTA-3'; anti-sense, 5'-TTCTGACAGCAGGTTGACCA-3') at 94℃ for 1 minute, 55℃ for 1 minute, and 72℃ for 1 minute (30 cycles) [24]. DNA for PCR analysis was obtained from the feces and isolated using QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Isolation and passage of the virus
The fecal sample was homogenized (10% w/v) in phosphate-buffered saline (PBS) and subsequently clarified by centrifuging the solution at 2,800 rpm for 10 minutes. The supernatant was filtered through a 0.45-µm filter and then a 0.22-µm filter (Schleicher & Schuell, Keene, NH, USA) to obtain the viral fraction. The filtered supernatant was overlaid on a 70% monolayer of A72 canine fibroma cells in alpha-minimum essential medium supplemented with 2% fetal bovine serum, non-essential amino acids, and antibiotics (at a final concentration of 100 units/mL of penicillin G sodium, 0.1 mg/mL of streptomycin sulfate, and 29 µg/mL of amphotericin B). The cytopathic effect (CPE) of the isolate on A72 cells was observed at 3 days post-infection (dpi). The presence of CPV on infected cells was confirmed by indirect fluorescent assay (IFA) by using an anti-CPV monoclonal antibody (Genobiotech, Daejeon, Korea) and fluorescein isothiocyanate-conjugated anti-mouse IgG (Sigma-Aldrich, St. Louis, MO, USA). Then, the isolated virus was passaged 4 times on A72 cells. CPE-positive A72 cell samples in their fourth passage were alternatively frozen and thawed 3 times, and then centrifuged at 3,000 rpm for 10 minutes at 4℃. The presence of CPV in the supernatant was confirmed by PCR analysis with type 2b-specific primers and CPE generation on the A72 cell monolayer before performing the in vivo infection tests.
Determination of the type of the isolated CPV
To determine the type of the isolated CPV, PCR was performed using CPV-2b-specific primer pairs (sense, 5'-CTTTAACCTTCCTGTAACAG-3'; anti-sense, 5'-CATAGTTAAATTGGTTATCTAC-3') at 94℃ for 30 seconds, 55℃ for 2 minutes, and 72℃ for 40 seconds (30 cycles).
Cloning of VP2 from the clinical isolate
The VP2 gene of the clinical isolate was amplified using PCR with primer pairs (sense, 5'-ATGAGTGATGGAGCAGTTCAACC-3'; anti-sense, 5'-TTAGTATAATTTTCTAGGTGCTAG-3') at 94℃ for 30 seconds, 55℃ for 2 minutes, and 72℃ for 40 seconds (30 cycles). The PCR product was isolated by electrophoresis on a 1% agarose gel and purified using QIAquick gel extraction kit (Qiagen) according to the manufacturer's instructions. The purified sample was cloned into apCR2.1-TOPO vector (Invitrogen, Carlsbad, CA, USA) and used to transform competent JM109 cells. The colonies were grown for 18 hours at 37℃ on Luria broth agar plates containing ampicillin. The plasmids were extracted by using GENE ALL Plasmid SV mini kit (General Biosystem, Seoul, Korea), and the inserts were subjected to dideoxy chain-termination sequencing (Applied Biosystems, Foster City, CA, USA). Sequence homology was estimated using BLASTn of the National Center for Biotechnology Information.
Testing the protection abilities of the attenuated virus
Seven male beagle dogs, aged 6 to 8 weeks, were used as test animals. The absence of the parvoviral antigen and the anti-parvoviral antibody in these animals was confirmed by the parvoviral antigen detection kit (Isu Abxis, Seoul, Korea) and parvoviral antibody detection kit (ImmunoComb, Biogal-Galed Labs., Galed, Israel), respectively. These dogs were allocated to 3 groups: group I, group II, and group III, which comprised 2, 2, and 3 dogs, respectively. Group I served as the control group and were orally administered with 1 mL of PBS without any infective agents. The dogs in group II were orally administered with the same volume ofthe clinical isolate, and those in group III were orally administered with the attenuated virus 2 weeks before the oral challenge with the clinical isolate. The dosage of the virus was 10, 50% tissue culture infectious dose (TCID50) of CPV [16,25]. The clinical condition of each animal was monitored daily. Blood samples were obtained at 2-day intervals to analyze viremia and antibody levels each condition was detected using PCR and ImmunoComb (Biogal-Galed Labs.), respectively. Feces of each animal were also taken at 2-day intervals for PCR investigation of fecal shedding of CPV. On the day of death or 11 days after inoculation, 10 different anatomical samples were taken (from the spleen, mesenteric lymph node, thymus, cardiac muscle, liver, lung, kidney, duodenum, jejunum, and ileum) and analyzed by PCR. Histological changes in the jejunum and ileum were evaluated after hematoxylin and eosin staining.
Results
Isolation of a novel type of CPV
We studied CPV infection in a dog that had severe enteritis, which was later confirmed by using a parvoviral antigen detection kit (Isu Abxis). PCR analysis of the DNA isolated from the fecal samples with the universal primers also showed positive results, as expected (Fig. 1). To isolate the virus from the fecal samples, we inoculated the filtered viral fraction on the A72 cells. The infected A72 cells showed cytoplasmic atrophy and elongation, nuclear polymorphism, and degradation, as well as detachment from the bottom of the plate in their final stage. On average, these changes occurred at 3 dpi and peaked at 5 dpi. CPVs in CPE-positive A72 cells were successfully visualized using IFA. A significantly higher increase in fluorescence was observed in the CPV-infected A72 cells than in the negative control cells (Fig. 2). These results clearly show that the isolate was a CPV, and we characterized this isolated virus.
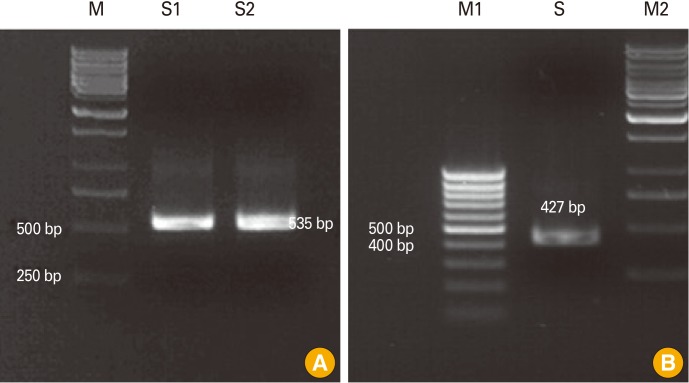
Fig. 1.
Identification of canine parvovirus (CPV) in fecal sample from a dog with enteritis. Polymerase chain reaction was performed with the universal primer pairs (A) and CPV-2b-specific primer pairs (B) to identify CPV in the fecal sample ofa dog with enteritis. (A) M, marker; S1 and S2, fecal samples from a dog. (B) M1, 100 bp marker; M2, marker; S, fecal sample.
Fig. 2.
Immunofluorescent assay (IFA) to verify the presence of the canine parvovirus (CPV). A72 cells were infected with the clinical isolate for 3 days, and the presence of CPV on infected cells was confirmed by IFA using anti-CPV monoclonal antibody and fluorescein isothiocyanate-conjugated anti-mouse IgG.
To identify the type of clinical isolate, we performed PCR with primer sets specific to CPV-2b. As shown in Fig. 1, the genomic size was found to be 472 bp, as expected, indicating that this clinical isolate belongs to the CPV-2b type. We then determined the nucleotide sequence of the virus and found that the isolate was a novel type since its VP2 region was not identical to that of any of the CPVs listed on GenBank. As shown in Table 1, this isolate has Asn at amino acid position 426 in VP2, while CPV-39 and CPV-133, which are classical CPV-2b strains, have Asp at that position.
Table 1.
Nucleotide and amino acid sequence analysis of the VP2 gene
CPV, canine parvovirus.
Protective capacity of the attenuated virus
In order to develop a new vaccine against CPV infection, we passaged the clinical isolate 4 times on A72 cells and performed the tests described in Materials and Methods with the attenuated virus to determine its protection abilities. The dogs in group II that were inoculated with the fecal viral sample alone experienced diarrhea at 3 dpi, and their symptoms immediately progressed to severe enteritis with anorexia, vomiting, diarrhea, dehydration, and dyspnea; they finally died at 6 dpi. In contrast, the 2 dogs in group III that were administered with attenuated virus prior to the oral challenge with the clinical isolate remained healthy until the termination of the experiment and did not show any clinical symptoms. The 2 dogs in group I, the negative control group without any artificial infection, were healthy for the first 6 days since the start of the experiment but experienced anorexia and diarrhea at 7 dpi, which progressed to severe diarrhea and dehydration by around 10 dpi. These pathological conditions of the dogs in group I might be attributed to the transmission of CPV from the dogs in group II.
The presence of CPV in the blood and feces was examined by PCR. Consistent with the clinical symptoms was the observation that the dogs in group II exhibited positive bands at 3-5 dpi; this indicated viremia and fecal shedding of CPV. The dogs in group I showed the presence of CPV at 9-11 dpi. However, the dogs in group III did not show viremia at all, although 2 of them (dog #5 and #7) showed fecal shedding of CPV at 5-9 dpi (Fig. 3).
Fig. 3.
Polymerase chain reaction (PCR) tests for detection of viremia and canine parvovirus (CPV) shedding. The (A) blood and (B) feces of dogs in the 3 different groups (group I, #1 and #2; group II, #3 and #4; group III, #5, #6, and #7) were taken at 1, 3, 5, 7, 9, and 11 days after inoculation and were subjected to PCR analysis to detect CPV.
To examine the development of antibody against CPV, the antibody levels in the blood were determined by using ImmunoComb; the results showed that antibody titers against CPV arose significantly at 7-11 dpi in the dogs of group III (Fig. 4). The dogs in the other groups, except dog #3 in group II, did not show such elevated antibody titers throughout the experiment. The antibody titer of dog #3 increased to 7 arbitrary units at 5 dpi, although this dog died at 6 dpi.
Fig. 4.
Changes in levels of antibody titers against canine parvovirus (CPV). Antibody titer levels against CPV at different times (days 0, 1, 3, 6, 7, 9, and 11 after inoculation) were determined by using ImmunoComb. The dogs in group II died at 6 dpi.
An important factor that should be studied to understand the virulence of this virus is its tropism. To examine this factor, we performed PCR to detect CPV in 10 different anatomical samples from the test animals. Most of the samples from each dog in groups I and II had CPVs, but in the group III dogs, CPVs were present in only 1or 2 different samples, including the ileum, mesenteric lymph nodes, spleen, and kidneys (Fig. 5).
Fig. 5.
Canine parvovirus (CPV) detection in different anatomical samples of the dogs after inoculation of the virus. On the day of death or 11 days after inoculation, 10 different anatomical samples from each dog were screened for the virus by polymerase chain reaction. Jej, jejunum; mLN, mesenteric lymph nodes; Thy, thymus; Lun, lung; Spl, spleen; Ile, ileum; Duo, duodenum; cMu, cardiac muscle; Liv, liver; Kid, kidney.
Histological examination of the jejunum and ileum of the dogs in groups I and II showed severe destruction of the villi and mucosal layers, including loss of surface epithelium, dilated crypts, shortening and blunting of villi, and inflammatory and hemorrhagic infiltration into the lamina propria (Fig. 6). No such significant histologic lesions were found in the intestines of the dogs in group III.
Fig. 6.
Histopathological changes in the small intestine of dogs in group II. Hematoxylin and eosin staining was performed on the samples from duodenum and jejunum of the group II dogs that showed typical clinical signs of canine parvovirus infection. Histopathological changes in 2 dogs (dogs #3 and #4) were similar, and representative changes are shown here.
Discussion
In this study, we successfully isolated a new type of CPV from a dog with severe enteritis, and the attenuated form of the virus conferred complete protection against infection with homologous CPV. This suggests that it is highly possible to develop a new vaccine against CPV in dogs.
Molecular characterization of the clinical isolate revealed that the virus had Asn at position 426 (426-Asn) and Val at position 555 (555-Val) in VP2. The amino acid sequences in VP2 of the clinical isolate are different from those in either CPV-2a or CPV-2b. These substitutions are of importance, since a single-nucleotide substitution in CPV may result in dramatic modulation of antigenicity [19,22,23]. Although there were obvious differences in amino acid sequences in VP2, we could not differentiate the isolate from the standard CPV-2b by using the differential PCR assay developed by Pereira et al. [12]. This was because the CPV-2b-specific primers were designed to test for 2 single-nucleotide polymorphisms-A4062G and A4449G-which indicate the replacement of amino acids at positions 426 and 555 in CPV-2b. Each of the type 2b-specific primers has 1 of the 2 mutations at the 3' end therefore, any nucleotide mismatches at these locations could effectively prevent primer extension. However, in our case, the mismatch occurred only at position 4062 in the isolate, and the amplification of the isolate was not completely inhibited by the 2b-specific primers. This phenomenon was studied by Decaro et al. [21], and the CPV subgroup with 426-Asn and 555-Val in VP2, a category that includes the isolate, was designated as the novel type-2a CPV.
The dogs in group III remained healthy until the end of the test, without any signs of illness, and induced strong humoral immune responses, indicating that the attenuated virus exhibits potential immunogenicity. It is noteworthy that the virulence of the clinical isolate disappeared on just 4 passages and its immunogenicity was retained. Our results indicate that the virulence of the clinical isolate had been rapidly attenuated during the course of cellular adaptation, and that the antibodies induced by the cell-adapted CPVs had a strong protective effect against the homologous CPV. The high efficacy of a low-passage, high-titer modified live CPV vaccine in pups with maternally derived antibodies was studied by Hoare et al. [25]. In their study, the low-passage CPV was more immunogenic than conventional modified live CPV strains, thereby overcoming the vaccine break because of relatively higher levels of maternally derived antibodies.
In this study, we successfully isolated a CPV from a dog and found that this strain was a novel type 2b. However, we cannot speculate on the prevalence of this type of virus in the dog population in Korea. Although the vaccine developed in this study provided protection against homologous CPV, its efficacy in conferring protective capacity against other types of CPV in dogs should be determined. In this regard, epidemiological studies on CPV strains in the domestic dog population are urgently needed because they will improve the management of infections resulting from the swift evolution of the continually progressing CPV strains.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Appel MJ, Scott FW, Carmichael LE. Isolation and immunisation studies of a canine parvo-like virus from dogs with haemorrhagic enteritis. Vet Rec. 1979;105:156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- 2.Parrish CR, Have P, Foreyt WJ, Evermann JF, Senda M, Carmichael LE. The global spread and replacement of canine parvovirus strains. J Gen Virol. 1988;69(Pt 5):1111–1116. doi: 10.1099/0022-1317-69-5-1111. [DOI] [PubMed] [Google Scholar]
- 3.Parrish CR. Host range relationships and the evolution of canine parvovirus. Vet Microbiol. 1999;69:29–40. doi: 10.1016/s0378-1135(99)00084-x. [DOI] [PubMed] [Google Scholar]
- 4.Truyen U, Gruenberg A, Chang SF, Obermaier B, Veijalainen P, Parrish CR. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binn LN, Lazar EC, Eddy GA, Kajima M. Recovery and characterization of a minute virus of canines. Infect Immun. 1970;1:503–508. doi: 10.1128/iai.1.5.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael LE, Binn LN. New enteric viruses in the dog. Adv Vet Sci Comp Med. 1981;25:1–37. [PubMed] [Google Scholar]
- 7.Carmichael LE, Schlafer DH, Hashimoto A. Minute virus of canines (MVC, canine parvovirus type-1): pathogenicity for pups and seroprevalence estimate. J Vet Diagn Invest. 1994;6:165–174. doi: 10.1177/104063879400600206. [DOI] [PubMed] [Google Scholar]
- 8.Buonavoglia D, Cavalli A, Pratelli A, et al. Antigenic analysis of canine parvovirus strains isolated in Italy. New Microbiol. 2000;23:93–96. [PubMed] [Google Scholar]
- 9.Mochizuki M, Harasawa R, Nakatani H. Antigenic and genomic variabilities among recently prevalent parvoviruses of canine and feline origin in Japan. Vet Microbiol. 1993;38:1–10. doi: 10.1016/0378-1135(93)90070-n. [DOI] [PubMed] [Google Scholar]
- 10.Parrish CR, O'Connell PH, Evermann JF, Carmichael LE. Natural variation of canine parvovirus. Science. 1985;230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- 11.Parrish CR, Aquadro CF, Strassheim ML, Evermann JF, Sgro JY, Mohammed HO. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J Virol. 1991;65:6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira CA, Monezi TA, Mehnert DU, D'Angelo M, Durigon EL. Molecular characterization of canine parvovirus in Brazil by polymerase chain reaction assay. Vet Microbiol. 2000;75:127–133. doi: 10.1016/s0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- 13.Steinel A, Venter EH, Van Vuuren M, Parrish CR, Truyen U. Antigenic and genetic analysis of canine parvoviruses in southern Africa. Onderstepoort J Vet Res. 1998;65:239–242. [PubMed] [Google Scholar]
- 14.Truyen U, Platzer G, Parrish CR. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Vet Rec. 1996;138:365–366. doi: 10.1136/vr.138.15.365. [DOI] [PubMed] [Google Scholar]
- 15.Truyen U, Steinel A, Bruckner L, Lutz H, Mostl K. Distribution of antigen types of canine parvovirus in Switzerland, Austria and Germany. Schweiz Arch Tierheilkd. 2000;142:115–119. [PubMed] [Google Scholar]
- 16.Wang HC, Chen WD, Lin SL, Chan JP, Wong ML. Phylogenetic analysis of canine parvovirus VP2 gene in Taiwan. Virus Genes. 2005;31:171–174. doi: 10.1007/s11262-005-1791-0. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Mochizuki M, Naito R, et al. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology. 2000;278:13–19. doi: 10.1006/viro.2000.0653. [DOI] [PubMed] [Google Scholar]
- 18.Hirayama K, Kano R, Hosokawa-Kanai T, et al. VP2 gene of a canine parvovirus isolate from stool of a puppy. J Vet Med Sci. 2005;67:139–143. doi: 10.1292/jvms.67.139. [DOI] [PubMed] [Google Scholar]
- 19.Martella V, Cavalli A, Pratelli A, et al. A canine parvovirus mutant is spreading in Italy. J Clin Microbiol. 2004;42:1333–1336. doi: 10.1128/JCM.42.3.1333-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura M, Nakamura K, Miyazawa T, Tohya Y, Mochizuki M, Akashi H. Monoclonal antibodies that distinguish antigenic variants of canine parvovirus. Clin Diagn Lab Immunol. 2003;10:1085–1089. doi: 10.1128/CDLI.10.6.1085-1089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decaro N, Elia G, Campolo M, et al. New approaches for the molecular characterization of canine parvovirus type 2 strains. J Vet Med B Infect Dis Vet Public Health. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 22.Chang SF, Sgro JY, Parrish CR. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truyen U, Parrish CR, Harder TC, Kaaden OR. There is nothing permanent except change. The emergence of new virus diseases. Vet Microbiol. 1995;43:103–122. doi: 10.1016/0378-1135(95)92531-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirasawa T, Kaneshige T, Mikazuki K. Sensitive detection of canine parvovirus DNA by the nested polymerase chain reaction. Vet Microbiol. 1994;41:135–145. doi: 10.1016/0378-1135(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 25.Hoare CM, DeBouck P, Wiseman A. Immunogenicity of a low-passage, high-titer modified live canine parvovirus vaccine in pups with maternally derived antibodies. Vaccine. 1997;15:273–275. doi: 10.1016/s0264-410x(96)00184-3. [DOI] [PubMed] [Google Scholar]