Abstract
Background
Formation of new blood vessels, by either angiogenesis or vasculogenesis, is critical for normal wound healing. Major processes in neovascularization include (i) growth-promoting or survival factors, (ii) proteolytic enzymes, (iii) activators of multiple differentiated and progenitor cell types, and (iv) permissible microenvironments. A central aim of wound healing research is to “convert” chronic, disease-impaired wounds into those that will heal.
The problem
Reduced ability to re-establish a blood supply to the injury site can ultimately lead to wound chronicity.
Basic/Clinical Science Advances
(1) Human fetal endothelial progenitor cells can stimulate wound revascularization and repair following injury, as demonstrated in a novel mouse model of diabetic ischemic healing. (2) Advances in bioengineering reveal exciting alternatives by which wound repair may be facilitated via the creation of vascularized microfluidic networks within organ constructs created ex vivo for wound implantation. (3) A “personalized” approach to regenerative medicine may be enabled by the identification of protein components present within individual wound beds, both chronic and acute.
Clinical Care Relevance
Despite the development of numerous therapies, impaired angiogenesis and wound chronicity remain significant healthcare problems. As such, innovations in enhancing wound revascularization would lead to significant advances in wound healing therapeutics and patient care.
Conclusion
Insights into endothelial progenitor cell biology together with developments in the field of tissue engineering and molecular diagnostics should not only further advance our understanding of the molecular mechanisms regulating wound repair but also offer innovative solutions to promote the healing of chronic and acute wounds in vivo.

Ira M. Herman
Background
Angiogenesis, the formation of new blood vessels from the preexisting vasculature, is indispensable for successful wound healing. Postinjury, microvascular endothelial cells (ECs) that line the inner surface of blood vessels are activated by low oxygen tension/hypoxia and proangiogenic factors, including vascular endothelial growth factor (VEGF). In turn, ECs degrade their surrounding extracellular matrix, begin migration and cell division (proliferation), and then reestablish cell–cell contacts, forming new capillaries.1 Another critical process required for wound revascularization is vasculogenesis, which relies on the mobilization of endothelial progenitor cells (EPCs) from the bone marrow to areas of regenerating or healing tissues. EPCs (both embryonic and adult) possess several stem-cell like surface markers, including the cell surface glycoprotein CD133, and can differentiate into several cell types.2 Although stem cell applications have proven beneficial in cardiovascular settings,3 EPCs' efficacy in fostering diabetic wound healing remains problematic, because diabetic patients possess both diminished and dysfunctional EPCs4,5 as well as unfavorable wound microenvironments. As a need for molecular and cellular therapeutics remains evident, alternative pathways are being explored, including the development of implantable bioengineered microvascular networks that would enhance wound bed perfusion.6 Ultimately, understanding patient-specific metabolic and molecular profiles7 should provide potent diagnostic tools, advancing the field toward a personalized regenerative medicine-based approach.
Target Articles.
1. Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, and Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of wnt signaling. Circ Res 2009; 104: 1095.
2. Borenstein JT, Megley K, Wall K, Pritchard EM, Troung D, Kaplan DL, Tao SL, and Herman IM: Tissue equivalents based on cell-seeded biodegradable microfluidic constructs. Materials 2010; 3: 1833.
3. Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, Krieg T, Shannon JD, and Fox JW: Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res 2010; 9: 4758.
Clinical Problem Addressed
Cells and tissues are inherently dependent on the vasculature to supply critical nutrients and oxygen in exchange for metabolites. Impaired wound revascularization can impede healing, just as insufficient perfusion is a hallmark of chronic wounds. Several approaches including delivery of proangiogenic growth factors8 have been employed to enhance wound healing; however, current treatments remain technologically or biologically hampered.9 Thus, the challenge persists to create innovative and effective therapeutics for acute and chronic wound repair.
Basic Science Context
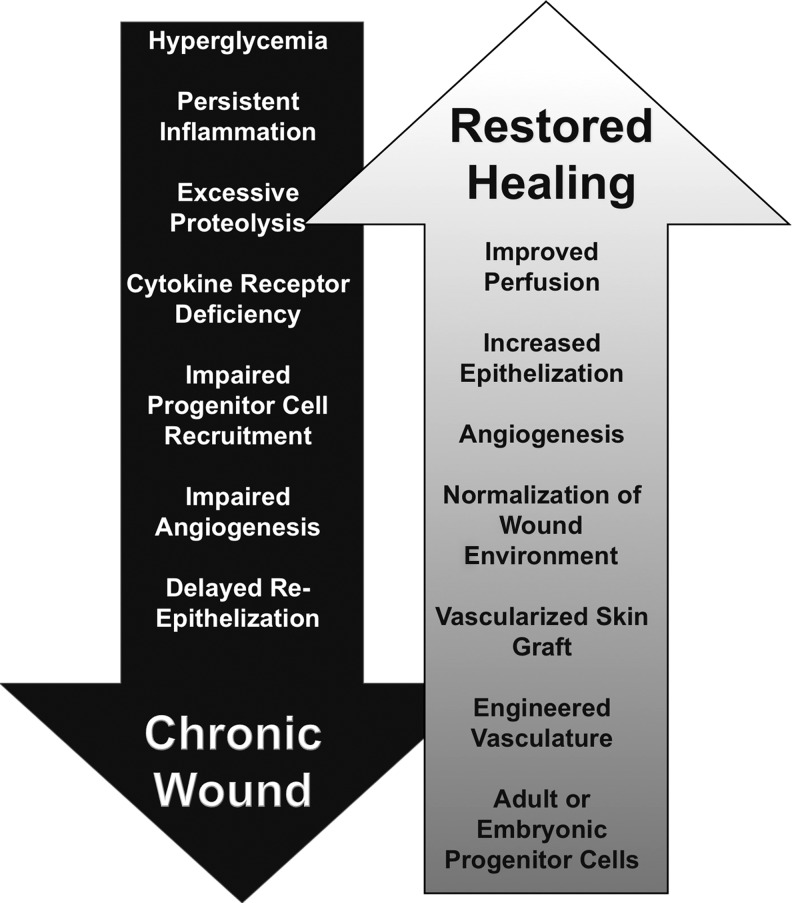
A growing body of evidence has advanced our understanding of the mechanisms leading to nonhealing, chronic wounds.10 These include, but are not limited to, reduced bioavailability of growth factors and receptors, abnormal production/modification of matrix proteins, diminished proliferative capacity of resident cells, and insufficient or impaired wound perfusion (Fig. 1). The latter stems from EC dysfunction as well as impaired recruitment of EPCs to the injury site.11 One method to circumvent this issue is the application of exogenous adult or fetal progenitor cells (FPCs) to chronic wound beds.12 Commonly expressing the EPC surface markers CD133, CD34, and VEGF receptor-2 (KDR), these cells can differentiate into both vascular endothelial and perivascular (mural) cells.2 In addition, CD133-positive cells generate proangiogenic soluble mediators, such as VEGF,2 and are able to promote revascularization following pathologic injury, including ischemic heart disease.13 Although fetal or adult endothelial progenitor cells may potentially offer benefits for chronic wound sufferers, further study will be needed to ensure safety and efficacy. Also, alternative strategies for wound revascularization are being investigated including the use of bioengineered vascularized scaffolds.6 Linked to this, successful wound revascularization will require a permissive microenvironment, which could be adjusted in a patient-specific manner based on wound conditions determined using modern proteomics or point-of-care diagnostics.7
FIG. 1.
Chronic wounds are often characterized by hyperglycemia, persistent inflammation, and growth factor and cytokine receptor deficiencies, which lead to impaired progenitor cell recruitment and angiogenesis and delayed epithelialization. Despite the progress achieved in the development of wound healing therapeutics, significant problems persist. Novel wound healing approaches that aim to include adult or embryonic progenitor cells or vascularized skin grafts seem promising, yet these methods will require further preclinical and clinical evaluation before normalization of the chronic wound microenvironment fosters the restoration of wound healing.
Experimental Model or Material—Advantages and Limitations
Treatment of ischemic wounds in mice using human FPCs
Isolating human fetal aortic progenitor cells represents a considerable challenge, technically and ethically,12,14 requiring rigorous cell selection, recovery, and propagation. For example, Barcelos and coauthors2,12 used EC-specific anti-CD31 to eliminate differentiated ECs and anti-CD133 antibody to enrich for progenitor cells. To evaluate the proangiogenic potential of these cells, a newly established mouse model of ischemic wound healing was used, which relies upon pharmacologic induction of diabetes, followed by ischemic insult. This model recapitulates both the hyperglycemia accompanying diabetes as well as the ischemia-reperfusion issues characteristic of chronic wounds. Therefore, this is an attractive model to test a variety of cellular and molecular-based healing modalities, including progenitor cells, under a more clinically relevant setting.
Creation of prevascularized microfluidic networks in biodegradable scaffolds
Microvascular networks with dimensions and features resembling the human dermal microvasculature are being constructed using biocompatible biomaterials.6 Such silk scaffolds can be seeded with micro- or macrovascular-derived ECs and perfused ex vivo. Moreover, these cellularized microfluidic networks can be layered using other dermal compartment cells or overlaid with epithelial cellular components. Clearly, further work will be necessary to ensure optimal host engraftment in vivo of such vascularized “organ equivalents.”
Analysis of protein profiles of acute and chronic wounds
Wound exudates were noninvasively collected from either acute or chronic (venous) wounds.7 Representative samples were subjected to protein identification analysis using mass spectrometry. The subsequently identified proteins, not previously linked to wound healing or its impairment, have been further characterized using immunohistochemistry. Challenges include both the intensive effort of data analysis as hundreds of proteins are identified in this manner, and a likely omission of potentially important yet underrepresented targets undetectable using mass spectrometry techniques.
Take-Home Message.
Basic science advances
• Human embryo–derived CD133+ progenitor cells promote both angiogenesis and wound healing in diabetic ischemic ulcers in mice.
• The wound healing effects of live human embryo–derived CD133+ progenitor cells can be replicated in the absence of cells by the use of cell-free media containing soluble factors, such as VEGF produced by CD133+ cells (conditioned media).
• Angiogenic stimulation is achieved via soluble factor–mediated increase in endothelial survival, proliferation, and migration.
• Nonimmunogenic silk-based channels can support survival and growth of microvascular ECs.
• The wound exudates derived from acute and chronic wounds have different protein composition.
• The molecules that were exclusively found in healing wounds include several types of collagens, thrombin, and heparan sulfate proteoglycan—all critical for wound revascularization.
Clinical science advances
• Proangiogenic embryo-derived CD133+ progenitor cells or the cell-conditioned media have robust wound healing potential in vivo.
• Microfluidic devices might provide a tool for revascularization of chronic wounds and stimulation of their healing.
Relevance to clinical care
• Noninvasive probing of wound microenvironment using modern proteomics together with CD133+ cells and vascularized skin grafts may provide useful tools for the personalized medicine.
Discussion of Findings and Relevant Literature
During normal wound healing, EPCs are effectively recruited to the remodeling microcirculation, thus leading to wound revascularization and timely healing. This response is likely to be dampened in diabetic ulceration. Indeed, it has been recently demonstrated that EPCs from normal but not diabetic patients contribute to postischemic revascularization.4 Diabetic EPCs are both lower in number5 and dysfunctional, displaying a shift toward a proinflammatory phenotype.15 Normal adult and/or FPCs (i) can differentiate into several cell types and (ii) have stimulatory effects on biological processes and are therefore likely to be beneficial for chronic wound patients and those with impaired perfusion. However, successful utilization of stem cells for chronic wound healing still needs further development and optimization. In a recent study, fetal CD133-positive cells (FPC133+), isolated from human fetal aortas and expanded as previously described,2 were used to stimulate ischemic wound healing in diabetic mice using a collagen-based delivery system.12 Both FPC133+ and FPC133+-conditioned media accelerate rates of wound closure, increase EC proliferation, and promote wound revascularization. Strikingly, a minimal number of vascular-associated FPC133+ are observed in wounds at 3 days postinjury/transplantation, yet the proangiogenic effects persisted for 7 days, suggesting an indirect (paracrine) mechanism that sustains FPC functionality postinjury. In fact, several soluble mediators, including VEGF, interleukin-6, interleukin-8, monocyte chemoattractant protein, and granulocyte-colony stimulating factor, have been validated as produced by FPC133+.12 This observation provides an important insight into the mechanisms of stem cell–mediated repair. Of note, CD133+ cells are found in several human cancers16; therefore, their delivery to wounds might be undesirable. On the other hand, proangiogenic cytokines generated by CD133+ cells provides a means to replace live progenitor cells with their conditioned media. Although cell-free preparations containing bioactive molecules demonstrate wound healing potential in animal models,12 a major obstacle to this therapy is the insufficient growth factor receptor density and responsiveness of cells residing within the wound bed, as is often the case among chronic wound sufferers.17 Alternative strategies include modification of patient-specific EPCs, themselves, through inhibition of certain cell signaling pathways to increase reparative function.18 All together, progenitor cell therapy and/or appropriate modifications could prove beneficial to promote a chronic healing phenotype to an acute response. Additional work is needed to delineate cell type, modification protocol, and mode of delivery.
Currently, no consensus exists regarding the safety and efficacy of exogenous/endogenous stem cell applications for wound healing. As an alternative, the delivery of biomaterial scaffolds to foster host-specific recovery has been suggested. For example, silk fibroin, prepared as previously described,6 is routinely used for adult progenitor cell growth and differentiation.19 Moreover, such biomaterials can be fabricated into complex designs that mimic vascular branching patterns in vivo. In turn, the silk-based scaffolds can be populated with competent differentiated ECs, with fluid flow as a patterning guide. Described by Borenstein and coauthors,6 biodegradable microfluidic constructs were seeded with human microvascular ECs, which remained viable and retained their cell surface markers for over a week. Additional modifications include coculturing with perivascular cells as well as inclusion of a keratinocyte cell layer to create tissue equivalents for implantation. Although the successful implantation of such microfluidic devices into a wound bed in vivo has not been yet described, beneficial effects on wound revascularization and healing are anticipated.
Successful wound revascularization is largely dependent on a permissive wound microenvironment. However, the identification and standardization of the molecular profiles of the individual wounds remains challenging. Recently, a concept of “wound bar coding” was proposed20 to provide a classification scheme based on gene expression patterning by resident or peripheral wound bed cells. The levels of gene activity can be assessed using modern RNA microarray technologies and presented in a “heat map” style, depicting both up- and downregulated genes. This strategy provides potent information, which may both guide wound debridement and lead to appropriate selection of treatment strategies based on individual patient needs (personalized medicine). However, several potential drawbacks include the requirement of multiple biopsies from numerous locations and the inability to assay for specific proteins within a wound. Therefore, a more conducive option might be the noninvasive probing of the wound environment from wound exudates as was reported by Eming and coauthors.7 This test not only enables repetitive monitoring of gene expression patterns but also determines protein composition of wound microenvironments and thus might provide powerful predictions of the actual conditions within the wound. A recent study of comparative proteomics of acute and chronic wounds using this approach7 has demonstrated that the nonhealing ulcers have decreased amount of several extracellular matrix components, namely, heparan sulfate proteoglycan and collagen type I, both of which are critical for normal wound revascularization.21,22 Moreover, this study confirms a previously established and significant increase in production of multiple proteases, which are detrimental for both wound extracellular matrix components and proangiogenic growth factors.23 In addition, it identifies species that are exclusively present in acute healing, such as the serine protease thrombin and the antimicrobial dermicidin. Finally, these authors report the identification of certain proteins, which have never been previously associated with wound healing, including the matrix molecule olfactomedin-4. Together, these results shed light on how the normalization of wound microenvironment in a patient-specific manner, which in turn could be directed by microarray20 and proteomic analysis,7 would culminate in the promotion of wound revascularization or healing.
Innovation
In recent decades, embryonic and adult endothelial progenitor cells have been proposed to have therapeutic potential. However, it remains to be shown whether these cells are applicable for treatment of ischemic diabetic wounds. The work of Barcelos and coauthors12 demonstrates that, in fact, FPC133+ have proangiogenic properties in this model and thus might be a useful tool for treatment of ischemic wounds in diabetic patients. Further, endothelialized microvascular networks based on degradable silk scaffolds6 may provide a valuable platform for future development of vascularized biomaterials. Finally, identification of protein components within wound microenvironments advances the field reach of the development of personalized approaches to wound care.
Caution, Clinical Remarks, and Recommendations
Although undifferentiated human-derived FPC133+ could be considered a helpful tool for chronic wound revascularization, significant risks to the patients, including risk of cancer development and infectious disease transmission, exist. Additional research remains to clarify progenitor cell biological processes and prevent undesirable effects of cell-based therapies.
• Progenitor cells can be differentiated in vitro and delivered into the wounds within biomaterial-based scaffolds, thus reducing the risk of undesirable cell differentiation in vivo.
• Combination of RNA- and protein-based diagnostic tools could be used to direct successful wound revascularization and healing.
Future Development of Interest
The use of adult and/or embryonic endothelial progenitor cells in combination with biocompatible artificial microvascular networks could provide a useful tool for revascularization and enhancement of wound healing. Optimized protocols designed to obtain and expand patient-compatible stem cells should be devised and would continue to add to the regimen of personalized medicine. Additional studies using novel animal models will determine the cellular and molecular mechanisms that underlie acute and chronic healing and provide a better understanding of the basic biology underlying stem cell application, which would demonstrate both the safety and efficacy of this treatment for the clinic. Insightful creation of therapeutic interventions as well as powerful predictive tools would positively affect the outcomes for managing and healing chronic wounds. Ultimately, these approaches should be based on personalized approaches to wound diagnosis and innovative treatment modalities in efforts to decrease the chronic wound burden of the population.
Abbreviations and Acronyms
- EC
endothelial cell
- EPC
endothelial progenitor cell
- FPC
fetal progenitor cells
- FPC133 +
CD133-positive fetal progenitor cells
- VEGF
vascular endothelial growth factor
Acknowledgements and Funding Sources
This work was funded by NIH grants (EY15125 and EY19533).
Author Disclosure and Ghostwriting
I.M. Herman is a consultant for Healthpoint, Inc.
References
- 1.Singer AJ. Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Invernici G. Emanueli C. Madeddu P. Cristini S. Gadau S. Benetti A, et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Caballero S. Sengupta N. Afzal A. Chang KH. Li Calzi S. Guberski DL, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner S. Hoellerl F. Schmid-Kubista KE. Zeiler F. Schernthaner G. Binder S, et al. Circulating angiopoietic cells and diabetic retinopathy in T2DM patients with and without macrovascular disease. Invest Ophthalmol Vis Sci. 2011;52:4655. doi: 10.1167/iovs.10-6520. [DOI] [PubMed] [Google Scholar]
- 6.Borenstein JT. Megley K. Wall K. Pritchard EM. Truong D. Kaplan DL, et al. Tissue equivalents based on cell-seeded biodegradable microfluidic constructs. Materials. 2010;3:1833. [Google Scholar]
- 7.Eming SA. Koch M. Krieger A. Brachvogel B. Kreft S. Bruckner-Tuderman L, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9:4758. doi: 10.1021/pr100456d. [DOI] [PubMed] [Google Scholar]
- 8.Leahy PJ. Lawrence WT. Biologic enhancement of wound healing. Clin Plast Surg. 2007;34:659. doi: 10.1016/j.cps.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Schultz GS. Davidson JM. Kirsner RS. Bornstein P. Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZJ. Velazquez OC. Hyperoxia, endothelial progenitor cell mobilization, and diabetic wound healing. Antioxid Redox Signal. 2008;10:1869. doi: 10.1089/ars.2008.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barcelos LS. Duplaa C. Krankel N. Graiani G. Invernici G. Katare R, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of wnt signaling. Circ Res. 2009;104:1095. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler DS. Lazarus H. Nair R. Goldberg JL. Greco NJ. Lassar T, et al. Safety and efficacy of bone marrow-derived autologous CD133+ stem cell therapy. Front Biosci (Elite Ed) 2011;3:506. doi: 10.2741/e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gucciardo L. Lories R. Ochsenbein-Kolble N. Done’ E. Zwijsen A. Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116:166. doi: 10.1111/j.1471-0528.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 15.Loomans CJ. van Haperen R. Duijs JM. Verseyden C. de Crom R. Leenen PJ, et al. Differentiation of bone marrow-derived endothelial progenitor cells is shifted into a proinflammatory phenotype by hyperglycemia. Mol Med. 2009;15:152. doi: 10.2119/molmed.2009.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monzani E. Facchetti F. Galmozzi E. Corsini E. Benetti A. Cavazzin C, et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan ST. Quattrini C. Jeziorska M. Malik RA. Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30:3058. doi: 10.2337/dc07-1421. [DOI] [PubMed] [Google Scholar]
- 18.Bhatwadekar AD. Guerin EP. Jarajapu YP. Caballero S. Sheridan C. Kent D, et al. Transient inhibition of transforming growth factor-beta1 in human diabetic CD34+ cells enhances vascular reparative functions. Diabetes. 2010;59:2010. doi: 10.2337/db10-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman GH. Diaz F. Jakuba C. Calabro T. Horan RL. Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 20.Brem H. Stojadinovic O. Diegelmann RF. Entero H. Lee B. Pastar I, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13:30. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringer SE. The role of heparan sulphate proteoglycans in angiogenesis. Biochem Soc Trans. 2006;34(Pt 3):451. doi: 10.1042/BST0340451. [DOI] [PubMed] [Google Scholar]
- 22.Dalton SJ. Whiting CV. Bailey JR. Mitchell DC. Tarlton JF. Mechanisms of chronic skin ulceration linking lactate, transforming growth factor-beta, vascular endothelial growth factor, collagen remodeling, collagen stability, and defective angiogenesis. J Invest Dermatol. 2007;127:958. doi: 10.1038/sj.jid.5700651. [DOI] [PubMed] [Google Scholar]
- 23.Lauer G. Sollberg S. Cole M. Flamme I. Sturzebecher J. Mann K, et al. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]