Abstract
Background
First described in 1994, fibrocytes are now appreciated to participate in different inflammatory and fibrogenic processes as well as in wound healing. Fibrocytes are unique in their expression of both myeloid and connective tissue products, which include a distinct cytokine and surface marker expression profile. Recent studies suggest their clinical utility as predictive biomarkers and as targets for therapeutic intervention.
The Problem
Fibrocytes are involved in physiological and beneficial processes such as wound repair. Their involvement in detrimental processes such as aberrant collagen deposition in different fibrosing diseases reveals the sensitive balance in which these bone marrow progenitors have to be maintained.
Basic/Clinical Science Advances
The enumeration of circulating fibrocytes and correlation with clinical severity of different fibrosing disorders is one of the most promising advantages in recent fibrocyte research. Besides their potential as a biomarker, fibrocytes may be therapeutically targeted by serum amyloid P, which inhibits their differentiation. In addition, recent murine studies supported the immunomodulatory potential of fibrocytes and demonstrated that these cells are controlled by CD4+ T cells.
Clinical Care Relevance
The potential prognostic utility of quantifying circulating fibrocytes in patients suffering from fibrotic diseases is one of the most promising aspects in possible clinical applications. In addition, controlling the number and differentiation of fibrocytes by therapeutic regulation of known differentiation by specific T-cell immunosuppression may open new avenues for the treatment of fibrosing diseases.
Conclusion
Further detailed understanding of fibrocyte biology and their regulation in different disorders is desirable to advance new therapies for the treatment of chronic fibrosing disorders such as interstitial lung disease and to promote wound repair.

Gerrit Grieb
Background
In 1994, fibrocytes were first described as fibroblast-like, peripheral blood cells that migrate into regions of tissue injury.1 Today, accumulated data indicate that these cells are involved in different fibrotic processes that have an inflammatory or autoimmune etiology, such as atherosclerosis, airway remodeling in asthma, interstitial pulmonary fibrosis, the stromal response to tumor invasion, wound healing, and hypertrophic scarring.2 Fibrocytes secrete a unique cytokine and chemokine profile that differs from monocytes, macrophages, T lymphocytes, dendritic cells, fibroblasts, and endothelial cells.3 They express different fibroblast proteins such as collagen I and III, fibronectin, vimentin, the hematopoietic stem cell marker (CD34), and the leukocyte common antigen (CD45).1 Further, fibrocytes have a distinct morphology and show small cytoplasmic extensions that are intermediate in size between pseudopodia and microvilli.1 Approximately 0.5% of leukocytes in the peripheral blood are fibrocytes; in culture, they can differentiate from CD14+ cells into a phenotype characterized by collagen expression. It was demonstrated that fibrocytes are able to migrate to cutaneous wounds in response to secondary lymphoid chemokines, which function as a ligand for chemokine receptor 7.4 Moreover, recent clinical data suggest the potential of enumerating circulating fibrocytes as a biomarker for disease progression in different fibrotic disorders such as asthma, pulmonary fibrosis, atherosclerosis, nephrogenic systemic fibrosis, and hypertrophic scarring.5,6 A greater understanding of the mediators that influence fibrocyte biology may offer new opportunities for therapeutic manipulation of fibrocytes in these fibrotic disorders.
Target Articles.
1. Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O'Byrne PM, Strieter RM, and Kolb M: Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009; 179: 588.
2. Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, Reilkoff RA, Bucala RJ, and Herzog EL: Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Investig 2010; 90: 812.
3. Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, and Mack M: CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA 2009; 106: 17892.
Clinical Problem Addressed
Fibrocytes are known to participate in tissue responses to invasion and injury. Different studies have demonstrated that these unique cells are involved in physiological processes (e.g., wound healing), but also in pathological collagen deposition, which can result in different fibrotic disorders.7 These include pulmonary fibrosis, nephrogenic systemic fibrosis, cardiac disease, liver fibrosis, renal fibrosis, and hypertrophic scarring.6 Recent data obtained from such clinical settings suggest that the enumeration of circulating fibrocytes may be a biomarker for disease progression and outcome.8
Relevant Basic Science Context
Fibrocytes secrete a combination of different chemokines, cytokines, and growth factors that create a milieu favorable for tissue repair.9 The initially expressed hematopoietic stem cell marker, CD34, expressed by fibrocytes, decreases over time during the wound healing process.10 This phenomenon appears together with the increased expression of prolyl-4-hydroxlase, which is an enzyme that is important for stabilization of collagen triple helices.11 CD45 also is downregulated as fibrocytes differentiate.12 This transition of fibrocyte phenotype also is observed in the context of increased expression of transforming growth factor beta (TGF-β),11 which plays an important role in normal tissue repair as well as fibrosis.13 TGF-β accelerates fibrocyte differentiation into cells that appear phenotypically similar to fibroblasts and myofibroblasts in vitro.4 However, increased levels of serum amyloid P (SAP) can inhibit lung fibrosis by downregulating profibrotic monocyte responses as well as fibrocyte differentiation without affecting the levels of TGF-β1.13 High levels of interleukin (IL)-1β, which occur as a consequence of tissue injury and monocyte/macrophage and stromal cell activation, induce fibrocytes to secrete tumor necrosis factor (TNF)-α, macrophage colony stimulating factor, IL-6, IL-10, macrophage inflammatory protein (MIP)-1α, and MIP-β.14 Fibrocytes also are potent antigen-presenting cells and are capable of initiating T-cell immunity; they express surface proteins required for antigen presentation, including class II major histocompatability complex molecules and the costimulatory molecules CD80 and CD86.10,15 Thus, fibrocytes exhibit a high antigen-presenting capability, which likely underlies their role in the chronic fibrotic responses associated with autoimmunity, such as in granulomas or in scleroderma.
Experimental Model And Material—Advantages and Limitations
The first study of fibrocytes in a wound repair model was conducted by Bucala et al.1 Wound chambers were surgically implanted into the subcutaneous tissue of mice and within 2 days the exudative fluid of the wound chambers revealed a fibroblast-like, CD34+/Col+ cell that was adherent and spindle shaped and therefore termed the “fibrocyte.”1 Subsequently, the isolation of fibrocytes was found to be achieved from a CD14+-enriched mononuclear cell population isolated from the buffy coat of the peripheral blood.4,8 This isolation procedure provides facile acquisition of fibrocytes for further experiments or analysis by fluorescent activated cell sorting.16 Niedermeier et al. isolated fibrocytes from a CD11b+ CD115+ Gr1+ monocyte subpopulation under the control of CD4+ T cells, which further supports the role of the adaptive immune response in modulating fibrocyte differentiation.7
For verification that fibrocytes do indeed develop in the bone marrow, Mori et al. used an in vivo model of sex-mismatched bone marrow chimera mice.12 These observations were confirmed by Fathke et al., who transplanted bone marrow from enhanced green fluorescent protein (EGFP) transgenic mice into wild-type mice. In the skin, diverse EGFP+ cells and bone marrow–derived cells showed strong expression for CD45 and demonstrated a typical fibrocyte phenotype.17
Discussion of Findings and Relevant Literature
Recent proceedings in fibrocyte research are characterized by two landmarks.
Enumeration of fibrocytes
Clinical management of idiopathic pulmonary fibrosis (IPF) remains complex and challenging until today. Still, no effective drug therapy exists for this disease. Fibrocytes as mesenchymal circulating progenitor tissue cells are involved in fibrosing disorders and might be potentially used as a biomarker for progression of fibrosis. Moeller et al. recently showed that these cells are an indicator for disease activity of IPF.5 For quantification, circulating cells expressing CD45 and collagen I were defined as fibrocytes and counted in patients with stable IPF and during the acute exacerbation of the disease. The number of fibrocytes was compared with different clinical parameters and with the survival rate. Patients with acute respiratory distress syndrome and healthy age-matched volunteers were used as control groups. In patients with IPF, the number of circulating fibrocytes was significantly elevated. This was even more apparent when comparing patients during acute exacerbation with healthy controls (p < 0.001).5 Patients suffering from acute respiratory distress syndrome did not differ from healthy controls or stable IPF patients with respect to the circulating number of fibrocytes. In addition, fibrocyte numbers were an independent predictor of early mortality (p < 0.001).5 Taken together, this study showed that fibrocytes might be a useful biomarker for IFP disease progression and for early mortality in IFP patients.
Fibrocytes also have been proposed to be involved in the pathogenesis of systemic sclerosis. An enumeration of circulating fibrocytes under these circumstances and comparison of these data to healthy control has not been performed until recently. A recent study by Mathai et al. demonstrated that in patients suffering from interstitial lung disease associated with systemic sclerosis, the number of circulating fibrocytes was significantly increased when compared with healthy controls.8 Cultured CD14+ monocytes from these patients also revealed a profibrotic phenotype characterized by expression of CD163 and enhanced secretion of CCL18 and IL-10 in response to inflammatory stimulation. In addition, plasma levels of IL-10, MCP-1, IL-1RA, and TNF-α were significantly elevated in these patients.8 This study further revealed that healthy aged subjects (>60 years) had higher numbers of circulating fibrocytes. Overall, these findings support a role for fibrocytes in the pathophysiology of the interstitial lung disease that develops as a consequence of systemic sclerosis and suggest a role for these cells in either tissue homeostasis or certain sequelae of normal aging.
Immunodifferentiation of fibrocytes
The differentiation of monocytes into fibrocytes is controlled by cells of the immune system, such as CD4+ T cells. The report that T cells might have a role in fibrocyte maintenance or differentiation has long been suspected by the observation that, during in vitro culture, T cells may persist for several days in close association and cell–cell contact with fibrocytes.4 Niedermeier et al. established that the development of murine fibrocytes from a CD11b+ CD155+ Gr1+ monocyte subpopulation is dependent on the control of CD4+ T cells.7 In the absence of CD4+ cells, the differentiation of fibrocytes was decreased in vitro and in vivo. In the presence of CD4+ cells, T-cell activation determined the development of fibrocytes. Activation of CD4+ T cells induced the release of IL-2, TNF, IFN-γ, and IL-4, which prevented the differentiation and outgrowth of fibrocytes. In a murine unilateral ureteral obstruction model, IL-2 and TNF also reduced the number of fibrocytes and the severity of fibrosis.7 Using a calcineurin inhibitor, activation of CD4+ T cells led to significantly increased outgrowth of fibrocytes and renal deposition of collagen I. In summary, it has been demonstrated that the differentiation of fibrocytes is critically dependent on CD4+ T cells and that the circumstances of T-cell activation may either support or block the development and differentiation of fibrocytes. These findings are noteworthy in light of the central role of adaptive T-cell responses in the fibrogenic sequela of autoimmunity and in the pathologic progression of interstitial lung disease, glomerulonephritis, and scleroderma. These data also suggest potential avenues for evaluating specific pharmacological interventions to ameliorate fibrosis.
Innovation
Besides the involvement of fibrocytes in wound healing and scar formation, which was introduced more than 15 years ago,11 these recent findings suggest additional innovation in fibrocyte research. The enumeration of circulating fibrocytes in patients suffering from different fibrotic diseases may serve as a biomarker for severity and progression of the disease. These findings could apply to lung diseases such as asthma or pulmonary fibrosis, liver fibrosis, and renal fibrosis.6 The fact that the differentiation of the circulating progenitor into mature fibrocytes may be critically governed by activated T cells also opens new perspectives for influencing and therapeutically inhibiting fibrocyte differentiation.7 This potential application could be clinically useful in the treatment of fibrosing disorders or in enhancing tissue repair and wound healing by the selective activation and differentiation of fibrocytes. Further innovations in fibrocyte research are improved serum-free culture conditions for differentiation of human and murine fibrocytes.18 In this context, fibrocyte isolation is possible via the differentiation of a CD14+ monocyte population, providing cell preparation procedures that are less complex and increase the number of isolated fibrocytes for in vitro experiments.18
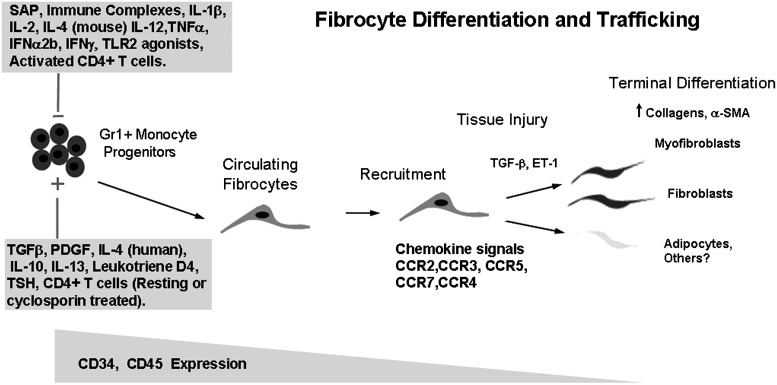
Summary Illustration
The figure shows regulation of fibrocyte differentiation. As monocytic progenitors, circulating fibrocytes are recruited via different chemokine signals to the site of injury. The differentiation of the circulating progenitors into mature cells is stimulated or decreased by various factors. In this context, CD4+ T cells have a prominent role in the control of fibrocyte differentiation.
Take-Home Message.
Basic science advances
• Fibrocytes are controlled by immune cells, for example, CD4+ T cells.
Clinical science advances
• Enumeration of circulating fibrocytes correlates with disease severity of fibrosing disorders. Fibrocytes play a prominent role in blocking TGF-β lung fibrosis by SAP.
Relevance to clinical care
• Fibrocytes could serve as a biomarker for fibrotic disease progression and outcome. Controlling fibrocytes opens potential avenues for treatment of fibrosing diseases.
Cautions, Critical Remarks, and Recommendations
Our knowledge of fibrocyte biology and involvement in wound healing and different fibrotic chronic diseases has substantially grown. These cells have been shown to secrete a unique profile of cytokines and to control different processes in wound healing and collagen deposition. In addition, the angiogenic capabilities of fibrocytes have been described. The exact processes governing how fibrocytes are mobilized from the bone marrow, how they exit the circulation, and how they further differentiate as local cells in the tissue need to be further elucidated. A better understanding of these mechanisms will allow further insight into the biology of these unique cells and their potential role as a biomarker and involvement in different fibrotic disorders.
Future Development of Interest
Recent data obtained from clinical settings suggest that the enumeration of circulating fibrocytes may be a biomarker for disease progression and outcome. This would be of great value for the prognosis and outcome of different chronic fibrotic diseases. There is currently no effective therapy for fibrosing diseases and a better understanding of fibrocytes and their regulatory mechanisms could lead to the pharmacologic manipulation of these cells to ameliorate or reverse the fibrotic, tissue remodeling responses that occur in different disease states. In addition, in vitro expansion of fibrocytes and re-administration into the patient could be of therapeutic potential, for instance, in retarded or disturbed wound healing. The delicate balance in the regulatory pathways that control appropriate wound repair versus pathologic fibrosing disorders must be further elucidated to achieve optimal therapeutic benefit and clinical outcome.
Abbreviations and Acronyms
- α-SMA
alpha smooth muscle actin
- CCR
chemokine receptor
- CXCR
CXC chemokine receptor
- COL
collagen
- EGFP
enhanced green fluorescent protein
- IFN
interferon
- IL
interleukin
- IPF
idiopathic pulmonary fibrosis
- MIP
macrophage inflammatory protein
- SAP
serum amyloid P
- TGF-β1
transforming growth factor beta 1
- TNF
tumor necrosis factor
Acknowledgements and Funding Sources
G. Grieb was supported by the “START” program of the RWTH Aachen University and by travel funds from the Deutsche Forschungsgemeinschaft (DFG; GR-3724/1-1). R. Bucala is supported by the NIH.
Author Disclosure and Ghostwriting
R. Bucala is on the Scientific Advisory Board of Promedior, Inc., which seeks to prevent pathologic fibrosis and scarring by administering serum amyloid P. This article was not written by any writer other than the authors.
References
- 1.Bucala R. Spiegel LA. Chesney J. Hogan M. Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71. [PMC free article] [PubMed] [Google Scholar]
- 2.Bucala R, editor. Fibrocytes: New Insights into Tissue Repair and Systemic Fribrosis. Hackensack, NJ: World Scientific; 2007. No. 18. [Google Scholar]
- 3.Pilling D. Fan T. Huang D. Kaul B. Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. 2009;4:7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe R. Donnelly SC. Peng T. Bucala R. Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 5.Moeller A. Gilpin SE. Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 6.Herzog EL. Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010;38:548. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedermeier M. Reich B. Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106:17892. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathai SK. Gulati M. Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Investig. 2010;90:812. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesney J. Bucala R. Peripheral blood fibrocytes: novel fibroblast-like cells that present antigen and mediate tissue repair. Biochem Soc Transac. 1997;25:520. doi: 10.1042/bst0250520. [DOI] [PubMed] [Google Scholar]
- 10.Blakaj A. Bucala R. The role of fibrocytes in wound healing. Adv Wound Care. 2009;1:266. [Google Scholar]
- 11.Aiba S. Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 12.Mori L. Bellini A. Stacey MA. Schmidt M. Mattoli S. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Murray LA. Qingsheng C. Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P. Int J Biochem Cell Biol. 2011;43:154. doi: 10.1016/j.biocel.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Chesney J. Metz C. Stavitsky AB. Bacher M. Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419. [PubMed] [Google Scholar]
- 15.Chesney J. Bacher M. Bender A. Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan TE. Bucala R. Culture and analysis of circulating fibrocytes. Methods Mol Med. 2007;135:423. doi: 10.1007/978-1-59745-401-8_28. [DOI] [PubMed] [Google Scholar]
- 17.Fathke C. Wilson L. Hutter J, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilling D. Vakil V. Gomer RH. Improved serum-free culture conditions for the differentiation of human and murine fibrocytes. J Immunol Methods. 2009;351:62. doi: 10.1016/j.jim.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]