Abstract
Objective
We evaluated vaginal defensin concentrations and levels of BV-associated bacterial species in pregnant women.
Study Design
Self-collected vaginal swabs from two visits during pregnancy were tested with qPCR for nine bacterial species. Beta defensin 2 (HBD2), HBD3 and alpha defensins 1–3 (HNP1–3) were measured by ELISA.
Results
Our 126 participants were primarily African American (60%), had a mean gestational age at enrollment of 10 weeks (±3) and at follow-up of 25 weeks (± 6). At enrollment, prevalence of BV was 74% (94/126), which decreased to 60% (75/126) at follow-up. At enrollment, HBD3 concentrations were significantly lower in women with BV (2.64 + 0.91 vs. 3.25 + 0.99 log10 pg/mL; p = 0.003). Higher concentrations of Atopobium vaginae, BVAB1 and BVAB2 were associated with significantly lower concentrations of HBD3 (p < 0.01).
Conclusions
BV was associated with lower vaginal concentrations of HBD3, but not HBD2 or HNP1–3, in pregnant women.
Keywords: Bacterial vaginosis, Defensins, Innate immunity in pregnancy
Introduction
Bacterial vaginosis (BV) is a vaginal syndrome characterized by a vaginal microbiota with a diverse community of anaerobic species. BV in pregnancy has been associated with a two-fold increase in preterm birth,1–3 presumably due to ascending infection and inflammation.4 BV is characterized by complex and heterogeneous bacterial communities5 and it is unclear if particular bacterial species are associated with increased risk of preterm birth. Current therapy for BV has been unsuccessful in reducing rates of preterm birth.6 If a particular pathogen could be identified as more inflammatory or more pathogenic, it might allow more targeted therapy to reduce preterm deliveries.
Defensins are cationic antimicrobial peptides produced as part of the innate immune response at mucosal surfaces.7 The human beta defensins (HBD) are primarily produced by epithelial cells, and have been shown to be elevated in the amniotic fluid of women with intrauterine infection and preterm labor.8,9 However, there are mixed data about the response of beta-defensins to BV – some studies have shown an increase10 and others no change.11 Alpha-defensins (also known as human neutrophil peptides or HNP) are primarily produced by neutrophils, and have also been shown to be elevated in the amniotic fluid of women with intrauterine infection and preterm labor, but data about levels in women with and without BV are mixed.12–15
We hypothesized that bacterial species associated both with BV and with preterm birth in a pregnant cohort,16 such as Gardnerella vaginalis and Megasphaera species, would increase beta-defensin levels, but not alpha-defensin levels, as neutrophils are usually absent in women with BV. We evaluated this association in a cohort study of pregnant women with a high prevalence of BV.
Materials and Methods
This analysis was conducted on a subset of pregnant women enrolled in a prospective cohort study of vaginal bacteria and preterm birth in Philadelphia, PA (ProjectBABIES). English or Spanish speaking pregnant women were eligible for enrollment in the parent study if they were less than 16 weeks gestation based on self-reported last menstrual period, lived in Philadelphia, and contributing multiple vaginal swabs to measure various aspects of BV. At enrollment, women completed an interview and self-collected vaginal swabs, which were stored at −80C until processing.17 Vaginal fluid self-collected were spread on a glass slide and transported, in batches, to the clinical microbiology lab at the University of Pennsylvania for gram staining and BV identification using the Nugent criteria. All slides were examined and interpreted by a single individual during the course of the study. BV was diagnosed for the study by Nugent score of 7–10.18 Women were not treated for BV as part of this study. These procedures were repeated at a follow-up visit scheduled prior to 28 weeks gestation. At follow-up women were asked about interim diagnoses of sexually transmitted infections or BV, as well as any antibiotic treatment. Women were included in the substudy if they were enrolled between June 2008 and January 2010 and had both an enrollment and a follow-up swab available for analysis.
Swabs were thawed, and then eluted into 1mL PBS, which was then centrifuged at 10,000 × g for 10 minutes. The resulting cell pellet underwent DNA extraction using the MoBio BiOstic Bacteremia kit, and testing using species-specific qPCR assays for the BV-associated species Gardnerella vaginalis, Atopobium vaginae, Leptotrichia spp, Sneathia spp, Mobiluncus spp, Megasphaera spp, Bacterial Vaginosis Associated Bacterium (BVAB) 1, BVAB2 and BVAB3.19,20 As previously described, all samples also underwent qPCR testing for the human 18S gene to confirm contact with a mucosal surface, and evaluation for PCR inhibition using an amplification control.21, 22 Mock swabs were put through DNA extraction and PCR as a negative control to detect reagent contamination. Samples that had undetectable levels of bacteria were assigned the value of lower limit of the assay (250 copies16S rRNA/swab).
Commercial ELISA kits were used to test the swab supernatant for HBD2, HBD3 (Alpha Diagnostic International, San Antonio, TX) and HNP1–3 (Hycult Biotech, Plymouth Meeting, PA). Samples with undetectable levels of defensins were assigned the value of the lower limit of detection for each assay (HBD2 12.5 pg/mL, HBD3 50 pg/mL, HNP1–3 156 pg/mL).
All comparisons of mean concentrations of defensins or bacteria were performed as a cross-sectional analysis at one time point: either enrollment or follow-up. Quantities of bacteria and defensins were log transformed for analysis. Mean quantities of bacteria at enrollment and follow-up were compared using a paired student’s t-test. Mean quantities of defensins were compared between women with and without BV using student’s t-test. Differences in defensin concentrations across quartiles of bacterial concentrations were compared using ANOVA. Associations between defensin concentrations and demographic factors were assessed using linear regression with robust standard errors. We also conducted a longitudinal analysis using a multivariable linear regression model, controlling for race. Percent change in quantity of bacteria between enrollment and follow-up was used as the independent variable, and percent change in defensin concentration as the dependent variable.
Results
A total of 1560 pregnant women were enrolled in the parent study, and 126 were selected for this sub-analysis, giving 252 samples for analysis. Participants were primarily young, African-American women, with a high school education or less. (Table 1). Women were mostly enrolled in the first trimester (mean 10 weeks ± 3, range 4–19 weeks), and returned for follow-up in the mid second trimester, at a mean gestational age of 25 weeks (± 6 weeks; range 15–38 weeks). Two women (8%) were diagnosed with twin pregnancy between their enrollment and follow-up visit. There were no significant differences in race, education, pregnancy history or prevalence of BV between women in the sub-analysis and the larger cohort. (data not shown)
Table 1.
Demographic and clinical information at enrollment
| N = 126 | |
|---|---|
| Age (mean + SD) | 24 ± 6 |
| Gravidity (median, IQR) | 1 (0, 3) |
| Parity (median, IQR) | 1 (0, 2) |
| Race (n, %) | |
| Black | 76 (60%) |
| White | 8 (6%) |
| Asian | 2 (2%) |
| Hispanic | 36 (29%) |
| Other | 4 (3%) |
| Education (n, %) | |
| Some high school, or less | 54 (43%) |
| High school graduate (or GED) | 47 (37%) |
| Some college or vocational training | 21 (17%) |
| College graduate | 4 (3%) |
| Marital status (n, %) | |
| Single | 97 (77%) |
| Married (or living as married) | 27 (21%) |
| Separated | 2 (2%) |
| Smoker (n, %) | 26 (21%) |
| Gestational age at enrollment (mean + SD) | 10 ± 3 weeks (range 4–19) |
| Nugent score (n, %) | |
| 0–3 | 8 (6%) |
| 4–6 | 20 (16%) |
| 7–10 | 93 (74%) |
| Diagnosed with GC/CT this pregnancy, prior to enrollment (n, %) | 7 (6%) |
| Detectable bacteria present at baseline? (n, %) | |
| G. vaginalis | 116 (92%) |
| Atopobium | 106 (84%) |
| Leptotrichia/Sneathia | 89 (71%) |
| Mobiluncus | 73 (58%) |
| Megashpaera | 83 (66%) |
| BVAB1 | 50 (40%) |
| BVAB2 | 77 (61%) |
| BVAB3 | 50 (40%) |
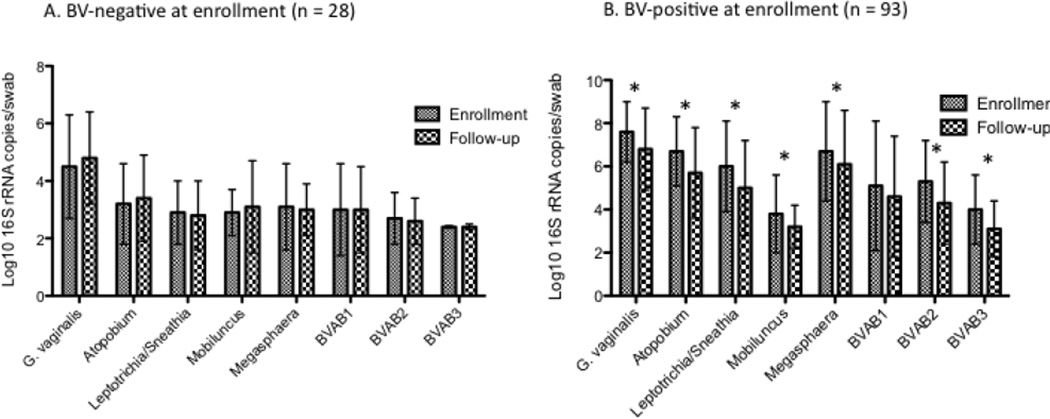
Most bacterial species were detected in the majority of the population at enrollment, with the exception of BVAB1 and BVAB3, which were only detected in 40% of women (Table 1). This is likely due to the high prevalence of BV in 74% (94/126) of participants. At follow-up, the prevalence of BV had decreased to 60%(75/126). Only two women reported being diagnosed with BV by their physician between enrollment and follow-up, both of whom reported treatment with antibiotics. Of those with BV at follow-up, only 4(5%) had incident BV, while 69 (95%) had persistent or recurrent BV by Nugent score. In keeping with the decrease in prevalence of BV, the quantity of BV-associated bacteria present decreased between enrollment and follow-up among women with BV at enrollment, but stayed constant among women with normal or intermediate flora at baseline. (Figure 1). Quantities of each bacteria were significantly higher among women with BV at enrollment compared to those without BV (data not shown).
Figure 1.
Mean concentrations of bacterial species at enrollment and follow-up visits in women with and without BV at enrollment.
*signifies a significant difference between enrollment and follow-up by paired t-test, p < 0.05
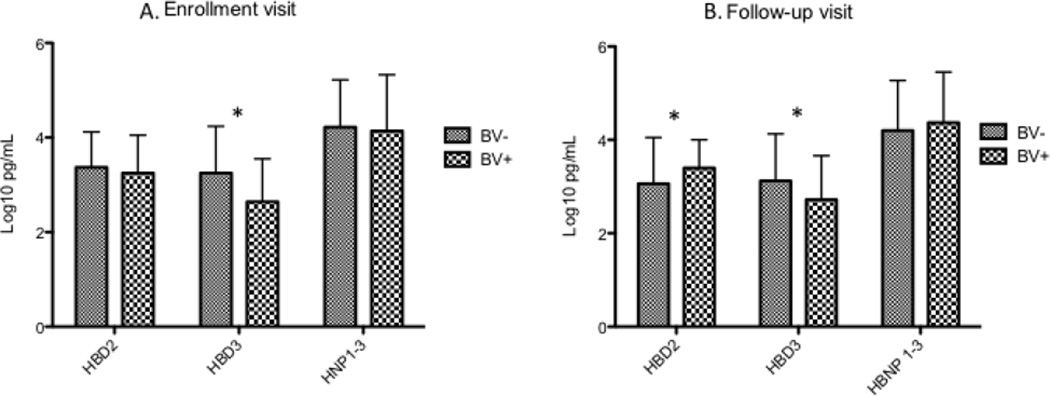
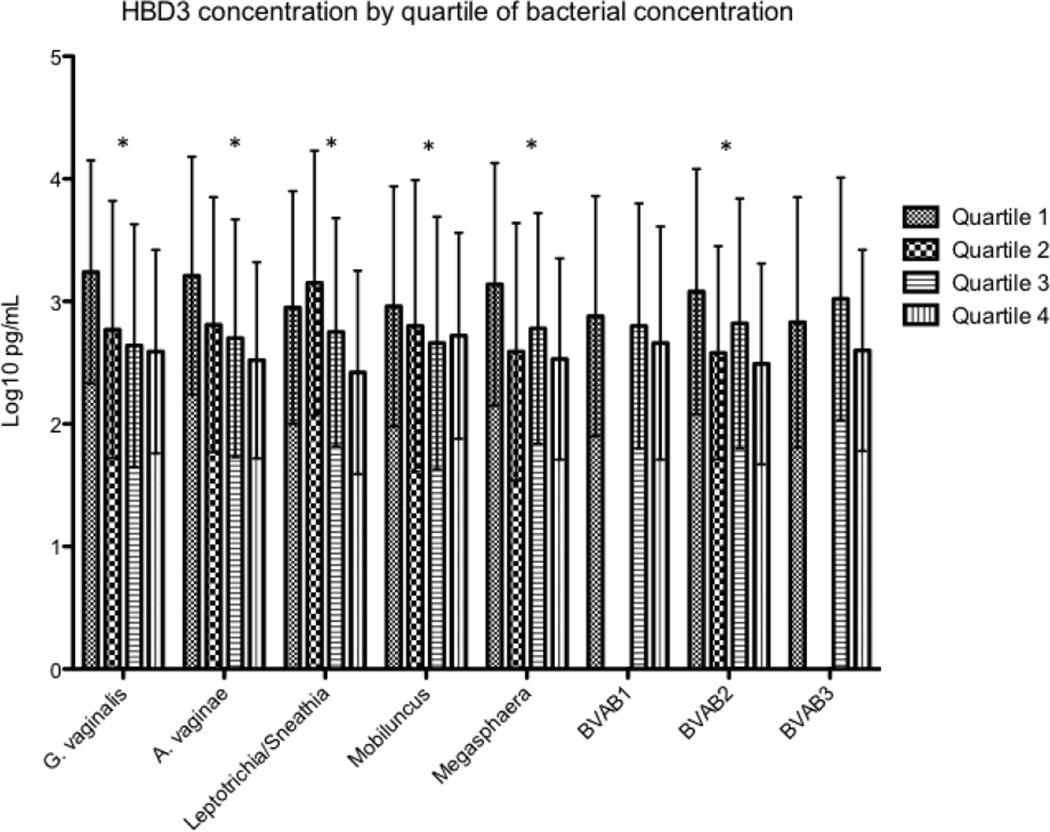
HBD2 was detectable in 110 (87%) of women at enrollment, HBD3 in 76 (60%), and HNP1–3 in 105 (83%). Concentrations of HBD2 and HNP1–3 were not different between women with and without BV at enrollment, while HBD3 concentrations were significantly lower in women with BV. (Figure 2) For each increasing quartile of bacterial concentration at enrollment, HBD3 concentrations were significantly lower (Figure 3) for all species except Mobiluncus, BVAB1 and BVAB3. No significant associations were seen between concentrations of individual bacterial species and HBD2 or HNP1–3. At follow-up, HBD2 was detectable in 104 (83%) of women, HBD3 in 80 (63%), and HNP1–3 in 107 (85%). At the second visit, HBD2 was significantly higher in women with BV, while HBD3 concentrations were lower in women with BV and HNP1–3 was not different between women with and without BV. (Figure 2) A similar trend toward lower HBD3 concentrations with increasing quartiles of bacterial concentrations was seen at the follow-up visit, but only reached statistical significance for BVAB2 (data not shown). The four women who developed incident BV between enrollment and follow-up had significantly lower levels of HBD3 at enrollment than the 24 women who remained BV negative through the whole study (median 50 pg/mL vs/ 4855 pg/mL; p = 0.001), as well as HNP1–3 (median 3199 pg/mL vs. 37582 pg/mL; p = 0.001).
Figure 2.
Mean concentrations of vaginal defensins in women with and without BV
* signifies a significant difference between women with and without BV by t-test, p < 0.05
Figure 3.
Mean concentrations of HBD3 by quartile of bacterial concentration for each species at enrollment.
* signifies a significant difference in HBD3 concentrations between quartiles, as measured by linear regression, p < 0.05. Samples with no detectable bacteria or defensin are included in calculations of mean or quartile values with a value at the lower limit of detection of the assay
Defensin concentrations were not significantly associated with gestational age, either at enrollment or follow up (Table 2), nor with maternal age or smoking status. However, African-American and Hispanic women had lower concentrations of HNP1–3 compared to white women (p-value < 0.001). For this reason, in our regression model looking at the association between change in bacterial concentrations between visits and the change in defensin concentrations, we only adjusted for race. In that multivariate model, with change in defensin concentrations as the dependent variable, increased concentrations of Atopobium vaginae, BVAB1 and BVAB2 were associated with decreased concentrations of HBD3 but not HBD2 or HNP1–3. (Table 3) Adjusting for gestational age at enrollment, or time between visits did not significantly change the results of this analysis (data not shown).
Table 2.
Association between demographic factors and defensin concentrations.
| HBD2 | HBD3 | HNP 1–3 | |
|---|---|---|---|
| Age at enrollment | 0.01 (−0.02, 0.04) | 0.02 (−0.01, 0.05) | −0.01 (−0.04, 0.03) |
| Gestational age at enrollment | −0.03 (−0.07, 0.01) | 0.002 (−0.05, 0.06) | −0.01 (−0.07, 0.06) |
| Gestational age at follow-up | −0.02 (−0.04, 0.01) | −0.02 (−0.05, 0.01) | 0.02 (−0.01, 0.06) |
| Smoker at enrollment | 0.01 (−0.24, 0.42) | 0.32 (−0.15, 0.79) | −0.02 (−0.58, 0.54) |
| Race at enrollment | |||
| White | Ref | Ref | Ref |
| Black | −0.15 (−0.46, 0.16) | 0.10 (−0.55, 0.75) | −0.89 (−1.26, −0.53) |
| Hispanic | −0.20 (−0.55, 0.15) | −0.04 (−0.73, 0.64) | −1.04 (−1.49, −0.59) |
| Asian | 0.18 (−0.14, 0.50) | −0.50 (−1.53, 0.52) | 0.13 (−0.65, 0.90) |
Associations measured by linear regression, reported as regression coefficient (95% CI). This can be interpreted as the log10 difference in defensin concentration between groups, or with each increase in week gestation.
Bold values are statistically significant with p < 0.05.
Table 3.
Association between percent change in bacterial concentrations and percent change in defensin concentrations between the enrollment and follow-up visit.
| HBD2 | HBD3 | HNP | |
|---|---|---|---|
| Gardnerella vaginalis | 0.15(−0.001, 0.31) | −0.15 (−0.38, 0.07) | −0.02 (−0.23, 0.18) |
| Atopobium vaginae | 0.12 (−0.06, 0.30) | −0.26 (−0.49, −0.02) | −0.02 (−0.18, 0.15) |
| Leptotrichia/Sneathia | 0.01 (−0.11, 0.13) | −0.14 (−0.29, 0.01) | 0.11 (−0.17, 0.38) |
| Mobiluncus | 0.20 (−0.06, 0.47) | −0.05 (−0.32, 0.22) | 0.15 (−0.08, 0.37) |
| Megashpaera spp | 0.15 (−0.01, 0.30) | −0.10 (−0.27, 0.06) | 0.10 (−0.11, 0.31) |
| BVAB1 | 0.01 (−0.08, 0.10) | −0.13 (−0.25, − 0.003) | 0.06 (−0.16, 0.27) |
| BVAB2 | 0.03 (−0.12, 0.18) | −0.23 (−0.45, −0.01) | 0.13 (−0.11, 0.37) |
| BVAB3 | −0.13 (−0.37, 0.11) | −0.04 (−0.31, 0.23) | 0.18 (−0.08, 0.44) |
Associations assessed by linear regression, adjusted for race.
This can be interpreted as the percent difference in defensin concentration associated with each percent increase in bacterial concentration between visits.
Bold values are statistically significant with p < 0.05.
Comment
In this study of pregnant women we found that BV was associated with lower vaginal concentrations of HBD3, but not HBD2 or HNP1–3. In addition, among all women (with and without BV), multiple individual bacterial species (Atopobium vaginae, BVAB1 and BVAB2) were associated with lower concentrations of HBD3. Other BV-associated bacterial species showed a trend to association with decreased quantities of HBD3, but did not reach statistical significance. Our study was not designed to evaluate the causal association between defensins and bacteria, thus we cannot state whether low levels of HBD3 predispose to BV and colonization with BV-associated bacterial species, or whether that colonization leads to downregulation of or increased proteolysis of HBD3.
Few studies have compared levels of all three defensins between women with and without BV. Valore et al showed that non-pregnant women with BV had lower levels of HBD2 and HNP1–3 compared to women with normal microbiota, and that levels increased after treatment.13 Our study also measured defensins at two time points, but did not show similar low levels of HNP 1–3 or HBD2 with BV, though we did see lower levels of HBD3. It is possible that pregnancy modifies the interaction between bacteria and the host mucosa, which may account for differences between our two studies. In pregnant women, Balu et al showed that women with an intermediate Nugent score had higher levels of HNP1–3 than women with a normal Nugent score, but that women with BV did not have significantly different concentrations compared to normal.14 Xu et al showed no difference in concentration of HNP1–3 in pregnant women with and without BV.15 However, that study did show lower vaginal levels of HNP1–3 in African American women compared to white women, as we demonstrated in this analysis. Our results add to these analyses by evaluating individual species of BV-associated bacteria and demonstrating no significantly different association with defensin concentrations for any single species, suggesting that associations between BV and immune response are not driven by the presence of individual species, but rather the altered microbial community as a whole.
It is unclear why HBD3 and not HBD2 was associated with BV and concentrations of BV-associated bacterial species. Although HBD2 and HBD3 are both beta-defensins, they have different biochemical properties, different antimicrobial efficacy, and different chemotactic roles. HBD3 is more stable in high salt concentrations and has better efficiency for killing Staphylococcus aureus.23 Both have similar efficacy for killing E. coli, but their antimicrobial effect on BV-associated species has not been well studied. HBD3 is chemotactic for macrophages, while HBD2 attracts memory T-cells and immature dendritic cells.23 HBD3 has also been associated with anti-inflammatory effects on toll-like receptor signaling.24 While both HBD2 and HBD3 are induced via the TLR2 pathway, it appears that they are regulated by different signaling cascades, which may explain their differential expression.25 It is not surprising to us that HNP1–3 were not associated with BV, as neutrophils are not classically present in the mucosa or vaginal fluid of women with BV.
Our findings that BV and higher concentrations of BV-associated bacteria were associated with lower levels of HBD3 were contrary to our original hypothesis, but concordant with data from other mucosal sites. In patients with periodontitis, which is also characterized by a complex microbial community and inflammation, lower levels of HBD3 were seen in gingival fluid compared to patients with healthy gums.26 Several groups have demonstrated that bacterial proteases can degrade defensins.27 There is some evidence in respiratory epithelium that Klebsiella pneumoniae can inhibit cells from producing HBD3 by suppressing toll-like receptor activation.28 It is possible that BV-associated bacterial species can reduce HBD3 concentrations through either mechanism. Alternatively, individuals may have different numbers of copies of beta-defensin genes.29,30 Higher numbers of gene copies have been associated with higher expression of the protein,31 thus women with lower HBD3 levels may be biologically more at risk for BV due to lower levels of antimicrobial defensins - as suggested by the four women in our study who developed incident BV and had lower levels of HBD3 at enrollment.
Bacterial vaginosis has been linked to preterm birth, likely through induction of an inflammatory response.1–4 It is still unclear whether this is related to the type of bacterial stimulus, or the genotype and inflammatory potential of the host. Gomez et al showed that women with polymorphisms in three genes associated with inflammatory response had a higher risk of preterm delivery if they had BV in pregnancy than women without the polymorphisms, though whether these polymorphisms were associated with increased or decreased inflammation is not specified.32 Polymorphisms in the gene for TNF-a have also been associated with increased risk of preterm delivery in women with BV.33 Interestingly, Simhan et al have shown that early in pregnancy, lower levels of pro-inflammatory cytokines are associated with increased rates of preterm birth (possibly due to decreased ability to defend against bacterial ascent in to the uterus)34. Some species of bacteria, such as Ureaplasma, have been associated with increased risk of preterm birth35 while others (Mycoplasma, Atopobium, Fusobacterium) are commonly found in the amniotic membranes at the time of preterm delivery,36 suggesting that some bacterial species may have more potential to cause adverse outcomes than others. Our study did not evaluate pregnancy outcomes, but does suggest that either some bacterial species may suppress the innate immune response, or that women with lower levels of antimicrobial peptides such as HBD3 may be more susceptible to bacterial vaginosis and possibly its complications.
Our study is limited by a relatively small sample size, but our population was quite diverse and had a high prevalence of BV, making it an excellent choice for studying these interactions. This community has previously been reported to have a much higher rate of BV than what is reported in the general population.37 We only collected data at two time points during the pregnancy, which gives a limited understanding of the course of the immune response during pregnancy. Our requirement that women have two samples to be included in the substudy excludes women who dropped out of the study due to late miscarriage or early preterm delivery, populations of significant clinical interest. However, our focus for this analysis was the association between individual bacterial species and vaginal defensin concentrations in pregnancy rather than on outcomes. Our analysis of the vaginal microbiota was limited to eight individual species or genera. Using the species-specific qPCR assays allows us to correlate quantity of bacteria with quantity of defensins, which is not possible when using the broad-range community profiling techniques capable of detecting additional species. Defensin concentrations were measured in eluate from vaginal swabs, which may not collect a standard amount of secretions with each sampling, however self-collection of swabs facilitated participation in the study and was more acceptable to participants than speculum exam and vaginal lavage.
Our observations that the beta defensins are associated with BV-associated bacterial species in pregnant women, and that HBD3 concentrations are lower in women with BV raise several questions about the regulation of the mucosal immune response: are bacteria able to downregulate gene expression to escape innate immunity? Or are lower levels of defensins a marker of genetic or biologic vulnerability? Additionally, the lack of racial differences in beta-defensin concentrations, but the presence of lower neutrophil-related defensins in African American and Hispanic women raises further questions about the role of race in the immune response, especially during pregnancy.
Acknowledgments
Funding: This study was supported in part by pilot funding from the University of Washington Institute for Translational Health Sciences, as well as by R01HD038856 from NICHD (DN). Dr. Mitchell is supported by a K08 award (NIAID, 1K08AI087969)
Footnotes
This study was conducted in Philadelphia, PA
Disclosure: None of the authors report any conflict of interest.
References
- 1.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. New Eng J Med. 1995 Dec 28;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 2.Holst E, Goffeng AR, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994 Jan;32(1):176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009 Sep;116(10):1315–1324. doi: 10.1111/j.1471-0528.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008 Jan 5;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. New Eng J Med. 2005 Nov 3;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 6.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. New Eng J Med. 2000 Feb 24;342(8):534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 7.Doss M, White MR, Tecle T, Hartshorn KL. Human defensins and LL-37 in mucosal immunity. J Leukocyte Biol. 2010 Jan;87(1):79–92. doi: 10.1189/jlb.0609382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during pregnancy. Placenta. 2007 Nov-Dec;28(11–12):1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Soto E, Espinoza J, Nien JK, Kusanovic JP, Erez O, Richani K, et al. Human beta-defensin-2: a natural antimicrobial peptide present in amniotic fluid participates in the host response to microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2007 Jan;20(1):15–22. doi: 10.1080/14767050601036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan SR, Liu XP, Liao QP. Human defensins and cytokines in vaginal lavage fluid of women with bacterial vaginosis. Int J Gynecol Obstet. 2008 Oct;103(1):50–54. doi: 10.1016/j.ijgo.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Levinson P, Kaul R, Kimani J, Ngugi E, Moses S, MacDonald KS, et al. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS (London, England) 2009 Jan 28;23(3):309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 12.Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, et al. Bacterial vaginosis, vaginal fluid neutrophil defensins, and preterm birth. Obstet Gynecol. 2003 May;101(5 Pt 1):862–868. doi: 10.1016/s0029-7844(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 13.Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun. 2006 Oct;74(10):5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balu RB, Savitz DA, Ananth CV, Hartmann KE, Miller WC, Thorp JM, et al. Bacterial vaginosis and vaginal fluid defensins during pregnancy. Am J Obstet Gynecol. 2002 Nov;187(5):1267–1271. doi: 10.1067/mob.2002.126989. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Holzman CB, Arvidson CG, Chung H, Goepfert AR. Midpregnancy vaginal fluid defensins, bacterial vaginosis, and risk of preterm delivery. Obstet Gynecol. 2008 Sep;112(3):524–531. doi: 10.1097/AOG.0b013e318184209b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson DB, Hanlon A, Hassan S, Britto J, Geifman-Holtzman O, Haggerty C, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med. 2009;37(2):130–134. doi: 10.1515/JPM.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson DB, Bellamy S, Gray TS, Nachamkin I. Self-collected versus provider-collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. J Clin Epidemiol. 2003 Sep;56(9):862–866. doi: 10.1016/s0895-4356(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 18.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Mirobiol. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in Vaginal Bacterial Concentrations with Intravaginal Metronidazole Therapy for Bacterial Vaginosis as Assessed by Quantitative PCR. J Clin Microbiol. 2009 Mar;47(3):721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan S, Liu C, Mitchell CM, Fiedler TL, Thomas KK, Agnew KJ, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell CM, Hitti JE, Agnew KJ, Fredricks DN. Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria. BMC Infect Dis. 2009;9:89. doi: 10.1186/1471-2334-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis. 2008;8:73. doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Mysliwy J, Spudy B, Lorenzen I, Reiss K, Gelhaus C, et al. Human beta-defensin 2 and beta-defensin 3 chimeric peptides reveal the structural basis of the pathogen specificity of their parent molecules. Antimicrob Agents Ch. 2011 Mar;55(3):954–960. doi: 10.1128/AAC.00872-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semple F, MacPherson H, Webb S, Cox SL, Mallin LJ, Tyrrell C, et al. Human beta-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur J Immunol. 2011 Nov;41(11):3291–3300. doi: 10.1002/eji.201141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scharf S, Zahlten J, Szymanski K, Hippenstiel S, Suttorp N, N'Guessan PD. Streptococcus pneumoniae induces human beta-defensin-2 and-3 in human lung epithelium. Exp Lung Res. 2012 Mar;38(2):100–110. doi: 10.3109/01902148.2011.652802. [DOI] [PubMed] [Google Scholar]
- 26.Brancatisano FL, Maisetta G, Barsotti F, Esin S, Miceli M, Gabriele M, et al. Reduced human beta defensin 3 in individuals with periodontal disease. J Dent Res. 2011 Feb;90(2):241–245. doi: 10.1177/0022034510385686. [DOI] [PubMed] [Google Scholar]
- 27.Eley BM, Cox SW. Proteolytic and hydrolytic enzymes from putative periodontal pathogens: characterization, molecular genetics, effects on host defenses and tissues and detection in gingival crevice fluid. Periodontol. 2003;31:105–124. doi: 10.1034/j.1600-0757.2003.03107.x. 2000. [DOI] [PubMed] [Google Scholar]
- 28.Moranta D, Regueiro V, March C, Llobet E, Margareto J, Larrarte E, et al. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect Immun. 2010 Mar;78(3):1135–1146. doi: 10.1128/IAI.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fode P, Stegger M, Andersen PS. Human beta-defensin 3 (DEFB103) and its influence on Staphylococcus aureus nasal carriage. Int J Infect Dis. 2011 Jun;15(6):e388–e394. doi: 10.1016/j.ijid.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Hardwick RJ, Machado LR, Zuccherato LW, Antolinos S, Xue Y, Shawa N, et al. A worldwide analysis of beta-defensin copy number variation suggests recent selection of a high-expressing DEFB103 gene copy in East Asia. Hum Mutat. 2011 Jul;32(7):743–750. doi: 10.1002/humu.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groth M, Wiegand C, Szafranski K, Huse K, Kramer M, Rosenstiel P, et al. Both copy number and sequence variations affect expression of human DEFB4. Genes Immun. 2010 Sep;11(6):458–466. doi: 10.1038/gene.2010.19. [DOI] [PubMed] [Google Scholar]
- 32.Gomez LM, Sammel MD, Appleby DH, Elovitz MA, Baldwin DA, Jeffcoat MK, et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am J Obstet Gynecol. 2010 Apr;202(4):386.e1–386.e6. doi: 10.1016/j.ajog.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 33.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF., 3rd A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004 Jun;190(6):1504–1508. doi: 10.1016/j.ajog.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Simhan HN, Krohn MA. First-trimester cervical inflammatory milieu and subsequent early preterm birth. Am J Obstet Gynecol. 2009 Apr;200:377.e1–377.e4. doi: 10.1016/j.ajog.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 35.Breugelmans M, Vancutsem E, Naessens A, Laubach M, Foulon W. Association of abnormal vaginal flora and Ureaplasma species as risk factors for preterm birth: a cohort study. Acta Obstet Gynecol Scand. 2010;89(2):256–260. doi: 10.3109/00016340903418769. [DOI] [PubMed] [Google Scholar]
- 36.Jones HE, Harris KA, Azizia M, Bank L, Carpenter B, Hartley JC, et al. PLoS One. 2009 Dec 8;4(12):e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson DB, Bellamy S, Clothier BA, Macones GA, Nachamkin I, Ruffin A, et al. Characteristics and Pregnancy Outcomes of Pregnant Women Asymptomatic for Bacterial Vaginosis. Mat Child Health Jl. 2008;12(2):216–222. doi: 10.1007/s10995-007-0239-7. [DOI] [PubMed] [Google Scholar]