Abstract
Purpose
We report on the prevalence of benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTS) among men of Jeju Island, representing a coastal and insular area, using a cross-sectional community-based survey.
Materials and Methods
A total of 553 participants in a prostate health screening campaign on Jeju Island were subjected to measurements of the International Prostate Symptom Score (IPSS), prostate volume, uroflowmetry, postvoiding residual urine volume, and prostate-specific antigen levels. Eliminating 58 participants who were suspected of having prostate cancer, we analyzed the data from 495 participants. The definition of BPH was a combination of moderate IPSS (8~19) to severe IPSS (>19) and prostate enlargement (>30 g on transrectal ultrasonography).
Results
The prevalence of BPH was 21.0% overall: 11.6% among subjects aged 50~59 years, 18.1% for those aged 60~69, 30.8% for those aged 70~79 and 50.8% among those aged 80 years or more. Compared with previous studies in urban or rural areas, the prevalence was slightly lower. The prevalence of BPH and of moderate to severe LUTS increased with age and showed significant differences between age groups (p=0.028 and 0.033, respectively). A positive correlation was found between the IPSS and quality of life score. Among subunits of IPSS, the nocturia score contributed most to the severity of LUTS and had the highest correlation with a quality of life score.
Conclusions
The overall prevalence of BPH in this study was 21.0%, which is slightly lower than in previous studies in urban or rural areas.
Keywords: Prostatic hyperplasia, Prevalence
INTRODUCTION
Benign prostatic hyperplasia (BPH) is one of the most common urologic diseases in men over 50 years of age. Enlargement of the prostate, manifestation of lower urinary tract symptoms (LUTS), and bladder outlet obstruction lead to various voiding symptoms, as the incidence increases rapidly with advancing years. There are differences in the reported prevalence of LUTS and BPH among countries, possibly arising from cultural or linguistic differences.1 Because this finding suggests that the results in one country might not be applicable to others, it is necessary to investigate the natural history of LUTS in each one.
As aging of the Korean population has increased, the management of BPH has become more important in overall public health care policy. However, there have only been a few epidemiological surveys about BPH in Korea.2-7 Furthermore, because previous data were not collected using standardized criteria and clinical definitions, the diagnostic and epidemiological studies carried out to date have limited value. There are only a few epidemiological reports on urban and rural areas, whereas there are no data for coastal or insular areas. Like rural areas, these contain a high proportion of elderly people. Therefore, the present study was carried out to determine the prevalence of BPH and LUTS in men living in Jeju Island as representing a coastal and insular area. We used a cross-sectional community-based study to establish statistical associations between the International Prostate Symptom Score (IPSS), prostate volume, peak urinary flow rate (Qmax), postvoiding residual urine volume (PVR) and quality of life (QoL) score, and analyzed changes in these variables with age.
MATERIALS AND METHODS
From September to December 2010, we conducted a cross-sectional community-based epidemiological study on men aged 50 years or over living in Jeju Island and the islands annexed to Korea. The study was conducted in cooperation with the community health centers of Jeju-do and the Korea Prostate Health Council, Inc. In total, 553 participants in a prostate health screening campaign were invited to participate in a check-up on their physical condition. To keep the study as representative as possible, we included them uniformly according to age and location. According to location and age, participants of no single administrative district or decade of age were over 40% of the total sample. Those who provided written informed consent to participate were included in the study. The participants were interviewed by a health care coordinator to obtain basic demographic information. All participants were also assessed for the serum level of prostate-specific antigen (PSA), Qmax using a portable uroflowmeter, and PVR by bladder ultrasound scans. They were given a linguistically validated version of the IPSS, with assistance provided whenever necessary. They underwent a physical examination, including a digital rectal examination (DRE) and measurement of prostate volume by transrectal ultrasound (TRUS) (SonoAce X6, Medison Inc., Seoul, Korea). Those requiring intervention were treated appropriately. Among them, 58 participants were excluded because of a history of prostate cancer or a PSA level of more than 4 ng/ml, or because of findings suggesting prostate cancer from the DRE or TRUS examinations. In the end, 495 participants were analyzed. The severity of LUTS was evaluated by IPSS and QoL and the men were categorized into four groups by years of age: 50~59, 60~69, 70~79, and 80 or older.
The questionnaire consisted of a Korean-language translation of the IPSS, which included seven LUTS questions (incomplete emptying, frequency, intermittency, urgency, weak urinary stream, hesitancy, and nocturia) and one QoL question. Each symptom was scored as a value of 0 to 5 (0, not at all; 1, less than one time in five; 2, less than half the time; 3, about half the time; 4, more than half the time; and 5, almost always during the preceding month). A symptom score of 0 to 35 was calculated by adding the scores the patient gave to each of the seven symptoms. A storage symptom score was obtained by adding the frequency, urgency, and nocturia values, while a voiding symptom score was calculated by adding the scores of incomplete emptying, intermittency, weak stream, and straining symptoms. Next, the symptom scores were categorized into three levels of severity from 'mild' to 'severe' (0~7, mild; 8~19, moderate; and 20~35, severe). The QoL question was utilized to score the overall discomfort to patients caused by their current urinary symptoms, from 0 to 6 (0, delighted; 1, pleased; 2, mostly satisfied; 3, about equally satisfied and dissatisfied; 4, mostly dissatisfied; 5, unhappy; and 6, terrible).
We defined clinical BPH as that seen in men showing moderate to severe symptoms (total IPSS score ≥8 points) with a prostate volume ≥30 g estimated from TRUS.8 In accordance with this definition, we investigated the prevalence rates of BPH and of moderate to severe LUTS according to age group. We also calculated age-adjusted prevalence of BPH with the direct standardization method, in which the prevalence was adjusted by age and gender to the population within the same age range in Jeju Island based on the community health survey (Ministry of Healh & Welfare, 2010). In addition, we studied the correlation between age, Qmax, prostate volume, QoL, and IPSS.
Statistical analyses were performed using SPSS, version 12.0 (SPSS Inc., Chicago, IL, USA) using one-way ANOVA, chi-squared tests, multiple regression analysis, and Pearson correlation analysis. p<0.05 and p<0.001 were considered statistically significant, respectively.
RESULTS
1. Prevalence of BPH and LUTS
The mean age of participants was 72.4±5.6 years (range 50~92). There were 164 (33%) men aged 50~59 years, 149 (30%) aged 60~69, 123 (25%) aged 70~79, and 59 (12%) aged 80 years or more. Mean values for prostate volume, Qmax, PVR, and PSA levels were 33.3±15.8 g, 20.5±1.3 ml/s, 13±5 ml, and 0.95±0.8 ng/ml, respectively. The IPSS total score and QoL score as an evaluation of LUTS and QoL for all participants were 12.5±7.6 points and 1.8±1.2 points, respectively. In comparing the groups, the total IPSS and QoL scores increased significantly according to the age group (p=0.016 and p=0.002, respectively). None of the following factors showed statistically significant differences among age groups: prostate size, Qmax, PVR, or serum PSA level (Table 1). Of the overall group, 104 men (21.0%) were determined to have clinically confirmed BPH using our definitions listed above. There was a slightly lower prevalence of BPH in coastal and insular areas than in urban and rural areas. The prevalence of BPH was 11.9% (19/164) of men aged 50~59 years, 18.0% (27/149) of those aged 60~69, 30.8% (38/123) of those aged 70~79, and 50.8% (30/59) of men aged 80 or more. These changes with age were statistically significant (p=0.028) (Table 1). We calculated that the age-adjusted prevalence of BPH was 23.3% (95% confidence interval [CI]: 19.8~25.6) overall. According to age, the prevalence was 16.8% (95% CI: 12.5~20.4) of men aged 50~59 years, 21.7% (95% CI: 18.5~23.9) of those aged 60~69, 24.4% (95% CI: 20.8~26.6) of those aged 70~79, and 28.1% (95% CI: 22.2~34.8) of men aged 80 or more.
Table 1.
Population demographics and the prevalence of BPH
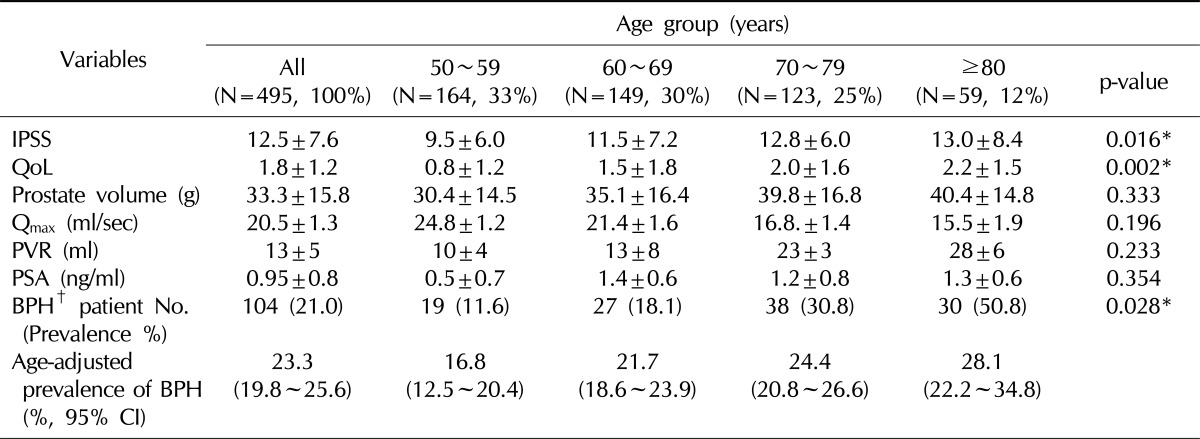
BPH: benign prostate hyperplasia, IPSS: International Prostate Symptom Score, QoL: quality of life, Qmax: peak uroflow rate, PVR: postvoiding residual urine volume, PSA: prostate-specific antigen, CI: confidence interval.
*Statistically significant. †Definition of BPH: IPSS ≥8 and prostate volume ≥30 g.
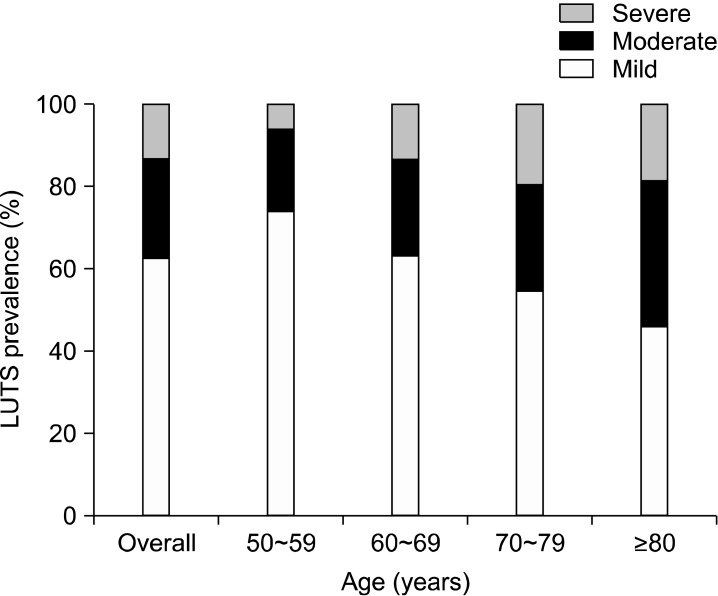
The incidence of mild LUTS was 62.4% (309/495), that of moderate LUTS was 24.4% (121/495), and that of severe LUTS was 13.1% (65/495). The overall prevalence of moderate to severe LUTS was 37.6% (186/495). It was found in 26.2% (43/164) of men aged 50~59 years, 36.9% (55/149) of men aged 60~69, 45.5% (56/123) of men aged 70~79, and 54.2% (32/59) of men aged 80 or more. These age-related increases were statistically significant (p=0.033) (Fig. 1).
Fig. 1.
Prevalence and severity of lower urinary tract symptoms (LUTS) in different age groups studied. The prevalence of moderate to severe LUTS increased significantly with age.
2. Correlations between IPSS and other parameters
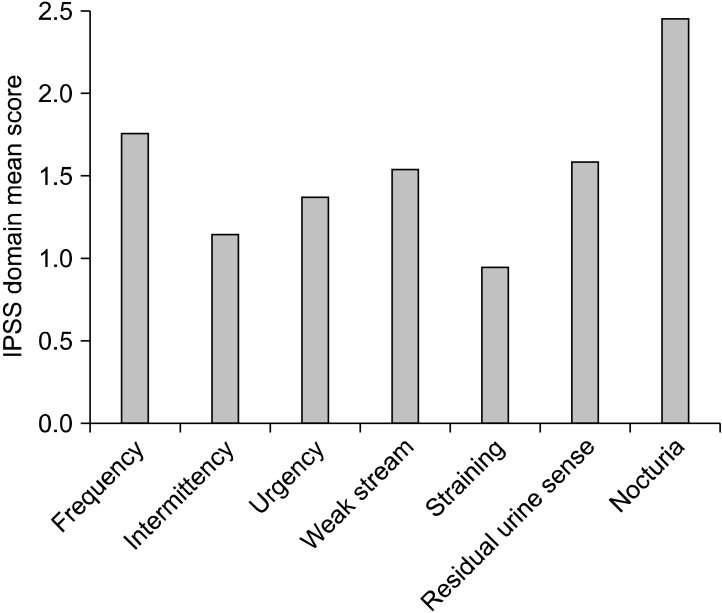
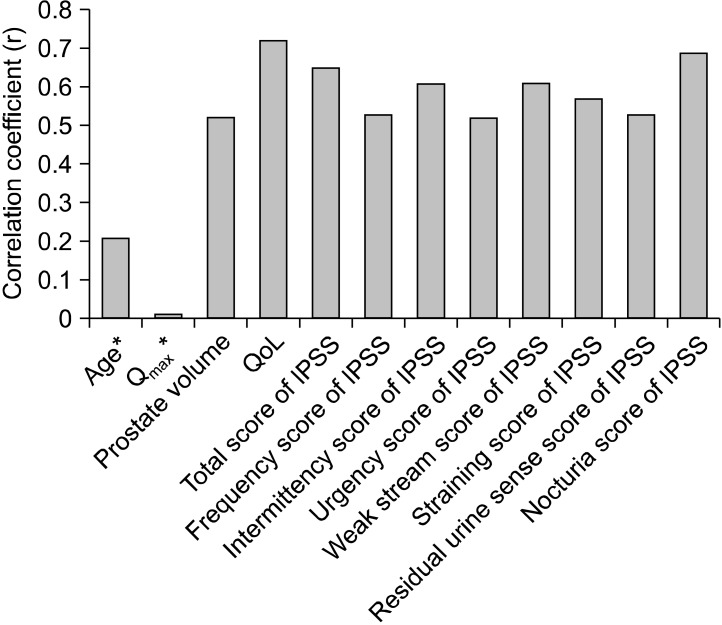
To evaluate each subunit in the IPSS domain of severity for moderate to severe LUTS, nocturia was the highest at 2.5, followed by 1.8 for urinary frequency. The scores were lower for a sense of residual urine (1.7), weak stream (1.6), urgency (1.4), and straining (1.0) (Fig. 2). We searched for a correlation between IPSS and each parameter such as QoL, age, Qmax and prostate size. Multiple regression analysis showed the IPSS and prostate volume to be a significant predictor of a QoL score (Pearson correlation coefficient r=0.67, 0.54; p<0.001, respectively). However, age and Qmax showed a weak correlation. Each subunit of the IPSS was found to be closely related to the total IPSS score and QoL score, and there were statistically significant correlations. Further, stepwise multiple regression analysis against the seven symptoms in the IPSS showed that nocturia was the strongest predictor of a QoL score (Pearson correlation coefficient r=0.71, p<0.001; Fig. 3).
Fig. 2.
Severity of subunits of lower urinary tract symptom (LUTS) categories in International Prostate Symptom Score (IPSS) questionnaire. The score for nocturia was highest followed by frequency.
Fig. 3.
Pearson correlation coefficients of the IPSS with each parameter. Age* and Qmax* were weak correlation factors but the rest were strong correlation factors (p<0.001). IPSS: International Prostate Symptom Score, Qmax: peak urinary flow rate, QoL: quality of life.
DISCUSSION
The diagnosis of BPH includes taking a medical history of the patient and recording the IPSS, DRE, Qmax, PVR, and TRUS results, but it is difficult to define BPH from these tests. Furthermore, it is impossible to choose any measure that is superior to the others in terms of the efficacy of treatment. Hence, researchers have reported data on the prevalence of BPH according to their own definitions. It is difficult to develop diagnostic criteria of BPH based on a standard method. Thus, Garraway et al9 defined clinical BPH as a prostate volume of ≥20 g measured by TRUS and a Qmax of ≤15 ml/sec. The prevalence of BPH in their study was 14% of men in their 40s and 40% of men in their 70s. Bosch et al10 reported a 19% prevalence of BPH, which was defined as a prostate volume of ≥20 g by TRUS and an IPSS of ≥8 with moderate to severe LUTS. In Korea, Rhew et al4 defined clinical BPH as an IPSS of ≥8 with moderate to severe LUTS and a Qmax ≤10 ml/sec; Chung et al3 defined it as an IPSS of ≥8 with moderate to severe LUTS, a prostate size of ≥20 g measured by DRE, and a Qmax of ≤10 ml/sec. Thus, there have been no consistent criteria for diagnosing BPH clinically. Previous epidemiologic studies in Korea have tended to consider three variables, namely IPSS, Qmax, and prostate volume. However, in the present study, clinical BPH was defined as an IPSS of ≥8 with moderate to severe LUTS and a prostate volume of ≥30 g measured by TRUS. This is why there were no statistically significant differences in the prevalence of BPH when using the two variables of IPSS and prostate size (12%) from that of BPH diagnosed using the three variables of IPSS, prostate size, and Qmax (10%).6,11 A recent study in Korea, using a good epidemiological study design, applied the same two variables.6 In addition, Park et al6 claimed that Qmax varies according to voiding urine volume in individuals and there were large errors in the criteria to determine whether or not BPH was present by Qmax ≤10 ml/sec or ≤15 ml/sec uniformly. We agreed with Park and decided to base our criteria for clinical BPH on those adopted in Park's study.
To investigate the prevalence of any disease, epidemiological studies should be conducted using random sampling according to population distribution ratios. We attempted to maintain the representative nature of each area in this study, and we made an effort to assign populations using a constant ratio; however, there are limitations when enrolling voluntary participants. Nevertheless, given the large socioeconomic cost and difficulties encountered in sampling and collecting data in such epidemiological studies, we believe that our study is valid.
The present study showed that the prevalence of clinical BPH was slightly lower, at 21.0% (104/495 men), than in other reports from Korea. The reported prevalence rates of BPH were 40% in the urban area of Seongnam in Gyeonggi-do province and 25.5% in Busan.4,6 The prevalence was 23.2% in the rural area of Yeoncheon in Gyeonggi-do, and 27.7% in the inland area of Chungcheongbuk-do.5,7 The prevalence in Seongnam was much higher than that of the other areas, but the subjects of that study were men aged 65 years and older, which might have contributed to the increased prevalence of BPH. In addition, it is possible that men in Seongnam had more time and resources to obtain health information; therefore, the degree of understanding might have been better than in other areas. Unlike the results for urban areas, the prevalence of BPH was very low at 11.1% in the rural area of Jeongeup in Jeollabuk-do province.3 This was probably because prostate volume was not checked by TRUS but by DRE, which is not an objective measurement and one that tends to underestimate the real prostate volume and give a spuriously low prevalence of BPH, but with low reliability. Compared with domestic data, the prevalence of BPH was 27.6% (in men aged over 50 years) in Japan, 30% (55~75 years) in The Netherlands and 23% (50~80 years) in Canada. Data from other countries are within the range of 25~30%.12-14
The present study showed that the prevalence of mild LUTS was 62.4% (309/495), that of moderate LUTS was 24.4% (121/495), and that of severe LUTS was 13.1% (65/495). The overall prevalence of moderate to severe LUTS was 37.6% (186/495). This was lower than that of the other reports (>50%). In Korea, the reported incidence rates of LUTS were 53% in Seongnam, 63.6% in Busan, 50.1% in Chungcheongbuk-do province, and 49.4% in Jeongeup in Jeollabuk-do province. Data for other countries including USA, Japan, and Sweden were similar.15-17
To conclude, the present study showed that prevalence rates of clinical BPH and that of moderate to severe LUTS in Jeju Island were lower than in other areas of Korea and in other countries. In our opinion, we think that one cause might lie in the residents' diet, consisting primarily of healthy seafood, contrasting with areas with high meat consumption. Second, Jeju Island is separated from the mainland, so residents have continued to maintain their genetic traits as a coastal and insular area. Third, cultural differences in these areas mean that BPH is simply regarded as a natural process of aging and not a serious disease. Finally, we believe there was some difficulty in communications caused by old age.
Our results were similar to previous studies in that there were close correlations between QoL and the IPSS total score, and between QoL and prostate volume, but weak correlations between QoL and age, and between QoL and Qmax. Thus, the QoL and IPSS measures increased significantly with age but Qmax and PVR did not. Rha et al18 reported a statistically significant difference in prostate volume with age; we also found that prostate size increased with age but this lacked statistical significance.
We investigated the correlation of each subunit of the LUTS category in the IPSS domain. Nocturia was the most common LUTS and showed the highest correlation with QoL. These results were similar to those reported from Jeongeup in Jeollabuk-do province and other reports with the exception of a weak stream having the highest correlation in Seongnam.3,6 This suggests that nocturia increased more rapidly with age than other subunits of LUTS. This has a great effect on the QoL and is the most common cause for visiting urologic clinics.19 The physical and mental fatigue caused by nocturia is associated with other physical and psychological problems and requires clearer explanations and education for those who experience it.
There were several limitations in the present study. First, the subjects were not a random sample of the population taken according to the distribution ratio, but voluntary participants among whom we made an effort to adjust for population variations according to a constant ratio. Second, we did not use an objective voiding diary, but a subjective questionnaire. Third, we did not consider the effect of previous medication or underlying diseases, such as a neurogenic bladder, urethral strictures, or an overactive bladder. Therefore, these factors should be considered when interpreting this study.
CONCLUSIONS
The overall prevalence of BPH among men from Jeju Island was 21.0% in men aged 50 years and older. It was 11.6% among those aged 50~59 years, 18.1% in those aged 60~69, 30.8% in those aged 70~79, and 50.8% in men aged 80 years or more. The incidences of BPH and moderate to severe LUTS increased with age but were slightly lower than in previous studies in urban or rural areas of Korea. Healthy dietary habits, maintaining inherited traits thanks to geographical separation, cultural differences, and a difficulty in communications caused by old age might have influenced these results. Nocturia was the most common LUTS in the IPSS domain and worsened the QoL. Therefore, we suggest that Jeju Island, representing a coastal and insular area, should be considered in future Korean public health policy on the clinical management of men with BPH.
References
- 1.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee E, Yoo KY, Kim Y, Shin Y, Lee C. Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur Urol. 1998;33:17–21. doi: 10.1159/000019529. [DOI] [PubMed] [Google Scholar]
- 3.Chung TG, Chung J, Lee MS, Ahn H. Prevalence of benign prostatic hyperplasia in Jeong-Eup area: community-based study. Korean J Urol. 1999;40:52–58. [Google Scholar]
- 4.Rhew HY, Koo JH, Cho SS, Kang JS, Lee CK, Kim JC, et al. The prevalence of BPH in Busan city over age 40. Korean J Urol. 2001;42:223–227. [Google Scholar]
- 5.Lee HL, Seo JW, Kim WJ. The prevalence of benign prostatic hyperplasia: community-based study in Chungbuk province. Korean J Urol. 1999;40:1500–1505. [Google Scholar]
- 6.Park HK, Park H, Cho SY, Bae J, Jeong SJ, Hong SK, et al. The prevalence of benign prostatic hyperplasia in elderly men in Korea: a community-based study. Korean J Urol. 2009;50:843–847. [Google Scholar]
- 7.Lee ES, Lee C, Kim Y, Shin Y. Estimation of benign prostatic hyperplasia prevalence in Korea: an epidemiological survey using international prostatic symptom score(IPSS) in Yonchon county. Korean J Urol. 1995;36:1345–1352. [Google Scholar]
- 8.Barry MJ, Fowler FJ, Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The Measurement Committee of the American Urological Association. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 9.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 10.Bosch JL, Kranse R, van Mastrigt R, Schröder FH. Reasons for the weak correlation between prostate volume and urethral resistance parameters in patients with prostatism. J Urol. 1995;153:689–693. doi: 10.1097/00005392-199503000-00039. [DOI] [PubMed] [Google Scholar]
- 11.Blanker MH, Groeneveld FP, Prins A, Bernsen RM, Bohnen AM, Bosch JL. Strong effects of definition and nonresponse bias on prevalence rates of clinical benign prostatic hyperplasia: the Krimpen study of male urogenital tract problems and general health status. BJU Int. 2000;85:665–671. doi: 10.1046/j.1464-410x.2000.00570.x. [DOI] [PubMed] [Google Scholar]
- 12.Ukimura O, Kojima M, Inui E, Ochiai A, Hata Y, Watanabe M, et al. A statistical study of the American Urological Association symptom index for benign prostatic hyperplasia in participants of mass screening program for prostatic diseases using transrectal sonography. J Urol. 1996;156:1673–1678. [PubMed] [Google Scholar]
- 13.Bosch JL, Hop WC, Kirkels WJ, Schröder FH. The International Prostate Symptom Score in a community-based sample of men between 55 and 74 years of age: prevalence and correlation of symptoms with age, prostate volume, flow rate and residual urine volume. Br J Urol. 1995;75:622–630. doi: 10.1111/j.1464-410x.1995.tb07421.x. [DOI] [PubMed] [Google Scholar]
- 14.Norman RW, Nickel JC, Fish D, Pickett SN. 'Prostate-related symptoms' in Canadian men 50 years of age or older: prevalence and relationships among symptoms. Br J Urol. 1994;74:542–550. doi: 10.1111/j.1464-410x.1994.tb09181.x. [DOI] [PubMed] [Google Scholar]
- 15.Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsukamoto T, Kumamoto Y, Masumori N, Miyake H, Rhodes T, Girman CJ, et al. Prevalence of prostatism in Japanese men in a community-based study with comparison to a similar American study. J Urol. 1995;154:391–395. doi: 10.1097/00005392-199508000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Andersson SO, Rashidkhani B, Karlberg L, Wolk A, Johansson JE. Prevalence of lower urinary tract symptoms in men aged 45-79 years: a population-based study of 40 000 Swedish men. BJU Int. 2004;94:327–331. doi: 10.1111/j.1464-410X.2004.04930.x. [DOI] [PubMed] [Google Scholar]
- 18.Rha KH, Choi YD, Hong SJ, Song JS, Kim BH, Choi HS, et al. Prostate volume variation with age: community-based survey in namhae region. Korean J Androl. 2001;19:119–123. [Google Scholar]
- 19.Kim BS, Lee JW, Kim YT, Park HY, Kwon SW, Lee TY. The prevalence and risk factors of nocturia for males participating in a prostate examination survey. Korean J Urol. 2008;49:818–825. [Google Scholar]