Abstract
Context
Patients with chronic heart failure have impaired long-term survival, but their own expectations regarding prognosis have not been well studied.
Objectives
To quantify expectations for survival in heart failure patients, compare patient expectations to model predictions, and identify factors associated with an optimistic prognostic outlook.
Design, Settings, Participants
We carried out a prospective face to face survey with patients in a heart failure disease management program. Patient-predicted life expectancy (Patient-PL) was obtained using a visual analog scale. Model-predicted life expectancy (Model-PL) was calculated using the Seattle Heart Failure Model. Actuarial-predicted life expectancy (Actuarial-PL), based on age and sex alone, was calculated using life tables. Observed survival was determined from review of medical records and search of the Social Security Death Index.
Main Outcome Measure
Patient predicted life expectancy (Patient-PL) compared to model predicted life expectancy (Model-PL and Actuarial-PL) and observed survival. The ratio of Patient-PL to Model-PL was defined as the Optimism Index (OI).
Results
The cohort consisted of 122 patients (mean age 62 years, 47% African-American, 42% NYHA class III or IV). On average, patients overestimated their life expectancy relative to model-predicted life expectancy (median Patient-PL = 13.0 yrs, Model-PL = 10.0 yrs). The median OI was 1.4. Younger age, ischemic etiology, lower ejection fraction, less depression, and increased NYHA class were the most significant predictors of greater optimism. During a median follow up of 3.1 years, 29% of the original cohort died. There was no association between the degree of optimism and observed survival.
Conclusions
Ambulatory patients with heart failure tended to substantially overestimate their life expectancy compared with model-based predictions for survival. Because differences in perceived survival could affect decision-making regarding advanced therapies and end of life planning, the causes of these prognostic misperceptions warrant further study.
Keywords: Heart failure, prognosis, risk-assessment, attitude to health, questionnaires
INTRODUCTION
Heart failure accounts directly for 55,000 deaths and indirectly for an additional 230,000 deaths in the United States each year.1 Despite advances in care, the prognosis for patients with symptomatic heart failure remains grim, with median life expectancy of less than 5 years.2 For those with the most advanced disease, mortality rates approach 90% at 1-year.3, 4 About half of these deaths are due to progressive pump failure while the remainder are sudden.5 Prognosis is highly dependent on a multitude of patient characteristics, and a number of prognostic models have been developed to help predict survival in heart failure patients.6–9
Given the progressive nature of this disease, its high mortality rate, and its predilection for the elderly, end-of-life issues should be at the forefront of heart failure management. In recognition of this, practice guidelines from major cardiovascular societies all include sections on end of life considerations, which advocate ongoing patient and family education regarding prognosis for quality of life and survival.10–12 Despite these guidelines, data on end of life issues in heart failure are sparse, especially in comparison to other morbid chronic diseases such as cancer.13, 14 The extent to which heart failure patients understand their prognosis remains poorly defined, and the few existing studies addressing end of life in heart failure have largely involved resuscitation preferences.15–18 Studies focusing specifically on patient perceptions of life expectancy in heart failure are absent from the literature, although examples do exist in other disease settings.19, 20
Patient perception of prognosis is important because it fundamentally influences medical decision making around medications, devices, transplantation, and end of life care. Prior study in the field of oncology has shown that patients who believe they have a better chance for survival are more likely to favor aggressive therapy.21 With the increasing availability of potentially life-saving therapies which are also costly, invasive, and often morbid (e.g. left ventricular assist devices), understanding patient perceptions of prognosis is vital for making appropriate care decisions.
The goals of the current study were to quantify patient expectations of life expectancy in ambulatory patients with heart failure, compare those expectations to model estimations and to observed survival, and to identify patient-related factors which are associated with discrepancies between patient-predicted and model-predicted prognosis.
METHODS
Patients
This study was conducted between July and December 2004 at the Duke University Heart Failure Disease Management Program in Durham, NC, a clinical program that provides heart failure care to a broad range of patients in the Durham community. On days determined by interviewer availability, all patients were consecutively approached and asked to participate in the study, which involved completing a questionnaire administered by a physician or nurse practitioner. Patients were excluded if they did not speak English, if they were cognitively impaired, if the interview would have occurred on the day of their initial encounter with the disease management program, or if they had previously participated in the survey. Patients had not previously met the interviewer. Interviews lasted approximately 15 minutes. All patients gave signed informed consent. The study was approved by the Duke University Institutional Review Board.
Questionnaire Design and Validity
The questionnaire was composed of 63 multiple-choice and short-answer questions, as well as visual analog scales (VAS). Basic language was used to maximize understanding. Sections included characterization of patient-perceived prognosis,22–24 heart failure symptom severity,25 perceived quality of life,26 resuscitation preferences,15 and demographic information. Patients were screened for depression using the two question Patient Health Questionnaire 2 (PHQ-2).27–29 Religiosity was characterized using a modified Duke University Religion Index.30 Self reported race was collected through a multiple-choice item on the questionnaire to assess whether patient expectations about prognosis differed based on racial background. Recent clinical, medication, laboratory, and imaging data were obtained from the electronic medical record.
Patient perceptions of life expectancy were addressed with sequential questions. After a short introduction to the concept of prognosis, patients were asked, “What do you think the eventual outcome will be from your heart failure?” Possible answers were: (1) it will be cured, (2) I will have a normal life expectancy but will have heart failure the rest of my life, (3) heart failure will likely shorten my life, and (4) I don’t know or refuse to answer. To quantify patient-perceived life expectancy, patients were provided the following instructions: “While no one can ever say how much longer he/she might live, sometimes patients with chronic illnesses do think about this question. If you had to guess, how much longer do you think you will live?” Two sequential redundant responses were then recorded. In the first, quantitative ranges of patient-predicted remaining life expectancy were assessed with the following multiple choice answers: >10 years, 5–10 years, 2–5 years, 1–2 years, 0–1 years, and I don’t know or refuse to answer.24 Immediately following, a visual analog scale of life expectancy was provided.22 The scale provided a timeline composed of a 10 cm horizontal line with major hatch marks at each cm labeled in 10 year increments from 0 to 100 and minor hatch marks at each mm. Patients given these instructions: “Put one mark on the line to indicate your current age. Put another mark on the line to indicate how old you think you might be when you die.” The difference between these two marks was recorded as the “patient-predicted life expectancy” (Patient-PL).
Model-Predicted Life Expectancy and Observed Survival
The Seattle Heart Failure Model (SHFM), a well-validated prognostic tool in chronic heart failure, was used to estimate mean remaining life expectancy for each study subject.6, 31–33 We used the electronic medical record to collect data on all variables required to calculate the SHFM score, including clinical characteristics (age, sex, NYHA functional class, systolic blood pressure, and weight), medications (angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, statin, aldosterone blocker, loop diuretic dose, and allopurinol), device therapies (implantable cardiac defibrillator, cardiac resynchronization therapy), and results of diagnostic testing (ejection fraction, sodium, hemoglobin, lymphocyte %, uric acid, and total cholesterol). The most recent test results from within 12 months of the interview were used. Missing variables were set as the mean for all patients in the dataset. The mean life expectancy provided by the SHFM for each individual subject was recorded as the “model-predicted life expectancy” (Model-PL).
Actuarial-predicted life expectancy (Actuarial-PL), based on age and sex alone, was calculated for each patient in the cohort using life expectancy data from www.mortality.org (University of California, Berkeley). Observed survival was assessed through a search of the Duke electronic medical record and the Social Security Death Index (SSDI) on February 12, 2008. To account for possible lag in recently deceased patients being available in the SSDI, we classified patients absent from the SSDI search as alive as of 6 months prior to our SSDI search or at their last documented clinical encounter, whichever was later.
Statistical Analysis
Baseline variables were reported as medians and interquartile ranges (IQR) for continuous variables or percentages for categorical variables. The internal consistency of prognostic assessments on the questionnaire was assessed by calculating Cronbach’s alpha for intra-subject correlation of Patient-PL from the visual analog scale compared with life expectancy as expressed using the multiple choice item. The relationship of Patient-PL to Model-PL and Actuarial-PL was evaluated using linear regression analysis. The “optimism index” (OI) was defined as the simple ratio of Patient-PL to Model-PL for each individual subject. We assessed the statistical power of our sample size to reject the null hypothesis that the geometric mean of OI was 1.0, assuming the observed coefficient of variation in the existing data and an exact 2-sided alpha of 0.05. We found that 122 patients provided an 80% power to detect an OI geometric mean as small as 1.28, a difference we considered clinically relevant.
For descriptive purposes, patients were grouped into categories based on ranges of OI that were felt to define clinically meaningful categories: “pessimistic” (OI < 0.7), “realistic” (OI 0.7–1.3), and “optimistic” (OI > 1.3). The univariate relationships of baseline patient characteristics with descriptive categories of OI were evaluated with Cochran-Mantel-Haenszel statistics for dichotomous variables, and non-parametric Wilcoxon rank sum statistics for continuous variables.
In order to evaluate the validity of the SHFM in our population, we calculated the model accuracy (c-statistic) in our cohort at 1 year and 3 years of follow up, and also assessed calibration of the model in our cohort by comparing predicted mortality to observed mortality by quintiles of predicted risk.
To identify characteristics associated with a more optimistic outlook, we preformed multivariable linear regression with the log transformed OI as the dependent variable. Based on cohort size, candidate variables considered for the model were limited to 12, and were selected using clinical assumptions of importance. These included age, sex, race, education status, economic status, depression, NYHA class, recent hospitalization, length of diagnosis, ejection fraction, etiology of heart failure, and presence of implantable cardiac defibrillator. To identify non-linear relationships between continuous covariates and OI, we first examined plots of each continuous covariate versus log transformed OI to visually assess whether a non-linear relationship was present, and then evaluated the contribution of higher order terms (squared and cubed) using a bivariate linear model; where higher order terms were statistically significant at p<0.1, we retained these for multivariable modeling. Backward stepwise selection was applied. Variables were retained in the model if the regression coefficient was significantly associated at the α = 0.10 level.
Finally, Cox proportional hazards analysis was used to assess the relationship between categories of OI and observed mortality after adjustment for other known predictors of mortality in heart failure (age, sex, race, ischemic etiology, NYHA functional class, and ejection fraction). We performed backwards selection with OI forced into the model, retaining covariates in the final model with p<0.1 or where removal of the covariate resulted in a 10% change in the parameter estimate for OI. All statistical analyses were performed using SAS software (version 9.1.3, Cary, NC).
RESULTS
Cohort Characteristics
During the 6 months of study enrollment, 154 patients were approached to participate in the study and 148 agreed. Of these 148 subjects, 26 were unable or unwilling to estimate their life expectancy using the VAS, and were therefore excluded from the current analysis. On the related multiple choice answer for life expectancy, 21 of these excluded patients indicated that they felt unable to predict their life expectancy, and 5 answered that they would live >10 years. In comparison to the final study cohort, the 26 excluded patients were more likely to be African-American (77%, N=20) and female (61%, N=16); observed mortality over median follow up of 3 years was 31%, which was similar to the main cohort.
Baseline characteristics for the 122 patients in the final cohort are presented in Table 1. Our study cohort was racially diverse (47% African-American) and included a significant proportion of elderly patients (45% > 65 years old). The cohort included a broad spectrum with regard to disease severity (58% NYHA class I/II, 42% NYHA class III/IV). Most patients had long-standing chronic heart failure (83% with heart failure for > 1 year), and the utilization of guideline-recommended medical therapy for heart failure was high. Comorbidities were common, with hypertension, hyperlipidemia, and diabetes present in the majority of patients.
Table 1.
Baseline characteristics for the study cohort stratified by categories of optimism index
| Categories of Optimism Index (OI) |
||||||
|---|---|---|---|---|---|---|
| N | Overall | Pessimistic | Realistic | Optimistic | P | |
| [OI < 0.7] | [OI 0.7–1.3] | [OI > 1.3] | value | |||
| Variable | (N=26) | (N=32) | (N=64) | |||
| Demographics | ||||||
| Age (years) | 122 | 61 (53–74) | 74 (60–80) | 60 (58–77) | 56 (49–66) | <0.01 |
| Male | 122 | 62% (76) | 62% (16) | 66% (21) | 61% (39) | 0.90 |
| Race | 122 | 0.02 | ||||
| Caucasian | 50% (61) | 65% (17) | 63% (20) | 20% (24) | ||
| African American | 47% (57) | 27% (7) | 38% (12) | 59% (38) | ||
| Other | 3% (4) | 8% (2) | 0% (0) | 3% (2) | ||
| College educated | 122 | 50% (61) | 77% (20) | 41% (13) | 44% (28) | <0.01 |
| Comorbidities | ||||||
| Hypertension | 122 | 82% (100) | 85% (22) | 81% (26) | 81% (52) | 0.93 |
| Diabetes | 122 | 54% (66) | 58% (15) | 50% (16) | 55% (35) | 0.84 |
| Atrial fibrillation | 122 | 30% (36) | 23% (6) | 47% (15) | 23% (15) | 0.04 |
| Stroke | 122 | 16% (20) | 15% (4) | 31% (10) | 9% (6) | 0.02 |
| COPD | 122 | 29% (35) | 23% (6) | 41% (13) | 25% (16) | 0.22 |
| Cancer other than skin | 122 | 11% (14) | 8% (2) | 9% (3) | 14% (9) | 0.63 |
| Active smoker | 122 | 12% (15) | 12% (3) | 13% (4) | 13% (8) | 0.99 |
| Heart failure | ||||||
| NYHA Class | 122 | 0.60 | ||||
| I | 11% (13) | 19% (5) | 6% (2) | 9% (6) | ||
| II | 48% (58) | 38% (10) | 56% (18) | 47% (30) | ||
| III | 34% (42) | 35% (9) | 34% (11) | 34% (22) | ||
| IV | 7% (9) | 8% (2) | 3% (1) | 9% (6) | ||
| Ejection fraction | 121 | 0.26 (0.20–0.35) | 0.30 (0.25–0.35) | 0.30 (0.20–0.40) | 0.25 (0.20–0.35) | 0.10 |
| Ischemic etiology of heart failure | 122 | 38% (46) | 61% (16) | 50% (16) | 22% (14) | <0.01 |
| Heart failure diagnosis ≥ 1 year | 122 | 83% (101) | 88% (23) | 91% (29) | 77% (49) | 0.16 |
| Hospitalization in past 12 months | 120 | 61% (73 | 62% (16) | 50% (15) | 66% (42) | 0.35 |
| Current therapies | ||||||
| Implanted cardiac defibrillator | 122 | 25% (30) | 27% (7) | 28% (9) | 22% (14) | 0.76 |
| Biventricular pacemaker | 122 | 14% (17) | 20% (5) | 13% (4) | 13% (8) | 0.64 |
| ACE inhibitor or ARB | 122 | 93% (114) | 96% (25) | 94% (30) | 92% (59) | 0.30 |
| Beta-blocker | 122 | 86% (105) | 96% (25) | 84% (27) | 83% (53) | 0.24 |
| Furosemide equivalents, mg/24 hr | 122 | 80 (20–160) | 80 (20–160) | 40 (15–120) | 80 (40–160) | 0.62 |
| Statin | 122 | 55% (67) | 73% (19) | 59% (19) | 45% (29) | 0.05 |
| Antidepressant | 122 | 28% (34) | 38% (10) | 25% (8) | 25% (16) | 0.40 |
| Exam and laboratory data | ||||||
| Systolic blood pressure (mmHg) | 121 | 124 (110–140) | 129 (114–138) | 130 (116–150) | 120 (100–134) | 0.01 |
| Heart rate (bpm) | 122 | 75 (66–84) | 71 (66–80) | 73 (62–78) | 78 (68–91) | 0.05 |
| Weight (kg) | 121 | 92 (78–109) | 91 (73–106) | 89 (73–106) | 94 (81–117) | 0.45 |
| Sodium, serum (mEq/L) | 118 | 140 (138–142) | 139 (138–141) | 141 (138–142) | 140 (136–142) | 0.30 |
| Creatinine, serum (mg/dL) | 118 | 1.2 (1.0–1.6) | 1.2 (0.9–1.4) | 1.3 (1.0–1.5) | 1.2 (0.9–1.6) | 0.60 |
| Hemoglobin (g/dL) | 122 | 12.8 (11.6–14.1) | 13.5 (12.4–14.6) | 12.9 (11.8–14.2) | 12.3 (11.2–14.1) | 0.08 |
| White blood cell count, (109/L) | 121 | 6.7 (5.6–8.2) | 7.3 (5.5–9.4) | 6.7 (5.9–8.8) | 6.5 (5.5–8.0) | 0.69 |
| Lymphocyte percent (%) | 102 | 23 (16–31) | 25 (18–32) | 24 (16–30) | 22 (16–32) | 0.91 |
| Uric acid (mg/dL) | 76 | 7.8 (6.1–9.5) | 6.8 (5.6–9.2) | 8.2 (6.9–9.8) | 7.8 (6.3–9.6) | 0.28 |
| Cholesterol, total (mg/dL) | 118 | 166 (142–194) | 168 (152–199) | 163 (142–186) | 166 (127–204) | 0.70 |
| NT-proBNP, serum (ng/mL) | 99 | 1132 (370–2638) | 1106 (407–2381) | 1297 (491–2495) | 1116 (360–3044) | 0.95 |
| Psychosocial | ||||||
| Quality of life, (0=low, 100=high) | 122 | 59 (40–75) | 50 (38–75) | 55 (45–75) | 60 (48–80) | 0.44 |
| Depression (0=low, 7=high) | 122 | 1 (0–3) | 2 (1–4) | 1 (0–3) | 1 (0–2) | 0.13 |
| Religiosity (0=low, 22=high) | 107 | 16 (13–19) | 16 (12–19) | 16 (13–19) | 17 (15–19) | 0.56 |
| Financially stable, subjective | 122 | 34% (41) | 31% (8) | 34% (11) | 34% (22) | 0.94 |
P values were calculated for categorical covariates using Cochran-Mantel-Haenszel statistics, whereas p values for continuous variables were calculated using non-parametric Wilcoxon rank sum statistics. Continuous variables are expressed as medians and interquartile ranges [IQR]. OI = optimism index; NYHA = New York Heart Association class; mmHg = millimeters mercury; bpm = beats per minute; mEq/L = milliequivalents per liter; mg/dL = milligrams per deciliter; mg/mL – nanograms per milliliter; ICD = implantable cardiac defibrillator; furosemide equivalents were estimated with the use of Micromedex as previously described6
Data capture for baseline variables among the 122 patients in the final cohort was relatively complete; variables used in the SHFM were present for 97% of the study subjects (N ≥ 118), with the exception of serum uric acid level (N=76) and lymphocyte percent (N=102) (see Table 1). Data used for the calculation of the SHFM score was relatively recent, with 78% of data obtained at the time of enrollment and 87% of data from within 1 month of enrollment.
Patient and Model Predicted Life Expectancies
The median Patient-PL was 13.0 years (IQR 8–21, range 1 to 54 years). The intra-subject reliability for patient-predicted life expectancy was good (standardized Cronbach’s alpha 0.92 for correlation between visual analog scale and multiple-choice determined life expectancy). In response to the qualitative question addressing the eventual outcome of their heart failure, 9% of study subjects answered “It will be cured” (N=11), 51% answered “I will have a normal life expectancy but will have heart failure the rest of my life” (N=62), 36% answered “heart failure will likely shorten my life” (N=44), and 4% refused to answer (N=5).
The median survival predicted by the SHFM (Model-PL) was 10.0 years (IQR 7.2–13.3, range 2.0 to 25.2 years). Using actuarial life tables based on age and sex alone (without consideration for heart failure status), the median Actuarial-PL was 20.5 years (IQR 11.5–26.3, range 3.5 to 52.6 years).
On average, patients had an optimistic prognostic outlook, and the majority of patients (63%) overestimated their life expectancy when compared to that predicted by the SHFM. The median OI (ratio of Patient-PL to Model-PL) was 1.4 (IQR 0.8–2.5; range 0.1 to 9.6). There was little relationship between Patient-PL and Model-PL (R = 0.02, slope = 0.06, intercept = 15.6 years, p = 0.80; Figure 1A). Patient predictions of life expectancy were more similar to those predicted by empirically-derived actuarial life tables based on age and sex alone, without regard for the presence of heart failure (R = 0.53, slope = 0.59, intercept = 4.2 years, p <0.0001; Figure 1B).
Figure 1.
Scatter plot of patient predicted versus Seattle Heart Failure Model predicted life expectancy (A) and patient predicted versus actuarial predicted (based on age and sex alone) life expectancy (B)
Observed Survival
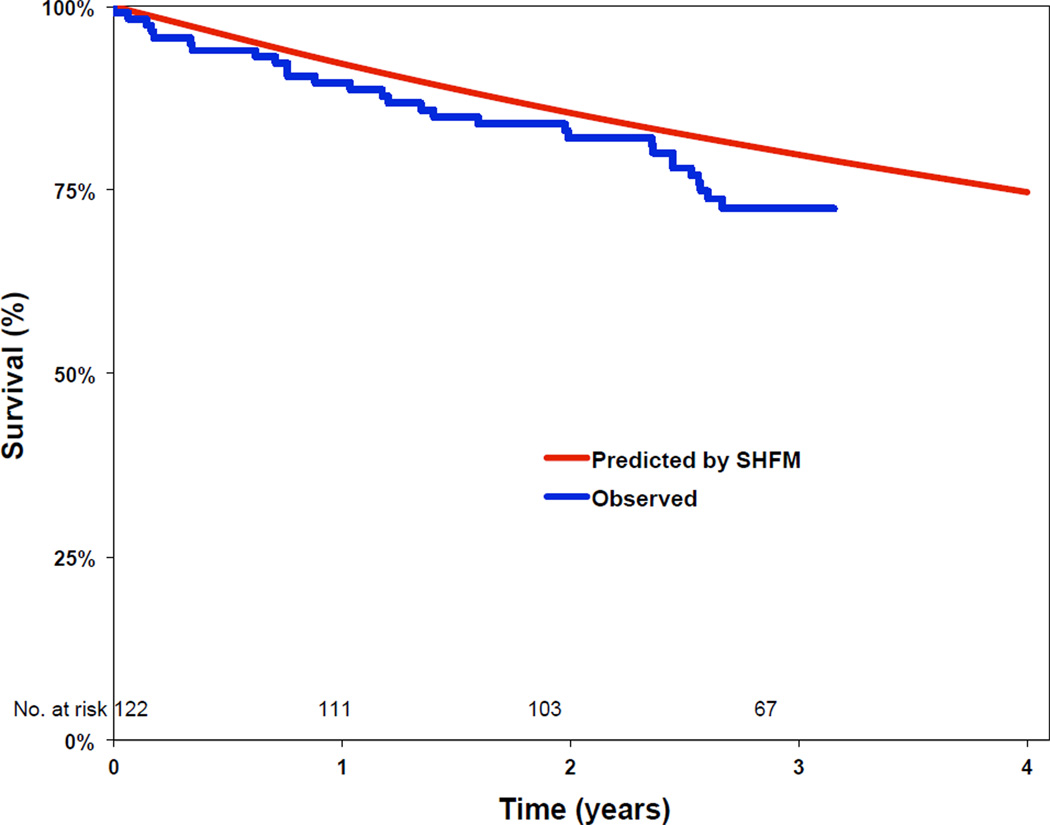
Twenty-nine percent of patients died (N=35, 95% CI 21–37%) over a median follow-up period of 3.1 years (IQR 2.7–3.3). Two patients underwent heart transplantation and one patient received a left ventricular assist device during follow-up. In order to assess the validity of the SHFM in our population, we analyzed the accuracy and calibration of SHFM predictions in the 35 patients who died during follow-up. Overall, the SHFM had accuracy in our population (c-statistic 0.73 for 1 year survival, 0.64 for 3 year survival) that was comparable to previously published validation cohorts.6 Observed survival was similar to that predicted by the SHFM at 1 year (90% observed vs. 92% predicted) and 3 years (72% observed vs. 80% predicted). Comparison of observed survival vs. that predicted by the SHFM in our cohort is shown in Figure 2. In an exploratory analysis in the subgroup of patients who died, there was no relationship between Patient-PL and observed survival (r = 0.17, p = 0.32, slope = 1.54, intercept = 11.1).
Figure 2.
Survival curves comparing the observed survival in our cohort to that predicted by the Seattle Heart Failure Model.
In order to evaluate whether an optimistic outlook was associated with better survival during follow-up, we employed Cox proportional hazards analysis to examine the relationship between survival and OI category (using realistic OI as the reference group). After adjusting for other likely predictors of outcome (age, gender, race, ejection fraction, NYHA class, and heart failure etiology), there was no relationship between OI category and survival (adjusted hazard ratio for optimistic OI 1.05, 95% CI 0.46–2.42, p=0.91; pessimistic OI 0.45, 95% CI 0.17–1.21, p=0.11). Kaplan-Meier curves of observed survival stratified by categories of OI are shown in Figure 3.
Figure 3.
Kaplan-Meier curves of observed survival during follow up stratified by groupings of OI.
Patient Factors Associated with Prognostic Outlook
Given the discordance of patient predictions with model predictions or observed survival, we used linear regression modeling to identify specific patient factors associated with a more optimistic prognostic outlook (i.e., higher OI). Of the 12 candidate variables considered, all the continuous variables had a linear relationship with OI except ejection fraction, which we modeled using quadratic and cubic terms to maximize the fit of the model. In the final multivariable model, the most significant predictors of higher log transformed OI were younger age (p < 0.001), ischemic etiology of heart failure (p = 0.0003), lower ejection fraction (p = 0.002), lower measures of depression (p = 0.001), and higher NYHA class (p = 0.01). The final multivariable model for predictors of log transformed OI is shown in Table 2.
Table 2.
Multivariable Model of Independent Predictors of Higher Optimism Index*
| Clinical Characteristic | Parameter Estimate |
95% Confidence Intervals |
Chi- square |
P-value |
|---|---|---|---|---|
| Younger age (per year) | 0.028 | 0.018–0.038 | 28.7 | <0.0001 |
| Ischemic etiology | 0.50 | 0.23–0.77 | 12.8 | 0.0003 |
| Lower ejection fraction (%) | 0.29 | 0.12–0.47 | 10.6 | 0.001 |
| (Ejection fraction)2 | 0.0083 | 0.0032–0.014 | 10.1 | 0.002 |
| (Ejection fraction)3 | −0.0001 | −0.0000–0.0001 | 9.6 | 0.002 |
| Lower depression score | 0.11 | 0.044–0.18 | 10.6 | 0.001 |
| Higher NYHA class | 0.22 | 0.046–0.40 | 6.1 | 0.01 |
| Lack of college education | 0.22 | −0.29–0.47 | 3.0 | 0.08 |
The log transformed OI was used as the dependent (outcome) variable with 12 clinical covariates considered in the initial model. The final reduced model is shown. Quadratic (ejection fraction)2 and cubic (ejection fraction)3 terms were included in the final model due to the non-linear relationship between ejection fraction and OI.
Of the potential predictors of OI, age had the most significant association with OI (p<0.001). There was a linear relationship between decreasing age and increasing Patient-PL, with progressively younger patients estimating progressively longer life expectancy despite model predictions that were similar across age groups (Figure 4A). In contrast, model predictions were much more influenced by measures of disease severity (heart failure etiology, ejection fraction, and NYHA class) than by age. Figure 4B shows the association between Patient-PL, Model-PL, and NYHA class, with higher NYHA classes having progressively decreasing model predictions of survival despite patient predictions that were relatively static. Notably, patients predicted their life expectancy essentially without regard for symptom severity, with the same median Patient-PL of 12 years for patients who were minimally symptomatic (NYHA class I) as for those with advanced symptoms (NYHA class IV).
Figure 4.
Patient predicted life expectancy vs. model predicted life expectancy stratified by patient age (A) and by NYHA class (B).
Since specific communication about prognosis between the clinician and the patient may have impacted understanding of likely prognosis, we examined OI for patients who reported having previously spoken to their clinician about prognosis (n = 45) compared to those who had not (n = 76), and found no significant difference in OI between these two groups (median OI = 1.34 vs. OI = 1.46, respectively; p =0.55).
COMMENT
In ambulatory patients with heart failure, self assessment of prognosis was on average significantly more optimistic than that predicted by a validated heart failure-specific model. When quantified as the ratio of patient predictions to model predictions of life expectancy (i.e., the optimism index), the median overestimation of survival in the population was 40% (median OI = 1.4). Overall, patient predictions of life expectancy correlated better with actuarial predictions (based on life tables using age and sex alone) than they did with expected survival based on the heart failure model. Taken as a whole, these data suggest that many patients with heart failure have survival expectations that differ markedly from the anticipated natural history of their disease.
In examining patient specific factors associated with greater optimism, we identified younger age, greater disease severity (ischemic heart failure etiology, lower ejection fraction, and higher NYHA class), and measures of less depression to be the most significantly associated with higher OI. Patient expectations about their survival varied dramatically with differences in age (younger patients predicting much longer survival), as shown in Figure 4A. Conversely, patients appeared to predict similar life expectancy regardless of the objective severity of their heart failure (as shown in Figure 4B for NYHA class). These two associations resulted in particularly discordant predictions in younger patients and those with more severe disease, and explain the seemingly paradoxical relationship between greater disease severity and greater optimism. Notably, both younger age34 and greater disease severity14 have previously been identified as being associated with greater discordance between patient and physician expectations about prognosis in patients with cancer. We also identified an association between lower measures of depression and greater degree of optimism, a relationship that seems intuitively valid (patients who are more depressed may be less likely to be optimistic). The complex biologic relationship between depression and heart failure has been increasingly recognized in recent years, and is a subject of ongoing randomized trials.35, 36
These results underscore the complexity of the identifying underlying reasons for the observed discordance between patient expectations and objective predictions of survival. One possible explanation of our findings may be that differences in perception about prognosis result from inadequate communication between clinicians and patients. The SUPPORT trial showed a high degree of incongruity between patients and their health care providers on end of life decisions.15 The lack of effective communication about prognosis may be due in part to physicians’ self-perceived inability to predict risk of mortality in advanced heart failure, as recently reported by Hauptman et al.37 Recent data from the ESCAPE study suggest that nurses were better than physicians at estimating 6 month mortality in patients with advanced heart failure.38 Although we did not identify a clear relationship between OI and prior physician-patient discussions regarding prognosis, it seems likely that issues of communication play a significant role in the way patients understand and interpret information about prognosis.
An alternative explanation for our findings may be that individuals’ predictions of longer life expectancy for themselves may simply reflect hope. Study of cancer patients has shown patient perspective to be important in estimations of prognosis. When patients with advanced malignancy are asked whether they themselves stand to benefit from participating in a phase I trial, they provide a much higher expected rate of benefit than when they are asked whether another person with the same level of disease is likely to benefit.39 The implication is that even when patients have a good understanding of prognosis, they may choose not to apply that information to themselves. In this study we asked patients only about self-assessed life expectancy, without regard to how they perceived prognosis for other individuals in the same situation.
The oncology literature provides a foundation for end of life expectations in chronic illness. However, little work has been done in this area in heart failure, despite a long-term prognosis comparable to many advanced forms of cancer. Previous work has shown that heart failure patients are less educated about their disease process than are lung cancer patients.40 In a qualitative study comparing illness trajectories of patients with Class IV heart failure and those with metastatic lung cancer, heart failure patients had less understanding about their disease and their prognosis.40 Whereas lung cancer patients thought about dying as a direct result of their illness, heart failure patients tended to think about dying more in the context of aging. This is concordant with our findings that heart failure patients estimated their survival to be more similar to actuarial survival based on age than that predicted by heart failure specific models.
Physicians and family members alike frequently counsel patients on the importance of maintaining a positive, optimistic outlook in the face of significant illness such as heart failure or cancer. While such an outlook may have clear psychological benefits for both patients and their families, the extent to which an optimistic outlook may impact observed survival in chronic illness has not been well studied. Although limited by the relatively small number of deaths in our cohort, the level of optimism about prognosis was not associated with observed survival in patients in our study.
Our results suggest that the communication of prognostic information between doctors and patients with heart failure is an area in need of additional attention, both in terms of clinical care and research. Improvements in patient understanding of prognosis may refine decision-making around resuscitation preferences, adherence to medical therapy, and consideration of advanced heart failure therapies such as implantable cardiac defibrillators, cardiac transplantation, or mechanical cardiac support. Efforts to better integrate palliative care into the treatment of selected patients with heart failure will certainly require a more sophisticated appreciation of patient-perceptions of prognosis.41 The increasing availability of web based clinical prediction tools, such as the SHFM, may facilitate improved communication about prognosis between patients and clinicians. In other diseases such as early stage breast cancer, the use of prognostic models and decision tools has been shown to increase patient understanding of prognosis and treatment options, leading to higher degrees of satisfaction with the process of care.42
Our study has several important limitations. It included a relatively small single-center sampling of heart failure patients in a university disease management program, which may limit the generalizability of our findings to other settings. Importantly, however, our cohort included a broad spectrum of disease severity as well as a significant proportion of elderly and African-American patients, and was generally similar to other unselected ambulatory cohorts with heart failure.32, 43 This study was performed in an ambulatory setting, and patient perceptions of life expectancy could be different during hospitalization for heart failure, a time when decisions regarding palliative care and advanced therapies are often made. Prior work in the acute setting has characterized predictors of referral to hospice, but has not included patient perceptions of prognosis.44
We excluded 26 patients from our analysis who were unwilling or unable to provide an estimation of their life expectancy, and such patients may have differed in relevant ways from study participants. Prior studies such as the SUPPORT study have also found that a significant proportion of patients are unwilling or unable to address expectations around end of life, suggesting that this potential for selection bias may be an unavoidable limitation of this type or research.21 We did not perform repeat questionnaires over time or other measures to validate the stability or consistency of the patient estimations of life expectancy; however, measures of the internal reliability of patient estimations of life expectancy on our questionnaire were quite high.
Although we did not identify a relationship between the degree of optimism and observed survival in our study, the relatively small number of events limited our power to assess this relationship. While patient predictions vs. observed survival would be of great interest, we did not have sufficient events to assess this relationship with any degree of statistical power; a study design that assessed this relationship over the full range of actual survival would require very long-term follow-up in order to capture actual mortality in a substantial majority of the study population. Finally, our study did not assess clinician estimates of patient’s life expectancy, so we were not able to contrast the expectations of patients with their providers. Despite these limitations, our study is the first to systematically and quantitatively examine the relationship between heart failure patients’ expectations about their prognosis, published model predictions for persons with heart failure, and observed survival.
CONCLUSIONS
In conclusion, in ambulatory patients with heart failure the self assessment of prognosis was substantially more optimistic than that predicted by heart failure survival models. This discordance was particularly marked in younger patients and those with more severe disease. The exact reasons for this incongruity are unknown, but may result from inadequate communication between clinicians and their patients about prognosis, or might simply reflect hope. Because differences in expectations about prognosis could affect decision-making regarding advanced therapies and end of life planning, further research into both the extent and the underlying causes of these differences is warranted. Whether interventions designed to improve communication of prognostic information between clinicians and patients would improve the process of care in heart failure should be tested in appropriately designed clinical trials.
Acknowledgements
The University of Washington holds the copyright for the Seattle Heart Failure Model. Dr. Levy has received royalties from the Seattle Heart Failure Model. There are no other conflicts of interest to disclose. Dr. Allen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More 'malignant' than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001 Jun;3(3):315–322. doi: 10.1016/s1388-9842(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003 Jun;9(3):180–187. doi: 10.1054/jcaf.2003.24. [DOI] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med. 2001 Nov 15;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Anker SD, Anand I, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007 Jul 24;116(4):392–398. doi: 10.1161/CIRCULATIONAHA.106.687103. [DOI] [PubMed] [Google Scholar]
- 6.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006 Mar 21;113(11):1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997 Jun 17;95(12):2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003 Nov 19;290(19):2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005 Feb 2;293(5):572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 10.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 11.Heart Failure Society Of A. HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006 Feb;12(1):e1–e2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Nieminen MS, Bohm M, Cowie MR, et al. Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology. Eur Heart J. 2005 Feb;26(4):384–416. doi: 10.1093/eurheartj/ehi044. [DOI] [PubMed] [Google Scholar]
- 13.Eidinger RN, Schapira DV. Cancer patients' insight into their treatment, prognosis, and unconventional therapies. Cancer. 1984 Jun 15;53(12):2736–2740. doi: 10.1002/1097-0142(19840615)53:12<2736::aid-cncr2820531233>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Lee SJ, Fairclough D, Antin JH, Weeks JC. Discrepancies between patient and physician estimates for the success of stem cell transplantation. JAMA. 2001 Feb 28;285(8):1034–1038. doi: 10.1001/jama.285.8.1034. [DOI] [PubMed] [Google Scholar]
- 15.Krumholz HM, Phillips RS, Hamel MB, et al. Resuscitation preferences among patients with severe congestive heart failure: results from the SUPPORT project. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Circulation. 1998 Aug 18;98(7):648–655. doi: 10.1161/01.cir.98.7.648. [DOI] [PubMed] [Google Scholar]
- 16.Formiga F, Chivite D, Ortega C, Casas S, Ramon JM, Pujol R. End-of-life preferences in elderly patients admitted for heart failure. QJM. 2004 Dec;97(12):803–808. doi: 10.1093/qjmed/hch135. [DOI] [PubMed] [Google Scholar]
- 17.Wachter RM, Luce JM, Hearst N, Lo B. Decisions about resuscitation: inequities among patients with different diseases but similar prognoses. Ann Intern Med. 1989 Sep 15;111(6):525–532. doi: 10.7326/0003-4819-111-6-525. [DOI] [PubMed] [Google Scholar]
- 18.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001 Sep;20(9):1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 19.Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007 Apr 10;25(11):1357–1362. doi: 10.1200/JCO.2006.08.3170. [DOI] [PubMed] [Google Scholar]
- 20.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995 Nov 22–29;274(20):1591–1598. [PubMed] [Google Scholar]
- 21.Weeks JC, Cook EF, O'Day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998 Jun 3;279(21):1709–1714. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 22.Chappell NL. Awareness of death in the disengagement theory: A concerptialization and an empirical investigation. Omega. 1975;6:325–343. [Google Scholar]
- 23.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med. 2006 Dec;20(8):745–754. doi: 10.1177/0269216306073112. [DOI] [PubMed] [Google Scholar]
- 24.Sulmasy DP, Terry PB, Weisman CS, et al. The accuracy of substituted judgments in patients with terminal diagnoses. Ann Intern Med. 1998 Apr 15;128(8):621–629. doi: 10.7326/0003-4819-128-8-199804150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Nomenclature and Criteria for Diagnosis of Diseases fo the Heart and Great Vessels. 9th ed. Littel, Brown & Co; 1994. Criteria Committee of the American Heart Association Affiliate NYC. [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003 Nov;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Friedman B, Conwell Y, Fiscella K. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. J Am Geriatr Soc. 2007 Apr;55(4):596–602. doi: 10.1111/j.1532-5415.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 29.Lowe B, Kroenke K, Grafe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2) J Psychosom Res. 2005 Feb;58(2):163–171. doi: 10.1016/j.jpsychores.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Koenig H, Parkerson GR, Jr, Meador KG. Religion index for psychiatric research. Am J Psychiatry. 1997 Jun;154(6):885–886. doi: 10.1176/ajp.154.6.885b. [DOI] [PubMed] [Google Scholar]
- 31.Levy WC. [Date accessed August 21, 2007];Seattle Heart Failure Model Calculator. 2006 http://depts.washington.edu/shfm/app.php?accept=1&enter=Enter.
- 32.May HT, Horne BD, Levy WC, et al. Validation of the Seattle Heart Failure Model in a community-based heart failure population and enhancement by adding B-type natriuretic peptide. Am J Cardiol. 2007 Aug 15;100(4):697–700. doi: 10.1016/j.amjcard.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 33.Dardas T. Evaluation of discrimination and calibration of the Heart Failure Survival Score and Seattle Heart Failure Model in ambulatory patients evaltuated for cardiac transplant; Paper presented at: American Heart Association Scientific Sessions; Orlando. 2007. [Google Scholar]
- 34.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001 Jun 19;134(12):1096–1105. doi: 10.7326/0003-4819-134-12-200106190-00009. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Kuchibhatla M, Clary GL, et al. Relationship between depressive symptoms and long-term mortality in patients with heart failure. American Heart Journal. 2007;154(1):102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 36.Joynt KE, Whellan DJ, O'Connor CM. Why is depression bad for the failing heart? A review of the mechanistic relationship between depression and heart failure. J Card Fail. 2004 Jun;10(3):258–271. doi: 10.1016/j.cardfail.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Hauptman PJ, Swindle J, Hussain Z, Biener L, Burroughs TE. Physician attitudes toward end-stage heart failure: a national survey. Am J Med. 2008 Feb;121(2):127–135. doi: 10.1016/j.amjmed.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Yamokoski LM, Hasselblad V, Moser DK, et al. Prediction of rehospitalization and death in severe heart failure by physicians and nurses of the ESCAPE trial. J Card Fail. 2007 Feb;13(1):8–13. doi: 10.1016/j.cardfail.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Weinfurt KP, Castel LD, Li Y, et al. The correlation between patient characteristics and expectations of benefit from Phase I clinical trials. Cancer. 2003 Jul 1;98(1):166–175. doi: 10.1002/cncr.11483. [DOI] [PubMed] [Google Scholar]
- 40.Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002 Oct 26;325(7370):929. doi: 10.1136/bmj.325.7370.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauptman PJ, Havranek EP. Integrating palliative care into heart failure care. Arch Intern Med. 2005 Feb 28;165(4):374–378. doi: 10.1001/archinte.165.4.374. [DOI] [PubMed] [Google Scholar]
- 42.Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004 Jul 28;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 43.Maggioni AP, Opasich C, Anand I, et al. Anemia in Patients With Heart Failure: Prevalence and Prognostic Role in a Controlled Trial and in Clinical Practice. Journal of Cardiac Failure. 2005;11(2):91–98. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Hauptman PJ, Goodlin SJ, Lopatin M, Costanzo MR, Fonarow GC, Yancy CW. Characteristics of patients hospitalized with acute decompensated heart failure who are referred for hospice care. Arch Intern Med. 2007 Oct 8;167(18):1990–1997. doi: 10.1001/archinte.167.18.1990. [DOI] [PubMed] [Google Scholar]