Abstract
Background
Impaired wound healing remains a major clinical problem with many etiologies. Altering gene expression to enhance healing is an innovative therapeutic approach. In recent years, we have developed a means to topically silence genes at the post-transcriptional level to locally alter wounds and improve the healing process.
The Problem
Many types of chronic wounds have been associated with alterations in the expression of genes that mediate healing. Targeting the expression of these genes in a way that can improve healing while limiting systemic side effects has been very challenging.
Basic/Clinical Science Advances
Our laboratory's recent work has focused on the use of topically applied small interfering ribonucleic acid (siRNA) to inhibit messenger RNA expression of certain mediators involved in healing in two different types of cutaneous injury—radiation-induced cutaneous injury and the diabetic excisional wound. By successfully inhibiting specific gene mediators with topical siRNA, we reversed downstream signaling pathways, which led to expedited wound healing in diabetic wounds and restoration to a more normal phenotype in radiation-induced skin injuries.
Clinical Care Relevance
The signaling pathways and gene mediators that we targeted and inhibited in murine models are present in humans. Applying parallel treatment strategies in humans may provide novel means of treating these burdensome and costly conditions.
Conclusion
Our novel method for local gene silencing is effective in treating various types of cutaneous murine wounds. Topical gene silencing with siRNA obviates the side effects of systemic medication and has the potential to be effective in healing or preventing a wide array of cutaneous human conditions.

Pierre B. Saadeh
Background
Aberrations in the mechanisms of wound repair result in a wide spectrum of impaired healing. Grossly, pathologies can range from impaired wound healing as seen in persistent diabetic wounds, to a scarring phenotype as seen in radiation-induced skin fibrosis. Impaired healing conditions have been associated with distinct aberrations in messenger RNA (mRNA) and protein expression profiles. Therapies that could potentially target wound healing pathways at the cellular level are attractive.
Since the discovery of RNA interference in 1998,1 and its subsequent application in human cell lines in 2001,2 RNA interference through the use of small interfering ribonucleic acid (siRNA) has offered a new approach to manipulating gene expression. Briefly, siRNA is a double-stranded RNA homolog of a gene of interest that can be synthesized, modified, and administered to cells both in culture and in vivo. Once in the cytoplasm, siRNA interacts with RNA-induced silencing complexes, unwinds into single stranded RNA, and then degrades its endogenous mRNA target. This process “knocks down” the quantity of synthesized protein in the cytoplasm, which then alters any cellular signaling that involves the target gene. The specificity of siRNA therapy offers intriguing intervention modalities and we have focused its use in preventing radiation-induced skin fibrosis and in enhancing diabetic wound closure.
Target Articles.
Lee J, Tutela J, Zoumalan R, Thanik V, Nguyen P, Varjabedian L, et al.: Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surgery 2010; 136: 714.
Nguyen P, Tutela J, Thanik V, Knobel D, Allen R, Chang C, et al.: Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen 2010; 18: 553.
Clinical Problem Addressed
Pathologic wound states are associated with a lower quality of life and are a common cause of morbidity and mortality in patients. Chronic diabetic wounds are often complicated by infection and sepsis and contribute significantly to healthcare cost. Also, radiation-induced fibrosis, a common adverse effect of cancer therapy, complicates subsequent surgical intervention, leads to strictures, and heals poorly. Impairments in healing in the aforementioned cutaneous injuries are currently understood, at least in part, on a cellular pathway level.3–6 Thus, we focused our efforts on mitigating two different pathologic phenotypes by altering mRNA expression with siRNA specifically targeted to the genetic dysregulations, which characterize these pathways.
Relevant Basic Science Context
Early damage in irradiated skin consists of erythema, desquamation, and ulceration, whereas more long-term effects include skin atrophy, decreased elasticity, fibrosis, microvascular damage, and impaired wound healing.7 Transforming growth factor β (TGF-β) is a central regulator for the inflammation and fibrosis that results following radiation.3,4 Recent studies have demonstrated that the fibrosis is largely mediated by an intercellular mediator, mothers against decapentaplegic homolog 3 (SMAD3), which is downstream to activation of the TGF-β receptor.8 Once activated by the TGF-β receptor complex, SMAD3 is transported into the nucleus, where it acts as a transcription factor for genes coding for multiple types of collagen and extracellular matrix proteins.9 Recent studies revealed that Smad3 knockout mice are protected from radiation-induced cutaneous injury.10 In our investigation, we attempted to achieve similar protective effects by knocking down SMAD3 locally with topical siRNA to SMAD3. In addition, by limiting therapy to the epidermis and dermis, we attempted to obviate the untoward side effects of systemic gene knockdown.
In our second line of investigation, we aimed to improve wound closure in diabetic wounds. Recent advances in the understanding of the pathogenesis of diabetic wound healing correlated hyperglycemia, poor wound healing, and higher levels of apoptosis.11 Hyperglycemia is associated with a diminished hypoxic response, which is critical in inducing and attracting the appropriate inflammatory mediators to a wound.5 This hypoxic response is normally mediated by hypoxia-inducible factor alpha (HIF-1α), a transcription factor that plays a significant role in wound healing; HIF-1α is dramatically reduced in diabetic wound.5 Studies have also revealed a role for the tumor suppressor gene p53 in promoting the proteasomal degradation of HIF-1α.12 Tumor protein 53 (p53) is responsible for both cell arrest and apoptosis.6 As p53 (and apoptosis) is increased during diabetic wound healing,13 we postulated that locally silencing p53 expression in the diabetic wound would inhibit apoptosis and stimulate the HIF1-α dependent pathways, ultimately leading to improved wound healing.
Experimental Model or Material: Advantages and Limitations
Murine skin is composed of epidermal and dermal architecture similar to human skin.14 Further, the mechanisms of wound healing and scarring in mouse skin are also very similar to those in human skin.
One limitation to rodent wound models is that due to the laxity of their skin and the mobility of the underlying panniculus carnosus, their primary mechanism of wound healing is by epithelial contracture healing (by primary intention). In contrast, large wounds in humans heal via re-epithelialization and granulation tissue (by secondary intention).15 We circumvented this criticism by using our previously established splinted wound model16 composed of a stent enforced with sutures around a given wound, thus preventing wound contracture and allowing healing by secondary intention.
With regard to siRNA, we have developed a topical agarose matrix-based siRNA delivery system that reproducibly delivers siRNA into the epidermis and through the dermis,17 not penetrating beyond the dermis. One advantage of this is the fact that gene suppression occurs locally, thus obviating systemic delivery and undesirable side effects. Weekly application was necessary for a sustained effect as the molecules are active for 5–7 days.17 Administering topical siRNA to an open wound has potential clinical applicability for human use. We have extended this technology for use on intact skin by disrupting the stratum corneum with a detergent, which then allows siRNA penetration. This current strategy is limited by the fact that the detergent used is irritating and painful to skin; nanoparticle-based delivery systems are being devised to circumvent this limitation. Ultimately, these technologies will require additional refinements in larger animal models, particularly the porcine model, before the consideration of human use.
Discussion of Findings and Relevant Literature
Using a published model of skin-only radiation injury, topical SMAD3 siRNA treatment before irradiation demonstrated near-complete inhibition of Smad3 in the epidermis and dermis by week 1 with an effect limited to the area of application on the skin. The area of SMAD3 knockdown was limited to the treatment area. After weekly application of siRNA, SMAD3 inhibition persisted and remained limited to the dermis. Figure 1 illustrates a schematic of topical siRNA application. After 4 weeks, quantitative measurements of epidermal thickness on histology revealed treated skin to be markedly thinner compared with controls. Histology also revealed siRNA-treated skin to have dermal collagen architecture similar to that of nonirradiated skin, whereas irradiated control specimens displayed dense collagen architecture. Moreover, a comparison of tissue elasticity quantified by tensiometry determined SMAD3 siRNA-treated skin to be significantly more elastic than nonsense siRNA-treated controls, approaching the elasticity of normal, nonirradiated skin.
FIG. 1.
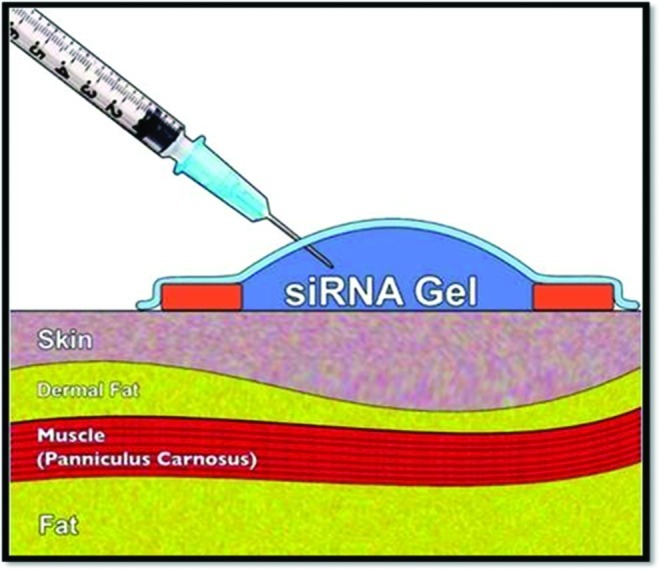
A schematic of topical small interfering ribonucleic acid (siRNA) application on intact skin. A complex of siRNA with agarose is applied over a designated treatment area of skin. siRNA penetrates through the epidermis and has intracellular effects in the epidermis and dermis. Color images available online at www.liebertpub.com/wound
By inhibiting SMAD3 expression in irradiated skin, downstream pathways responsible for collagen and extracellular matrix protein synthesis were attenuated and skin fibrosis following radiation was minimized.
In our p53-suppressed diabetic wound investigation, wounds received a complex of siRNA to p53 in agarose on postwound days 1 and 8. Complete wound closure occurred in 18 days, and robust granulation tissue was already present at 10 days. In comparison, untreated diabetic wounds healed in 28 days (wild-type nondiabetic wounds healed in 14 days).
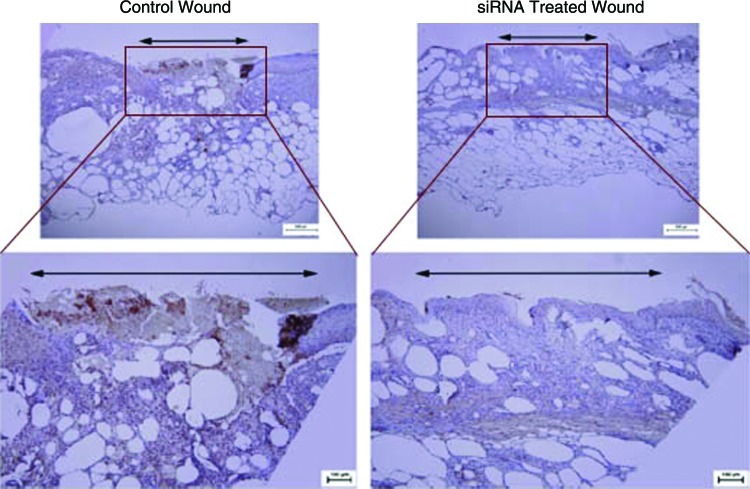
The level of p53 mRNA was shown to be suppressed three-fourths in p53 siRNA-treated wounds at 10 days postwounding. Immunohistochemistry showed near-complete knockdown of p53 protein expression as well (Fig. 2). p53 inhibition was accompanied with an increase in mRNA and protein expression of multiple vasculogenic cytokines at postwounding day 10. Specifically, there was a near 2-fold increase in HIF1-α and stromal cell-derived factor 1 (SDF-1) mRNA, and a 1.5-fold increase in vascular endothelial growth factor (VEGF) mRNA in p53 siRNA-treated wounds compared with control wounds. Parallel increases in HIF1-α, SDF-1, and VEGF protein expression were noted. Finally, p53 siRNA-treated wounds demonstrated increased vascularity (CD31 staining). Illustrating the transient effect of siRNA, p53 mRNA levels in healed wounds returned to low levels equal to controls at day 30. Moreover, rewounded formerly p53 siRNA-treated wounds and nonsense siRNA-treated (control) wounds demonstrated similar levels of p53 mRNA in their healing wounds at all healing time points.
FIG. 2.
Immunohistochemical staining for p53. Reduced expression of p53 (brown staining) is demonstrated within the p53-silenced wound beds compared with control wound beds at postwounding day 10. Color images available online at www.liebertpub.com/wound
By suppressing p53 expression in a diabetic wound, expression of HIF1-α, SDF-1, and other important mediators in the hypoxic response increased and was associated with a more normal wound healing interval and phenotype.
Better understanding of normal and pathologic wound healing pathways will provide even more selective targets for siRNA therapy. Additionally, new approaches to siRNA delivery will improve local siRNA knockdown and broaden the therapeutic scope of siRNA to more systemic pathologies.
Take-Home Message.
Basic science advances
Wound healing pathologies are grossly and mechanistically diverse. Many cutaneous conditions have been previously associated with specific cellular aberrations. Thus, cutaneous diseases or poor wound healing conditions offer an excellent opportunity for the modification of gene expression through topically applied siRNA. We showed that targeting a single upstream modulator can be an efficient and effective way to alter the expression of a target gene and the expression of the downstream products in associated cellular pathways. With the proper target, these changes can restore intracellular and extracellular environments to those that more closely resemble the normal healing state. Further, these modifications are transient, localized to the treated area, and avoid off-target effects.
Clinical science advances
Strategic modulation of wound healing pathways using transcutaneous siRNA improves healing of cutaneous pathologies. More thorough understanding of the pathways involved in wound healing will improve our outcomes through more specific targeting of downstream modulators. Further, siRNA therapy involving multiple targets may prove to be more effective than the current single-target approach.
Relevance to clinical care
Improving wound healing remains paramount to improving patient quality of life and limiting morbidity and mortality. Local manipulation of molecular targets using topically applied siRNA can yield favorable wound healing outcomes in murine models. Work is required to assure minimal patient risk in future clinical trials that employ parallel treatment strategies.
Innovation
In both investigation arms, a central cellular mediator was successfully suppressed and phenotypic pathologies in wound healing were reversed to a more normal phenotype. Our findings shed light on the potential diversity of clinical applicability of this therapeutic modality. Locally administered siRNA could potentially be used to downregulate any gene product of a known molecular target. This innovative technology can be applied to most skin pathology for which known genes are responsible, including contact hypersensitivity, autoimmune alopecia, psoriasis, scleroderma, and skin cancer ranging from basal cell carcinoma to squamous cell carcinoma to melanoma. For example, one candidate for local gene knockout with siRNA is an integrin-linked kinase, which regulates angiogenesis in melanoma18; in theory, downregulating the kinase would slow disease progression.
Caution, Critical Remarks, and Recommendations
Although our findings supporting the use of transcutaneous siRNA for the modulation of pathologic wounds are promising, a better understanding of the cellular components of the biologic pathways of normal wound healing and various states of pathologic wound healing is necessary to better target siRNA therapy. Certainly, silencing a tumor suppressor gene such as p53 even topically has theoretical risks and more selective downstream effectors of diabetic wound healing may represent more clinically relevant targets.
Finally, our transcutaneous siRNA model for intact skin (in the irradiated murine model) required a pretreatment regimen to disrupt the stratum corneum to allow siRNA penetration. Although effective, this process can be cumbersome and labor intensive. Less-invasive delivery systems need to be developed before this technology becomes more clinically applicable. Current efforts to circumscribe this problem include nanoparticle-based delivery systems.
Future Development of Interest
Truly individualized medicine will begin to evolve as technologies improve. Therapies that can safely and effectively alter gene expression will play an important role in individualized medicine. One might imagine that in the future a patient with a specific condition may be able to have their genetic data analyzed, the etiology of their pathology determined, and a specific siRNA target molecule synthesized to abrogate their disease process.
Abbreviations and Acronyms
- HIF-1α
hypoxia-inducible factor alpha
- mRNA
messenger RNA
- p53
tumor protein 53
- RNA
ribonucleic acid
- SDF1
stromal cell-derived factor 1
- siRNA
small interfering ribonucleic acid
- SMAD3
mothers against decapentaplegic homolog 3
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
This work was supported by grant 1UL1RR029893 from the National Center for Research Resources (NIH) and CORE grant 2009 from the Plastic Surgery Education Foundation/American Academy of Otolaryngology—Head and Neck Surgery Foundation.
Author Disclosure and Ghostwriting
J.L., S.W., S.M.W., and P.B.S. have no commercial associations and no financial disclosures. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
References
- 1.Fire A. Xu S. Montgomery MK. Kostas SA. Driver SE. Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Elbashir SM. Harborth J. Lendeckel W. Yalcin A. Weber K. Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 3.Muller K. Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35:96. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Martin M. Lefaix JL. Delanian S. TGF-beta 1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 5.Mace K. Yu D. Paydar K. Boudreau N. Young D. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 6.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 7.Dormand E-L. Banwell PE. Goodacre TEE. Radiotherapy and wound healing. Int Wound J. 2005;2:112. doi: 10.1111/j.1742-4801.2005.00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datto MB. Frederick JP. Pan LH. Borton AJ. Zhuang Y. Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SJ. Yuan WH. Lo ST. Trojanowska M. Varga J. Interaction of Smad3 with a proximal Smad-binding element of the human alpha 2(I) procollagen gene promoter required for transcriptional activation by TGF-beta. J Cell Physiol. 2000;183:381. doi: 10.1002/(SICI)1097-4652(200006)183:3<381::AID-JCP11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Flanders KC. Sullivan CD. Fujii M. Sowers A. Anzano MA. Arabshahi A, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J. Jiaojun D. Takami Y. Tanaka H. Yamaguchi R. Jingping G, et al. Protective effects of a free radical scavenger, MCI-186, on high-glucose-induced dysfunction of human dermal microvascular endothelial cells. Wound Repair Regen. 2004;12:607. doi: 10.1111/j.1067-1927.2004.12607.x. [DOI] [PubMed] [Google Scholar]
- 12.Ravi R. Mookerjee B. Bhujwalla ZM. Sutter CH. Artemov D. Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34. [PMC free article] [PubMed] [Google Scholar]
- 13.Kane CD. Greenhalgh DG. Expression and localization of p53 and bcl-2 in healing wounds in diabetic and nondiabetic mice. Wound Repair Regen. 2000;8:45. doi: 10.1046/j.1524-475x.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- 14.Sundberg P. Skin, Adnexa of the Laboratory Mouse. San Diego, CA: Elsevier Academic Press; 2004. [Google Scholar]
- 15.Wong VW. Sorkin M. Glotzbach JP. Longaker MT. Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol. 2011 doi: 10.1155/2011/969618. Article No. 969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galiano R. Michaels J. Dobryansky M. Levine J. Gurtner G. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 17.Thanik VD. Greives MR. Lerman OZ. Seiser N. Dec W. Chang CC, et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Therapy. 2007;14:1305. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 18.Wani AA. Jafarnejad SM. Zhou J. Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-κB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778. doi: 10.1038/onc.2010.644. [DOI] [PubMed] [Google Scholar]