Abstract
Background
Complex skin defects, such as burns and acute cutaneous trauma, are life-threatening injuries, often requiring temporary allograft placement to maintain fluid homeostasis and prevent infection until permanent wound closure is possible.
The Problem
The current standard of care for the management of full-thickness wounds that are unable to be closed in a single surgical stage is temporary coverage with cadaver allograft until an acceptable wound bed has been established. This approach has limitations including limited availability of human cadaver skin, the risk of disease transmission from cadaveric grafts, and inconsistent cadaver allograft quality.
Basic/Clinical Science
Near-diploid neonatal human keratinocyte cell line (NIKS)-based human skin tissue is a full-thickness, living human skin substitute composed of a dermal analog containing normal human dermal fibroblasts and a fully-stratified, biologically and metabolically active epidermis generated from NIKS keratinocytes, a consistent and unlimited source of pathogen-free human epidermal progenitor cells.
Clinical Care Relevance
NIKS-based human skin tissue is a living bioengineered skin substitute (BSS) intended to provide immediate wound coverage and promote wound healing through sustained expression by living cells of wound healing factors.
Conclusion
A phase I/IIa clinical trial found that NIKS-based BSS was well tolerated and comparable to cadaver allograft in the ability to prepare full-thickness complex skin defects prior to autografting. There were no deaths and no adverse events (AE) associated with this BSS. Exposure of the study subjects to the skin substitute tissue did not elicit detectable immune responses. Notably, this tissue remained viable and adherent in the wound bed for at least 7 days.

B. Lynn Allen-Hoffmann
Background
Human skin is the interface with the external environment and, as such, protects against chemicals and toxins, prevents local infection from microorganisms, regulates body temperature, and maintains fluid homeostasis. Disruption of cutaneous barrier function following significant skin trauma results in loss of fluid homeostasis and leaves the patient vulnerable to invasive bacterial infections. Complex skin defects often contain elements of both full-thickness and partial-thickness wounds admixed within the same wound areas. Although many partial-thickness wounds heal without the need for autografting, the time required to heal deep partial-thickness burns is more than three weeks.1 During this time, these wounds are at risk for infection and other complications that can delay definitive wound closure.1–3 Due to significant morbidity in terms of healing time, infection, and scarring, deep partial-thickness and full-thickness burns are treated as a single clinical entity.1,4–6 Although the current management for these complex skin defects attempts to provide coverage with split-thickness skin graft (STSG) as quickly as possible, this is not always possible. In those cases, the standard of care is temporary coverage with cadaver allograft until the wound can be definitively closed with STSG.1,7
A living, full-thickness, allogeneic, human skin substitute generated from NIKS cells (NIKS-based BSS) to provide immediate wound coverage and promote wound healing has been recently developed and is being clinically tested.8,9 This review will focus on the first-in-humans study of this BSS. NIKS cells are a near-diploid neonatal human epidermal keratinocyte progenitor cell line that exhibit keratinocyte-specific growth and differentiation characteristics yet maintain an extended lifespan in vitro.10 When cultured at the air–medium interface, NIKS generate a fully stratified epithelium with barrier function and tensile strength similar to intact skin. NIKS-based BSS consists of metabolically active cells that should serve as a source of sustained expression of wound healing factors and may reduce the need for autografting.
Target Articles.
1. Schurr MJ, Foster KN, Centanni JM, Comer AR, Wicks A, Gibson AL, Thomas-Virnig CL, Schlosser SJ, Faucher LD, Lokuta MA, and Allen-Hoffmann BL: Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma 2009; 66: 866.
2. Centanni JM, Straseski JA, Wicks A, Hank JA, Rasmussen CA, Lokuta MA, Schurr MJ, Foster KN, Faucher LD, Caruso DM, Comer AR, and Allen-Hoffmann BL: StrataGraft skin substitute is well tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg 2011; 253: 672.
Clinical Problem Addressed
It is estimated that complex skin defects, such as burns and acute trauma, affect 1.25 million people in the United States each year.11 Approximately 50,000 are hospitalized annually for burns, and 13,000 require STSG.12 Burns have accounted for 5% of the combat casualties sustained in the Iraq and Afghanistan conflicts13 and historically account for up to 20% of combat casualties.14
The current management for full-thickness and deep partial-thickness defects attempts to provide coverage with STSG as quickly as possible. In cases where there is insufficient healthy, intact skin or the condition of the patient or wound precludes STSG placement, the standard of care is temporary coverage until definitive closure with STSG can occur.1,7 In such cases, although additional products are available, the “gold standard for achieving temporary wound coverage is allograft cadaver skin.”7 Temporary coverage reduces fluid loss and infection1,15 and maintains a moist wound environment thereby promoting wound healing.1,16,17 This approach is limited by the availability of human cadaver skin, the non-trivial risk of disease transmission from cadaveric grafts,18,19 and the relatively rapid rate at which these grafts begin to be rejected.20 In patients for whom cadaver allograft is not available, other temporizing wound treatments must be used.
This chapter will focus on Food and Drug Administration (FDA) approved products. Other promising allogeneic and autologous technologies are under development and have been discussed elsewhere.21,22 Currently available products, Integra DRT® and AlloDerm®, are composed of acellular dermal analogs and have no epidermal component. AlloDerm is made from decellularized human cadaver skin, and since each lot is derived from a different donor, the risk of disease transmission varies from lot to lot. Safety concerns are associated with this type of allogeneic product.23–25 Since AlloDerm provides no barrier function, it is primarily used as a matrix for STSG. Integra DRT is composed of bovine collagen and shark glycosaminoglycan covered with a silastic membrane. While the membrane provides a barrier to water vapor loss, it has little effect on reducing the risk of infection. There is increasing concern that products of bovine origin may transmit infectious agents such as those responsible for bovine spongiform encephalopathy.
BSS composed of viable keratinocytes on dermal analogs containing living fibroblasts reproduce many structural and biological features of intact human skin and are believed to be more advantageous than acellular or biosynthetic substitutes.26 Currently approved products include Apligraf® and OrCel® (no longer produced or marketed). The keratinocytes in OrCel are not organized into a fully stratified epidermal layer and therefore do not exhibit a competent epidermal barrier.20 The only currently available, full-thickness BSS approved in the US is Apligraf.20,27 Apligraf contains keratinocytes grown on a dermal analog of bovine collagen containing living human fibroblasts. Safe and reliable human cell sourcing is a well-recognized problem.28 New cell banks must be generated, tested, and qualified for clinical use as existing cell banks are depleted. Additionally, Apligraf is difficult to work with20,29 and has documented problems with suspected wound infection.30 This product is indicated for chronic skin wounds only after first line therapies fail and has not been approved for the management of complex skin defects resulting from severe burns or other large skin trauma.
Autologous keratinocyte cultures, such as Epicel® from Genzyme Biosurgery, consist of thin sheets of poorly differentiated keratinocytes which provide no dermal component nor barrier function. Epicel is fragile, difficult to handle, requires several weeks to prepare, has a shelf life of 24 hours, and is associated with failure rates of 50%. There are no full-thickness autologous skin substitutes approved for the treatment of severe burns or other complex skin defects. A significant medical need exists for the development of innovative, therapeutic BSS that recapitulate the normal barrier function of intact human skin, speed wound re-epithelialization, and reduce time to wound closure.
Relevant Basic Science Context
Skin substitutes encompass a wide range of biomaterials, which may or may not contain living cells. The materials and cells used to generate skin substitutes determine what anatomical structures and functional features of human skin will be delivered to the wound to facilitate healing and tissue repair. Shevchenko and coworkers have recently reviewed the commercially available skin substitute products which can be classified according to the anatomical structure engineered (dermo-epidermal, epidermal, dermal), the duration of coverage (permanent, semi-permanent, temporary), and type of biomaterial (biological: autologous, allogeneic, xenogeneic; synthetic: biodegradable, non-biodegradable).31 To date, the most advanced products contain living human cells that generate the dermal and epidermal architecture of human skin as well as key functions of skin. Living BSS contain epidermal keratinocytes which provide protective barrier function through terminal differentiation and stratification, and promote wound healing through sustained expression of cytokines, host defense peptides (HDP), and other wound healing factors.20,26,29,32 Human keratinocytes express major histocompatibility complex (MHC) class I antigens, but lack constitutive expression of MHC class II antigens33,34 and therefore are considered “non-professional” antigen-presenting cells.35–37 Because these types of skin substitutes do not contain professional antigen-presenting cells, such as Langerhans cells, acute rejection is not anticipated, and replacement by patient keratinocytes over time is expected.38 Extensive clinical experience with Apligraf and other allogeneic treatments containing both epithelial cells as well as dermal fibroblasts show a lack of acute immune responses; however, it has also been shown that the cellular components of these treatments are lost within 6 to 8 weeks of placement.39,40
Experimental Model or Material: Advantages and Limitations
Although a number of skin substitutes have been developed and marketed to treat various skin defects, barriers to clinical success, ranging from limited efficacy to economic concerns, remain. The physical and functional characteristics of an ideal biologic skin substitute, originally described by Pruitt and Levine over 25 years ago41 have recently been expanded upon by others.29,32 The skin substitute should be elastic yet possess tensile strength, be translucent to permit direct observation of the healing process, and conform to the wound bed with rapid and sustained adherence to the wound surface. To increase the rate of healing and enhance the probability of wound closure, a number of desired functional parameters are noted. The skin substitute should not be antigenic, exhibit systemic or local toxicity, or increase the rate of infection. It should possess barrier function similar to that of normal skin and, ideally, inhibit the growth of microorganisms. Patient discomfort and the need for additional nursing care should be minimized. Finally, it should be an affordable treatment option with a long shelf life under convenient storage conditions. An abundant and consistent source of genetically uniform, pathogen-free human epidermal progenitor cells would facilitate the generation of skin substitute tissues with the physical and functional properties described above.
A limitation of all cell-based BSS therapies, regardless of the use of allogeneic or autologous cells, is the high cost of manufacturing. However, the potential to shorten hospitalization times due to reduced need for STSG harvesting and the possibility for improved wound healing may offset this limitation. In addition, because the NIKS-based BSS is produced from a long-lived cell source, it is anticipated that reduced cell testing requirements will reduce cost below that of other cell-based BSS. Because of the allogeneic nature of all cell-based BSS therapies, there remains a possibility for a directed immune response. However, extensive data for other cellular therapies (e.g., Apligraf, cultured epithelial keratinocytes, Dermagraft, OrCel, and ICX-SKN) suggest that cultured skin tissue is unlikely to be acutely rejected yet does get replaced over time.42–47 The precise mechanism for this is unclear, but it may be related to the fact that cultured skin substitute tissue lacks many of the cell types (e.g., endothelial or Langerhans cells) that play a role in cadaver allograft rejection, while still expressing minor histocompatibility antigens on the allogeneic cells.43,48,49
Discussion of Findings and Relevant Literature
A phase I/IIa, multicenter, randomized, safety and dose escalation trial was conducted to compare the ability of NIKS-based BSS to cadaver allograft (the standard of care) to condition and serve as a temporary covering for the full-thickness component of complex skin defects prior to subsequent autografting. Results of this trial demonstrate that this BSS tissue, a biologically active, fully stratified, temporary wound covering generated using NIKS keratinocytes, was well tolerated and showed no evidence of safety concerns.8,9
Expression of the host defense peptide, human beta defensin-3, is increased in NIKS-based BSS
Due to the increasing importance of HDPs in combating infection and modulating host immune responses, prior to clinical evaluation the expression of human beta defensin-3 (HBD-3) was examined. Quantitative polymerase chain reaction analysis revealed an approximately 8-fold increase in HBD-3 in NIKS-based BSS when compared to skin tissue from either primary human epidermal keratinocytes or the parental keratinocytes from which NIKS arose. Furthermore, when tissue was incubated in the presence of Staphylococcus carnosus, a bacteria known to be sensitive to antimicrobials, the bacterial number was reduced by >50%.
Clinical trial design
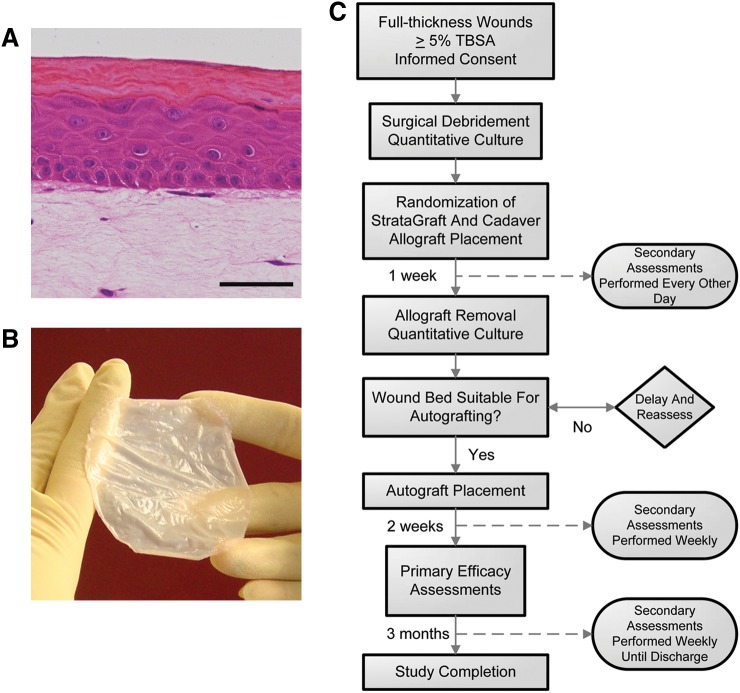
Fifteen patients with complex skin defects of ≥5% total body surface area resulting from burns, infections, and/or degloving injuries were enrolled in this study. NIKS-based BSS and cadaveric allograft were placed side-by-side in the full-thickness component of these complex skin defects. One week after placement, both allografts were removed and the wound bed was autografted when it was judged by the physician to be suitable to accept an autograft. The primary endpoint was percentage of autograft take 2 weeks after autograft placement. Autograft healing and wound site infection were assessed at 1, 2, 3, 4, and 8 weeks after autograft placement and then again at 3 months (study completion). Secondary endpoints included incidence of infection, appearance of the allograft tissues and underlying wound beds prior to autografting, and assessment of immunological responses to the cellular components of NIKS-based BSS. Safety assessments included monitoring of AE based on a modified World Health Organization AE grading scale developed for use in cancer patients,50 vital signs, hematologic parameters, incidence of wound infection, and immunologic responses.
Condition of the wound bed was comparable between NIKS-based BSS and cadaver allografts
Evaluation of the wound bed condition two weeks after autograft placement confirmed that autograft take was equivalent and nearly 100% in wound sites treated with skin substitute tissue or cadaver allograft. Furthermore, no significant differences in autograft appearance were noted. Prior to autografting, additional secondary endpoints were assessed. Like cadaver allograft, skin substitute tissue remained intact, preserving its physical characteristics after placement on wounds. Notably, the appearance of NIKS-based BSS on the wound bed was significantly better than cadaver allograft (p<0.010) as judged by appearance, color, and adherence. After allograft removal, the appearance of the wound bed and suitability for autografting was found to be equivalent for both allografts. Presence of infection was evaluated visually and through quantitative cultures of the wound bed at allograft removal. No differences were found and wound beds were not clinically infected (>105 organisms/gram tissue).
BSS tissue remained viable one week after placement
After 7 days in the wound bed, structure, composition, and viability of NIKS-based BSS was assessed and compared to cadaver allograft. Both allograft types maintained appropriate tissue architecture with defined basal and differentiated keratinocyte layers. To ascertain viability, immunohistochemical (IHC) staining for Ki67, found only in replicating cells,51 was performed. In NIKS-based BSS, Ki67 staining was observed in both basal and suprabasal keratinocytes, consistent with cellular proliferation during wound healing,52 In contrast, Ki67 staining in cadaver allografts was highly variable.
Allograft tissues did not induce acute inflammatory infiltrate in vivo
As a potential indication of graft rejection, IHC staining for T lymphocytes, B lymphocytes, and Langerhans cells was performed on the allografted tissues which had been placed in the wound bed for 1 week. No acute inflammatory infiltrate was found in any of the allografted tissues. The data presented9 show that after placement of NIKS-based BSS for 1 week in full-thickness wounds, normal tissue architecture was maintained with no substantial inflammatory infiltrate seen. These data are suggestive that this BSS did not induce an acute inflammatory response in patients with full-thickness skin loss and is in agreement with clinical experience with other allogeneic products as described above.
Skin substitutes generated from NIKS cells do not express key costimulatory molecules in vitro
Other studies have shown that human keratinocytes do not constitutively express MHC class II or co-stimulatory molecules and are therefore less likely to induce an immunological response unless stimulated.37 Preclinical studies demonstrated that NIKS-based BSS is negative for MHC class II human leukocyte antigen DR (HLA-DR) staining.9 Moreover, expression of the co-stimulatory molecules B7-1, B7-2, and CD40 was not detectable. Similar to other skin substitutes,53 in vitro exposure of tissues to γ-interferon (IFN-γ) was sufficient to enhance MHC class I HLA-ABC expression and induce the expression of class II HLA-DR and CD40. However, even at high concentrations of IFN-γ, expression of the key co-stimulatory molecules B7-1 or B7-2 was not detected. This suggests that although NIKS cells will likely be recognized as allogeneic by immunocompetent recipients through expression of class I antigens and minor histocompatibility antigens, they lack robust constitutive antigen presentation capability typically involved in acute rejection.
MHC class II expression is not detectable in epidermal cells after placement in full-thickness wounds
In order to examine acute inflammatory responses directed against the allograft tissues after placement on the wound bed for seven days, IHC staining for MHC class I and II molecules was completed. Staining showed expression of HLA-ABC on the keratinocytes; however, few HLA-DR positive cells were found in the epidermis of either skin substitute or cadaver allografts. Moreover, the morphology of the few HLA-DR positive cells seen within the epidermis was indicative of dendritic cells and not of keratinocytes. The data presented suggest the NIKS keratinocytes are not likely to act as antigen presenting cells and activate acute cellular immune responses directly.9 This observation is in agreement with data shown for other allogeneic therapies as discussed above.
Patients did not develop antibody responses targeted to the HLA types of NIKS or dermal fibroblasts found in BSS tissue
For clinical evaluation of a cell-based, biologic therapy, it is critical to evaluate the immunogenicity of the product during patient exposure. The panel reactive antibody (PRA) levels were measured prior to allograft placement, at the time of allograft removal, and after 3 months at study completion. Six of the 15 patients exhibited elevated PRA values for at least one time point; however, all these patients developed antibody reactivities to HLA antigens that are not present in NIKS-based BSS. One patient developed antibodies to a single NIKS HLA allele, however, it is unlikely to be specifically directed against the cells of the NIKS-based BSS since that individual also developed antibody specificities against other HLA alleles not expressed in BSS tissue. In addition, the individual did not develop antibodies to other HLA antigens on the NIKS keratinocytes. These data indicate that NIKS-based BSS is not acutely antigenic and does not elicit a potent antibody response.
Innovation
As previously mentioned, the need for repeated cell sourcing is a well-recognized problem in the field of biological therapies.28 Although advances in tissue engineering have enabled development of therapeutic human skin substitutes, the use of primary keratinocytes necessitates continual cell sourcing as existing cell banks are depleted. Once produced, each new bank must undergo expensive and exhaustive pathogen screening and tumorigenicity testing to be qualified for clinical use. Furthermore, due to the differences in individuals from which epidermal keratinocytes are harvested, genetic variability is a constant challenge. The use of NIKS epithelial progenitor cells offers cost advantages. As required by the Center for Biologics Evaluation and Research branch of the FDA, NIKS keratinocytes have been thoroughly examined and found to be non-tumorigenic and free of detectable viral or adventitious agents. The long-lived phenotype of the NIKS cells ensures an abundant and constant source of non-tumorigenic, pathogen-free cells.
As described in brief above, data from the Phase I/IIa trial showed no evidence of safety concerns after exposure to NIKS-based BSS for 7 days.8,9 Additional clinical testing is currently underway to assess longer exposure to NIKS-based BSS. Based on the initial study and in light of over 20 years of clinical experience with other cellularized, allogeneic skin therapies, it is anticipated that the allogeneic cells are not likely to persist and will eventually be replaced by the patient's own cells as the wound heals. Preclinical evidence supports this;54 however, the persistence and immunogenicity of NIKS-based BSS will need to be more thoroughly evaluated in a clinical setting.
Take-Home Messages.
Basic science advances
NIKS-based BSS is a full-thickness, living human skin substitute composed of a fibroblast-containing dermal analog and a fully stratified, biologically and metabolically active epidermis.
NIKS keratinocytes are a consistent and unlimited source of pathogen-free human epidermal progenitor cells.
Unlike primary keratinocytes, NIKS cells are amenable to genetic engineering using non-viral means.
Clinical science advances
A Phase I/IIa clinical trial found that NIKS-based BSS was well tolerated and comparable to cadaver allograft in the temporary coverage of full-thickness complex skin defects prior to autografting.
There were no deaths and no AE deemed to be associated with the use of NIKS-based BSS.
Exposure to NIKS-based BSS did not elicit any detectable acute immune response in the study subjects.
NIKS-based BSS remained viable and adherent in the wound bed for at least 7 days.
Relevance to clinical care
BSS tissue is intended to provide immediate wound coverage and promote wound healing through sustained expression of wound healing factors.
The NIKS cells are uniquely suited for genetic engineering approaches to produce genetically modified skin substitute tissues designed to secrete elevated levels of specific therapeutic factors.
The ability to specifically target known deficiencies or obstacles to healing presents an important new therapeutic option for the treatment of these life-threatening injuries.
Summary Illustration
NIKS-based BSS consists of a fully stratified epidermal layer overlayed onto a dermal equivalent containing human dermal fibroblasts (A). NIKS keratinocytes exhibit the differentiation characteristics of native human interfollicular epidermis including formation of appropriate tissue architecture, production, and organization of cell-type-specific proteins, development of a cornified envelope, and production and assembly of the extracellular lipid lamellae essential for normal barrier function. The epidermal layer of NIKS-based BSS exhibits tissue-specific stratification and possesses basal, spinous, granular, and cornified layers. A single layer of cuboidal basal cells rests at the junction of the epidermis and the dermal equivalent. Following mitotic division, cells in the basal layer move upward and undergo normal epidermal differentiation, ultimately producing enucleated squames. The basal cell layer continues to produce differentiating cells through normal mitosis as long as cultures are maintained in a suitable growth environment. Basal cells also produce adhesion proteins and form normal desmosomes and hemidesmosomes that permit tight cell-cell and cell-matrix adherence. The well-developed cornified layer confers an epidermal permeability barrier comparable to that of normal human epidermis. These features enable production of a durable, suturable skin substitute with excellent handling characteristics that is amenable to mechanical meshing prior to placement (B). For clinical evaluation, NIKS-based BSS was manufactured according to current good manufacturing practices requirements. The clinical study briefly overviewed above was designed to compare the ability of NIKS-based BSS to cadaver allograft to condition and temporarily cover the full-thickness component of complex skin defects prior to autografting (C). In this study, the primary endpoint was the percentage of autograft take 2 weeks after autograft placement.
Caution, Critical Remarks, and Recommendations
The study that was the subject of the reviewed articles was designed to assess safety. Further clinical evaluation is needed to determine whether NIKS-based BSS is effective in promoting wound healing in patients with complex skin defects resulting from burns or other acute trauma. Although the standard of care differs for full-thickness and partial-thickness wounds, almost all full-thickness wounds exist within the context of surrounding partial-thickness injuries. In addition, some areas of these wounds are indeterminate in depth and some areas of partial-thickness injury may convert to full-thickness injury after initial clinical assessment. Therefore, complex wounds are best viewed as a continuum that requires effective management of both full and partial-thickness components. Although the clinical trial discussed above is focused on the temporary coverage of full-thickness skin defects prior to autografting, it will be necessary to assess the safety and efficacy of NIKS-based BSS for the treatment of partial-thickness wounds for longer periods of time.
Future Development of Interest
Specific production of factors known to enhance wound healing or combat infection could be employed to further enhance therapeutic efficacy.55 Unlike primary keratinocytes, human NIKS keratinocytes are amenable to genetic engineering using non-viral means. This eliminates the regulatory challenges associated with traditional viral approaches. Subsequent selection of clonally pure populations of stably transfected cells ensures a consistent, genetically enhanced BSS with the potential to further improve wound healing through the secretion of elevated levels of specific therapeutic factors. Using this approach, skin tissues engineered to produce enhanced levels of antimicrobial factors have already been created.56 The ability to specifically target known deficiencies or obstacles that delay healing of complex skin defects presents an important new therapeutic option for the treatment of these life-threatening injuries.
Abbreviations and Acronyms
- AE
adverse events
- BSS
bioengineered skin substitute
- HBD-3
human beta defensin-3
- HDP
host defense peptide
- HLA
human leukocyte antigen complex
- IFN-γ
γ-interferon
- IHC
immunohistochemical
- MHC
major histocompatibility complex
- NIKS
near-diploid neonatal human keratinocyte cell line
- PRA
panel reactive antibody
- STSG
split-thickness skin graft
Acknowledgments and Funding Sources
The studies described within this chapter were sponsored by Stratatech Corporation, Madison, Wisconsin. Please note that this research was also funded in part by Howard Hughes Medical Institute, University of Wisconsin Department of Surgery, National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R43-AR47499, R41-AR050349, R42-AR050349, R44-AR47499) and National Heart, Lung, and Blood Institute (R01-HL74284).
Author Disclosure and Ghostwriting
Mary Lokuta, Cathy Rasmussen, and Christina Thomas-Virnig are employees of Stratatech Corporation. B. Lynn Allen-Hoffmann is the founder and CEO of Stratatech Corporation. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
References
- 1.Kagan RJ. Peck MD. Ahrenholz DH. Hickerson WL. Holmes JH. Korentager RA, et al. American Burn Association White Paper: Surgical Management of the Burn Wound and Use of Skin Substitutes. American Burn Association. 2009:1–44. doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- 2.Han A. Zenilman J. Melendez J. Shirtliff M. Agostinho A. James G, et al. The importance of a multifaceted approach to characterizing the microbial flora of chronic wounds. Wound Repair and Regen. 2011;19:532. doi: 10.1111/j.1524-475X.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarus G. Wound microbiology: tabula rosa, a blank slate. Wound Repair and Regen. 2011;19:531. doi: 10.1111/j.1524-475X.2011.00727.x. [DOI] [PubMed] [Google Scholar]
- 4.Dziewulski P. Barret JP. Assessment, operative planning and surgery for burn wound closure. In: Wolf SE, editor; Herndon DE, editor. Burn Care. Austin, TX: Landes Bioscience; 1999. pp. 19–52. [Google Scholar]
- 5.Pham TN. Gibran NS. Heimbach DM. Evaluation of the burn wound: management decisions. In: Herndon DN, editor. Total Burn Care. Galveston, TX: Saunders; 2007. pp. 119–126. [Google Scholar]
- 6.Muller M. Gahankari D. Herndon DN. Operative wound management. In: Herndon DN, editor. Total Burn Care. Galveston, TX: Saunders; 2007. pp. 177–195. [Google Scholar]
- 7.Hansbrough JF. Franco ES. Skin replacements. Clinics in Plastic Surgery. 1998;25:407. [PubMed] [Google Scholar]
- 8.Schurr MJ. Foster KN. Centanni JM. Comer AR. Wicks A. Gibson AL, et al. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma-Injury Infect Crit Care. 2009;66:866. doi: 10.1097/TA.0b013e31819849d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centanni JM. Straseski JA. Wicks A. Hank JA. Rasmussen CA. Lokuta MA, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds. Annals of Surgery. 2011;253:672. doi: 10.1097/SLA.0b013e318210f3bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen-Hoffmann BL. Schlosser SJ. Ivarie CA. Sattler CA. Meisner LF. O'Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114:444. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 11.Brigham PA. McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Mass casualties: burns. Centers for Disease Control and Prevention. www.bt.cdc.gov/masscasualties/burns.asp www.bt.cdc.gov/masscasualties/burns.asp
- 13.Kauvar DS. Wolf SE. Wade CE. Cancio LC. Renz EM. Holcomb JB. Burns sustained in combat explosions in Operations Iraqi and Enduring Freedom (OIF/OEF explosion burns) Burns. 2006;32:853. doi: 10.1016/j.burns.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Cancio LC. Horvath EE. Barillo DJ. Kopchinski BJ. Charter KR. Montalvo AE, et al. Burn support for Operation Iraqi Freedom and related operations, 2003 to 2004. J Burn Care Rehabil. 2005;26:151. doi: 10.1097/01.bcr.0000155540.31879.fb. [DOI] [PubMed] [Google Scholar]
- 15.Boyce ST. Kagan RJ. Meyer NA. Yakuboff KP. Warden GD. The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J Burn Care Rehabil. 1999;20:453. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 16.Svensjo T. Pomahac B. Yao F. Slama J. Eriksson E. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000;106:602. discussion 613–614. [PubMed] [Google Scholar]
- 17.Atiyeh BS. El-Musa KA. Dham R. Scar quality and physiologic barrier function restoration after moist and moist-exposed dressings of partial-thickness wounds. Dermatol Surg. 2003;29:14. doi: 10.1046/j.1524-4725.2003.29002.x. [DOI] [PubMed] [Google Scholar]
- 18.Archibald L. Jernigan D. Kainer M. Update: Allograft-associated bacterial infections—United States, 2002. Morbidity and Mortality Weekly Report. 2002;51:207. [PubMed] [Google Scholar]
- 19.Obeng MK. McCauley RL. Barnett JR. Heggers JP. Sheridan K. Schutzler SS. Cadaveric allograft discards as a result of positive skin cultures. Burns. 2001;27:267. doi: 10.1016/s0305-4179(00)00116-9. [DOI] [PubMed] [Google Scholar]
- 20.Eisenbud D. Huang N. Luke S. Silberklang M. Skin substitutes, wound healing: current status and challenges. Wounds. 2004;16:2. [Google Scholar]
- 21.Boyce ST. Kagan RJ. Greenhalgh DG. Warner P. Yakuboff KP. Palmieri T, et al. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006;60:821. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 22.Gravante G. Di Fede MC. Araco A. Grimaldi M. De Angelis B. Arpino A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Food and Drug Administration. Enforcement Report for April 2, 2008: AlloDerm recall
- 24.Food and Drug Administration. Enforcement Report for April 16, 2008: AlloDerm recall
- 25.Food and Drug Administration. Recall of Human Tissue Products-LifeCell Corp September 30, 2005: AlloDerm recall
- 26.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:874. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 27.Parenteau NL. Bilbo P. Nolte CJ. Mason VS. Rosenberg M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology. 1992;9:163. doi: 10.1007/BF02521744. [DOI] [PubMed] [Google Scholar]
- 28.Griffith LG. Naughton G. Tissue engineering: current challenges and expanding opportunities. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 29.Hrabchak C. Flynn L. Woodhouse KA. Biological skin substitutes for wound cover and closure. Expert Review of Medical Devices. 2006;3:373. doi: 10.1586/17434440.3.3.373. [DOI] [PubMed] [Google Scholar]
- 30.Novartis. www.pharma.us.novartis.com/product/pi/pdf/apligraf.pdf. 2002. www.pharma.us.novartis.com/product/pi/pdf/apligraf.pdf
- 31.Shevchenko RV. James SL. James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2009;7:229. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrenreich M. Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 33.Barnstable CJ. Jones EA. Crumpton MJ. Isolation, structure and genetics of HLA-A, -B, -C and -DRw (Ia) antigens. Br Med Bull. 1978;34:241. doi: 10.1093/oxfordjournals.bmb.a071504. [DOI] [PubMed] [Google Scholar]
- 34.Daar AS. Fuggle SV. Fabre JW. Ting A. Morris PJ. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984;38:287. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Daar AS. Fuggle SV. Fabre JW. Ting A. Morris PJ. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984;38:293. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Wikner NE. Huff JC. Norris DA. Boyce ST. Cary M. Kissinger M, et al. Study of HLA-DR synthesis in cultured human keratinocytes. J Invest Dermatol. 1986;87:559. doi: 10.1111/1523-1747.ep12455746. [DOI] [PubMed] [Google Scholar]
- 37.Morhenn VB. Benike CJ. Cox AJ. Charron DJ. Engleman EG. Cultured human epidermal cells do not synthesize HLA-DR. J Invest Dermatol. 1982;78:32. doi: 10.1111/1523-1747.ep12497875. [DOI] [PubMed] [Google Scholar]
- 38.Hu S. Kirsner RS. Falanga V. Phillips T. Eaglstein WH. Evaluation of Apligraf persistence and basement membrane restoration in donor site wounds: a pilot study. Wound Repair Regen. 2006;14:427. doi: 10.1111/j.1743-6109.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 39.Phillips TJ. Provan A. Colbert D. Easley KW. A randomized single-blind controlled study of cultured epidermal allografts in the treatment of split-thickness skin graft donor sites. Arch Dermatol. 1993;129:879. [PubMed] [Google Scholar]
- 40.Griffiths M. Livingstone R. Price R. Navsaria H. Survival of Apligraf in acute human wounds. Tissue Eng. 2004;10:1180. doi: 10.1089/ten.2004.10.1180. [DOI] [PubMed] [Google Scholar]
- 41.Pruitt BA., Jr. Levine NS. Characteristics and uses of biologic dressings and skin substitutes. Arch Surg. 1984;119:312. doi: 10.1001/archsurg.1984.01390150050013. [DOI] [PubMed] [Google Scholar]
- 42.Eaglstein WH. Iriondo M. Laszlo K. A composite skin substitute (graftskin) for surgical wounds. A clinical experience. Dermatol Surg. 1995;21:839. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 43.Trent JF. Kirsner RS. Tissue engineered skin: Apligraf, a bi-layered living skin equivalent. Int J Clin Pract. 1998;52:408. [PubMed] [Google Scholar]
- 44.OrTec International I: Summary of Safety and Effectiveness Data for OrCel. 2001.
- 45.Brain A. Purkis P. Coates P. Hackett M. Navsaria H. Leigh I. Survival of cultured allogeneic keratinocytes transplanted to deep dermal bed assessed with probe specific for Y chromosome. BMJ. 1989;298:917. doi: 10.1136/bmj.298.6678.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advanced BioHealing: Dermagraft® Human Fibroblast-Derived Dermal Substitute. 2007.
- 47.Boyd M. Flasza M. Johnson PA. Roberts JS. Kemp P. Integration and persistence of an investigational human living skin equivalent (ICX-SKN) in human surgical wounds. Regen Med. 2007;2:363. doi: 10.2217/17460751.2.4.363. [DOI] [PubMed] [Google Scholar]
- 48.Wilkins LM. Watson SR. Prosky SJ. Meunier SF. Parenteau NL. Development of a bilayered living skin construct for clinical applications. Biotechnol Bioeng. 1994;43:747. doi: 10.1002/bit.260430809. [DOI] [PubMed] [Google Scholar]
- 49.Nemecek GM. Dayan AD. Safety evaluation of human living skin equivalents. Toxicol Pathol. 1999;27:101. doi: 10.1177/019262339902700118. [DOI] [PubMed] [Google Scholar]
- 50.Miller AB. Hoogstraten B. Staquet M. Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 51.Gerdes J. Lemke H. Baisch H. Wacker HH. Schwab U. Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710. [PubMed] [Google Scholar]
- 52.Patel GK. Wilson CH. Harding KG. Finlay AY. Bowden PE. Numerous keratinocyte subtypes involved in wound re-epithelialization. J Invest Dermatol. 2006;126:497. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- 53.Laning JC. DeLuca JE. Hardin-Young J. Effects of immunoregulatory cytokines on the immunogenic potential of the cellular components of a bilayered living skin equivalent. Tissue Eng. 1999;5:171. doi: 10.1089/ten.1999.5.171. [DOI] [PubMed] [Google Scholar]
- 54.Rasmussen CA. Gibson AL. Schlosser SJ. Schurr MJ. Allen-Hoffmann BL. Chimeric composite skin substitutes for delivery of autologous keratinocytes to promote tissue regeneration. Annals of Surgery. 2010;251:368. doi: 10.1097/SLA.0b013e3181c1ab5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mansbridge J. Tissue-engineered skin substitutes. Expert Opin Biol Ther. 2002;2:25. doi: 10.1517/14712598.2.1.25. [DOI] [PubMed] [Google Scholar]
- 56.Thomas-Virnig CL. Centanni JM. Johnston CE. He LK. Schlosser SJ. Van Winkle KF, et al. Inhibition of multidrug-resistant Acinetobacter baumannii by nonviral expression of hCAP-18 in a bioengineered human skin tissue. Mol Ther. 2009;17:562. doi: 10.1038/mt.2008.289. [DOI] [PMC free article] [PubMed] [Google Scholar]