Abstract
Background
Prior to 2009, research regarding the role of CXC receptor 3 (CXCR3) in cutaneous biology was primarily in the context of inflammatory reactions. Foundational research performed at that time demonstrated that, in addition to recruited inflammatory cells, cellular components of the skin, keratinocytes, fibroblasts, and endothelial cells, also express CXCR3 and are capable of expressing CXCR3 ligands, specifically CXC ligand 10 (CXCL10) and CXCL11. Surprisingly, in vitro experimentation demonstrated differential effects on the different cell types, suggesting that the CXCR3 signaling pathway may serve as a coordinator of wound remodeling. In support of this, a CXCR3 null mouse line and a mouse line abrogating CXCL11 expression in the epidermis demonstrated delayed wound closure and disordered dermal wound healing.
The Problem
These findings demonstrate the role of CXCR3 signaling in the latter stages of wounding healing and opened a new avenue of investigation into the molecular and cellular mechanisms of coordinating the events of cutaneous tissue regeneration.
Basic Science Advances
More recent investigation highlights the role of CXCR3 signaling in the dramatic vascular pruning events after the proliferative stage of wound healing and its importance in guiding remodeling of dermal collagen during cicatrix formation.
Conclusion
CXCR3 signaling plays a strong role in coordinating the actions of several cell types during cutaneous wound healing. The disruption of this signaling pathway results in delayed return to homeostasis and dystrophic scarring.

Arthur C. Huen
Background
Recent years have brought much attention to the mechanisms of cutaneous wound healing. The sources of this attention are rather diverse. With a shift toward an older population comes an increasing number of chronic wounds complicating chronic diseases including diabetes, peripheral vascular disease, and cardiovascular disease.1
On the other hand, demand for products and procedures preserving youthful appearance or promoting skin rejuvenation has created a growing cosmetics industry. Many of the surgical procedures, devices, and treatments used to achieve skin rejuvenation are based on selective or nonselective skin wounding and subsequent wound remodeling to achieve more normal and, hopefully, more youthful-appearing skin.1,2
At the gross level, major themes in wound healing have been already uncovered, including the systematic progression of cutaneous wounds through the phases of hemostasis, inflammation, proliferation, and remodeling. Many of the key cell types, the factors that act upon them, and the products produced by cells in the wound bed have also been identified. Yet, mechanisms and signals that dictate progression from one phase of wound healing to another are to be yet clearly defined. Further, a clear understanding of how to treat chronic wounds, achieve scar-less wound healing, or, to a greater extent, reconstitute limbs much like our smaller amphibious vertebrate counterparts remains elusive.
Two reports published last year completed an initial survey of a signaling pathway that coordinates the return of the wound environment to a homeostasis closely resembling the prewound condition.3,4 The interesting aspect of this pathway is that stimulation of the same receptor in various cell types was demonstrated to activate overlapping sets of the same downstream effectors, but with widely different cellular behaviors. The following will summarize role of chemokine receptor CXC receptor 3 (CXCR3) and its ligands, namely, CXC ligand 10 (CXCL10) and CXCL11, on the progression of wound healing from the proliferative to remodeling phases of wound healing.
Target Articles.
1. Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, and Wells A: Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 2010; 176: 1743.
2. Bodnar RJ, Yates CC, Rodgers ME, Du X, and Wells A: IP-10 induces dissociation of newly formed blood vessels. J Cell Sci 2009; 122 (Pt 12): 2064.
Clinical Problem Addressed
In the clinical setting, closure of acute and chronic wounds is often a primary endpoint. However, the remodeling of the wound continues for a long period of time after wound closure, with this remodeling ultimately determining whether the wound recurs or fibrotic scarring ensues. Understanding derangements of this phase of wound healing may help explain for the variability in the appearance and strength of the resolved wound as well as the pathogenesis of chronic wounds.
Relevant Basic Science Context
Initial studies demonstrated that wounded keratinocyte cultures express chemokines, namely, CXCL11 (also known as interferon-inducible T-cell alpha chemoattractant [ITAC] and inducible protein 9 [IP-9]), which serve as ligands for chemokine receptor CXCR3.5 Although this chemokine promoted migration of keratinocytes, it inhibited migration of fibroblasts. The signaling pathway for both of these paradoxical effects was via signaling CXCR3, with ultimate activation of calpains, intracellular signaling proteases5,6; of note, in keratinocytes, CXCR3 signaling activated μ-calpain to loosen the adhesions for migration, whereas, in fibroblasts, CXCR3 signaling blocked μ-calpain activation to prevent de-adhesion. These studies were extended by use of transgenic mouse models in which either CXCR3 was ablated or CXCL11 expression was silenced in keratinocytes. Although CXCR3-null mice are viable and are able to ultimately close full-thickness cutaneous wounds, the time to wound closure is delayed.7,8 These results suggested that the CXCR3 signaling pathway could potentially represent one of several redundant pathways that serve to coordinate behavior keratinocytes and fibroblasts. The role of this pathway on a third major component of the healing wound bed, the endothelial cells, remained to be determined. Taken together, these results formed the working hypothesis that paracrine signaling via CXCR3 could serve as a broad signal coordinating the entire wound bed to shift from proliferative state to prodifferentiation state.
Experimental Model or Material: Advantages and Limitations
To address the varying effects of CXCR3 signaling in the dynamic tissue state of the wound, mouse wound models were necessary to observe physiologic cellular responses in the in vivo milieu. To tease out the molecular mechanisms behind observed phenomenon, in vitro methods isolated molecular interactions from the myriad of other signals potentially present in the in vivo wound, including recruited inflammatory cells, infectious agents, and repeated or incidental trauma occurring over the long period of observation required for completion of wound remodeling. The highlighted reports utilize both full-thickness wound healing models in transgenic mouse as well as in vitro wound healing methods. Both reports use CXCR3 null mice to demonstrate the dramatic effect of loss of this chemokine on later stages of wound healing. Cells isolated from these tissues were subsequently used for in vitro studies. Isolation of interactions among keratinocytes, endothelial cells, and fibroblasts more cleanly tease out the molecular mechanisms behind the paracrine signaling effects observed in vivo.
The main disadvantage of the mouse models in studying wound remodeling lies in the inherent differences in wound closure mechanisms utilized by “loose-skinned” mammals. As such, correlates to human hypertrophic or keloid scar do not appear to naturally occur in rats or mice. However, derangement of the wound remodeling process resulting in features of both keloid and hypertrophic scar suggests possible pathogenic mechanisms.9
Discussion of Findings and Relevant Literature
Role of CXCR3 signaling pathway in dermal wound remodeling
Building on prior work that showed that loss of CXCR3 signaling resulted in wounds that featured dermal immaturity at 90 days after wounding even though the wound was closed and appeared near normal, Yates et al. looked at wound healing in the same model system at 180 days. Surprisingly, CXCR3 null mice retained a visible scar at 180 days after wounding, one which was more accentuated than earlier time points, whereas the epidermis and dermis in wounded wild-type mice were comparable to unwounded tissue.3
The persistent scars in CXCR3 null mice were characterized by hyperkeratosis and a thicker dermis. Similar to CXCR3 null mice at 90 days postwounding, dermal collagen was made up of short fibers arranged somewhat haphazardly throughout the dermis. Correspondingly, the tensile strength of the pelt was still weaker at 180 days compared with that of the wild-type mouse.
Histologically, the scars demonstrated epidermal hyperkeratosis and dermal thickness and hypercellularity. Both of these features suggested potential similarities to hypertrophic scar in humans. However, the molecular derangements, which include increased expression of matrix metalloproteinase-9, tenascin C, fibronectin, collagen I, collagen III, and decorin, suggested a mixed picture of both hypertrophic and keloid scar.
A third intriguing phenomenon that was observed was a reappearance of an inflammatory infiltrate in the wounded tissue at 6 months after initial wounding. This inflammation was sterile as there was no apparent epidermal breach or detectable microbes on histologic sections. Thus, this wound appeared to progress from a healing state to one of chronic inflammation and fibrosis, similar to human hypertrophic scars.
Role of CXCR3 signaling pathway in vascular remodeling in the wound bed
A few hypotheses have been developed to explain the near total but orderly pruning of the abundant nascent vessels produced during the proliferative phase of wound healing. As granulation tissue represents a provasculogenic environment, it is possible that regression of these vessels is due to the loss of angiogenic signals. Alternatively, there may exist a separate signal initiating regression of vessels. These hypotheses can be generally grouped into two broad categories that describe regression phenomenon as either a largely stochastic process by which certain vessels are retained while others are lost or an orderly progression by which vessels pared or, more metaphorically, pruned to reveal a final vascular network.
Certainly each of these has its merits. A recapitulation of the developmental pathway of vasculogenesis could be certainly plausible as a network of vascular channels are formed and only those that form contiguous pathway are stabilized while others are allowed to degenerate. Alternatively, a controlled disassembly of a vascular scaffold once a sufficient network of nascent vessels has been stabilized could also be imagined. The reality is likely a mixture of these processes.
Looking at the effect of CXCR3 signaling on endothelial cells during wound remodeling, Bodnar et al. demonstrated that signaling by chemokine CXCL10 via CXCR3 initiates a sequence of events that culminates in dissolution of newly formed blood vessels.4 The initial observation was an increased expression of CXCR3 on newly formed endothelial cell tubes both in vitro and in vivo wounds. As wound remodeling continued, CXCR3 expression levels on the vasculature decreased in maturing vessels several days after formation.
To test the hypothesis that stimulation of CXCR3 via ligand CXCL10 resulted in these dramatic changes in the vasculature, a series of experiments either abrogating CXCR3 by using the CXCR3-null mouse or exogenous addition of ligand CXCL10 was performed. Wounding in the CXCR3-null mouse resulted in persistence of the newly formed vessels, whereas the addition of exogenous CXCL10 into both in vitro and in vivo Matrigel wound model systems resulted in dissociation of endothelial tubes.
Looking more closely at the molecular mechanisms behind this phenomenon, Bodnar et al. demonstrated that signaling through the CXCR3 receptor resulted in activation of an enzyme cascade, resulting in cleavage of cell-substrate adhesion molecules via calcium-dependent protease μ-calpain, which leads to cessation of endothelial cell migration and dissociation of vessels.4,10 Interestingly, this is the same pathway that mediates keratinocyte migration while arresting fibroblast migration.5,6,11 The reason the keratinocytes do not undergo this detachment and death is the presence of many adhesion molecules not susceptible to such μ-calpain cleavage. These reports, taken together, show the differential effects of activation of CXCR3 on cell lineages in the healing wound bed, further demonstrating the role of CXCR3 as a coordinating signal that serves to shift granulation tissue from a proliferative state to a state promoting differentiation.
Innovation
The report by Yates et al. is the first to demonstrate a progressive and chronic hypertrophic scar in rodents, in addition to extending the prior finding that paracrine signaling from keratinocytes to underlying fibroblasts via CXCR3 and its ligands results in delayed wound remodeling. In addition, derangement in CXCR3 signaling also results in a late-appearing proinflammatory state that may also play a role in the development of this fibrosis, which could be comparable in certain aspects to hypertrophic or keloid scars seen in humans. This provides a much needed tool to develop new therapies for scar management.
Although much of the investigation of the role of CXCR3 in wound healing has focused mainly upon the crosstalk between keratinocytes and fibroblasts, Bodnar et al. demonstrates for the first time that endothelial cells in wounded skin are also regulated by this paracrine signaling pathway. In addition, the pathway of signaling is through the same receptor that modulates keratinocyte and fibroblast behavior. Further, the differential effects may be mediated through different isotypes of the CXCR3 receptors as endothelial cells predominantly express the CXCR3B isoform.
Take-Home Message.
-
• Signaling via CXCR3 synchronizes:
- promotion of keratinocyte migration,
- pruning of nascent vessels, and
- diminution of fibroblast migration during wound remodeling
• Derangement of CXCR3 signaling results in formation of a weak, fibrotic, and hypercellular wound in mouse models.
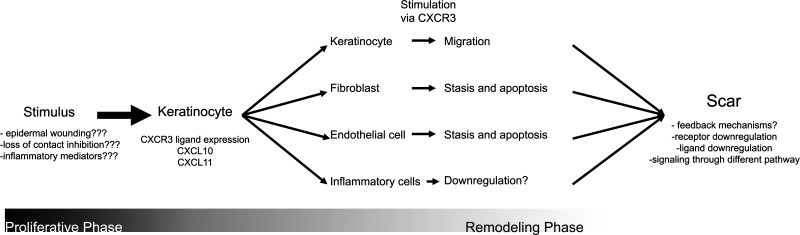
Summary Illustration
Schematic of the role of CXCR3 signaling in wound healing highlighting new research questions.
Caution, Critical Remarks, and Recommendations
The advances put forth by these two studies reveal how activities of different cell types of the remodeling wound could be coordinated via a single, albeit complex, signal transduction pathway. Stimulation via a single receptor, CXCR3, is responsible for migration of one cell type, while initiating cessation of migration and apoptosis in other cell types. In addition, disruption of this signaling cascade is located at what appears to be a critical juncture between proliferative and remodeling phases of cutaneous wound healing. Although CXCR3 signaling is not necessary for wound closure, closure is severely delayed in the CXCR3-null mouse model system. After wound closure is ultimately achieved, there is then a lack of progression in remodeling and possibly even a regression as there was a reappearance of inflammatory cells that could not be explained by repeat infection or repeat trauma. These results suggest a role of CXCR3 in activating additional pathways to coordinate later events in wound remodeling. In addition, CXCR3 signaling may also serve as part of a negative feedback loop regulating the earlier inflammatory and proliferative responses to trauma.
Future Development of Interest
Taken as a whole, these two reports in conjunction with the work leading up to them show CXCR3 signaling to be a coordinating signal involving keratinocytes, fibroblasts, as well as endothelial cells. Although initial work suggested that CXCR3 signaling may not be necessary for wound healing, as full-thickness wounds ultimately did close, the newer report demonstrating fibrotic, hypercellular scars in mice even 180 days postwounding may be evidence that this signaling pathway may, in fact, be necessary for proper wound healing during the later phases of remodeling.
These results now open several new avenues for investigation. Given the far-reaching effects of this paracrine signaling cascade—the number of cell types affected, the extended time course over which its effects can be seen, and the dramatic effects on scar formation—the control mechanisms that initiate and terminate this signaling is likely tightly controlled. These mechanisms remain to be discovered.
One aspect of wound healing that has been excluded from these studies is the contribution of immune regulators and inflammatory cells in the remodeling wound bed. It is well documented that CXCR3 is involved in inflammatory states including inflammatory dermatoses of the skin, response to infection, acute transplant graft rejection, and fibrosis in solid organs.12–16 The finding in the late remodeling wound by Yates et al. of the return of a nonspecific lymphohistiocytic response that does not appear to be due to infectious agents points to a potential immunoregulatory role of CXCR3 signaling.
Finally, looking more globally, these results also suggest that subtle derangements of this signaling pathway and possibly others like this may explain dystrophic wound healing such as keloid and hypertrophic scarring in humans. Understanding of this pathway and possible manipulation of its effects may allow for development of therapeutic interventions.
Abbreviations and Acronyms
- CXCL10
CXC ligand 10
- CXCL11
CXC ligand 11
- CXCR3
CXC receptor 3
Acknowledgments and Funding Sources
This work was supported by NIH 1K08GM095917-01.
Author Disclosure and Ghostwriting
The authors have nothing to disclose. No ghostwriters were used to write this article.
References
- 1.Wicke C. Bachinger A. Coerper S. Beckert S. Witte MB. Konigsrainer A. Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized wound care center. Wound Repair Regen. 2009;17:25. doi: 10.1111/j.1524-475X.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 2.Rendon MI. Berson DS. Cohen JL. Roberts WE. Starker I. Wang B. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32. [PMC free article] [PubMed] [Google Scholar]
- 3.Yates CC. Whaley D. Hooda S. Hebda PA. Bodnar RJ. Wells A. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen. 2009;17:34. doi: 10.1111/j.1524-475X.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodnar RJ. Yates CC. Rodgers ME. Du X. Wells A. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci. 2009;122(Pt 12):2064. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satish L. Yager D. Wells A. Glu-leu-arg-negative CXC chemokine interferon gamma inducible protein-9 as a mediator of epidermal-dermal communication during wound repair. J Invest Dermatol. 2003;120:1110. doi: 10.1046/j.1523-1747.2003.12230.x. [DOI] [PubMed] [Google Scholar]
- 6.Satish L. Blair HC. Glading A. Wells A. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol Cell Biol. 2005;25:1922. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yates CC. Whaley D. Kulasekeran P. Hancock WW. Lu B. Bodnar R, et al. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates CC. Whaley D. Y-Chen A. Kulesekaran P. Hebda PA. Wells A. ELR-negative CXC chemokine CXCL11 (IP-9/I-TAC) facilitates dermal and epidermal maturation during wound repair. Am J Pathol. 2008;173:643. doi: 10.2353/ajpath.2008.070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottrup F. Agren MS. Karlsmark T. Models for use in wound healing research: A survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8:83. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar RJ. Yates CC. Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. 2006;98:617. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiraha H. Glading A. Gupta K. Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouazzaoui A. Spacenko E. Mueller G. Miklos S. Huber E. Holler E, et al. Chemokine and chemokine receptor expression analysis in target organs of acute graft-versus-host disease. Genes Immun. 2009;10:687. doi: 10.1038/gene.2009.49. [DOI] [PubMed] [Google Scholar]
- 13.Geleff S. Draganovici D. Jaksch P. Segerer S. The role of chemokine receptors in acute lung allograft rejection. Eur Respir J. 2010;35:167. doi: 10.1183/09031936.00042309. [DOI] [PubMed] [Google Scholar]
- 14.Kohlmeier JE. Cookenham T. Miller SC. Roberts AD. Christensen JP. Thomsen AR, et al. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009;183:4378. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang D. Liang J. Hodge J. Lu B. Zhu Z. Yu S, et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flier J. Boorsma DM. van Beek PJ. Nieboer C. Stoof TJ. Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]