Abstract
Background
Adipose tissue is one of the richest sources of mesenchymal stem cells that exhibit an outstanding ability to regenerate skin.
The Problem
Although the anatomical sites of adipose-derived stem cells (ASCs) in the body are relatively oxygen-deficient (i.e., 1%–5% oxygen content), ASCs are usually cultured under normoxic conditions, and long-term culturing of ASCs in normoxia may induce their senescence.
Basic/Clinical Science Advances
The review is an overview of the cellular responses of ASCs during hypoxia, which collectively increase the wound-healing potential of ASCs. Furthermore, the mechanism of action for stimulation by hypoxia (i.e., a pivotal role of reactive oxygen species and related signal pathways) will be discussed.
Clinical Care Relevance
Hypoxia is a critical stimulatory factor for ASCs. Therefore, understanding the response and adaptation of ASCs to hypoxia may be invaluable for developing novel cell therapeutic strategies.
Conclusion
Culturing ASCs under hypoxia may uniquely benefit proliferation, stemness, migration, and growth factor secretion. Therefore, the preconditioning of ASCs by hypoxia shows a prominent wound-healing effect in clinical use.

Jong-Hyuk Sung
Background
Regenerative medicine uses the body's own stem cells and growth factors to repair damaged tissue, such as skin. Adipose-derived stem cells (ASCs) and their soluble factors reportedly have been used to regenerate skin, and they offer a potential solution for skin regeneration.1–4 For example, ASCs and their soluble factors accelerate wound healing, reduce wrinkling, improve pigmentation, and promote hair growth.2–5 In addition to differentiation, transplanted ASCs and their soluble factors induce the angiogenesis, increase the proliferation of fibroblasts and keratinocytes, and remodel the extracellular matrix.6 Recently, we found that culturing in hypoxia enhances the wound-healing function of ASCs by increasing the proliferation and secretion of growth factors for ASCs.7 Furthermore, the mechanism for the hypoxia-induced stimulation of ASCs was investigated that hypoxia generates reactive oxygen species (ROS), which activate platelet-derived growth factor receptor (PDGFR) pathways.8 Therefore, this review provides an overview of the cellular responses of ASCs during hypoxia and of the enhanced wound-healing potential under hypoxic culturing conditions. In addition, the mechanism of action for stimulation by hypoxia will be addressed.
Target Articles.
1. Kim JH, Park SH, Park SG, Choi JS, Xia Y, and Sung JH: The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev 2011; 20: 1753.
2. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, and Sung JH: Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and upregulation of VEGF and bFGF. Wound Repair Regen 2009; 17: 540.
3. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, and Park JS: Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007; 48: 15.
Clinical Problem Addressed
Vascular complications are commonly associated with problematic wounds, which lead to tissue hypoxia. In general, acute mild hypoxia supports adaptation and survival, whereas chronic severe hypoxia leads to tissue damage.9 Because the microenvironment near the wound is in an oxygen-deficient state, it is interesting to elucidate the effect of hypoxia on the wound-healing function of ASCs.
Low oxygen tension is reportedly an important characteristic of the stem cell niche, and hypoxia provides signals conducive to the maintenance of definitive stem cell properties.10,11 Therefore, understanding the response and adaptation of ASCs to hypoxic culturing may be invaluable for developing novel ASC therapies.
Relevant Basic Science Context
Most stem cells in the body exist in environments with a low, or very low oxygen supply. Additionally, in vitro, oxygen concentration is sensed by stem cells, and low PO2 modifies their phenotypes; therefore, use of PO2 matter should be more carefully monitored in stem cell culturing.12 For example, hypoxia can reduce spontaneous differentiation and maintain clonality of human embryonic stem cells, and it can enhance the generation of induced pluripotent stem cells.12–14 In addition, when mesenchymal stem cells are cultured under hypoxic conditions in vitro, their proliferative and self-renewal capacities are significantly improved.15 Likewise, ASCs favor hypoxia and their phenotypes are regulated by it.
Experimental Model or Material: Advantages and Limitations
The wound-healing effect was investigated in excision wound models of nude mice after topical application of ASCs and conditioned medium of ASCs (ASC-CM).16 To study the mechanism, primary dermal fibroblasts were cultured, and the effect of ASC-CM on their proliferation, migration, and collagen synthesis was measured. In addition, the effect of hypoxia on the proliferation and growth factor secretion of ASCs was examined using an hypoxia incubator.7 The ASC-CM was harvested in hypoxia (hypoCM) and the wound-healing potential was compared with normoxia (norCM).7 ROS generation by hypoxia was directly measured by 2′,7′-dichlorofluorescin diacetate intensity in a fluorescent activated cell sorter. Phosphorylation of PDGFR-β, a type of serine/threonine protein kinase (Akt), and extracellular signal-related kinase (ERK) was measured by Western blot analysis. Study of the inhibition of ROS generation was examined using N-acetyl-cysteine (NAC, ROS scavenger) and diphenyleneiodonium (DPI; NADPH oxidase [Nox] inhibitor).8
Discussion of Findings and Relevant Literature
ASCs promoted proliferation of dermal fibroblasts not only by cell-to-cell direct contact but also by paracrine factors. ASC-CM enhanced the secretion of type I collagen in dermal fibroblasts by regulating the mRNA levels of extracellular matrix proteins: upregulation of collagen and downregulation of matrix mataloproteinase-1. Moreover, ASC-CM showed a stimulatory effect on the migration of dermal fibroblasts. In addition to this in vitro evidence, ASCs and ASC-CM significantly reduced the wound sizes and accelerated the re-epithelialization of the excision wound model. In addition to our study, other groups have demonstrated the wound-healing effect of ASCs and their mechanism of action, and found that locally transplanted ASCs accelerate wound healing not only by differentiation and vasculogenesis but also by paracrine factors.6,17 Induced pro-angiogenic potential of ASCs by hypoxia has been reported that the transplantation of ASCs cultured in hypoxia significantly increased the local blood flow and angiogenic gene expression was partially improved by hypoxia preconditioning.18,19
Incubation under hypoxic conditions enhanced the proliferation of ASCs in either the presence or absence of serum. Furthermore, mRNA and protein measurements showed that hypoxia upregulated the secretion of growth factors such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in ASCs. HypoCM significantly promoted collagen synthesis and the migration of human dermal fibroblasts, compared with norCM. In the animal studies, hypoCM significantly reduced the wound size and accelerated the healing compared with norCM. Inhibition of VEGF and bFGF in conditioned medium using neutralizing antibodies attenuated the wound healing in animal experiment, which indicates that VEGF and bFGF are involved in the enhanced wound-healing function by hypoxia.
Further investigation included a key mediator and a signal pathway involved in the stimulation of ASCs during hypoxia. Hypoxia significantly increased ROS generation, which was greatly reduced by NAC and DPI treatment. Likewise, the hypoxia-induced proliferation and migration of ASCs were reversed by NAC and DPI treatment, suggesting the involvement of ROS generation in ASC stimulation. In Western blot analysis, hypoxia increased the phosphorylation of PDGFR-β, which was followed by an activation of the Akt and ERK pathways. These results suggest a pivotal role for ROS generation in the stimulation of ASCs by hypoxia.
Innovation
Hypoxia and the generation of ROS serve as a second messenger in the intracellular signal transduction pathway.20,21 However, most studies focus on the negative aspects of hypoxia and ROS generation. Physiological levels of intracellular ROS are required to activate the DNA repair pathway for maintaining genomic stability in stem cells and to increase the proliferation and migration of stem cells. In addition, stem cells efficiently manage oxidative stress and have a high resistance to ROS-induced death.22,23 Therefore, attention must be paid to the positive effects of hypoxia and ROS generation in ASC physiology.
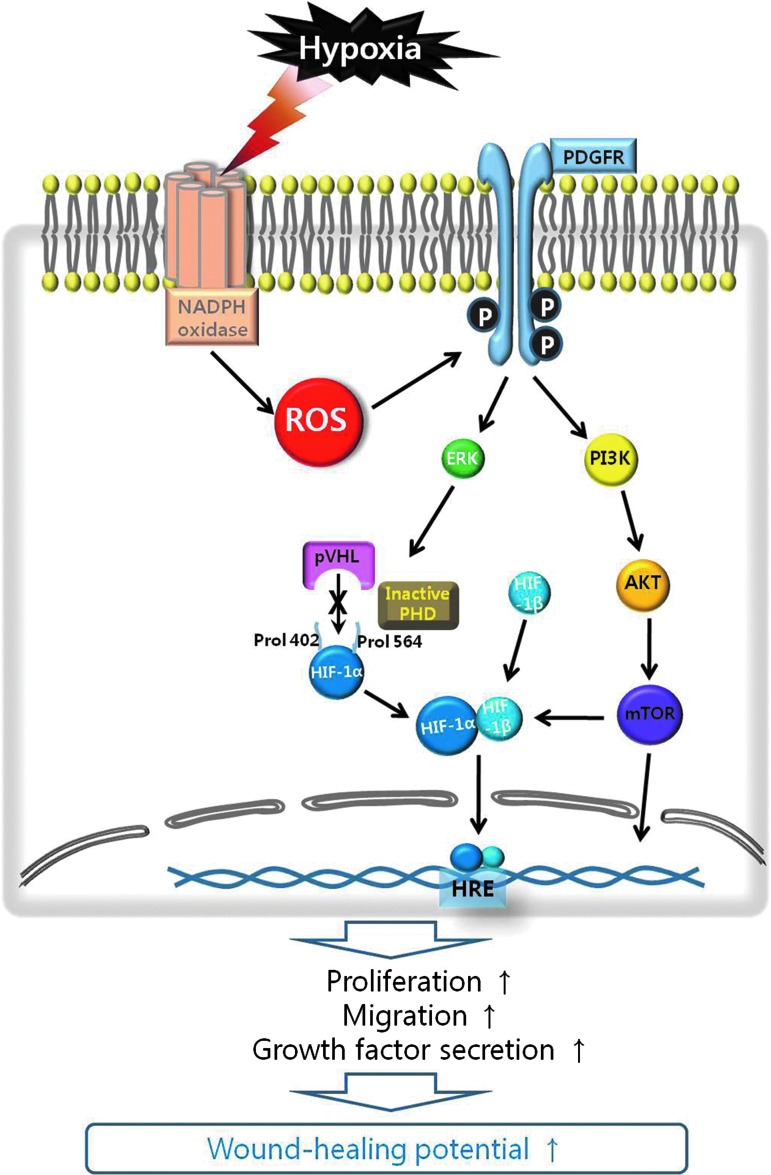
Summary Illustration
Hypoxia initiates ROS synthesis in ASCs via the Nox family. Increased intracellular ROS levels activate receptor-type or nonreceptor-type tyrosine kinases in ASCs. Of them, PDGFR-β was primarily phosphorylated. Activation of these tyrosine kinases subsequently increases the phosphorylation of PI3K/Akt/mammalian targets of the rapamycin (mTOR) and ERK1/2 signaling pathways. These events inhibit the degradation of hypoxia-inducible factor-1 alpha (HIF-1α) by the propyl-hydroxylation of the von Hippel Lindau tumor suppressor protein and an increase in cytosolic HIF-1α levels. HIF-1α translocates to the nucleus where it modulates the transcription of its target genes after binding to the hypoxia-responsive element in the nucleus. Upregulation of target genes may increase the proliferation, migration, and growth factor secretion of ASCs, which collectively enhances the wound-healing function of ASCs.
Caution, Critical Remarks, and Recommendations
Typically, ASCs are cultured under ambient or normoxic conditions. However, the O2 concentration in the physiological niches occupied by ASCs is much lower. Thus, hypoxia can function as a prophylactic signal, increasing proliferation, adhesion, and migration of ASCs by ROS generation. In addition to oxygen content, diverse culturing conditions (i.e., supplement of growth factors, quality of serum and its concentration, composition of extracellular matrix, and quality of culture wares) collectively influence the proliferation and regeneration potential of ASCs. Therefore, caution should be taken to optimize the expansion conditions for ASC therapies.
Take-Home Message.
Basic science advances
• ASCs and their soluble factors accelerate wound healing.
• Hypoxia enhances the wound-healing function of ASCs by increasing proliferation/migration of ASCs and secretion of paracrine factors.
• Hypoxia-enhanced ASC stimulation is mediated by generation of ROS and activation of PDGFR pathways.
Clinical science advances
• Low oxygen tension is an important characteristic of the stem cell niche and hypoxia provides signals conducive to the maintenance of definitive stem cell properties.
• The long-term culturing of ASCs in normoxia drives them to senescence, but hypoxia uniquely contributes to their expansion and wound-healing functions.
Relevance to clinical care
• Before ASC transplantation, adaptation of ASCs to hypoxia may be invaluable for developing a prominent ASC therapy.
Future Development of Interest
While hypoxia and ROS generation are involved in apoptosis, low and moderate intracellular ROS levels act as a signal transducer that activates ASCs. Because moderate ROS levels increase the proliferation and regenerative potential of ASCs, it is beneficial to expose ASCs to moderate oxidative stress during manipulation. Hypoxia or the addition of an ROS donor may negate the expense of culturing ASCs during expansion, and ROS preconditioning may enhance the wound-healing potential of ASCs in clinical use. Therefore, future study should address the efficiency of ROS donors and the optimal ROS concentrations for an ASC culture.
Abbreviations and Acronyms
- Akt
a type of serine/threonine protein kinase
- ASC
adipose-derived stem cells
- ASC-CM
conditioned medium of ASCs
- bFGF
basic fibroblast growth factor
- DPI
diphenyleneiodonium
- ERK
extracellular signal-related kinase
- HIF-1α
hypoxia-inducible factor-1 alpha
- mTOR
mammalian targets of the rapamycin
- Nox
NADPH oxidase
- PDGFR
platelet-derived growth factor receptor
- ROS
reactive oxygen species
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
Summary Illustration and the Reference section were kindly edited by Ji-Hye Kim. This review was partially supported by a grant (C10102410) from the Gyeonggi Technology Development Program funded by Gyeonggi Province and by a grant (2011–0019636) of the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST).
Author Disclosure and Ghostwriting
The authors have no competing interests. The authors were the only writers for this article.
References
- 1.Song SY. Chung HM. Sung JH. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther. 2010;10:1529. doi: 10.1517/14712598.2010.522987. [DOI] [PubMed] [Google Scholar]
- 2.Park BS. Jang KA. Sung JH. Park JS. Kwon YH. Kim KJ. Kim WS. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34:1323. doi: 10.1111/j.1524-4725.2008.34283.x. [DOI] [PubMed] [Google Scholar]
- 3.Kim WS. Park SH. Ahn SJ. Kim HK. Park JS. Lee GY. Kim KJ. Whang KK. Kang SH. Park BS. Sung JH. Whitening effect of adipose-derived stem cells: a critical role of TGF-beta 1. Biol Pharm Bull. 2008;31:606. doi: 10.1248/bpb.31.606. [DOI] [PubMed] [Google Scholar]
- 4.Kim WS. Park BS. Kim HK. Park JS. Kim KJ. Choi JS. Chung SJ. Kim DD. Sung JH. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. J Dermatol Sci. 2008;49:133. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Won CH. Yoo HG. Kwon OS. Sung MY. Kang YJ. Chung JH. Park BS. Sung JH. Kim WS. Kim KH. Hair growth promoting effects of adipose tissue-derived stem cells. J Dermatol Sci. 2010;57:134. doi: 10.1016/j.jdermsci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Nie C. Yang D. Xu J. Si Z. Jin X. Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant. 2011;20:205. doi: 10.3727/096368910X520065. [DOI] [PubMed] [Google Scholar]
- 7.Lee EY. Xia Y. Kim WS. Kim MH. Kim TH. Kim KJ. Park BS. Sung JH. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH. Park SH. Park SG. Choi JS. Xia Y. Sung JH. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011;20:1753. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17:1. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eto H. Suga H. Inoue K. Aoi N. Kato H. Araki J. Doi K. Higashino T. Yoshimura K. Adipose injury-associated factors mitigate hypoxia in ischemic tissues through activation of adipose-derived stem/progenitor/stromal cells and induction of angiogenesis. Am J Pathol. 2011;178:2322. doi: 10.1016/j.ajpath.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suga H. Eto H. Aoi N. Kato H. Araki J. Doi K. Higashino T. Yoshimura K. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg. 2010;126:1911. doi: 10.1097/PRS.0b013e3181f4468b. [DOI] [PubMed] [Google Scholar]
- 12.Wion D. Christen T. Barbier EL. Coles JA. PO(2) matters in stem cell culture. Cell Stem Cell. 2009;5:242. doi: 10.1016/j.stem.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y. Takahashi K. Okita K. Ichisaka T. Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Millman JR. Tan JH. Colton CK. The effects of low oxygen on self-renewal and differentiation of embryonic stem cells. Curr Opin Organ Transplant. 2009;14:694. doi: 10.1097/MOT.0b013e3283329d53. [DOI] [PubMed] [Google Scholar]
- 15.Wang DW. Fermor B. Gimble JM. Awad HA. Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184. doi: 10.1002/jcp.20324. [DOI] [PubMed] [Google Scholar]
- 16.Kim WS. Park BS. Sung JH. Yang JM. Park SB. Kwak SJ. Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH. Jung M. Kim HS. Kim YM. Choi EH. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol. 2011;20:383. doi: 10.1111/j.1600-0625.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 18.Efimenko A. Starostina E. Kalinina N. Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove CJ. Bovenkerk JE. Pell CL. Johnstone BH. Considine RV. March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 20.Chan EC. Jiang F. Peshavariya HM. Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122:97. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y. Lou MF. The regulation of NADPH oxidase and its association with cell proliferation in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:2291. doi: 10.1167/iovs.08-2568. [DOI] [PubMed] [Google Scholar]
- 22.Dernbach E. Urbich C. Brandes RP. Hofmann WK. Zeiher AM. Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 23.Valle-Prieto A. Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]