Abstract
Background
Mesenchymal stem cell (MSC) treatment of wounds results in accelerated wound closure, increased granulation tissue formation, and increased angiogenesis. These adult stem cells exert their therapeutic effects primarily by secreting soluble factors that regulate cellular responses to cutaneous injury.
The Problem
There is an urgent need for novel therapies for the treatment of wounds with delayed healing. Current treatment options for chronic nonhealing wounds and burns are limited and not always effective, despite significant advances in wound care including application of bioengineered skin equivalents and growth factors.
Basic/Clinical Science Advances
The three target articles advance the field by addressing critical gaps in knowledge about MSC function and mechanism during wound healing. The first target article provides the first evidence that MSCs regulate macrophage phenotype in wounds. The second target article demonstrates that diabetes mellitus impairs the ability of MSCs to promote wound healing and this can be rescued by a novel lipid mediator deficit in diabetic wounds. The final target article reports that the surgical technique of tissue expansion is enhanced by MSCs.
Clinical Care Relevance
MSC therapy suppresses inflammation in wounds and may be more effective when used in conjunction with other therapeutics that modulate the diabetic wound environment. It also shows potential as an adjunctive therapy for surgical tissue expansion.
Conclusion
MSCs represent promising emerging therapies for wounds with delayed healing such as chronic nonhealing wounds and burns.

Anne M. Hocking
Background
In the past decade, mesenchymal stem cells (MSCs) have emerged as a promising therapy for the treatment of wounds with delayed healing. Clinical trials are in progress by using MSCs for treatment of chronic nonhealing wounds.1 There is also interest in their therapeutic potential for accelerating thermal burn wound closure2 and for the treatment of severe radiation burns.3–4 MSC treatment of wounds results in accelerated wound closure, increased granulation tissue formation, and increased angiogenesis.5 Surprisingly, these positive outcomes are not due to MSCs differentiating to replace the damaged skin, instead it is now apparent that MSCs exert their therapeutic effects by secreting soluble factors that regulate cellular responses to cutaneous injury.5
Despite the excitement about MSC therapy for treatment of wounds, this field remains in its infancy with many challenges to be addressed before effective use in the clinic. Further investigation is required to define the interactions between MSC and the different cell types present in the wound. Such studies also need to identify the MSC-derived factors that are responsible for regulating the local cellular responses to injury. Also critical for optimal MSC therapy is an understanding of how MSCs are impacted by the wound environment. The three target articles selected for this chapter address these gaps in knowledge and highlight new directions for MSC treatment of wounds.
Target Articles.
1. Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, and Le AD: Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells 2010; 28: 1856.
2. Tian H, Lu Y, Shah SP, and Hong S: 14S, 21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem 2011; 286: 4443.
3. Yang M, Li Q, Sheng L, Li H, Weng R, and Zan T: Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Ann Surg 2011; 253: 202.
Clinical Problem Addressed
The development of MSC therapy targeting cutaneous wounds addresses an urgent need for novel therapies for the treatment of wounds with delayed healing. Current treatment options for chronic nonhealing wounds and burns are limited and not always effective, despite significant advances in wound care including application of bioengineered skin equivalents and growth factors. MSC therapy represents a new approach for accelerating wound closure. In addition, MSC therapy also shows potential as an adjunctive therapy for surgical techniques used for skin replacement after trauma, burn, or surgery.6
Relevant Basic Science Context
MSCs are multipotent cells with a fibroblast-like morphology. These adult stem cells were first isolated from bone marrow but are now known to be present in the stromal fraction of all adult tissues. Unfortunately, the MSC phenotype is difficult to define because of heterogeneity within a population and between populations. Both tissue source and lab-specific protocols for isolation, culture, and expansion contribute to the phenotype differences between populations. A consequence of this heterogeneity is that MSCs are being given many different names. They are commonly referred to as multipotent stromal cells, marrow stromal cells, mesenchymal stromal cells, and stromal progenitor cells. The predominant term in the recent literature is MSC, although it remains under debate whether they are true stem cells capable of self-renewal. In response to these challenges, The International Society for Cellular Therapy published two position statements clarifying nomenclature and establishing minimal criteria for defining MSC phenotype.7,8 The three criteria for defining human MSCs are (1) adherence to tissue culture plastic; (2) greater than 95% of the population positive for the cell surface markers CD73, CD90, and CD105 and greater than 98% of the population negative for CD11b, CD14, CD19, CD34, CD45, CD79a, and HLADR surface molecules; and (3) the ability of differentiate into osteoblasts, adipocytes, and chondroblasts under standard in vitro differentiating conditions.8
There is great interest in the therapeutic potential of MSCs because of their immunosuppressive properties and their ability to promote repair and regeneration of damaged tissue. MSCs have shown beneficial effects for the treatment of immune disorders such as diabetes mellitus, Crohn's disease, and graft versus host disease.9 MSC treatment also reduces tissue damage after injury in the heart, lung, kidney, liver, brain, and skin.9 MSCs exert these therapeutic effects primarily by secreting factors that regulate local and systemic responses to injury. MSC secretion of cytokines and growth factors promotes angiogenesis and reduces cell death, inflammation, and scar formation at the site of injury.9 MSCs also release immunosuppressive factors that inhibit proliferation of immune cells such as T-cells, B-cells and natural killer cells.10 In the context of wound repair, administration of exogenous MSCs results in accelerated wound closure with increased granulation tissue formation and increased angiogenesis.5 Further study suggests that MSCs elicit these effects by secreting factors that regulate responses to injury by macrophages, keratinocytes, dermal fibroblasts, and endothelial cells, which are the major cell types present in the wound.5
Experimental Model or Material: Advantages and Limitations
MSCs have a number of advantages for cell-based therapy targeting wounds with delayed healing. They are immunosuppressive and appear to lack significant immunogenicity, both being important properties for minimizing the risk of rejection. MSC isolation and expansion is relatively easy, which is an advantage for MSC therapy development.11 In addition, MSCs are adult stem cells, so their use is not burdened with the ethical issues associated with human embryonic stem cells. MSC engraftment defined by incorporation and persistence in the wound is a major limitation for MSC therapy targeting wounds. In non-wound injury models, engraftment appears to be affected by the protocol used for delivery of MSCs to the site of injury. From these studies, it is clear that timing of delivery, number of cells delivered, and the delivery site all impact the efficiency of MSC engraftment.9 Donor immunogenicity and the local wound environment may also contribute to engraftment efficiency. MSC therapy for wounds is also limited by the modest rate of MSC differentiation in the wound, although this is only a limitation for MSC therapy aimed at regeneration instead of scar formation in response to cutaneous injury. Lastly, there is an important caveat for the translation of MSC animal studies to human clinical trials, because there are key differences between human and mouse MSCs. For example, recent work demonstrates that human and mouse MSCs release different mediators of immunosuppression; human MSCs use indoleamine 2,3-dioxygenase, whereas mouse MSCs use nitric oxide.12
Discussion of Findings and Relevant Literature
Although there is evidence that MSCs recruit macrophages to wounds,13 little is known about the interaction of these two cell types during wound repair. Zhang et al. address this gap in knowledge and report that MSCs regulate macrophage phenotype in acute wounds.14 In vitro experiments for this study demonstrated that macrophages cocultured with human gingival-derived MSCs acquired an anti-inflammatory M2 phenotype. These macrophages had increased phagocytic activity and increased secretion of anti-inflammatory cytokines while reducing production of pro-inflammatory cytokines. Importantly, the macrophages cocultured with MSCs increased the expression of an accepted marker of M2 macrophages. These data using gingival-derived MSCs are consistent with recent studies using MSCs isolated from bone marrow.15–17 In addition, Zhang et al. found that neutralizing either interleukin-6 (IL-6) or granulocyte macrophage colony stimulating factor (GM-CSF) activity in the coculture medium inhibited MSC induction of the M2 phenotype, which suggests that MSCs' secretion of these specific cytokines is critical for reprogramming the macrophage phenotype.
Zhang et al. also provide important evidence that MSCs regulate macrophage phenotype in cutaneous wounds. In these in vivo experiments, human gingival-derived MSCs were systemically injected into mice via the tail vein at 1 day after generating a full-thickness excisional wound. Consistent with previous reports,5 MSCs homed to the site of cutaneous injury and promoted wound healing with accelerated wound closure, increased granulation tissue formation, and increased angiogenesis. Importantly, quantification of the number of M2 macrophages in the wounds confirmed the in vitro data: more M2 macrophages were present in MSC-treated wounds compared with controls. MSC-treated wounds also showed suppression of the inflammatory response with significant reductions in neutrophil infiltration and pro-inflammatory cytokine production. Conversely, the anti-inflammatory cytokine IL-10 was increased in MSC-treated wounds. Collectively, these data suggest that the accelerated wound closure, granulation tissue formation, and angiogenesis observed in MSC-treated wounds may result from MSC induction of the M2 macrophage phenotype. This is based on previous work reporting that the M2 macrophage phenotype in cutaneous wounds is associated with the resolution of the inflammatory phase and its replacement with angiogenesis and tissue remodeling.18 Lastly, Zhang et al. also provide data that suggest that the accelerated closure of MSC-treated wounds may be due to reduced neutrophil levels, because wound closure is accelerated in neutrophil-depleted mice.19
In the second target article, Tian et al. investigate the effects of diabetes mellitus on MSC function.20 Such studies are essential for the development of MSC therapy targeting diabetic complications such as chronic nonhealing wounds. To my knowledge, Tian et al. provide the first evidence that diabetes directly impairs the ability of bone marrow-derived MSCs to promote wound healing. Compared with MSCs isolated from nondiabetic mice, MSCs from diabetic mice were significantly less effective at accelerating epithelialization, increasing granulation tissue formation, and increasing angiogenesis in healing diabetic wounds. In addition, in vitro experiments showed that diabetic MSCs are also less effective at inducing dermal microvascular endothelial cell chemotaxis and endothelial tube formation.
Tian et al. also report that the diabetic wound environment has a direct impact on MSC function.20 They demonstrate that diabetes inhibits formation of a novel lipid mediator, 14S,21R-dihydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S,21R-diHDHA), in cutaneous wounds.20 Previous work by the authors reports that 14S,21R-diHDHA is derived from the omega-3 fatty acid docosahexaenoic acid and its formation in nondiabetic mice is induced by wounding.21 Interestingly, 14S,21R-diHDHA treatment of wounds accelerated wound closure and increased both granulation tissue formation and angiogenesis in both nondiabetic and diabetic mice.20,21 Importantly, the present study found that restoring 14S,21R-diHDHA levels in the diabetic wound has a positive impact on MSC therapy for cutaneous wounds.20 Efficacy of both diabetic and nondiabetic MSCs was significantly improved when administered to diabetic wounds in combination with 14S,21R-diHDHA. Indeed, 14S,21R-diHDHA appears to rescue the impaired function of diabetic MSCs. Further investigation demonstrated that 14S,21R-diHDHA activated p38 signaling in wounds and MSCs.20
The final target article by Yang et al. describes the application of MSCs to tissue expansion, a procedure routinely used to grow extra skin for reconstructive surgery.6 In a rat model of tissue expansion, bone marrow-derived MSCs were injected into the expanded skin flap. The transplanted MSCs increased the inflation volume and shortened the time to achieve the intended inflation volume (27 vs. 42 days for the controls). Further analyses of the expanded flap indicated that MSCs promoted skin regeneration. Administration of MSCs increased epidermal thickness, cell proliferation, angiogenesis, and collagen synthesis and also appeared to prevent thinning of the skin, an undesired outcome of repeated tissue expansion. MSC transplantation also resulted in increased synthesis of the growth factors epidermal growth factor (EGF) and vascular endothelial growth factor in the expanded flap. Specific silencing of EGF expression in MSCs using RNA interference attenuated the enhanced tissue expansion. These experiments suggest that MSC secretion of EGF is critical for the beneficial effects of MSCs on tissue expansion.
Innovation
The three target articles advance the field by addressing critical gaps in knowledge about MSC function and mechanism during wound healing. Zhang et al. provide the first evidence that MSCs convert macrophages into the anti-inflammatory M2 phenotype in cutaneous wounds.14 This study is also the first to suggest that MSC reprogramming of macrophage phenotype is mediated by the cytokines, IL-6 and GM-CSF. Tian et al. advance the field by clearly demonstrating that diabetes impairs the ability of MSCs to promote wound healing.20 Interestingly, altering the diabetic wound environment by rescuing a lipid deficit significantly improved the efficacy of both nondiabetic and diabetic MSCs. This novel study has direct implications for the use of autologous MSCs for treatment of chronic nonhealing wounds in patients with diabetes. It also suggests that controlling the metabolic environment of diabetic wounds may be necessary for optimal MSC therapy. The final study by Yang et al. describes a new application for MSC therapy targeting wounds.6 It demonstrates that MSCs are beneficial for the tissue expansion technique used for reconstructive surgery. MSC transplantation had a positive impact on both the amount of skin expansion and the time to achieve the desired expansion.
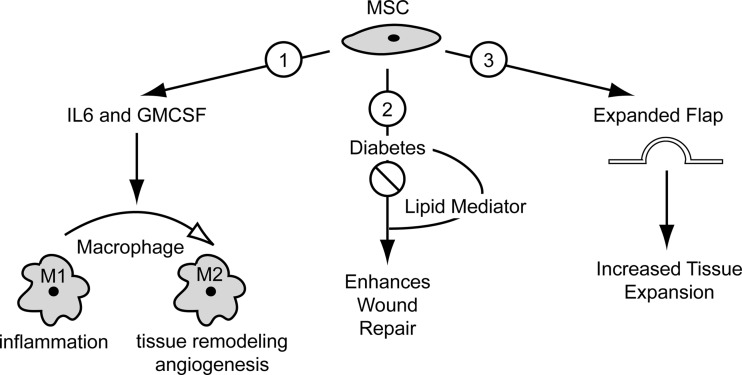
Summary Illustration
The summary illustration schematically depicts the take-home message of each target article. Target article 1 shows that MSC secretion of the cytokines, IL-6 and GM-CSF, converts macrophages to an M2 phenotype. In contrast to M1 macrophages, which are abundant in the inflammatory response to injury, M2 macrophages are the predominant macrophage during angiogenesis and tissue remodeling. Target article 2 shows that diabetes reduces the efficacy of MSCs in enhancing wound repair. Interestingly, MSC efficacy was rescued when administered to diabetic wounds in combination with a novel lipid mediator, 14S,21R-diHDHA. Lastly, target article 3 shows that injection of exogenous MSCs into an expanded skin flap increases the amount of skin expansion while shortening the time to achieve expansion, which indicates potential as an adjunctive therapy for surgical techniques such as tissue expansion used for skin replacement after trauma, burn, or surgery.
Caution, Critical Remarks, and Recommendations
Although MSCs have shown promise for treatment of wounds, much work needs to be done before they can be therapeutically used in the wound care clinic. A number of outstanding questions remain about MSC homing, engraftment, function, mechanism, and therapy optimization.5,9 Understanding MSC homing to wounds is directly relevant to successful engraftment. Determining whether MSC secretion of cytokines and growth factors is temporally regulated during wound repair will provide insight into whether MSC supernatant is as effective as living MSCs in promoting wound repair. It is also important to understand the impact of the wound environment and any underlying disease environment on the efficacy of MSC therapy. Monitoring MSC fate in vivo is also essential, because MSC differentiation into specific skin phenotypes is likely to alter their therapeutic effects.5 Addressing these questions is essential for safe and effective MSC therapy targeting wounds with delayed healing. This increased knowledge may also contribute to the development of a new generation of MSC therapy designed to promote wound repair without scar formation.
Take-Home Message.
Basic science advances
• MSCs induce macrophage conversion into the anti-inflammatory M2 phenotype and attenuate the inflammatory response to injury in acute wounds. Administration of exogenous MSCs resulted in a significant increase in the number of M2 macrophages in the wound and a significant reduction in both neutrophil infiltration and pro-inflammatory cytokine production. In vitro experiments suggest that MSC induction of M2 macrophages is mediated by the cytokines IL-6 and GM-CSF.
• Diabetes impairs the efficacy of MSCs in promoting wound healing. MSCs from diabetic mice were significantly less effective than nondiabetic MSCs at accelerating epithelialization, increasing granulation tissue formation, and increasing angiogenesis in diabetic wounds.
• The diabetic wound environment has a direct impact on MSC function. Diabetes inhibits formation of a novel lipid mediator, 14S,21R-diHDHA, in cutaneous wounds. Restoring 14S,21R-diHDHA levels in the diabetic wound significantly improved the efficacy of both diabetic and nondiabetic MSCs in enhancing wound repair.
• MSC therapy is beneficial for the surgical technique of tissue expansion. MSCs increase skin growth and reduce the time needed to achieve the desired amount of extra skin. These beneficial effects on tissue expansion appear to be mediated by MSC secretion of EGF.
Clinical science advances
• MSC therapy shows potential as an adjunctive therapy for surgical techniques used for skin replacement after trauma, burn, or surgery. Application of MSC therapy to the tissue expansion technique increases skin expansion and reduces the time needed to acquire the extra skin. It also appears to prevent thinning of the new skin, an undesired outcome of repeated tissue expansion.
Relevance to clinical care
• MSCs represent promising emerging therapies for wounds with delayed healing such as chronic nonhealing wounds and burns.
• The immunomodulatory properties of MSCs such as their effects on macrophage phenotype may be critical for the treatment of wounds with persistent or excessive inflammation.
• Understanding the effect of diabetes mellitus on MSC function is essential for effective autologous and nonautologous MSC therapy.
• MSC therapy may be more effective when used in conjunction with other therapeutic interventions that modify the diabetic wound environment. For example, a diet rich in omega-3 fatty acids may be beneficial for MSC therapy for chronic nonhealing wounds due to diabetes.
• The application of MSC therapy to tissue expansion shows promise, particularly in overcoming the challenges associated with repeated expansion for replacement of large areas of skin damage.
Future Development of Interest
Genetic engineering appears to be a future direction for MSC therapy. Engineering is being used to improve MSC homing to site of tissue damage in addition to promoting MSC survival and engraftment at the site of injury.11 It is also being used to tailor the release of MSC factors that regulate tissue repair and regeneration.11
The three target articles advance our understanding of how MSC function in skin and wounds. Collectively, they underscore the need to understand mechanism and the impact of the wound environment. All three studies also forge new directions in the field. Future investigation is warranted to determine whether the effect of MSCs on macrophages is required for the positive outcomes on epithelialization, angiogenesis, and tissue remodeling. Future studies aimed at understanding how diabetes impairs MSC function in wounds are also justified. The tissue expansion study also provides a rationale for determining whether current medical and surgical interventions for wound care can be enhanced by MSC therapy. These new directions may significantly contribute to the translation of MSC therapy from the research laboratory to the wound care clinic.
Abbreviations and Acronyms
- 14S,21R-diHDHA
14S,21R-dihydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid
- EGF
epidermal growth factor
- GM-CSF
granulocyte macrophage colony stimulating factor
- IL
interleukin
- MSC
mesenchymal stem cell
Acknowledgments and Funding Sources
The author expresses gratitude to Lara Muffley for excellent assistance with the preparation of the summary illustration and to Jeffrey Bradley for critical comments on the chapter. The author's research on MSCs and wound healing has been supported by a grant (number: R01 GM073624) from the NIH National Institute of General Medicine and by a grant (number: P30 DK017047) from the NIH National Institute of Diabetes and Digestive and Kidney Diseases.
Author Disclosure and Ghost Writing
The author has no commercial associations that could lead to a conflict of interest. The author is solely responsible for writing the article and did not use ghostwriters.
References
- 1.U.S. National Institutes of Health Registry of Clinical Trials. http://clinicaltrials.gov http://clinicaltrials.gov
- 2.Butler KL. Goverman J. Ma H. Fischman A. Yu YM. Bilodeau M. Rad AM. Bonab AA. Tompkins RG. Fagan SP. Stem cells and burns: review and therapeutic implications. J Burn Care Res. 2010;31:874. doi: 10.1097/BCR.0b013e3181f9353a. [DOI] [PubMed] [Google Scholar]
- 3.Francois S. Bensidhoum M. Mouiseddine M. Mazurier C. Allenet B. Semont A. Frick J. Sache A. Bouchet S. Thierry D. Gourmelon P. Gorin N-C. Chapel A. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 4.Benderitter M. Gourmelon P. Bey E. Chapel A. Clairand I. Prat M. Lataillade JJ. New emerging concepts in the medical management of local radiation injury. Health Phys. 2010;98:851. doi: 10.1097/HP.0b013e3181c9f79a. [DOI] [PubMed] [Google Scholar]
- 5.Hocking AM. Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M. Li Q. Sheng L. Li H. Weng R. Zan T. Bone marrow-derived mesenchymal stem cells transplantation accelerates tissue expansion by promoting skin regeneration during expansion. Ann Surg. 2011;253:202. doi: 10.1097/SLA.0b013e3181f9ba1ah. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM. Le Blanc K. Dominici M. Mueller I. Slaper-Cortenbach I. Marini FC. Deans RJ. Krause DS. Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M. Le Blanc K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop DJ. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Ankrum J. Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med. 2010;16:203. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA. Goolaerts A. Howard JP. Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38(Suppl 10):S569. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkinson CP. Gomez JA. Mirotsou M. Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren G. Su J. Zhang L. Zhao X. Ling W. L'huillie A. Zhang J. Lu Y. Roberts AI. Ji W. Zhang H. Rabson AB. Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 13.Chen L. Tredget EE. Wu PY. Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang QZ. Su WR. Shi SH. Wilder-Smith P. Xiang AP. Wong A. Nguyen AL. Kwon CW. Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J. Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggini J. Mirkin G. Bognanni I. Holmberg J. Piazzón IM. Nepomnaschy I. Costa H. Cañones C. Raiden S. Vermeulen M. Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtaki H. Ylostalo JH. Foraker JE. Robinson AP. Reger RL. Shioda S. Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deonarine K. Panelli MC. Stashower ME. Jin P. Smith K. Slade HB. Norwood C. Wang E. Marincola FM. Stroncek DF. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovi JV. He LK. DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448. doi: 10.1189/jlb.0802406. [DOI] [PubMed] [Google Scholar]
- 20.Tian H. Lu Y. Shah SP. Hong S. 14S, 21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds. J Biol Chem. 2011;286:4443. doi: 10.1074/jbc.M110.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y. Tian H. Hong S. Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing. J Lipid Res. 2010;51:923. doi: 10.1194/jlr.M000059. [DOI] [PMC free article] [PubMed] [Google Scholar]