Abstract
Background
Complex skin defects resulting from acute skin trauma and chronic, nonhealing wounds are life-threatening injuries. Infection is one of the most common obstacles to the healing of these types of wounds. Host defense peptides (HDPs) possessing a broad spectrum of activity against microorganisms and serving as innate immune modulators have emerged as potential treatment strategies for infected wounds.
The Problem
The increase in multidrug-resistant clinical bacterial isolates highlights the need for new and innovative anti-infective therapies for the treatment of both acute and chronic skin wounds.
Basic/Clinical Science
To address the critical need for new therapeutic options to reduce infection and improve wound healing, a bioengineered skin substitute (BSS) tissue has been created to act as an anti-infective living human skin tissue that provides enhanced expression of the endogenous HDP, cathelicidin. To generate a BSS exhibiting these antimicrobial properties, the clinically tested NIKS progenitor cells were employed to provide a source of genetically uniform, nontumorigenic, pathogen-free human keratinocytes that are amenable to genetic engineering using nonviral means.
Clinical Care Relevance
Pathogenic bacterial strains are increasingly developing antibiotic resistance, thereby forcing the clinician to use potent antibiotics with deleterious effects on keratinocyte viability and migration. Therefore, an urgent need exists for new wound therapies that can circumvent many of the problems associated with current antibiotic treatments.
Conclusion
Enhanced expression of cathelicidin in a genetically engineered human BSS has been shown to inhibit the bacterial growth of a multidrug-resistant clinical strain of Acinetobacter baumannii in vivo, creating a new and innovative therapeutic option for combating these debilitating wound infections while also promoting healing.

B. Lynn Allen-Hoffmann
Background
Skin is an effective and essential barrier to toxins, irritants, and microorganisms. Damage to skin's barrier function can lead to dehydration, increased metabolic load, entry of toxic substances, immunosuppression, and wound infection. Normal skin is composed of three basic parts: an underlying matrix of fibroblasts, collagen, and other extracellular matrix glycoproteins that provide resiliency and elasticity (dermis); an upper layer of gradually desquamating keratinocytes that prevent water loss and exposure to foreign invaders (epidermis); and skin appendages. Squames are eventually rubbed off by friction, cleaning, and other minor trauma. Bacteria that attempt to invade through the skin are captured among these dead cells and are subsequently sloughed off. In addition, the innate immune response prevents invasion of microbial organisms if the outermost layer of the skin barrier is penetrated. Macrophages and neutrophils engulf invading microorganisms and produce reactive oxygen intermediates that kill microbial agents. Antimicrobial HDPs, naturally expressed and localized to the upper layers of the epidermis, are essential effectors in this innate immune response.
Target Article.
Thomas-Virnig CL, Centanni JM, Johnston CE, He LK, Schlosser SJ, Van Winkle KF, et al.: Inhibition of multidrug-resistant Acinetobacter baumannii by nonviral expression of hCAP-18 in a bioengineered human skin tissue. Mol Ther 2009; 17: 562.
Clinical Problem
Acute burn wounds affect an estimated 1.25 million Americans each year.1 Compounding this problem, the majority of patients with full-thickness or deep partial thickness wounds require autografting, resulting in painful donor site wounds that are susceptible to fluid loss and are at increased risk of infection. Chronic nonhealing wounds affect an additional 4.5 million people in the United States and 18 million people worldwide, with increased projections as the U.S. population ages.2 Left untreated, chronic wounds result in morbidity, discomfort, pain, and significant disability. These persistent wounds are prone to re-injury and infection that can result in life-threatening complications and amputations. Approximately 1.5 million diabetics in the United States are treated for skin ulcers annually, many of which become infected and result in over 80,000 amputations each year.2
Bioengineered skin substitutes (BSSs) are not currently approved for management of acute wounds. Current management of acute full-thickness wounds involves temporary coverage with freshly harvested or cryopreserved cadaver skin or impermeable dressings to protect from infection and dehydration to allow for wound bed vascularization. In the case of acute injuries, wound bed vascularization is critical for subsequent autologous skin graft take. This overall approach suffers from significant drawbacks, including limited availability of human cadaver skin, risk of transmission of pathogens to the patient from the cadaver grafts, and eventual graft rejection due in part to the presence of cadaveric vascular endothelial cells and immune cells.3
In contrast to acute wounds, the FDA has approved BSSs to treat chronic wounds that are unresponsive to other treatment options. However, BSSs are contraindicated for treatment of infected chronic wounds. Overall, infection is one of the most common reasons for impaired wound healing.4 Infected nonhealing cutaneous wounds are often seen in chronic wounds such as diabetic ulcers and lower extremity ulcers of vascular etiology as well as large surgical wounds, especially in trauma patients. Multidrug-resistant strains of bacteria have recently become a significant issue in the treatment of nonhealing cutaneous wounds. These nonresolved infections result in the inability to achieve wound closure with continued destruction of the underlying tissue due to the potent exotoxins that many bacteria secrete.5,6 Healing is impeded until the infective level can be reduced to below 105 organisms/g of tissue.4
Relevant Basic Science
Host defense peptides (HDPs) display antimicrobial activity against many common pathogens and have been shown play important role in skin homeostasis and cutaneous wound healing.7,8 However, severe burns and chronic skin ulcers are often depleted of HDPs.7,9,10 The cationic and amphiphilic characteristics of HDPs enable these peptides to function antimicrobially by binding and inserting into the cell membrane of microbes. Although there is debate as to the exact antimicrobial mechanism that HDPs utilize, many models focus upon the disruption of the microbial target membrane by these cationic peptides.11 While many HDPs have been identified, the two most prominent types in human skin are the defensins and cathelicidins. To date, one cathelicidin, hCAP-18, has been found in humans. Because cathelicidin is an endogenous human peptide and viral vectors were not used to generate the hCAP-18-expressing BSS, therapeutic application is unlikely to elicit an antibody response. The full-length hCAP-18 is cleaved to release the cathelin domain from the LL-37-containing carboxy terminus.11 Mature LL-37 can be further processed to release peptides with enhanced antimicrobial activity.11 Cathelicidin possesses antimicrobial activity against a broad range of bacteria, fungi, and viruses, and is a critical component of innate defenses against wound infection by enhancing leukocyte recruitment and activation.12 Studies to amplify the expression of HDPs in keratinocytes have had successful antimicrobial outcomes; however, transition of these approaches to clinical use is precluded by their use of karyotypically unstable cell lines or primary cells with limited replication potential.13–16 Many of these studies utilize the HaCaT cell line, which is chromosomally unstable, and exhibits an abnormal karyotype both numerically and structurally. In addition, the HaCaT cells were isolated from the periphery of a malignant melanoma. Although they have never been shown to be tumorigenic in immunocompromised mice, they do exhibit a transformed phenotype in a soft agar assay. In addition, most of these studies utilized viral transfection methods that are problematic due to preexisting immunogenicity toward adenoviruses in the population, and regulatory challenges in adopting viral approaches for clinical use. Gene therapy approaches in rats using viral transfer of hCAP-18 into infected burn wounds,17 or by ex vivo transfer of LL-37 into free skin flaps,18 have also proven successful. These approaches were able to reduce bacterial burden of Pseudomonus aeruginosa or Staphlococcus aureus in their respective studies, further establishing the efficacy of cathelicidin to combat infection in vivo.
Experimental Model: Advantages and Limitations
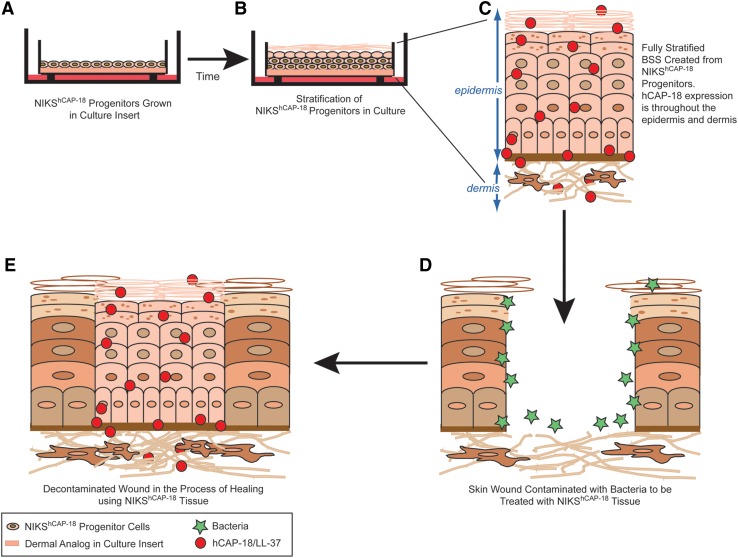
Organotypic culture of human keratinocytes has enabled development of BSSs for treatment of cutaneous wounds.3,19 These therapeutics reproduce many of the structural and biological features of intact human skin and are FDA approved to treat chronic wounds although none have been approved for management of severe burns or other large skin trauma. In addition, the primary human cell strains used to create these skin substitutes must be continually sourced from different human tissue donors. The NIKS human keratinocyte cell line is ideally suited for cell therapy and tissue engineering applications because they provide an abundant and consistent source of nontumorigenic, pathogen-free, genetically uniform human epidermal progenitor cells.20 These unique cells possess the capacity for self-renewal and are long-lived in culture, but undergo normal epidermal differentiation to generate a fully stratified interfollicular epithelium (Fig. 1). It is this self-renewal capacity that enables stable transfection of NIKS keratinocytes with nonviral vectors and selection of clonally pure populations of stably transfected cells.21
Figure 1.
A schematic of the production and usage of a NIKShCAP-18-based BSS. (A) NIKS progenitor cells, genetically modified to produce the host defense peptide, hCAP-18, are plated and cultured in an insert. The cells are fed on the outside of the insert with media (dark pink). (B) After lifting the cells in the insert so they are in contact with the air, signals are produced for the cells to differentiate into a fully stratified skin tissue. This process takes ∼14 days to produce mature tissue. (C) Enlarged schematic of BSS grown in the insert. The BSS produces hCAP-18 (red circles) throughout the epidermis and hCAP-18 is secreted into the dermis. (D) Wounded native skin that has lost all layers of the epidermis. Bacteria (green stars) have contaminated the wound bed. Placement of the fully stratified BSS can occur on top of this open wound bed. (E) Grafted wound is decontaminated of bacteria by the NIKShCAP-18 tissue and is in the process of healing.
Potential limitations to use of a BSS-expressing cathelicidin include the allogeneic nature of the human cells used in its creation and the possibility of enhanced immunomodulatory as well as chemotactic activity due to increased cathelicidin levels.22,23 However, clinical findings with BSS therapies suggest that cultured skin tissue is unlikely to be acutely rejected in humans.24–28 Skin substitutes are gradually replaced by the patient's skin with no excessive inflammation or other signs of acute rejection.25–28 Finally, a limitation of all cell-based BSS therapies is the high cost of manufacturing. However, this may be counterbalanced by an improved wound healing timeline, which would result in shorter patient hospitalization.
Take-Home Message.
Basic science advances
A consistent, pathogen-free source of keratinocytes (NIKS) was isolated that can recapitulate three dimensional skin tissue.
Nonviral genetic engineering enables cell-based therapies to target specific wound pathophysiologies.
The use of HDPs has emerged as an exciting new therapeutic approach to enhance innate host defense mechanisms against invading microorganisms.
NIKS tissue engineered to produce cathelicidin significantly reduced the growth of a multidrug-resistant isolate of A. baumannii in an in vivo infected burn wound by 99%, to subinfective levels (<105/g tissue).
Clinical science advances
Full-thickness skin substitutes containing viable allogeneic human fibroblast and keratinocyte cells isolated from donated human infant foreskin have been successfully marketed. However, these therapeutics are contraindicated for use in infected wounds.
A Phase I/IIa clinical trial to assess safety of a full-thickness skin substitute tissue produced using NIKS keratinocytes in the temporary management of traumatic skin wounds was successfully completed. No therapy-related adverse events occurred or acute immune responses to NIKS cells were found in these patients.
Topical treatment of HDPs has been found to be relatively safe.
Relevance to clinical care
New cell-based therapies that specifically target infection may provide a new and innovative way to promote wound healing.
Systemic and topical antibiotics are the standard of care for the treatment of wound infections; however, bacterial clinical isolates are becoming multidrug resistance at an alarming rate.29 Concern over multidrug-resistant organisms has escalated as many are now resistant to most commercially available antibiotics. To combat these multidrug-resistant pathogens, potent antibiotics with undesirable side effects have become the last resort for physicians. The human body's natural antimicrobials are the HDPs. It is difficult for microbes to acquire resistance to the highly negatively charged HDP peptides that kill microbes by interacting with their membranes.30,31 In order to gain resistance, bacteria must alter the composition of their membranes. There are reports of reduced susceptibility to HDPs due to enhanced bacterial proteolysis and increased expression of proteins that promote bacterial colonization and dissemination of infection, although bacteria that have become partially resistant to HDPs display reduced virulence or are inhibited in their growth.32,33 The use of a genetically engineered BSS could alleviate many of these concerns by providing a topical continuous supply of HDPs directly to the wound site, thereby reducing the need for powerful systemic antibiotics.
Discussion of Findings
Recently, the NIKS keratinocytes were used to produce the first genetically modified human cell-based therapy with nonviral vectors (Fig. 1).21 The genetically modified NIKS (NIKShCAP-18) was constructed with the human CAMP cDNA encoding the full-length hCAP-18 protein. Expression was targeted to the epidermis by an epithelial-specific human promoter, thereby providing a continuous topical supply of the cathelicidin HDP (hCAP-18/LL-37) to cutaneous wounds. NIKShCAP-18 tissue may fulfill a critically unmet medical need for the treatment of nonhealing skin wounds as evidenced by the tissue's (1) continuous tissue-specific synthesis of cathelicidin that is processed by skin cells into active forms; (2) comparable tissue architecture to native skin; (3) favorable safety assessment; and (4) proven in vitro and in vivo antimicrobial efficacy.
A continuous supply of cathelicidin and its mature cleavage products
The production of cathelicidin RNA and protein by NIKShCAP-18 tissue was assessed using quantitative polymerase chain reaction, immunoblotting, enzyme-linked immunosorbant assay, immunofluorescence, and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF/TOF) mass spectrometry. The assessment revealed that the NIKShCAP-18 clone produced ∼9,000-fold more full-length cathelicidin mRNA and expressed > 100-fold more hCAP-18/LL-37 protein compared to unmodified tissue. Immunoblot analysis confirmed that these tissues contained higher levels of both tissue-associated and secreted hCAP-18 and LL-37 when compared to unmodified tissues. Thus, the protein secretion supports the therapeutic use of NIKShCAP-18 tissue in providing an antimicrobial barrier and in reducing infection in the wound bed. In addition, MALDI-TOF/TOF mass spectrometry revealed the presence of the processed LL-37 peptide in the NIKShCAP-18 tissue lysates.
For the protein to have therapeutic use, the full-length cathelicidin preproprotein (hCAP-18) needs to be proteolytically processed intra- and extracellularly for the active LL-37 peptide to be released. Two proteases (proteinase-3 and kallikreins) are known to process hCAP-18 to its mature forms in skin.11 Immunoblot analyses verified that hCAP-18 produced by the NIKShCAP-18 tissue was cleaved in the presence of proteinase-3 in a dose-dependent manner, indicating that processing to the active peptides is possible given the presence of enzymes found in wounds.
NIKShCAP-18 cells produce a skin tissue containing all layers of human epidermis
Histologically, the NIKShCAP-18 tissue was indistinguishable from unmodified NIKS tissue and possessed all epidermal layers found in native human epidermis. Indirect immunofluorescence analysis using an antibody that detects hCAP-18/LL-37 revealed that a low level of hCAP-18/LL-37 was present throughout the epidermis of the unmodified NIKS skin tissue. In contrast, strong staining of hCAP-18/LL-37 was evident in both the dermis and epidermis of the NIKShCAP-18 skin tissue. Thus, the elevated levels of hCAP-18/LL-37 produced by the tissue have the potential to diffuse into a treated wound bed through the dermal compartment.
Safety assessments
An important criterion of any cells targeted for therapeutic use is that they must not be tumorigenic. Tumorigenicity studies on the NIKShCAP-18 cells have shown that they do not exhibit anchorage-independent growth in vitro. As confirmation of the anchorage-independent growth data, an in vivo tumorigenicity study was also performed on the NIKShCAP-18 cells. No tumors were observed in this 12-week study in mice injected with the NIKShCAP-18 cells.
In vitro bioactivity assessment
The antimicrobial potential of the NIKShCAP-18 tissues was initially evaluated in vitro. Bioactivity of the NIKShCAP-18 tissue was measured in an antibacterial assay using tissue extracts that were incubated with Staphylococcus carnosus, a nonpathogenic bacterium used due to its susceptibility to antimicrobials. The bioactivity assay revealed an inhibition of bacterial growth by over 78% relative to unmodified NIKS tissue extracts.
In vivo bioactivity assessment
To further characterize the antimicrobial properties of NIKShCAP-18 tissue, a series of studies was performed in a well-characterized murine model of acute infected skin wounds caused by a scald injury. An inoculum of a multidrug-resistant clinical isolate of Acinetobacter baumannii was applied to the burn wound. The following day, eschar from the burn wound was removed and BSS from unmodified NIKS or NIKShCAP-18 was applied. About 72 h after treatment, the BSS was removed and the bacteria present in the wound bed were quantified. NIKShCAP-18 tissue significantly reduced the A. baumannii growth in the burn wound by 99% compared to unmodified NIKS (p < 0.05).
Innovation
The well-characterized growth properties, thorough testing history and amenability to genetic engineering, combined with their successful clinical evaluation, make NIKS cells an ideal source for translational research studies focused on innovative cell-based therapies. The unique approach utilizing therapeutic biologically active skin substitutes provides a constant supply of naturally produced, endogenously processed, and regulated antimicrobial peptides directly into the wound bed. This approach should be more efficacious than the use of synthetic or modified peptides. Topically administered antimicrobial peptides must be applied repeatedly at high concentrations to compensate for drug loss due to protein degradation and loss on wound dressings. Sustained synthesis and topical delivery of LL-37 by a tissue will eliminate the need for serial applications.
Caution, Critical Remarks, and Recommendations
Use of HDPs and their derivatives as innovative antimicrobial drugs has proven difficult because of the complex interactions of these cationic peptides with membranes and with each other.34,35 Traditional pharmaceutical approaches for developing commercially viable HDP drug candidates have encountered the following challenges: (1) high cost associated with synthesizing and/or purifying large quantities of HDPs, (2) conservation of the appropriate secondary structure and maintenance of biological activity in recombinant expression systems, and (3) suboptimal potency associated with topical application, re-application, and degradation. Despite these drawbacks, a small number of antimicrobial peptide trials remain active.36 Topical use of HDPs has proven to be relatively safe; however, very little research has been performed to elucidate if HDPs can be used systemically.31,35
Future Development of Interest
The strategy to generate a human BSS expressing enhanced levels of cathelicidin yielded very favorable results that support the clinical testing of this novel cell-based therapeutic approach for the treatment of nonhealing skin wounds. Based on the proof-of-concept studies, NIKShCAP-18 tissue is moving into clinical development.
We believe that the treatment of complex skin defects will evolve to a multi-faceted approach. Bioengineered, cell-based therapies of the future will provide a dynamic response to the changing environment of the healing wound. In the near-term, NIKS tissue will be engineered to deliver enhanced levels of not only HDPs but also other wound healing factors. Next-generation skin-substitute tissues may be programmed to react specifically to endogeneous signals within the wound bed and coordinate the release of a host of defined wound healing factors or those that have yet to be investigated or identified. The future holds the possibility of advancing beyond simply closing and repairing severe skin damage to stimulating the regeneration of tissue in and around the wound site.
Abbreviations and Acronyms
- BSS
bioengineered skin substitute
- HDP
host defense peptide
- MALDI TOF/TOF
matrix-assisted laser desorption/ionization time-of-flight
- NIKS
neonatal human keratinocyte cell line
Acknowledgments and Funding Sources
We thank Thomas Cleven, Sandy Schlosser, Barry Steiglitz, and Melissa Taylor for their editorial assistance with this chapter. We also thank Cathy Rasmussen for help with figure preparation. The work presented in this chapter was supported by the National Institutes of Health (NIH) SBIR Grant NIH/NIDDK (grant no. R44 DK069924).
Author Disclosures
All authors are employed by and/or have a financial interest in Stratatech Corporation.
References
- 1.Brigham PA. McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Crandall MA. Wound care markets: volume 1: skin ulcers. In: Heffner S, editor. Wound Care Markets. New York, NY: Kalorama Information; 2003. pp. 103–118. [Google Scholar]
- 3.Eisenbud D. Huang N. Luke S. Silberklang M. Skin substitutes and wound healing: current status and challenges. Wounds. 2004;16:2. [Google Scholar]
- 4.Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77:637. doi: 10.1016/s0039-6109(05)70572-7. [DOI] [PubMed] [Google Scholar]
- 5.Melstrom KA., Jr. Smith JW. Gamelli RL. Shankar R. New perspectives for a new century: implications of pathogen responses for the future of antimicrobial therapy. J Burn Care Res. 2006;27:251. doi: 10.1097/01.BCR.0000216291.68192.54. [DOI] [PubMed] [Google Scholar]
- 6.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 7.Heilborn JD. Nilsson MF. Kratz G. Weber G. Sorensen O. Borregaard N, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 8.Ong PY. Ohtake T. Brandt C. Strickland I. Boguniewicz M. Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 9.Milner SM. Ortega MR. Reduced antimicrobial peptide expression in human burn wounds. Burns. 1999;25:411. doi: 10.1016/s0305-4179(98)00192-2. [DOI] [PubMed] [Google Scholar]
- 10.Poindexter BJ. Bhat S. Buja LM. Bick RJ. Milner SM. Localization of antimicrobial peptides in normal and burned skin. Burns. 2006;32:402. doi: 10.1016/j.burns.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner J. Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 12.Steinstraesser L. Koehler T. Jacobsen F. Daigeler A. Goertz O. Langer S, et al. Host defense peptides in wound healing. Mol Med. 2008;14:528. doi: 10.2119/2008-00002.Steinstraesser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carretero M. Del Rio M. Garcia M. Escamez MJ. Mirones I. Rivas L, et al. A cutaneous gene therapy approach to treat infection through keratinocyte-targeted overexpression of antimicrobial peptides. FASEB J. 2004;18:1931. doi: 10.1096/fj.04-1515fje. [DOI] [PubMed] [Google Scholar]
- 14.Ito A. Takahashi T. Kawabe Y. Kamihira M. Human beta defensin-3 engineered keratinocyte sheets constructed by a magnetic force-based tissue engineering technique. J Biosci Bioeng. 2009;108:244. doi: 10.1016/j.jbiosc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Supp DM. Gene therapy to fight infection in skin transplants. Pharmacogenomics. 2007;8:483. doi: 10.2217/14622416.8.5.483. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y. Inokuchi S. Takazawa K. Umezawa K. Saito T. Kidokoro M, et al. Introduction of human beta-defensin-3 into cultured human keratinocytes and fibroblasts by infection of a recombinant adenovirus vector. Burns. 2011;37:109. doi: 10.1016/j.burns.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen F. Mittler D. Hirsch T. Gerhards A. Lehnhardt M. Voss B, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12:1494. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 18.Ghali S. Bhatt KA. Dempsey MP. Jones DM. Singh S. Aarabi S, et al. Treating chronic wound infections with genetically modified free flaps. Plast Reconstr Surg. 2009;123:1157. doi: 10.1097/PRS.0b013e31819f25a4. [DOI] [PubMed] [Google Scholar]
- 19.Parenteau NL. Bilbo P. Nolte CJ. Mason VS. Rosenberg M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology. 1992;9:163. doi: 10.1007/BF02521744. [DOI] [PubMed] [Google Scholar]
- 20.Allen-Hoffmann BL. Schlosser SJ. Ivarie CA. Sattler CA. Meisner LF. O'Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114:444. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas-Virnig CL. Centanni JM. Johnston CE. He LK. Schlosser SJ. Van Winkle KF, et al. Inhibition of multidrug-resistant Acinetobacter baumannii by nonviral expression of hCAP-18 in a bioengineered human skin tissue. Mol Ther. 2009;17:562. doi: 10.1038/mt.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Y. Chen Q. Schmidt AP. Anderson GM. Wang JM. Wooters J, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lande R. Gregorio J. Facchinetti V. Chatterjee B. Wang YH. Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 24.Centanni JM. Straseski JA. Wicks A. Hank JA. Rasmussen CA. Lokuta MA, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumaticwounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg. 2011;254:672. doi: 10.1097/SLA.0b013e318210f3bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curran MP. Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs. 2002;16:439. doi: 10.2165/00063030-200216060-00005. [DOI] [PubMed] [Google Scholar]
- 26.Trent JF. Kirsner RS. Tissue engineered skin: Apligraf, a bi-layered living skin equivalent. Int J Clin Pract. 1998;52:408. [PubMed] [Google Scholar]
- 27.Hu S. Kirsner RS. Falanga V. Phillips T. Eaglstein WH. Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006;14:427. doi: 10.1111/j.1743-6109.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips TJ. Manzoor J. Rojas A. Isaacs C. Carson P. Sabolinski M, et al. The longevity of a bilayered skin substitute after application to venous ulcers. Arch Dermatol. 2002;138:1079. doi: 10.1001/archderm.138.8.1079. [DOI] [PubMed] [Google Scholar]
- 29.Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2006;43(Suppl 2):S100. doi: 10.1086/504487. [DOI] [PubMed] [Google Scholar]
- 30.Schroder JM. Epithelial peptide antibiotics. Biochem Pharmacol. 1999;57:121. doi: 10.1016/s0006-2952(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 31.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 32.Gryllos I. Tran-Winkler HJ. Cheng MF. Chung H. Bolcome R., 3rd Lu W, et al. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proc Natl Acad Sci USA. 2008;105:16755. doi: 10.1073/pnas.0803815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hornef MW. Wick MJ. Rhen M. Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002;3:1033. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- 34.Koczulla AR. Bals R. Antimicrobial peptides: current status and therapeutic potential. Drugs. 2003;63:389. doi: 10.2165/00003495-200363040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Marr AK. Gooderham WJ. Hancock RE. Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol. 2006;6:468. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L. Falla TJ. Potential therapeutic application of host defense peptides. Methods Mol Biol. 2010;618:303. doi: 10.1007/978-1-60761-594-1_19. [DOI] [PubMed] [Google Scholar]