Abstract
Background
Macrophages (Mφs) participate in wound healing by coordinating inflammatory and angiogenic processes. Mφs respond to environmental cues by adopting either “classically” activated (M1) proinflammatory or “alternatively” activated (M2a, M2b, M2c, M2d) wound healing phenotypes.
The Problem
Mφ polarization is essential for wound healing and aberrations in this process are linked to several pathologies. It is important to elucidate molecular mechanisms underlying Mφ polarization.
Basic/Clinical Science Advances
Mφs are categorized as proinflammatory (M1) or anti-inflammatory/wound healing (M2). M1 Mφs are observed in initial tissue damage responses, are induced by exogenous pathogen-associated molecular patterns or endogenous damage-associated molecular patterns, and exhibit increased phagocytosis and pro-inflammatory cytokine production, facilitating innate immunity and wound debridement. M2 Mφs predominate later in repair, express vascular endothelial growth factor, transforming growth factor beta, and interleukin 10 (IL-10), are activated by varied stimuli, assist in the resolution of inflammation, and promote tissue formation and remodeling. Recent work has characterized a novel “M2d” phenotype resulting from adenosine-dependent “switching” of M1 Mφs that exhibits a pattern of marker expression that is distinct from canonical IL-4/IL-13–dependent M2a Mφs. Recent studies have demonstrated important roles for specific transcriptional elements in M1 and M2a Mφ polarization, notably members of the interferon regulatory factor family interferon regulatory factor 5 (IRF5) and IRF4, respectively. The role of these IRFs in M2d polarization and wound healing remains to be determined.
Clinical Care Relevance
Knowledge of microenvironmental signals and molecular mechanisms that mediate Mφ polarization should permit their manipulation to regulate important physiological processes and resolve pathological conditions.
Conclusion
Proper Mφ polarization is essential to effective wound healing, and distinct phenotypes, such as the angiogenic M2d Mφ, may be of critical importance to this process. The IRF5 transcription factor has been shown to play a key role in M1 Mφ activation and the Jumonji domain containing-3-IRF4 pathway has been implicated in M2 Mφ activation.

Samuel Joseph Leibovich
Background
As immune effector cells, the role of macrophages (Mφs) in inflammation and host defense is well characterized. Additionally, Mφs are integral in the promotion of proper wound healing as well as the resolution of inflammation in response to pathogenic challenge or tissue damage. These diverse physiological functions stem from the remarkable plasticity of Mφs, which allows these cells to dramatically change their form and function in response to local environmental signals.1–3 Unstimulated Mφs are typically quiescent; stimulation of these cells, however, results in the development of markedly polarized phenotypes in response to molecular cues residing in the local microenvironment. Current classification of Mφs recognizes polarization into two distinct phenotypes, termed “classically” activated (M1) or “alternatively” activated (M2).2 M1 Mφs are induced by recognition of pathogen-associated molecular patterns, such as bacterial lipopolysaccharides (LPS) and peptidoglycan, or damage-associated molecular patterns, such as released intracellular proteins and nucleic acids, as well as stimulation by the T-cell–secreted cytokine interferon gamma (IFN-γ). M1 Mφs represent a proinflammatory phenotype, exhibiting increased phagocytic and antigen processing activity as well as increased production of proinflammatory cytokines (e.g., interleukin 1 [IL-1], IL-6, IL-12, and tumor necrosis factor alpha [TNF-α]) and oxidative metabolites (e.g., nitric oxide and superoxide) to promote host defense and removal of damaged tissue. In contrast, M2 Mφs are induced by a variety of stimuli (e.g., IL-4/IL-13, glucocorticoids) and represent a phenotype that is potentially important in the promotion of wound healing and tissue remodeling as well as the resolution of inflammation.1–3
Clinical Problem Addressed
The remarkable plasticity of Mφs has important implications for clinical science. Proper Mφ polarization is necessary in several important physiological processes including, but not limited to, wound healing, immune response, and nerve/muscle regeneration.1–5 Thus, it is not surprising that aberrations in Mφ polarization are associated with some of the pathology observed in defective wound healing, diabetes, muscular dystrophy, fibroproliferative diseases such as rheumatoid arthritis and liver and lung fibrosis, as well as tumor progression.1–4,6–8 Elucidating the specific microenvironmental signals that contribute to Mφ polarization could potentially lead to methods for the pharmacological manipulation of Mφ phenotypes to promote favorable processes (e.g., wound healing) or inhibit pathologic processes (e.g., fibroproliferative diseases and tumor growth).
Relevant Basic Science Context
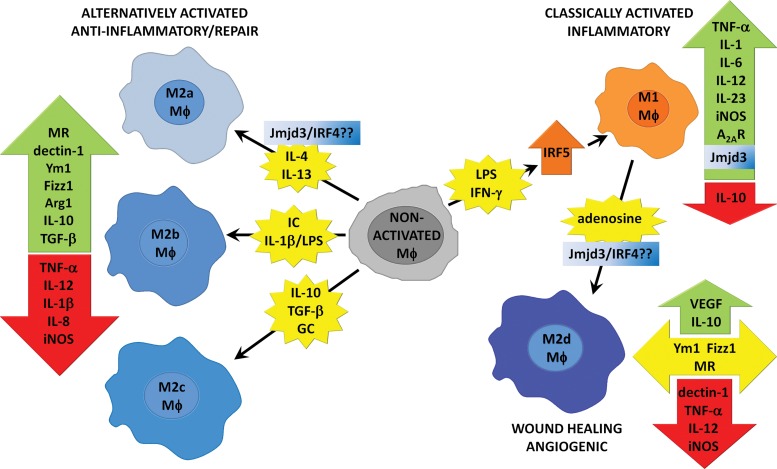
One of the hallmarks of Mφs is their remarkable plasticity, that is, the ability to alter their phenotype in response to different environmental stimuli. Two major categories of Mφs, those exhibiting proinflammatory and anti-inflammatory/wound healing phenotypes, are currently recognized and are termed M1 and M2, respectively.2 Considerable research has sought to both identify the wide variety of signals that induce these phenotypes as well as characterize the molecular profiles of M1 and M2 Mφs, as outlined in Fig. 1.1–3 However, as our knowledge of Mφ polarization becomes more complex, it has emerged that there is a broader set of signals that induce distinct Mφ phenotypes than the traditional M1/M2 classification accommodates. For instance, although IL-4/IL-13 signaling through the IL-4 receptor-α (IL-4Rα) represents the prototypical M2 Mφ activation pathway, recent research has demonstrated the presence of Mφs exhibiting M2-like characteristics even in the absence of this signaling.9 In addition, M2 Mφs induced by IL-4, although exhibiting reduced phagocytic activity, show markedly increased secretion of proinflammatory cytokines in response to LPS challenge.10 Finally, we recently defined a subtype of M2-like Mφs induced by costimulation of Mφs with Toll-like receptor (TLR) and adenosine A2A receptor (A2AR) agonists that display a distinct molecular signature. A2AR stimulation by adenosine in the presence of TLR agonists switches Mφs from a M1 phenotype into an angiogenic M2-like phenotype, which we have termed “M2d.”11 The discovery of this and other novel Mφ activation states underscores the importance of local extracellular signals in determining Mφ function. Thus, a more complete understanding of the spatiotemporal changes in signaling molecules during wound healing and their effect on Mφ function may allow for the enhancement of this critical process.
Figure 1.
Pathways of Mφ polarization. Nonactivated Mφs are polarized into distinct phenotypes by specific inducing agents and display typical changes in gene expression. Note that not all inducing agents are included and expression profiles for different M2 Mφ subtypes can differ based on the nature of induction.
Target Articles.
1. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, and Eming SA: Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184: 3964–3977.
2. Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, and Udalova IA: IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 2011; 12: 231–238.
3. Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, and Akira S: The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 2010; 11: 936–944.
Experimental Model or Material: Advantages and Limitations
Studies of the influence of Mφ polarization on wound healing have traditionally utilized in vitro culture of Mφs and in vivo models of wound healing in mice as experimental models. In vitro culture represents a good model for the identification of Mφ activation stimuli as well as elucidation of the molecular profiles of resultant Mφ populations, because of the ability to precisely control the extracellular milieu. However, the lack of other cell types that would normally be present, as well as other physiological limitations, could obscure important in vivo changes that may occur in the local environment that could affect Mφ polarization. In vivo models of wound healing certainly address some of these concerns and the use of mice allows for genetic manipulation to identify proteins and other factors that may contribute to Mφ polarization, but important limitations remain. In particular, significant species-specific differences in Mφ protein expression have been demonstrated, indicating the need for further elucidation of M1/M2 Mφ polarization in the human system.1
Discussion of Findings and Relevant Literature
Mφs are a population of immune cells that orchestrate a diverse array of functions including inflammation, tissue repair, and immune responses. This functional diversity is achieved by the remarkable heterogeneity of Mφs, which have the capacity to dramatically change their phenotype as a result of differentiated plasticity as well as local environmental cues. Mφs are generally classified as either classically (M1) or alternatively (M2) activated. M1 Mφs have a proinflammatory phenotype exhibiting increased phagocytic activity and secretion of proinflammatory cytokines that aid in the removal of pathogens and abnormal or damaged tissues. M2 Mφs have a polar opposite phenotype exhibiting high levels of anti-inflammatory cytokines and fibrogenic and angiogenic factors that serve to resolve inflammation and promote wound healing.1–3 Both M1 and M2 Mφs express distinct molecular markers as outlined in Fig. 1; however, further characterization of each phenotype has begun to demonstrate marked variability in Mφ molecular profiles as well as the activating agents that induce them.1–3,11 Thus, M2 Mφs are now classified in three distinct subgroups, termed M2a, M2b, and M2c, based upon the inducing agent and molecular marker expression (Fig. 1).1,2 In this classification, the M2a subtype represents the prototypical IL-4/IL-13–dependent, IL-4Rα–dependent M2 phenotype.1 Our laboratory has characterized an additional M2 subtype, which, unlike previously described M2 Mφs, involves “switching” from an inflammatory M1 into an angiogenic M2 phenotype. This subtype, which we termed M2d, is induced by A2AR signaling pursuant to initial stimulation by TLR agonists and is marked by decreased proinflammatory cytokine release concurrent with upregulation of traditional M2 cytokines such as IL-10 and the potent angiogenic molecule vascular endothelial growth factor (VEGF). We have recently characterized the phenotypic characteristics of this M2d Mφ population in comparison to M2a Mφs. M2d Mφs express high levels of IL-10 and VEGF and low levels of TNF-α and IL-12 and do not show increased expression of eosinophil chemotactic factor L (Ym1), found in inflammatory zone 1 (FIZZ1), mannose receptor (MR), or dectin.11
Efforts to establish a more complete picture of Mφ phenotypes have recently uncovered two transcriptional regulators that appear central to M1 and M2 Mφ polarization. These regulators, interferon regulatory factor 5 (IRF5) and IRF4, are members of the interferon regulatory factor family and have been recently reported to play important roles in M1 and M2 Mφ polarization, respectively. Krausgruber et al. demonstrated that IRF5 expression drives M1 polarization in Mφs, with IRF5 directly activating transcription of several M1 proinflammatory cytokines, such as IL-12 and IL-23, while repressing transcription of the anti-inflammatory cytokine IL-10, an established M2 cytokine. IRF5 is found at high levels in M1 Mφs, and its expression is induced by IFN-γ, LPS, or GM-CSF. Knockdown of IRF5 severely impairs the expression of inflammatory cytokines.12 IRF5 represents a potentially important clinical target, as genetic polymorphisms in IRF5 have been linked to several autoimmune diseases, including rheumatoid arthritis and multiple sclerosis.13,14 Likewise, Satoh et al. have implicated IRF4 as a crucial mediator of M2 Mφ polarization. IRF4 inhibits TLR signaling by interacting with MyD88, but the molecular mechanisms of M2 Mφ induction by IRF4 remain unknown.15,16 An upstream effector of IRF4-induced M2 polarization, the histone demethylase Jumonji domain containing-3 (Jmjd3), was also identified in this study. Jmjd3 and IRF4 are critically involved in the IL-4–dependent induction of a subset of genes expressed by M2a Mφs (arginase 1, Ym1, FIZZ1, and MR), whereas regulation of iNOS is independent of this Jmjd3/IRF4 pathway.16 The roles of IRF4 and IRF5 in the adenosine-dependent induction of the M2d phenotype are not yet known; however, studies from our lab clearly indicate that this induction is independent of IL-4/IL-13 and STAT6, and switching to the M2d phenotype occurs unimpeded in Mφs from IL-4Rα knockout mice. Interestingly, Jmjd3 expression is induced in Mφs by TLR signaling via nuclear factor-κB (NF-κB), and induction of the angiogenic M2d phenotype also requires TLR stimulation and NF-κB signaling.17,18
Wound healing is a complex and dynamic process, requiring the coordination of many different signals and cell types to promote effective scar tissue formation. The functional plasticity of Mφs in response to spatiotemporal changes in environmental signals underlies their ability to participate in diverse aspects of wound repair. Wound Mφs in the early stage of repair are more M1-like, when clearance of foreign/damaged matter is required, but M2 Mφs predominate in later stages of repair in response to the need for new tissue formation.9 Lucas et al. have recently demonstrated the essential role of Mφs in the early and middle phases of wound repair, characterized by inflammation and granulation tissue formation, respectively. In these studies, transgenic mice expressing the human diphtheria toxin receptor under the control of the CD11b promoter (CD11b-DTR mice) were used to enable selective depletion of Mφs from skin wounds at different phases of repair by administration of diphtheria toxin. Wounds depleted of Mφs during either the early or middle stages of wound repair demonstrate markedly attenuated wound repair. Loss of Mφs in the early stage impairs induction of granulation tissue formation, myofibroblast differentiation, and angiogenesis. Interestingly, these changes correlate with the impairment of M2 Mφ polarization, as evidenced by lack of Ym1/FIZZ1 expression and reduced secretion of transforming growth factor beta (TGF-β) and VEGF. Depletion of Mφs during the middle stage of wound repair resulted in similar pathologic alterations, with wounds displaying immature granulation tissue and impaired angiogenesis because of endothelial cell apoptosis. Likewise, this impaired healing was associated with the lack of the M2 cytokines TGF-β and VEGF, suggesting a role for M2 Mφ polarization in this process.19 These studies again strongly confirm the classical observations of Leibovich and Ross (1975) concerning the roles of Mφs in wound repair.20 With regard to Mφ polarization, however, it should be noted that this study used prototypical M2a markers to identify polarized Mφ phenotypes. Additional studies to analyze the presence and role of the M2d Mφ population are required. The M2d population expresses elevated levels of A2ARs and A2BRs, but does not express elevated MR, Ym1, FIZZ1, or dectin. These studies are currently in progress.
Take-Home Messages.
Basic science advances
• Local environmental cues influence the phagocytic and secretory behavior of Mφs to promote development of either an inflammatory Mφs phenotype (M1) or an anti-inflammatory/wound healing phenotype (M2).
• M2a Mφs are induced by IL-4Rα–dependent activation and express MR, Ym1, and FIZZ1; M2d Mφs are induced by TLR/adenosine A2A receptor-dependent activation, in an IL-4Rα-independent manner, and do not express elevated MR, Ym1, and FIZZ1.
• M2 Mφs play an essential role in early and middle stages of wound repair. Depletion of Mφs during healing attenuates TGF-β and VEGF signaling and delays the formation and maturation of new tissue. Further studies of the roles of M2a and M2d Mφs are required.
• IRFs play critical roles in the polarization of Mφs. IRF5 promotes polarization of Mφs into the M1, proinflammatory phenotype, whereas IRF4 influences M2a Mφ polarization in response to transcriptional regulation by Jmjd3 demethylation.
Clinical science advances
• Characterization of local environmental signals and subsequently induced Mφ subpopulations that regulate inflammatory and tissue repair phases of wound healing provides insight into potential mechanisms for therapeutic modulation. Therapeutic modulation of Mφ polarization presents novel opportunities for the treatment of conditions whose pathogenesis is linked to aberrant Mφ activation.
• The identification of adenosine “switching” of Mφs from an M1 to M2d phenotype provides a novel paradigm for analysis of Mφ polarization, underscoring the importance of signaling crosstalk in the complex processes of wound repair and disease pathogenesis.
• The identification of the roles of IRF4 and IRF5 in M1/M2 polarization of Mφs provides novel insights that should prove valuable for development of selective therapeutic modulation.
Innovation
Our discovery of the novel M2d Mφ subtype coupled with recent discoveries elucidating the essential role of Mφs in the early and middle stages of wound repair suggests an important role for adenosine-mediated “switching” in the regulation of angiogenesis during the repair process. Moreover, the identification of “adenosine switching” of Mφs highlights the importance of crosstalk among several signaling pathways in mediating Mφ polarization. The extracellular milieu of wounds represents a “primordial soup” of signaling cues, and it is quite likely that several stimuli act on Mφs at any given time. Thus, it becomes important to characterize the spatial and temporal changes in the wound environment and determine the differential contributions of the stimuli present to Mφ polarization. This could potentially enable the artificial manipulation of Mφs polarization to enhance normal physiological processes, such as wound repair, while combating pathological processes resulting from dysregulation of Mφ function. Our experimental model, along with recent knowledge of the central roles of IRF4 and IRF5 in Mφ polarization outlined above, presents a promising approach for analyzing relative contributions of disparate signals on Mφ polarization.
Caution, Critical Remarks, and Recommendations
Although significant progress has been made identifying factors underlying Mφs polarization, much work remains. It is important to remember that, despite traditional classification of Mφs as classically (M1) or alternatively (M2) activated, many novel phenotypes that do not fit the canonical molecular profile of these two groups have been identified. As our identification of the M2d phenotype has demonstrated, Mφ polarization can be influenced by the concurrent stimulation of several different signaling pathways. The extracellular milieu in a healing wound comprises different palettes of signaling cues based upon spatial location within the wound, temporal location during the repair process, and the organism's surrounding environment. The study of Mφ polarization at the transcriptional level, as observed with IRF4 and IRF5, should provide ways to observe the interplay of various signaling pathways on Mφ polarization. Additional benefits may result from the fact that factors regulating transcription appear to influence clusters of genes expressed during polarization, potentially providing insight into the varied molecular profiles observed during polarization.
Future Developments of Interest
Recent research has detailed the critical role of M2 Mφs in the early stages of wound healing. The discovery of the ability of adenosine signaling in the presence of TLR stimulation to induce phenotypic switching from M1 into M2d Mφs, coupled with evidence documenting impaired healing and, most notably, crippled angiogenesis due to decreased VEGF signaling in the absence of Mφs, underscores the importance of evaluating the effect of TLR/A2AR costimulation of Mφs on wound repair. Additionally, evaluation of the intracellular signaling pathways underlying M1 to M2 “switching” could aid in the development of therapeutics to regulate this switch, thereby allowing for the enrichment of certain Mφ populations to promote enhancement of selective physiological processes to aid in the treatment of infection and disease.
Abbreviations and Acronyms
- A2AR
adenosine A2A receptor
- FIZZ1
found in inflammatory zone 1
- IFN-γ
interferon gamma
- IL
interleukin
- IL-4Rα
interleukin-4 receptor alpha
- IRF5
interferon regulatory factor 5
- Jmjd3
Jumonji domain containing-3
- LPS
lipopolysaccharide
- MR
mannose receptor
- M1 Mφ
classically activated Mφ
- M2 Mφ
alternatively activated Mφ
- Mφ
macrophage
- NF-κB
nuclear factor-κB
- TGF-β
transforming growth factor beta
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
- Ym1
eosinophil chemotactic factor L
Acknowledgment And Funding Source
Mφ biology research in SJL's laboratory was supported by NIH (grant RO1-GM068636).
Disclaimer
The contents of this article are solely the responsibility of the author and do not necessarily represent the official views of the NIGMS or NIH.
Author Disclosure and Ghostwriting
The authors have no conflicts of interest. No ghostwriters were used in the writing of this article.
References
- 1.Martinez FO. Helming L. Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FO. Sica A. Mantovani A. Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X. Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villalta SA. Rinaldi C. Deng B. Liu G. Fedor B. Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20:790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kigerl KA. Gensel JC. Ankeny DP. Alexander JK. Donnelly DJ. Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sindrilaru A. Peters T. Wieschalka S. Baican C. Baican A. Peter H. Hainzl A. Schatz S. Qi Y. Schlecht A. Weiss JM. Wlaschek M. Sunderkötter C. Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olefsky JM. Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 8.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daley JM. Brancato SK. Thomay AA. Reichner JS. Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varin A. Mukhopadhyay S. Herbein G. Gordon S. Alternative activation of macrophages by IL-4 impairs phagocytosis of pathogens but potentiates microbial-induced signalling and cytokine secretion. Blood. 2010;115:353–362. doi: 10.1182/blood-2009-08-236711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinberg S. Hasko G. Wu D. Leibovich SJ. Suppression of PLCβ2 by endotoxin plays a role in the adenosine A2A receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am J Pathol. 2009;175:2439–2453. doi: 10.2353/ajpath.2009.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krausgruber T. Blazek K. Smallie T. Alzabin S. Lockstone H. Sahgal N. Hussell T. Feldmann M. Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 13.Dieguez-Gonzalez R. Calaza M. Perez-Pampin E. de la Serna AR. Fernandez-Gutierrez B. Castañeda S. Largo R. Joven B. Narvaez J. Navarro F. Marenco JL. Vicario JL. Blanco FJ. Fernandez-Lopez JC. Caliz R. Collado-Escobar MD. Carreño L. Lopez-Longo J. Cañete JD. Gomez-Reino JJ. Gonzalez A. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:1264–1274. doi: 10.1002/art.23426. [DOI] [PubMed] [Google Scholar]
- 14.Kristjansdottir G. Sandling JK. Bonetti A. Roos IM. Milani L. Wang C. Gustafsdottir SM. Sigurdsson S. Lundmark A. Tienari PJ. Koivisto K. Elovaara I. Pirttilä T. Reunanen M. Peltonen L. Saarela J. Hillert J. Olsson T. Landegren U. Alcina A. Fernández O. Leyva L. Guerrero M. Lucas M. Izquierdo G. Matesanz F. Syvänen AC. Interferon regulatory factor 5 (IRF5) gene variants are associated with multiple sclerosis in three distinct populations. J Med Genet. 2008;45:362–369. doi: 10.1136/jmg.2007.055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negishi H. Ohba Y. Yanai H. Takaoka A. Honma K. Yui K. Matsuyama T. Taniguchi T. Honda K. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc Natl Acad Sci U S A. 2005;102:15989–15994. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh T. Takeuchi O. Vandenbon A. Yasuda K. Tanaka Y. Kumagai Y. Miyake T. Matsushita K. Okazaki T. Saitoh T. Honma K. Matsuyama T. Yui K. Tsujimura T. Standley DM. Nakanishi K. Nakai K. Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 17.De Santa F. Totaro MG. Prosperini E. Notarbartolo S. Testa G. Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan M. Pinhal-Enfield G. Hao I. Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol Biol Cell. 2007;18:14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas T. Waisman A. Ranjan R. Roes J. Krieg T. Muller W. Roers A. Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 20.Leibovich SJ. Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]