Abstract
Prostate cancer is now ranked fifth in incidence among cancers in Korean adult males. This is attributable to the more Westernized dietary style which increases the morbidity of prostate cancer and the development of cancer diagnostic technologies, such as prostate-specific antigen and advanced medical systems, increasing the rate of prostate cancer diagnosis. Prostate cancer effects include not only erectile dysfunction caused by the disease itself, but also by psychiatric disorders caused by prostate cancer or its treatments. Prostate cancer by itself reduces sexual desire and the frequency of sexual intercourse. Additionally, surgery or hormonal therapy to block testosterone further increases the frequency of erectile dysfunction. Erectile dysfunction following radical prostatectomy is primarily attributable to nerve injury caused by intraoperative nerve traction, thermal injury, ischemic injury, and local inflammatory reactions. Additionally, the absence of nocturnal penile tumescence causes persistent hypoxia of the corpus cavernosum, which, secondarily, causes anatomical and functional changes in the corpus cavernosum. Preservation of erectile function is one of the most significant issues for patients with local prostate cancer. Erectile dysfunction following radical prostatectomy is known to have various prognoses, depending on preservation of the neurovascular bundle, patient age, and preoperative erectile status. Intracavernosal injections, PDE5 inhibitors, and penile rehabilitation therapy using a vacuum constriction device after radical prostatectomy are known to improve the recovery of erectile function. Recently, testosterone replacement therapy has also drawn attention as a treatment method.
Keywords: Prostate, Neoplasms, Erectile dysfunction, Therapy
INTRODUCTION
The prostate is an accessory sex gland that consists of glandular tissue and fibromuscular tissue surrounding the glandular tissue. It has a mean weight of approximately 20 g in normal adults. The gland body of the prostate is arranged concentrically, having the urethra in the center, from which 15~30 tubes originate, and are connected to the prostatic urethra. The fluid produced in the prostate accounts for 20~30% of the total semen volume. Non-peptides, such as citric acid, spermine, cholesterol, lipids, and zinc, and proteins, such as prostate-specific antigen, (PSA), human kallikrein 2, human kallikrein L1, prostatic acid phosphatase, and prostatespecific protein are synthesized in and secreted from the prostate.
Prostate cancer is the most common male cancer in the USA, and ranks in second place in terms of cancer-related mortality.1,2 Risk factors for prostate cancer include age, ethnicity, and a high-fat diet. The morbidity of prostate cancer is increasing in South Korea at one of the fastest rates in the world. Morbidity due to prostate cancer in adult males, aged ≥65 years, was reported to be 5.1% in 1990 and 9.1% in 2005, showing an approximately two-fold increase.3 In 2007, prostate cancer was ranked in fifth in morbidity among cancers that occur in Korean male adults. This is attributable to the Westernized dietary style increasing the morbidity of prostate cancer and the development of diagnostic technologies, such as PSA and advanced medical systems, increasing the rate of prostate cancer diagnosis.4,5
Prostate cancer effects include not only erectile dysfunction caused by the disease itself, but also caused by psychiatric disorders in turn caused by prostate cancer or its treatment(s). Prostate cancer in and of itself reduces sexual desire and the frequency of sexual intercourse. Additionally, surgery or hormone therapy to block testosterone further increases the frequency of erectile dysfunction. Erectile dysfunction following radical prostatectomy is primarily attributable to nerve injuries caused by intraoperative nerve traction, thermal injury, ischemic injury, and local inflammatory reactions.
Preservation of erectile function is one of the most significant interests of patients with local prostate cancer. Erectile dysfunction following radical prostatectomy is known to have various progonoses, depending on the preservation of the neurovascular bundle, patient age, and preoperative erectile status. Intracavernosal injections, PDE5 inhibitors, and penile rehabilitation therapy using vacuum constriction devices after radical prostatectomy can improve the recovery of erectile function. Because most previous reports were, however, fragmentary or peripheral, gaining an overall understanding of prostate cancer and sexual function has been difficult. Accordingly, the overall relationship between prostate cancer and sexual function is reviewed in this paper.
PROSTATE CANCER AND OCCURRENCE OF SEXUAL DYSFUNCTION
Although the prostate is close to the penis, there has been no report of sexual dysfunction caused directly by prostate cancer. However, if a growing prostate tumor causes lower urinary tract symptoms, such as prostate hypertrophy, it could decrease sexual function. That would be a secondary reduction in sexual function caused by severe lower urinary tract symptoms, rather than a direct effect of the prostate cancer. That is, prostate cancer does not directly cause abnormalities of the corpus cavernosum.
A sudden reduction in sexual function in patients with prostate cancer frequently occurs because of psychological instability caused by the occurrence of the cancer, and, in particular, depression.6,7 The occurrence of prostate cancer also often has a negative effect on the mental status of the female partner, causing a decrease in sexual function in the female partner.8 Because a diagnosis of prostate cancer simultaneously causes fear and anxiety in married couples regarding the effects of prostate cancer on their lives, it also creates an unstable mental status in their sex life, resulting in reduced sexual activity.
TREATMENT OF PROSTATE CANCER AND SEXUAL FUNCTION
Prostate cancer treatments include surgery, hormone therapy, radiotherapy, and chemotherapy. Each treatment method is selected primarily according to the disease stage of the patient. In prostate cancer treatment, as testosterone is involved in the prostate and its mechanism of action, erectile dysfunction often occurs as a critical complication regardless of the selection of a surgical treatment or hormonal therapy.
1. Radical prostatectomy and sexual function
Radical prostatectomy is the standard treatment method selected for the treatment of local prostate cancer limited to the intraprostate region. As endourological treatments using an endoscope or robot have been used more frequently recently, the frequency of radical prostatectomy has been decreasing. It is, however, still the most important treatment method for local prostate cancer.
Erectile dysfunction that occurs after curative radical prostatectomy is generally described to be caused by injuries of the cavernous nerve and blood vessels, and has been considered a postoperative complication that is unavoidable in conventional surgery. In the past, due to insufficient data regarding the neurovascular bundle near the prostate, the frequency of erectile dysfunction after radical prostatectomy was high. In 1982, Walsh and Donker9 reported that erectile dysfunction could be decreased by reducing injury of the neurovascular bundle that is located near the prostate and is connected to the corpus cavernosum. Since then, because the neurovascular bundle has been specifically preserved by the anatomical approach that has been introduced to curative radical prostatectomy, the frequency of erectile dysfunction has decreased substantially.10 In radical prostatectomy that preserves the nerve, however, it has been reported that, unlike urinary incontinence, erectile function does not show a rapid recovery, and its recovery may be delayed for up to 2 years after the surgery.11 Erectile dysfunction following radical prostatectomy is attributable primarily to nerve injury caused by intraoperative nerve traction, heat-induced injury, ischemic injury, and local inflammatory reactions, resulting in postoperative erectile dysfunction despite sexual stimulation, failure of penile erection during sleep, and persistent hypoxia of the corpus cavernosum. These problems then secondarily cause anatomical and functional changes in the corpus cavernosum.12
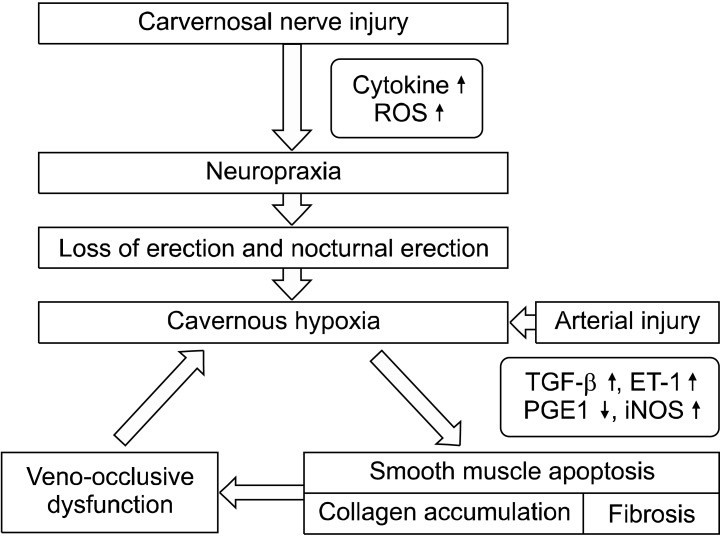
Previous studies using animal models reported that cavernosal nerve injury caused increased collagen in the corpus cavernosum, apoptosis in the cavernosal smooth muscle, and cavernosal fibrosis. Additionally, due to increased transforming growth factor-1 and decreased prostaglandin E1 (PGE1) and cyclic adenosine monophosphate (cAMP) in the corpus cavernosum, and the secretion of cytokines and endothelin-1 from injured nerves, veno-occlusive erectile dysfunction may then occur as a result of structural changes in the corpus cavernosum (Fig. 1).13 Direct injury of the neurovascular bundle during radical prostatectomy may also cause arterial erectile dysfunction due to injuries to the cavernosal artery.
Fig. 1.
Pathophysiology of erectile dysfunction following radical prostatectomy. ROS: reactive oxygen species, TGF-β: transforming growth factor-beta, ET-1: endothelin-1, PGE1: prostaglandin E1, iNOS: inductible nitric oxide synthase.
The incidence of erectile dysfunction occurring after radical prostatectomy has been reported to be 10~100%, depending on the study (Table 1).14 This range is likely attributable to differences in surgical methods, patient age, ethnicity, preoperative erectile status, data analysis methods, and points in time examined. Generally, erectile function is recovered within 1 year following radical prostatectomy, and within 2 years after surgery in the case of a delayed situation. Thereafter, the recovery of erectile function is difficult.15 Various factors are involved in the recovery of erectile function post-surgery. The recovery frequency has been reported to vary significantly depending on preoperative erectile function, patient age, postoperative changes in penile hemodynamics, preservation of the neurovascular bundle during surgery, and the surgeon's performance.16
Table 1.
Radical prostatectomy and consequences for erectile function after the operation
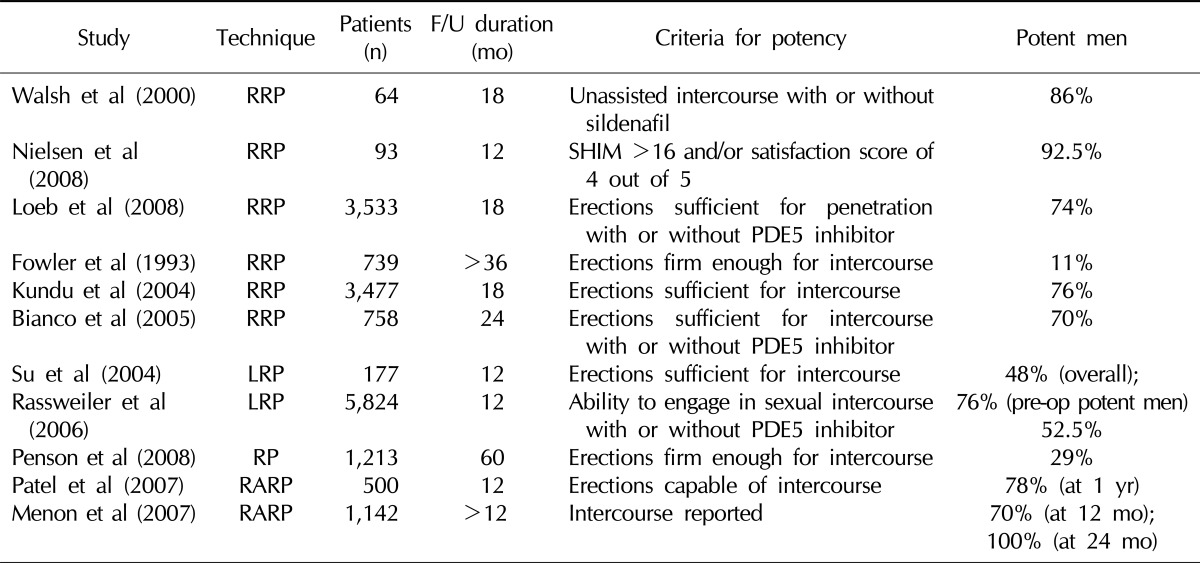
F/U: follow-up, RRP: retropubic radical prostatectomy, LRP: laparoscopic radical prostatectomy, RP: radical prostatectomy, RARP: robot-assisted radical prostatectomy, SHIM: sexual health inventory for men, Pre-op: pre-operative.
2. Radiation therapy and sexual function
Sexual dysfunction occurs in 20~80% of patients who undergo radiation therapy. Radiation therapy may cause injuries, including to nerves, if the radiation range expands to the trigone of the bladder, ectopic ureteral orifice, posterior and lateral rectal wall, and bulbo-membranous urethra. Complications that occur after radiation are classified as early and late. Early complications occur in tissues that undergo rapid mitosis, like the intestinal mucosa, and are typically reversible, whereas late complications occur due to injury of the microvessels, and are irreversible changes.
Generally, the main complications that occur after radiation therapy, such as proctitis, urethral stricture, edema of the penis and lower extremities, and erectile dysfunction, have been shown to occur at incidences of 10% or less.17 Additionally, the frequency of maintaining erectile function after radiation therapy has been reported to be approximately 50%, which is higher than that of radical prostatectomy.18 Formenti et al19 reported no increase in the frequency of sexual function abnormality after adjuvant radiation therapy following a nerve-preservation radical prostatectomy. Litwin20 reported no difference in quality of life among radical surgery, radiation therapy, and observation groups, but found that patient satisfaction with sexual function and voiding symptoms was lower in the radical surgery group than in the other two groups.
Few studies on the mechanism of erectile dysfunction following radiotherapy have been reported. The current theory is that radiation affects the penile bulb and neurovascular bundle. However, some studies have reported that the penile bulb has an insignificant role in priapism. The pathophysiological effect of radiation therapy on the neurovascular bundle has not been clearly defined. Zelefsky and Eid21 reported that the rate of arterial erectile dysfunction was significantly higher in the radiation therapy group than in the radical prostatectomy group in penile duplex ultrasonography conducted before and after prostaglandin administration. Radiation therapy also causes nerve injuries. A study using an animal model showed that the expression of nitric oxide synthase-containing nerves decreased after radiation therapy.22
3. Brachytherapy and sexual function
Unlike conventional radiotherapy, which may cause radiation-induced side effects, like inflammation in the tissues near the cancer tissue due to radiation, brachytherapy only irradiates the cancer tissue, reducing this complication.23 Brachytherapy has been widely used in the USA and Europe for the past 10 years. Now, it accounts for approximately 30% of the early treatment of prostate cancer. After prostate cancer surgery, incontinence, a common postoperative complication, occurs in 50~90% of patients who undergo the operation, and above all, erectile dysfunction may occur in 10~90% of patients who undergo such surgery. Thus, many patients who undergo such surgery suffer from inconvenience in their daily living after the surgery. In the case of brachytherapy, urinary incontinence and erectile dysfunction occur in 0.6% and 14~35% of patients, respectively, who undergo it. Thus, the frequency of erectile dysfunction is significantly lower in the brachytherapy group than in the conventional surgery group.
4. Cryosurgery
Cryosurgery is a treatment method that destroys cells using a cryogenic treatment. The basic mechanism of cryosurgery is as follows: if the cells are exposed to cold temperatures, ice crystals are formed in the extracellular fluid, but the formation of ice crystals in intracellular fluids is delayed due to the presence of various polymers. Due to the difference in osmotic pressure between the extracellular and intracellular fluids, cells undergo dehydration, leading to protein denaturation, and are eventually destroyed. The rapid decrease in temperature forms ice crystals in intracellular fluids, destroying membranes and tissues, leading to cell death. Additionally, the cold destroys capillary vessels, blocking micro blood flow, inducing cell ischemia, and eventually leading to cell death.
As cryosurgery is not commonly used in the treatment of prostate cancer, there are few relevant reports. Asterling and Greene24 reported that recovery of sexual function occurred in approximately 39% of patients who underwent cryosurgery. However, due to the small number of cases and reports, further studies are required. The mechanism of erectile dysfunction after cryosurgery is believed to be attributable to the cryogenic injury of the neurovascular bundle nearby the prostate, as seen in curative surgery.
5. Hormonal therapy (androgen-deprivation therapy)
In 1941, Huggins and Hodges conducted castration to treat patients with metastatic prostate cancer, and reported that blood phosphatase levels decreased and cancer-related symptoms improved.1 Thereafter, many studies reported a close relationship between prostate cancer and testosterone. Accordingly, androgen-deprivation therapy has been widely used as an important treatment in patients with prostate cancer. In androgen-deprivation therapy, prostate cancer is treated by inhibiting the proliferation of prostate cancer cells and inducing their necrosis by blocking the actions of testosterone. To do this, methods to inhibit the synthesis of testosterone or to block its binding to the testosterone receptor have been used.
The most problematic complication that occurs during androgen deprivation therapy for prostate cancer is sexual dysfunction. Most patients complain of erectile dysfunction and decreased sexual desire, although some patients maintain these functions. Reasons for these individual differences have not been clearly established, but are thought to be attributable to patient age before surgery, testosterone levels, and the preservation of physical function.25 If androgen deprivation therapy is conducted over a long period, decreased testis and penis size and fibrosis of the corpus cavernosum are observed.
TREATMENT OF SEXUAL DYSFUNCTION FOLLOWING THE TREATMENT OF PROSTATE CANCER
1. PDE5 inhibitors
An animal study on the long-term administration of a PDE5 inhibitor after cavernous nerve injury reported that venous leakage was improved and that corpus cavernosum tissues and erectile function were preserved.26 A number of studies have attempted to clinically validate this finding. Another animal study showed that long-term oral administration of vardenafil prevented physiological nerve block-induced dysfunction with penile vein occlusion. This result is likely to be attributable to the antifibrotic effects of PDE5 inhibitors and the upregulation of smooth muscle cell proliferation by the PDE5 inhibitor, with the preservation of corpus cavernosal smooth muscle cells.26 Similar results were obtained whether sildenafil or tadalafil was used. Additionally, when the bilateral cavernous nerve was injured in study animals, penile ischemia resulted. Continuous tadalafil administration recovered corpus cavernosum oxygenation and the smooth muscle/fibrosis ratio from a histological point of view. Sildenafil administration also prevented penile ischemia and reduced the expression of endothelin-I type B receptor, a precursor of fibrosis. The effects of these drugs improved with early administration.27 Since Mulhall et al28 first reported the results of penile rehabilitation using sildenafil in 2005, clinical studies have reported that PDE5 inhibitors have protective effects on smooth muscle and endothelial cells, nerve-modulating effects, and inducing effects on corpus cavernosum oxygenation. When sildenafil was administered daily to 76 patients with normal erectile function who had undergone bilateral nerve-sparing radical prostatectomy 48 weeks earlier, the recovery rate of erectile function was 24% in the 50 mg dose group, 33% in the 100 mg dose group, and 5% in the placebo group. Additionally, when sildenafil was administered to 40 male patients who had undergone bilateral nerve-sparing radical prostatectomies, and a biopsy was conducted before and 6 months after the surgery to compare the effect of sildenafil, no loss of the smooth muscle was seen in the 50 mg dose group, and increased smooth muscle tissue was seen in the 100 mg dose group.29 Based on these results, some researchers have suggested that penile rehabilitative treatment should be performed soon after surgery.
2. Intracavernosal injections
A single administration of alprostadil, a synthetic PGE1, in the corpus cavernosum, or the combined administration of alprostadil with papaverine or phentolamine is a treatment method for erectile dysfunction. Claro et al30 reported that when intracavernosal injection was conducted on patients who had normal sexual function before curative surgery, but who had postoperative erectile dysfunction, 40% of the patients showed a good result, and 94.6% showed erection sufficient to have sexual intercourse. In a long-term follow-up study conducted by Raina et al,31 one-third of patients who complained of erectile dysfunction after curative surgery selected intracavernosal injection as a primary treatment method; of them, 48% received a mean 3.7-year long-term treatment. Reasons for drop-out during treatment were as follows: inadequate erection 33%, preference for oral drugs 32%, afraid of injection 11%, inconvenience of injection 8%, priapism 1%, and recovery of normal erection 1%.
3. Vacuum constriction devices
A vacuum constriction device, one of the treatment methods for erectile dysfunction, induces an erection via artificial penis enlargement by covering the penis with the constriction device and applying negative pressure. The resulting erection differs from a physiological erection. It is not favored by Koreans, who tend to have a relatively small penis.
A previous clinical study on the treatment of erectile dysfunction following radical prostatectomy reported that when a vacuum constriction device was used for 9 months after the surgery, 80% of the patients were able to have sex using the device, but only 29% of the control group members were able to have sex. Another study reported that when a vacuum constriction device was used in 39 patients who had normal erectile function before surgery for 90 days after nerve-sparing radical prostatectomy, it was helpful.32 Also, erectile function was significantly lower in patients who used the device 6 months after surgery than in those who used the device 1 month after surgery, and that penis length decreased by 2 cm in patients who used the device 6 months after surgery compared with patients who used the device 1 month after surgery.33 However, no large-scale, randomized, controlled study has been reported due to insufficient patient numbers, although some investigations of methods of preventing penis dystrophy have been conducted. Thus, the available evidence on the effects of vacuum constriction devices in penis rehabilitation is insufficient.
4. Combination therapy
Combination therapy is used based on the theory that recovery of erectile function can be achieved through a synergistic effect of simultaneous application of two treatment methods. Alprostadil, which is used for intraurethral or cavernosal injections, primarily increases cAMP, whereas a PDE5 inhibitor increases cyclic guanosine monophosphate. Thus, combined therapy with these drugs could be helpful in the recovery of erectile function via different pathways. A small-scale study, in which the daily administration of 25 or 50 mg of sildenafil was combined with intracavernosal injection of alprostadil, reported that side effects decreased due to the decreased volume of the intracavernosal injection agent.34 Intracavernosal injection can use triple mixtures including papaverine and phentolamine as well as alprostadil monotherapy. When combined therapy with a vacuum constriction device and PDE5 inhibitor therapy was conducted for 3 months, an erection sufficient for sexual intercourse was observed in 56% of the patients who underwent nerve-sparing prostatectomy.35 Despite the theoretical feasibility of combination therapy, only small-scale, short-term clinical studies have been conducted to-date. Thus, the efficacy remains to be further validated. In addition, it should be considered that side effects, such as burden on the cardiovascular and gastrointestinal systems, may increase.
5. Penile prosthesis
A penile prosthesis is generally considered a last treatment method if a primary (oral administration) and a secondary treatment (intracavernosal injection) fail. Salonia et al36 reported that when a PDE5 inhibitor (daily administration or administration as needed) was administered to patients with erectile dysfunction following radical prostatectomy, 73% of the patients withdrew from treatment in an 18-month follow-up study. Reasons for patient withdrawal were unsatisfactory effect (84%) and no interest in sex (16%). A penile prosthesis can be helpful for patients who show poor results from primary or secondary treatments, and who have continued sexual desire. However, in actual clinical practice, use of a penile prosthesis following radical prostatectomy is rare. Stephenson et al37 reported that when a survey on treatment methods for erectile dysfunction was conducted in 1977 in prostate cancer patients who underwent radical prostatectomy or radiation therapy, 1.9% used a penile prosthesis. However, Menard et al38 reported that when satisfaction of 90 patients who underwent penile prosthesis surgery after radical prostatectomy and 131 who underwent penile prosthesis surgery due to vasculogenic erectile dysfunction was compared, patient satisfaction was higher in those who underwent penile prosthesis surgery after radical prostatectomy.
6. Testosterone replacement therapy
Testosterone is an essential element for male sexual function. In patients with prostate cancer, testosterone deficiency occurs not only because of hormonal therapy for the treatment of prostate cancer, but also as a result of the manifestation of male climacteric symptoms that occur during the aging process, independently of the prostate cancer. As most patients and doctors consider testosterone to be a substance involved in the occurrence of prostate cancer, they consider the use of testosterone for the treatment of sexual dysfunction following radical prostatectomy or male climacteric symptoms to be contraindicated. Although testosterone is involved in prostate cancer, however, no definite mechanism has as yet been identified. It has recently been reported that prostate cancer recurrence was insignificant in those who underwent testosterone replacement therapy to treat hypogonadism that occurs before and after radical prostatectomy or male climacteric symptoms (Table 2).39 In particular, for patients with local prostate cancer, it has been suggested that testosterone replacement therapy should be conducted if complete recovery is achieved by radical prostatectomy. However, the recurrence of prostate cancer remains controversial, so further validation is required.
Table 2.
Testosterone replacement therapy following radical prostatectomy and radiation treatment
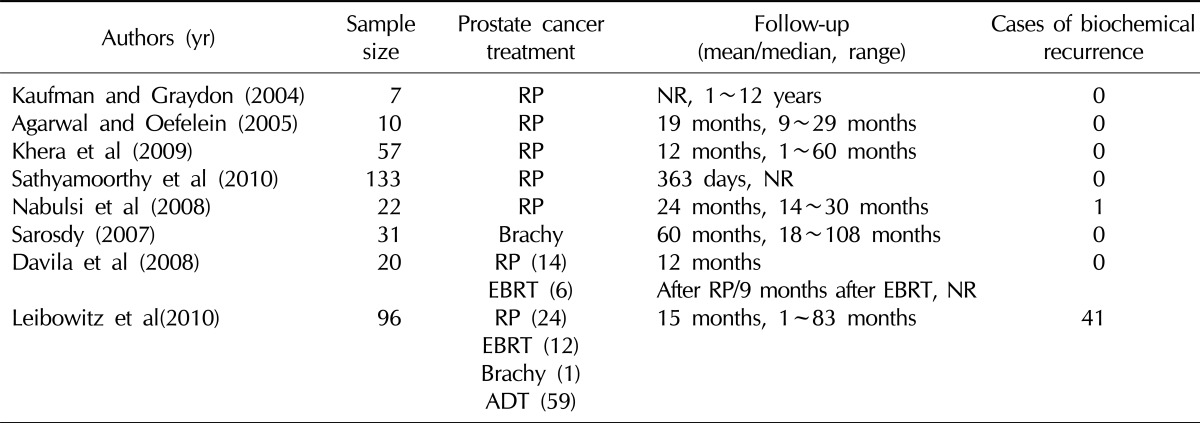
RP: radical prostatectomy, EBRT: external beam radiotherapy, ADT: androgen-deprivation therapy, NR: not reported.
CONCLUSIONS
Prostate cancer frequently causes sexual dysfunction. A prostate cancer diagnosis by itself reduces sexual desire and the frequency of sexual intercourse. Hormonal therapy to block testosterone and neurovascular bundle injury caused by radical prostatectomy further increase the frequency of erectile dysfunction. Preservation of erectile function is one of the most important issues to patients with local prostate cancer. Erectile dysfunction that occurs after radical prostatectomy is known to have various prognoses depending on preservation of the neurovascular bundle, patient age, and preoperative erectile status.
Intracavernosal injections, PDE5 inhibitors, and penile rehabilitation therapy using vacuum constriction devices, which are conducted after a radical prostatectomy, have been shown to be useful in the recovery of erectile function. Recently, testosterone replacement therapy has also drawn attention as a treatment method for the recovery of erectile function.
References
- 1.Giovannucci E, Platz EA. Epidemiology of prostate cancer. In: Vogelzang NJ, Scardino PT, Shipley WU, Debruyne FMJ, Linehan WM, editors. Comprehensive textbook of genitourinary oncology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 9–22. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Kim KI, Chang HJ, Cho YS, Youn TJ, Chung WY, Chae IH, et al. Current status and characteristics of hypertension control in community resident elderly Korean people: data from a Korean longitudinal study on health and aging (KLoSHa study) Hypertens Res. 2008;31:97–105. doi: 10.1291/hypres.31.97. [DOI] [PubMed] [Google Scholar]
- 4.Seo HK, Chung MK, Ryu SB, Lee KH Korean Urological Oncologic Society Prostate Cancer Study Group. Detection rate of prostate cancer according to prostate-specific antigen and digital rectal examination in Korean men: a nationwide multicenter study. Urology. 2007;70:1109–1112. doi: 10.1016/j.urology.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 5.Song C, Ro JY, Lee MS, Hong SJ, Chung BH, Choi HY, et al. Prostate cancer in Korean men exhibits poor differentiation and is adversely related to prognosis after radical prostatectomy. Urology. 2006;68:820–824. doi: 10.1016/j.urology.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Moore TM, Strauss JL, Herman S, Donatucci CF. Erectile dysfunction in early, middle, and late adulthood: symptom patterns and psychosocial correlates. J Sex Marital Ther. 2003;29:381–399. doi: 10.1080/00926230390224756. [DOI] [PubMed] [Google Scholar]
- 7.Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. J Sex Med. 2011;8:560–566. doi: 10.1111/j.1743-6109.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- 8.Kornblith AB, Herr HW, Ofman US, Scher HI, Holland JC. Quality of life of patients with prostate cancer and their spouses. The value of a data base in clinical care. Cancer. 1994;73:2791–2802. doi: 10.1002/1097-0142(19940601)73:11<2791::aid-cncr2820731123>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 10.Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145:998–1002. doi: 10.1016/s0022-5347(17)38512-9. [DOI] [PubMed] [Google Scholar]
- 11.Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58–61. doi: 10.1016/s0090-4295(99)00397-0. [DOI] [PubMed] [Google Scholar]
- 12.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 13.Burnett AL. Rationale for cavernous nerve restorative therapy to preserve erectile function after radical prostatectomy. Urology. 2003;61:491–497. doi: 10.1016/s0090-4295(02)02271-9. [DOI] [PubMed] [Google Scholar]
- 14.Magheli A, Burnett AL. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–427. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 15.Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648–657. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181:462–471. doi: 10.1016/j.juro.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Litwin MS, Flanders SC, Pasta DJ, Stoddard ML, Lubeck DP, Henning JM. Sexual function and bother after radical prostatectomy or radiation for prostate cancer: multivariate quality-of-life analysis from CaPSURE. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1999;54:503–508. doi: 10.1016/s0090-4295(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 18.Zelefsky MJ, Cowen D, Fuks Z, Shike M, Burman C, Jackson A, et al. Long term tolerance of high dose three-dimensional conformal radiotherapy in patients with localized prostate carcinoma. Cancer. 1999;85:2460–2468. doi: 10.1002/(sici)1097-0142(19990601)85:11<2460::aid-cncr23>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Formenti SC, Lieskovsky G, Skinner D, Tsao-Wei DD, Groshen S, Petrovich Z. Update on impact of moderate dose of adjuvant radiation on urinary continence and sexual potency in prostate cancer patients treated with nerve-sparing prostatectomy. Urology. 2000;56:453–458. doi: 10.1016/s0090-4295(00)00677-4. [DOI] [PubMed] [Google Scholar]
- 20.Litwin MS. Quality of life following definitive therapy for localized prostate cancer: potential impact of multiple therapies. Curr Opin Urol. 2003;13:153–156. doi: 10.1097/00042307-200303000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Eid JF. Elucidating the etiology of erectile dysfunction after definitive therapy for prostatic cancer. Int J Radiat Oncol Biol Phys. 1998;40:129–133. doi: 10.1016/s0360-3016(97)00554-3. [DOI] [PubMed] [Google Scholar]
- 22.Carrier S, Hricak H, Lee SS, Baba K, Morgan DM, Nunes L, et al. Radiation-induced decrease in nitric oxide synthase-containing nerves in the rat penis. Radiology. 1995;195:95–99. doi: 10.1148/radiology.195.1.7534430. [DOI] [PubMed] [Google Scholar]
- 23.Merrick GS, Butler WM, Wallner KE, Galbreath RW, Anderson RL, Kurko BS, et al. Erectile function after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:437–447. doi: 10.1016/j.ijrobp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Asterling S, Greene DR. Prospective evaluation of sexual function in patients receiving cryosurgery as a primary radical treatment for localized prostate cancer. BJU Int. 2009;103:788–792. doi: 10.1111/j.1464-410X.2008.08042.x. [DOI] [PubMed] [Google Scholar]
- 25.Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology. 2003;61(2 Suppl 1):32–38. doi: 10.1016/s0090-4295(02)02397-x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–435. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Vignozzi L, Morelli A, Filippi S, Vannelli GB, Mungai S, Marini M, et al. Effect of sildenafil administration on penile hypoxia induced by cavernous neurotomy in the rat. Int J Impot Res. 2008;20:60–67. doi: 10.1038/sj.ijir.3901596. [DOI] [PubMed] [Google Scholar]
- 28.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005;2:532–540. doi: 10.1111/j.1743-6109.2005.00081_1.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz EJ, Wong P, Graydon RJ. Sildenafil preserves intracorporeal smooth muscle after radical retropubic prostatectomy. J Urol. 2004;171:771–774. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 30.Claro J de A, de Aboim JE, Maríngolo M, Andrade E, Aguiar W, Nogueira M, et al. Intracavernous injection in the treatment of erectile dysfunction after radical prostatectomy: an observational study. Sao Paulo Med J. 2001;119:135–137. doi: 10.1590/S1516-31802001000400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raina R, Pahlajani G, Agarwal A, Zippe CD. The early use of transurethral alprostadil after radical prostatectomy potentially facilitates an earlier return of erectile function and successful sexual activity. BJU Int. 2007;100:1317–1321. doi: 10.1111/j.1464-410X.2007.07124.x. [DOI] [PubMed] [Google Scholar]
- 32.Dalkin BL, Christopher BA. Preservation of penile length after radical prostatectomy: early intervention with a vacuum erection device. Int J Impot Res. 2007;19:501–504. doi: 10.1038/sj.ijir.3901561. [DOI] [PubMed] [Google Scholar]
- 33.Köhler TS, Pedro R, Hendlin K, Utz W, Ugarte R, Reddy P, et al. A pilot study on the early use of the vacuum erection device after radical retropubic prostatectomy. BJU Int. 2007;100:858–862. doi: 10.1111/j.1464-410X.2007.07161.x. [DOI] [PubMed] [Google Scholar]
- 34.McMahon CG, Samali R, Johnson H. Treatment of intracorporeal injection nonresponse with sildenafil alone or in combination with triple agent intracorporeal injection therapy. J Urol. 1999;162:1992–1997. doi: 10.1016/S0022-5347(05)68085-8. [DOI] [PubMed] [Google Scholar]
- 35.Raina R, Agarwal A, Allamaneni SS, Lakin MM, Zippe CD. Sildenafil citrate and vacuum constriction device combination enhances sexual satisfaction in erectile dysfunction after radical prostatectomy. Urology. 2005;65:360–364. doi: 10.1016/j.urology.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Salonia A, Gallina A, Zanni G, Briganti A, Dehò F, Saccà A, et al. Acceptance of and discontinuation rate from erectile dysfunction oral treatment in patients following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;53:564–570. doi: 10.1016/j.eururo.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson RA, Mori M, Hsieh YC, Beer TM, Stanford JL, Gilliland FD, et al. Treatment of erectile dysfunction following therapy for clinically localized prostate cancer: patient reported use and outcomes from the Surveillance, Epidemiology, and End Results Prostate Cancer Outcomes Study. J Urol. 2005;174:646–650. doi: 10.1097/01.ju.0000165342.85300.14. [DOI] [PubMed] [Google Scholar]
- 38.Menard J, Tremeaux JC, Faix A, Pierrevelcin J, Staerman F. Erectile function and sexual satisfaction before and after penile prosthesis implantation in radical prostatectomy patients: a comparison with patients with vasculogenic erectile dysfunction. J Sex Med. 2011;8:3479–3486. doi: 10.1111/j.1743-6109.2011.02466.x. [DOI] [PubMed] [Google Scholar]
- 39.Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf) 2012;76:179–181. doi: 10.1111/j.1365-2265.2011.04233.x. [DOI] [PubMed] [Google Scholar]