Abstract
Background
Stress-induced disruption of hormonal balance in animals and humans has a detrimental effect on wound healing.
The Problem
After the injury, keratinocytes migrate over the wound bed to repair a wound. However, their nonmigratory phenotype plays a role in pathogenesis of chronic wounds. Despite many therapeutic approaches, there is a dearth of treatments targeting the molecular mechanisms mediated by stress that prevent epithelization.
Basic/Clinical Science Advances
Recent studies show that epidermal keratinocytes synthesize stress hormones. During acute wound healing, cortisol synthesis in the epidermis is tightly controlled. Further, a key intermediate molecule in the cholesterol synthesis pathway, farnesyl pyrophosphate (FPP), can bind glucocorticoid receptor (GR) and activate GR. Additionally, keratinocytes express beta-2-adrenergic-receptor (β2AR), a receptor for the stress hormone epinephrine. Importantly, migratory rates of keratinocytes are reduced by cortisol, FPP, epinephrine, and other β2AR agonists, thus indicating their role in the inhibition of epithelization. Topical inhibition of local glucocorticoid and FPP synthesis, as well as treatment with β2AR antagonists promotes wound epithelization.
Clinical Care Relevance
Modulation of local stress hormone production may represent an important therapeutic target for wound healing disorders. Topical administration of inhibitors of cortisol synthesis, statins, β2AR antagonists, and systemic beta-blockers can decrease cortisol synthesis, FPP, and epinephrine levels, respectively, thus restoring keratinocyte migration capacity. These treatment modalities could represent a novel therapeutic approach for wound healing disorders.
Conclusion
Attenuation of the local stress-induced hormonal imbalance in epidermis may advance therapeutic modalities, thereby leading to enhanced epithelization and improved wound healing.

Olivera Stojadinovic
Background
It is well recognized that stress, whether emotional or psychological, impairs wound healing.1 Stress stimulates the “fight or flight response,” causing systemic release of cortisol and epinephrine.
Epithelization is an important component of successful wound healing, which is impaired in chronic wounds. During acute healing, epidermal keratinocytes become activated. They proliferate, migrate, cover the wound bed and reconstruct a barrier that prevents infection and dehydration. Keratinocytes at the nonhealing wound edge are hyperproliferative and mitotically active, but they fail to migrate, thus creating hyperkeratotic tissue at the edge of the wound.2 Another factor contributing to the impaired epithelization in chronic wounds may be improper wound bed matrix. A fibrin matrix that is commonly seen in chronic wounds is not preferred by epithelial cells, and, therefore, their migration is compromised.
In addition to systemic release, recent studies demonstrate how locally induced stress hormones impair keratinocyte migration and wound healing. A novel finding that human epidermal keratinocytes (HEKs) in vitro and epidermis in vivo synthesize cortisol and express an enzyme indispensable for cortisol synthesis, 11-beta-hydroxylase (CYP11B1) and an enzyme that controls its negative feedback mechanism, 11-beta-hydroxysteroid dehydrogenase2 (11βHSD2) was recently discovered.3 Besides cortisol, an important intermediate in the cholesterol synthesis pathway, farnesyl pyrophosphate (FPP), can act as an agonist for the glucocorticoid receptor (GR) and inhibit keratinocyte migration as well. Elevation of another stress-induced hormone, epinephrine, also impairs healing. Elevated catecholamine (e.g., epinephrine) levels activate the keratinocyte beta-2-adrenergic receptor (β2AR), thus resulting in impairment of cell motility and wound re-epithelization.
Target Articles.
1. Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, and Tomic-Canic M: Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 2011; 286: 10265.
2. Vukelic S, Stojadinovic O, Pastar I, Vouthounis C, Krzyzanowska A, Das S, Samuels HH, and Tomic-Canic M: Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. J Biol Chem 2010; 285: 1980.
3. Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, and Isseroff RR: Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med 2009; 6: e12.
Clinical Problem Addressed
Chronic, nonhealing wounds contribute to a global disease burden, thus impacting both the health care system and economy, along with the patient's quality of life.4 These wounds fail to progress through the normal stages of wound healing and enter a state of chronic inflammation. Stress-induced hormones glucocorticoids (GCs) and catecholamines have been shown to interfere with keratinocytes ability to migrate during the normal wound healing process.3,5–8 Therefore, a potential therapeutic approach may be blocking the enzymes and/or receptors necessary for the local production and function of stress hormones.
Relevant Basic Science Context
GCs, key regulators of the physiological stress response, inhibit wound healing by modulation of diverse physiological processes, including metabolism, cell migration, proliferation, differentiation, and inflammation.8 HEKs synthesize cortisol, which is dependent on enzymes CYP11B1 and 11βHSD2. Interestingly, interleukin-1-beta (IL-1β), the first pro-inflammatory cytokine released by keratinocytes on epidermal injury, induces expression of CYP11B1 and increases cortisol production during early phases of wound healing, whereas inhibition of cortisol synthesis increases IL-1β production.3 This regulatory balance between GC synthesis and pro-inflammatory cytokines represents one possible mechanism through which GCs may prevent an excessive inflammatory response to epidermal injury, thus preventing wounds from entering a state of pathologic inflammation.
GCs are synthesized from cholesterol. The mevalonate pathway is essential for cholesterol synthesis in skin. A main branch point intermediate in the mevalonate pathway is FPP.9 FPP can bind GR, thus causing many of the same deleterious effects as GCs during the wound healing process. Each is shown to inhibit keratinocyte migration and wound epithelization through a similar mechanism by targeting expression of the early wound healing markers, keratin 6 and 16.3,9 Epinephrine, an additional regulator of the stress response, has also been implicated in the inhibition of wound healing. It is synthesized by keratinocytes7 and in burn wounds, due to a local up-regulation of the epinephrine-synthesizing enzyme phenylethanolamine-N-methyltransferase (PNMT). In conjunction with an increase in systemic levels, epinephrine impairs keratinocyte migration and wound re-epithelization. A nonmigratory keratinocyte phenotype on β2AR activation is shown to be due to the down-regulation of the AKT pathway, stabilization of the actin cytoskeleton, and an increase in focal adhesion formation.6
Experimental Model or Material: Advantages and Limitations
Various approaches are utilized to study keratinocyte migration and wound epithelization. The in vitro wound scratch assay allows direct examination of the effect of a substance on a specific cell type without influencing other tissue components. For example, primary human or animal keratinocytes and cells from animals in which a gene of interest is genetically deleted can be utilized. Wounding of keratinocytes by scratch creates a defect in cell monolayers, thus stimulating adjacent keratinocytes to migrate and repopulate the wounded area. It is important to discriminate between the effect of proliferation and migration of adjacent cells on wound closure; pretreatment with DNA synthesis inhibitor before wounding eliminates cell proliferation so that only migration rates are studied.
Ex vivo human skin experimental wound models have been validated in many studies. Similar expression patterns for multiple genes involved in epithelization and wound healing were confirmed when ex vivo wound models were compared with acute human wounds, thus proving the reliability and usefulness of ex vivo wounds in studying healing. The influence of multiple cell types during wound healing can be addressed by only using in vivo models.10 Excisional wounds in animals are widely used. Epithelization rates are usually measured by planimetry and compared.
The data generated from in vitro, ex vivo, and in vivo studies, in spite of its importance, do not always reflect the same process in humans. Unfortunately, the most important validation of the experimental data during acute wound healing in humans is limited due to difficulties in generating and obtaining acute human wound tissue.
Discussion of Findings and Relevant Literature
Stress hormones are a contributing culprit in the pathogenesis of delayed healing, but their mechanism of action has not been fully elucidated. Recent literature suggests that enzymes necessary for both cortisol and epinephrine synthesis are located in the epidermis, and keratinocytes synthesize both hormones, locally.3,6,11,12 Local cortisol and epinephrine synthesis impairs keratinocyte migration and ultimately delays epithelization.3,5 HEKs and epidermis in vitro and ex vivo, along with porcine epidermis in vivo, express CYP11B1, an enzyme necessary for the cortisol production, and produce clinically relevant levels of cortisol.
Injury-related molecules, such as pro-inflammatory cytokine IL-1β, contribute to controlling the local production of cortisol by keratinocytes. Cortisol production by keratinocytes can be induced by IL-1β during wound healing and, on the contrary, inhibition of cortisol synthesis can induce IL-1β production.3 Importantly, CYP11B1 expression and cortisol production is regulated during acute wound healing.3 This tightly regulated cortisol synthesis in the epidermis may serve as a local negative feedback loop to control the initial pro-inflammatory response and allow the acute wound healing process to proceed. However, in the setting of chronic wounds and persistent inflammation, one can speculate that this tightly controlled mechanism is deregulated.
FPP, an intermediate in cholesterol synthesis, can act as an agonist for the GR, and treatment with mevastatin, an inhibitor of cholesterol synthesis which blocks the formation of FPP, eliminates the activation of GR and enhances keratinocyte migration and wound epithelization in the acute wound setting.9 However, studies have shown that enhanced keratinocyte migratory potential is not mediated through the inhibition of cholesterol synthesis despite the disrupted skin barrier. This effect was due to the blockade of FPP's activation of the GR.9 Taken together, this finding establishes a second, upstream mechanism whereby the stress hormone pathway can be activated in epidermis. The role of FPP in the pathogenesis of chronic wounds and their nonmigratory keratinocytes needs further investigation.
Keratinocytes express β2AR for epinephrine, another stress hormone implicated in wound healing. Increased epinephrine levels are detrimental to wound healing. Activation of β2AR decreases human keratinocyte migratory speed, delays healing of an in vitro scratch wound assay, impairs healing of an excisional human skin wound ex vivo, delays healing of acute surgical wounds in vivo, and results in impairment of oral mucosal wound healing.5,13–17 Similar to CYP11B1, the epidermal level of the epinephrine synthesizing enzyme PNMT is up-regulated during burn wound injury. Burn wounding of skin induces local epidermal generation of epinephrine by up-regulation of the enzyme, PNMT, required for epinephrine synthesis in the peri-wound epidermis.6 Interestingly, the PNMT promoter has a GC response element,18 thus indicating the possibility of interaction between both stress hormone pathways during wound healing and, further, their synergistic role in inhibition of keratinocyte migration and epithelization. Although cross-talk between these two pathways may exist, further studies are needed to elucidate their role in wound healing impairment.
Comparable to GC treatment of cultured human and murine keratinocytes, treatment with levels of epinephrine equivalent to burn-stressed animals led to decreased migration rates. Activation of the β2AR in keratinocytes results in decreased signaling through the PI3K/AKT pathway and a consequential nonmigratory cell phenotype and decreased locomotory speed. This is accompanied by a stabilization of the actin cytoskeleton, an increase in focal adhesion formation, and an overall nonmigratory phenotype in HEK.
Inhibiting the production or action of stress hormones can reverse the deleterious effects on healing and keratinocyte migration. The application of metyrapone, a CYP11B1 inhibitor, significantly promotes the rate of epithelization and wound closure in in vivo and ex vivo wounds.3 Similarly, topical mevastatin, an 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor, reduces endogenous levels of FPP and increases epithelization in an ex vivo wound model.9 Further, inclusion of a β2AR antagonist, timolol, reverses the inhibitory effects of epinephrine on keratinocyte migration and wound epithelization.6 Systemic administration of a β2AR antagonist accelerates wound epithelization in an in vivo burn wound model. Taken together, these findings demonstrate the potential use of stress hormones as targets for future therapeutic approaches for burns and chronic wounds.
Take-Home Message.
Basic science advances
• Systemically released stress hormones, cortisol and epinephrine, are well known to inhibit wound healing, but until now, the mechanism of this action has not been well understood.
• The epidermis represents an extra-adrenal source of stress hormones, as keratinocytes produce both cortisol and epinephrine.
• Cortisol and FPP act as agonists to the GR. Both cortisol-GR and FPP-GR complexes target the K6 promoter to down-regulate K6 expression, thus decreasing keratinocyte migration.
• Activation of the bΒAR in keratinocytes results in decreased signaling through the PI3K/AKT pathway, thus resulting in a nonmigratory cell phenotype and decreased locomotory speed.
Clinical science advances
• Evidence from experimental wound healing models indicates that local production of stress-related hormones in keratinocytes contributes to delayed wound healing.
• Drugs such as statins and beta-blockers are widely used in the medical community, inexpensive, and maintain a good safety profile. Until recently, they have shown little benefit in wound healing.
• Mevastatin and timolol have recently shown promise in reversing the harmful effects of cortisol and epinephrine on keratinocyte migration and wound epithelization.
Relevance to clinical care
• Chronic, nonhealing wounds are a problem reaching epidemic proportions worldwide
• Stress hormones released either systemically or locally play an important role in the pathogenesis of delayed healing.
• Understanding the mechanisms responsible for impaired epithelization in chronic wounds will facilitate development of therapeutic targets
• In the future, statins and beta-blockers may be useful additions to the therapeutic armory for the treatment of delayed or impaired wound healing and burns
Innovation
The epidermis represents an extra-adrenal source of stress hormones, as keratinocytes produce cortisol and epinephrine. Cortisol and epinephrine have been shown to inhibit wound healing and are, therefore, important targets for therapeutic approaches. Two novel topical treatments, which are typically used to improve cardiovascular health, are proposed to improve wound healing: statins and beta-blockers.5,9,19 Additionally, topical treatment with metyrapone, an inhibitor of CYP11B1, enhanced keratinocyte migration and wound epithelization.
Although statins are known to lower cholesterol synthesis and reduce lipid levels, they may improve wound healing. Mevastatin reverses inhibition of epithelization by FPP and enhances the epithelization rate in acute wounds. Statins target the mevalonate pathway and have been proved to be beneficial in wound healing.19 Ongoing clinical trials in patients with burns and diabetic foot ulcers treated with statins will be helpful in evaluating the effects of systemic statins on healing outcomes.
β2AR antagonists, or beta-blockers, are widely used for the treatment of cardiac arrhythmias, cardio-protection after myocardial infarction, and hypertension. They could be a novel therapeutic option for wound healing, as beta-blockers, such as timolol, target the β2AR, and diminish the downstream effects of epinephrine during wound healing. Therefore, topical application of β2AR antagonists may represent a promising treatment for chronic wounds.
Further studies are needed to address these potential therapies in wound healing. One necessary question to be addressed is whether topical application of statins and beta-blockers is safe and whether these are clinically useful drugs for the treatment of patients with chronic, refractory wounds.
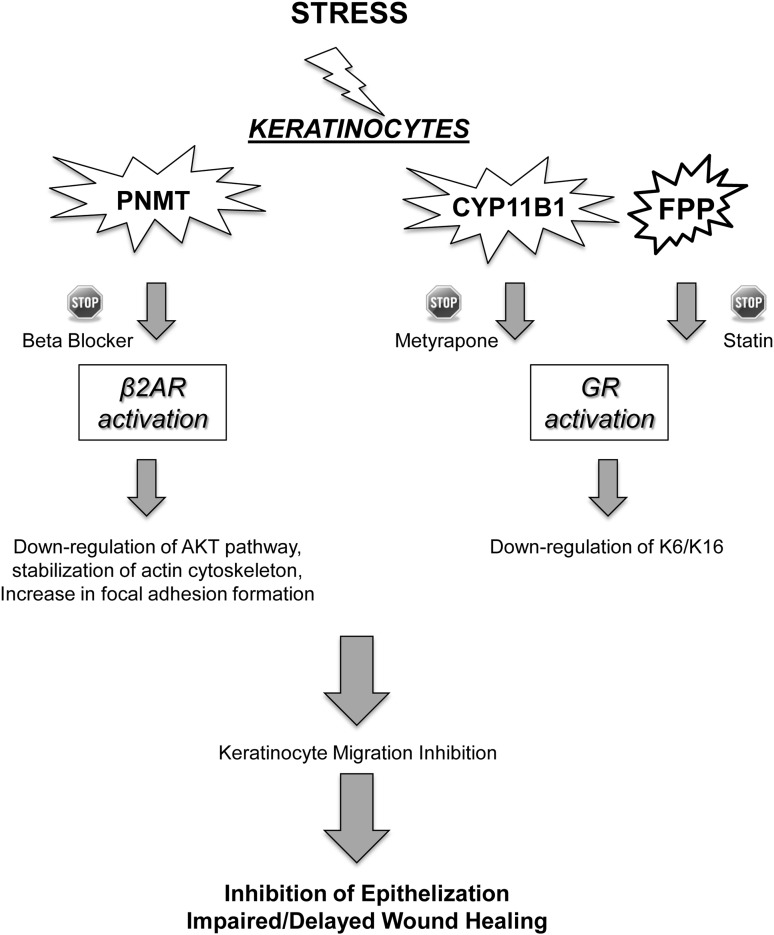
Summary Illustration
This illustration demonstrates the effect of local stress hormone production on keratinocytes migration and epithelization. Local stress hormones, cortisol, and epinephrine are produced by keratinocytes. They bind to their respective receptors, β2AR and GR, thus leading to down-regulation of the AKT pathway, stabilization of actin cytoskeleton, an increase in focal adhesion formation, and down-regulation of keratins 6 and 16. β2AR and GR activation lead to inhibition of keratinocyte migration and subsequent inhibition of epithelization and wound healing. However, inhibitors of cortisol synthesis, statins, β2AR antagonists, and systemic beta-blockers can decrease cortisol synthesis, FPP, and epinephrine levels, respectively, when topically applied to the skin, and restore keratinocyte migration capacity.
Caution, Critical Remarks, and Recommendations
Until recently, systemically released cortisol and epinephrine were recognized as the only mechanism through which stress hormones influenced wound healing.1 However, novel studies have shown that cortisol and epinephrine are also locally released during skin injury by resident keratinocytes.
IL-1β induced levels of locally released cortisol are postulated to play a role in the termination of the inflammatory phase of wound healing. It is possible that other inflammatory cytokines exert similar effects on cortisol release. Additionally, this novel finding poses a question relating to hormone regulation during chronic inflammatory states. Further research is needed to investigate this archetype of the inflammatory response present in wound healing and possibly in other inflammatory skin conditions.
In stressed individuals, elevated levels of systemic epinephrine could contribute to the inhibition of wound healing by delaying the re-epithelization in skin and cornea. However, it is likely that the dynamic process of re-epithelization is not solely dependent on the effects of epinephrine. For example, effects of statins investigated in patients with burns show that preinjury statin use is associated with an 83% reduction in the likelihood of death after burn injury.20 Therefore, the synergistic effects of cortisol and epinephrine on the inhibition of keratinocytes' locomotion during skin repair needs further exploration.
Future Development of Interest
It is well recognized that wound healing is delayed in the elderly. According to the World Health Organization (www.who.int/topics/ageing), the elderly population is growing faster than all other age groups. Nonhealing wounds, most commonly due to vascular disease, diabetes mellitus, or prolonged pressure, affect about 6.5 million people in the United States, with wounds in the elderly accounting for 85% of these.4 In addition to enormous suffering of patients, nonhealing wounds among the elderly represent a major area of unmet clinical need and lead to significant mortality. During recent years, experimental evidence emerged supporting new therapeutic revenues to accelerate epithelization, a defying parameter of wound healing. Among them, statins and beta-blockers, medications already widely used among the elderly population, may provide new therapeutic approaches for treatment of wound healing disorders. Clearly, further investigations are needed to determine the best administration route, concentration, and type of drug that wound lead to the best healing outcomes.
Abbreviations and Acronyms
- 11βHSD2
11-beta-hydroxysteroid dehydrogenase 2
- β2AR
beta-2-adrenergic receptor
- CYP11B1
11-beta-hydroxylase
- FPP
farnesyl pyrophosphate
- GR
glucocorticoid receptor
- GCs
glucocorticoids
- HEKs
human epidermal keratinocytes
- IL-1β
interleukin-1-beta
- PI3K
phosphatidylinositol 3-kinase
- PNMT
phenylethanolamine-N-methyltransferase
Acknowledgments snd Funding Sources
This work is supported by a grant from the National Institutes of Health DK086364-01.
Author Disclosure and Ghostwriting
The authors have no commercial associations to disclose. This article is not written by any writer other than the authors.
References
- 1.Gouin JP. Kiecolt-Glaser JK. The impact of psychological stress on wound healing: methods and mechanisms. Immunol Allergy Clin North Am. 2011;31:81. doi: 10.1016/j.iac.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojadinovic O. Brem H. Vouthounis C. Lee B. Fallon J. Stallcup M, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vukelic S. Stojadinovic O. Pastar I. Rabach M. Krzyzanowska A. Lebrun E, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen CK. Gordillo GM. Roy S. Kirsner R. Lambert L. Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steenhuis P. Huntley RE. Gurenko Z. Yin L. Dale BA. Fazel N, et al. Adrenergic signaling in human oral keratinocytes and wound repair. J Dent Res. 2011;90:186. doi: 10.1177/0022034510388034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sivamani RK. Pullar CE. Manabat-Hidalgo CG. Rocke DM. Carlsen RC. Greenhalgh DG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoghawala SY. Mannis MJ. Pullar CE. Rosenblatt MI. Isseroff RR. Beta2-adrenergic receptor signaling mediates corneal epithelial wound repair. Invest Ophthalmol Vis Sci. 2008;49:185. doi: 10.1167/iovs.07-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojadinovic O. Lee B. Vouthounis C. Vukelic S. Pastar I. Blumenberg M, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes: inhibition of apoptosis, interferon-gamma pathway, and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282:4021. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- 9.Vukelic S. Stojadinovic O. Pastar I. Vouthounis C. Krzyzanowska A. Das S, et al. Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor. J Biol Chem. 2010;285:1980. doi: 10.1074/jbc.M109.016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pastar I. Stojadinovic O. Krzyzanowska A. Barrientos S. Stuelten C. Zimmerman K, et al. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med. 2010;16:92. doi: 10.2119/molmed.2009.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannen RF. Michael AE. Jaulim A. Bhogal R. Burrin JM. Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. 2011;404:62. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Cirillo N. Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. 2011;112:1499. doi: 10.1002/jcb.23081. [DOI] [PubMed] [Google Scholar]
- 13.Orenberg EK. Pfendt EA. Wilkinson DI. Characterization of alpha- and beta-adrenergic agonist stimulation of adenylate cyclase activity in human epidermal keratinocytes in vitro. J Invest Dermatol. 1983;80:503. doi: 10.1111/1523-1747.ep12535068. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson DJ. Mahan JT. Influence of catecholamines on epidermal cell migration during wound closure in adult newts. Comp Biochem Physiol C. 1984;78:267. doi: 10.1016/0742-8413(84)90081-1. [DOI] [PubMed] [Google Scholar]
- 15.Chen J. Hoffman BB. Isseroff RR. Beta-adrenergic receptor activation inhibits keratinocyte migration via a cyclic adenosine monophosphate-independent mechanism. J Invest Dermatol. 2002;119:1261. doi: 10.1046/j.1523-1747.2002.19611.x. [DOI] [PubMed] [Google Scholar]
- 16.Pullar CE. Chen J. Isseroff RR. PP2A activation by beta2-adrenergic receptor agonists: novel regulatory mechanism of keratinocyte migration. J Biol Chem. 2003;278:22555. doi: 10.1074/jbc.M300205200. [DOI] [PubMed] [Google Scholar]
- 17.Pullar CE. Grahn JC. Liu W. Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J. 2006;20:76. doi: 10.1096/fj.05-4188com. [DOI] [PubMed] [Google Scholar]
- 18.Sabban EL. Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- 19.Stojadinovic O. Lebrun E. Pastar I. Kirsner R. Davis SC. Tomic-Canic M. Statins as potential therapeutic agents for wound healing disorders. Expert Rev Dermatol. 2010;5:689. [Google Scholar]
- 20.Fogerty MD. Efron D. Morandi A. Guy JS. Abumrad NN. Barbul A. Effect of preinjury statin use on mortality and septic shock in elderly burn patients. J Trauma. 2010;69:99. doi: 10.1097/TA.0b013e3181df61b1. [DOI] [PubMed] [Google Scholar]