Abstract
Purpose.
A majority of experimental data on dry eye disease (DED) immunopathogenesis have been derived from a murine model of DED that combines desiccating environmental stress with systemic muscarinic acetylcholine receptor (mAChR) inhibition. However, to our knowledge the effects of pharmacologic mAChR blockade on the pathogenesis of experimental DED have not been evaluated systemically. The purpose of our study was to investigate the differential effects of desiccating environmental stress and mAChR inhibition on the pathogenesis of DED.
Methods.
DED was induced in female C57BL/6 mice by exposure to a desiccating environment in the controlled-environment chamber or to systemic scopolamine, or by performing extraorbital lacrimal gland excision. Clinical disease was assessed using corneal fluorescein staining (CFS) and the cotton thread test (CTT). Corneal CD11b+ and conjunctival CD3+ T-cell infiltration were evaluated by flow cytometry. T-cells from draining cervical lymph nodes (CLN) and distant inguinal lymph nodes (ILN) were analyzed for Th1, Th2, Th17, and Treg responses by flow cytometry and ELISA.
Results.
Desiccating environmental stress and systemic mAChR blockade induced similar clinical signs of DED. However, desiccating environmental stress imparted higher conjunctival CD3+ T-cell infiltration, and greater Th17-cell activity and Treg dysfunction than mAChR blockade, while mAChR blockade decreased tear secretion to a greater extent than desiccating environmental stress. Systemic mAChR blockade attenuated Th17 activity and enhanced Th2 and Treg responses without affecting Th1 activity.
Conclusions.
In vivo inhibition of mAChRs variably affects CD4+ T-cell subsets, and desiccating environmental stress and systemic mAChR blockade induce DED through different primary pathogenic mechanisms.
Keywords: dry eye disease, controlled-environment chamber, scopolamine, CD4+ T-cells
Desiccating environmental stress and systemic mAChR blockade induce similar dry eye signs through different primary pathogenic mechanisms involving CD4+ T-cell response and tear production.
Introduction
Dry eye disease (DED) is the most common reason for patient presentation to ophthalmologists and optometrists in the United States.1,2 Clinical studies have demonstrated that CD4+ T helper (Th)–cell infiltration of the ocular surface is a prevalent finding in DED.3 Experimental evidence suggests that DED is an immunoinflammatory disorder of the ocular surface that involves effector Th-cell responses and regulatory T-cell (Treg) dysfunction.4–7 An experimental model used commonly to study the immunopathogenesis of DED utilizes a combination of desiccating environmental stress and pharmacologic inhibition of aqueous tear secretion.4,8,9 Desiccating environmental conditions are produced using either an air blower or a controlled-environment chamber (CEC) that allows for the continuous regulation of environmental conditions, such as temperature, humidity, and airflow.10 Exposing the ocular surface to dry conditions (e.g., low humidity and high airflow) increases tear evaporation and leads to the clinical signs of DED. Aqueous tear deficiency is induced by administering scopolamine (SCP), a tropane alkaloid that antagonizes muscarinic activity.
Scopolamine inhibits acetylcholine (ACh)-mediated stimulation of the lacrimal gland by blocking muscarinic ACh receptors (mAchR),11,12 thereby mimicking anti-M3 antibody-mediated Sjögren's syndrome.13 However, an independent, nonneuronal cholinergic system that modulates T-cell responses has been identified,14 necessitating the elucidation of SCP's pharmacologic effects on T-cells. It has been suggested that ACh regulates immune-neurohumoral crosstalk through its activity on T-cell–expressed mAChR and nicotinic ACh receptors.15 The activation of mAChRs has been shown to enhance intracellular Ca2+ signaling and upregulate c-fos mRNA expression,16 increase phytohemagglutinin-induced IL-2 production,17 enhance T-cell proliferation,18 and decrease IFN-γ synthesis.19 Conversely, inhibition of mAChRs decreases IL-6 secretion20 and suppresses leukocytic infiltration.18
To date, to our knowledge the role of mAChRs on each individual CD4+ T-cell subset in vivo has yet to be determined. Herein, we systemically investigated the effects of systemic mAChR blockade on Th1, Th2, Th17, and Treg responses. Furthermore, we analyzed the differential effects of desiccating environmental stress and mAChR inhibition on the pathogenesis of DED.
Materials and Methods
Animals
Female C57BL/6 mice 6 to 8 weeks old (Charles River Laboratories, Wilmington, MA) were used for this study. All animal experiments were approved by the Institutional Animal Care and Use Committee, and adhered to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
DED Induction
DED was induced in mice by either placing them in the CEC with a relative humidity below 15%, airflow of 10 L/min, and a constant temperature of 21°C to 23°C for 14 days,8 or subcutaneously administering 0.1 mL of 5 mg/mL scopolamine hydrobromide (SCP, formulated in normal saline; Sigma-Aldrich Corp., St Louis, MO) three times per day (9 AM, 1 PM, and 5 PM) on the dorsal surface of mice. For comparison of different models of tear-deficiency dry eye, DED was induced in one group by simultaneous SCP administration and CEC exposure (SCP + CEC), and in the other group by performing extraorbital lacrimal gland excision (LGE) and CEC exposure (LGE + CEC). Untreated age and sex-matched mice maintained in the standard vivarium were used as normal controls. To perform extraorbital lacrimal gland excision, mice were anesthetized, and the surgical site was marked approximately halfway between the lateral canthus and ear pinna. The extraorbital lacrimal gland was removed with blunt dissection, the cutaneous wound was closed with a single 8-0 nylon suture, and antibiotic ointment was applied to the surgical site. Several potential adverse effects of this procedure received consideration, including parotid gland damage (body weight loss associated with difficulty masticating or swallowing), facial artery damage (intraoperative or postoperative bleeding), facial nerve damage (blink reflex), and wound infection (wound inspection and behavior monitor). No cases with these adverse effects were identified in the study.
Clinical Evaluation
Corneal fluorescein staining (CFS) was used to evaluate corneal epithelial damage caused by DED. A dose of 1 μL of 2.5% fluorescein (Sigma-Aldrich Corp.) was applied into the lateral conjunctival sac of the mice, and 3 minutes later their corneas were examined using a slit-lamp biomicroscope (Topcon SL-D7; Topcon Corp., Tokyo, Japan) under cobalt blue light. Punctate staining was recorded in a masked fashion using the National Eye Institute grading system, scoring 0 to 3 for each of five areas of the cornea: central, superior, inferior, nasal, and temporal.21 The cotton thread test (CTT) was used to evaluate aqueous tear production as described previously.10 Briefly, a phenol red thread (Zone-Quick; Lacrimedics, Eastsound, WA) was placed in the lateral canthus of the conjunctival fornix of the right eye for 30 seconds after excess tears had been removed for a standard time of 4 seconds, and tear distance (in millimeters) was read under a microscope (Topcon SL-D7; Topcon Corp.). In SCP-treated mice, the CTT was performed half an hour before the SCP injection in the afternoon.
Flow Cytometry Analysis
Tissues were collected at day 14 of DED induction. Single-cell suspensions were prepared from cornea and conjunctiva by collagenase digestion. Briefly, tissues were removed and cut into small fragments, followed by digestion with 2 mg/mL collagenase type IV (Sigma-Aldrich Corp.) and 0.05 mg/mL DNase I (Roche, Basel, Switzerland) for 1 hour at 37°C with agitation. The suspension then was triturated through a 30-gauge needle to homogenize the remaining tissue and filtered through a 70-μm cell strainer (BD Biosciences, Bedford, MA). Corneal cells then were double-stained with PE-Cy5-conjugated anti-CD45 and Alexfluo 488-conjugated anti-CD11b (eBioscience, San Diego, CA). Conjunctival cells were stained with PE-conjugated anti-CD3e (eBioscience). Single-cell suspensions were prepared from lymph nodes using a 70 μm cell strainer. For Treg analysis, cells were stained with FITC-conjugated anti-CD4, PE-conjugated anti-CD25, and PE-Cy5–conjugated anti-Foxp3 (eBioscience). For effector T-cell analysis, cells first were stimulated with phorbol 12-myristate 13-acetate and ionomycin (Sigma-Aldrich Corp.) in the presence of GolgiStop (BD Biosciences), and then stained with FITC-conjugated anti-CD4. After fixation with IC Fixation Buffer (eBioscience), cells were permeabilized with Permeabilization Buffer (eBioscience) and stained with APC-conjugated anti–IFN-γ, PE-Cy7–conjugated anti–IL-4, and PE-conjugated anti–IL-17A (eBioscience). Control samples were stained with appropriate isotype-matched control antibodies (eBioscience). Stained cells were examined on an LSR II flow cytometer (BD Biosciences) and the results were analyzed using Summit v4.3 software (Dako Colorado, Inc., Fort Collins, CO).
Treg Suppression Assay
The suppressive function of Tregs was examined as described previously.6 Briefly, CD4+CD25+ Tregs and responder CD4+ T (Tresp) cells were isolated from lymph nodes by magnetic separation using Treg and CD4+ T-cell isolation kits (Miltenyi Biotec, Inc., Auburn, CA). The purity of sorted cells was >95%, and 97% of CD4+CD25+ Treg cells were Foxp3+ in all the groups as confirmed by flow cytometry analysis. Tresp cells (1 × 105) from normal mice were cocultured with Treg cells (5 × 104) from different experimental groups, T-cell–depleted syngeneic splenocytes (1 × 105, by CD90+ depletion kit; Miltenyi Biotec, Inc.) from normal mice, and 1 g/mL anti-CD3 Ab for 3 days. Proliferation of CD3-stimulated Tresp cells without adding Treg cells was considered control proliferation with 0% suppression. Proliferation was measured using the BrdU proliferation kit (Millipore, Billerica, MA), and percentage of suppression was calculated using the following formula: %suppression = ([Tresp proliferation without Treg − Tresp proliferation with Treg]/Tresp proliferation without Treg) × 100.
Cytokine Expression Analysis
Enriched CD4+ T-cells from lymph nodes were isolated by magnetic separation using CD4 T-cell isolation kit (Miltenyi Biotec, Inc.), and stimulated with PMA and ionomycin for 24 hours. The cytokines IFN-γ, IL-4, and IL-17 in cell supernatant were assayed with commercial ELISA kits (eBioscience).
Statistical Analyses
An unpaired, 2-tailed Student's t-test was used for data analysis with data normality examined by NormQuant.xls.22 Differences were considered significant at P < 0.05.
Results
Clinical Signs of DED Can Be Induced Effectively Using the CEC or SCP Alone
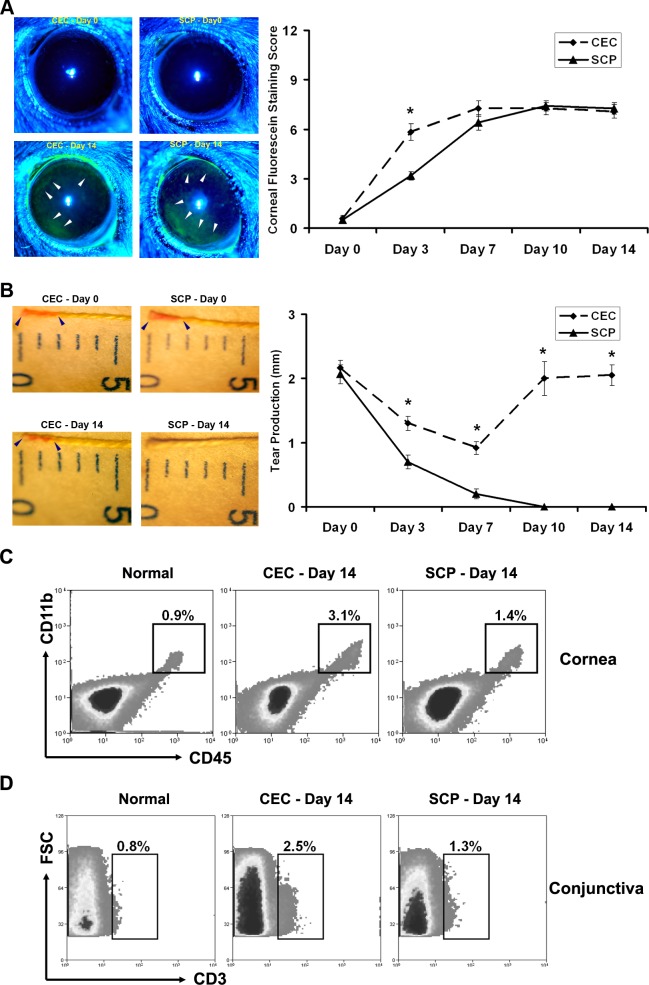
The DED-inducing effects of the controlled-environment chamber (CEC) alone have been reported;8 however, the ability of SCP alone to induce clinically evident DED has not been investigated to our knowledge. To determine if SCP alone can induce experimental DED as effectively as the CEC, mice were challenged with either CEC or SCP alone, and evaluated with CFS and CTT on days 0 (before challenge), 3, 7, 10, and 14. CEC and SCP generated comparable CFS scores and patterns at all time points except day 3, when the CEC group exhibited a significantly higher CFS score than SCP group (Fig. 1A). The more peripheral corneal staining patterns usually are seen in mild to moderate DED as presented here, while peripheral and central corneal staining is observed commonly in more severe DED.8 Aqueous tear production measurements revealed that SCP significantly inhibited tear secretion at early and late time points, while the CEC only caused a slight tear reduction at early time points that was not conserved at later time points (Fig. 1B). Examination of the ocular surface inflammation on day 14 revealed that SCP-induced DED was accompanied by lower corneal infiltration of CD11b+ cells (Fig. 1C) and lower conjunctival infiltration of CD3+ T-cells (Fig. 1D) in comparison with CEC-induced DED.
Figure 1.
Evaluation of DED. DED was induced using either a CEC or systemically administered SCP. (A) Corneal epithelial damage assessment by standard corneal fluorescein staining scores. Representative images of normal (day 0) and dry eye (day 14) corneas from each group are shown on the left. The corneal epithelial defect is exhibited as green staining (white arrowhead). *P < 0.05. (B) Aqueous tear production assessment by CTT. Representative images of normal (day 0) and dry eye (day 14) tear measurement from each group are shown on the left. The tear production is measured as the length of red staining (distance between the two blue arrowheads in each image). Data shown represent the mean ± SEM of a single representative experiment (12 eyes from 6 mice per group). *P < 0.05 between CEC and SCP groups. (C) Flow cytometry analysis of corneal CD11b+ cells. A total of 12 corneas was pooled in each group. Percentages represent CD45+CD11b+ cells as a proportion of total corneal cells. (D) Flow cytometry analysis of conjunctival CD3+ T-cells. A total of 12 conjunctivae was pooled in each group. Percentages represent CD3+ T-cells as a proportion of total conjunctival cells.
CEC and SCP-Induced DED Exhibit Comparable Th1 Responses
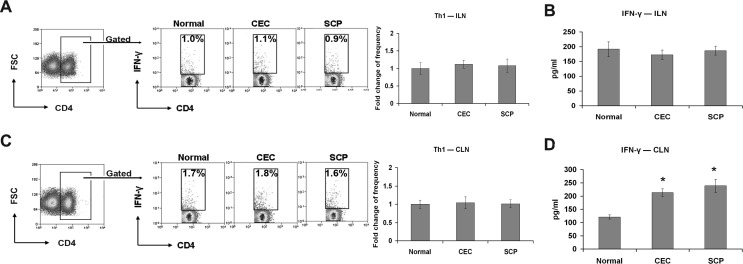
Draining cervical lymph nodes (CLN), including cervical and submandibular lymph nodes, are the primary sites of autoreactive T-cell priming and expansion in DED.5,6 Therefore, distant inguinal lymph nodes (ILN) were selected to determine the systemic effects of SCP on CD4+ T-cells. After 14 days of DED induction, ILN were harvested and examined using flow cytometry to measure T-cell frequency and ELISA to measure cytokine production. There were no significant differences in ILN IFN-γ+CD4+ T-cell frequency (Fig. 2A) or IFN-γ production (Fig. 2B) between the CEC, SCP, and naïve mice. Whereas, performing the same examinations on the CLN showed that there was a significant increase in IFN-γ production (2-fold) in the CEC and SCP groups compared to the normal group (Fig. 2D); however, there were no significant differences in IFN-γ+CD4+ T-cell frequencies (Fig. 2C), and this can be due to the enhanced cytokine secretion of IFN-γ+CD4+ T-cells, which are activated in DED draining lymph nodes. Furthermore, there were no significant differences in IFN-γ expression between the CEC and SCP groups.
Figure 2.
Th1 cell response evaluation. Distant ILN (A, B) and DED draining CLN (C, D) were harvested for Th1 frequency analysis and signature cytokine level quantification. The experiment was repeated twice with 6 animals in each group. (A, C) Representative flow cytometry dot plots from one of the experiments are located on the left. Percentages represent Th1 cells as a proportion of total CD4+ T-cells in normal, CEC-challenged, and SCP-challenged mice. The fold changes (mean ± SD) of Th1 cell frequencies in CEC and SCP groups relative to normal group are summarized for all the repeated experiments, and presented as a bar graph located on the right. (B, D) IFN-γ protein levels in each group were measured with ELISA. Data represent the mean ± SD of 6 mice from one representative experiment. *P < 0.05 when compared to normal group.
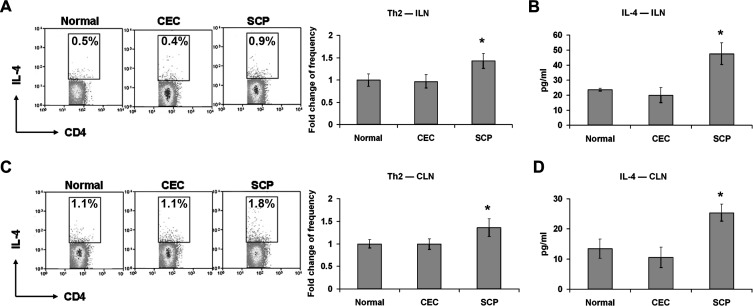
SCP Enhances Th2 Activity
Next, ILN and CLN were analyzed for Th2 responses. Although there were no significant changes in either IL-4 +CD4+ T-cell frequency or IL-4 level in CEC-derived ILN compared to normal ILN, IL-4 +CD4+ T-cell frequency and IL-4 levels in SCP-derived ILN significantly increased relative to normal levels (Figs. 3A, 3B). The same findings were observed in the CLN of SCP-treated mice (Figs. 3C, 3D), suggesting that these Th2 responses were the result of systemic SCP administration rather than the immune response to DED.
Figure 3.
Th2 cell response evaluation. Distant ILN (A, B) and DED draining CLN (C, D) were harvested for Th2 frequency analysis and signature cytokine level quantification. The experiment was repeated twice with 6 animals in each group. (A, C) Representative flow cytometry dot plots from one of the experiments are located on the left. Percentages represent Th2 cells as a proportion of total CD4+ T-cells for normal, CEC-challenged, and SCP-challenged mice. The fold changes (mean ± SD) of Th2 cell frequencies in CEC and SCP groups relative to normal group are summarized for all the repeated experiments, and presented as a bar graph located on the right. (B, D) IL-4 protein levels in each group were measured with ELISA. Data represent the mean ± SD of 6 mice from one representative experiment. *P < 0.05 when compared to normal group.
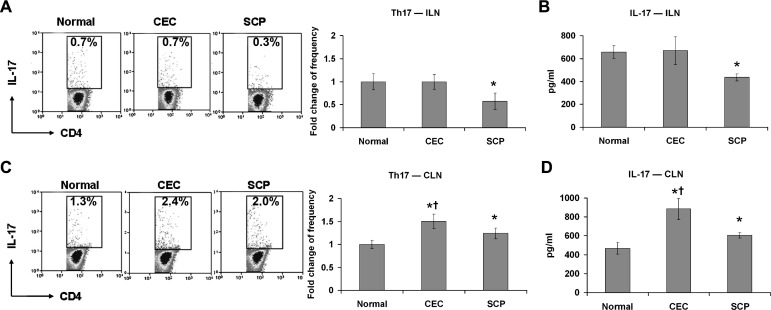
SCP Inhibits Th17 Responses
Th17 responses in the ILN and CLN of CEC- and SCP-treated mice were evaluated. In CEC-derived ILN, neither the IL-17+CD4+ T-cell frequency nor the IL-17 level differed significantly from normal. However, IL-17 +CD4+ T-cell frequency (Fig. 4A) and IL-17 level (Fig. 4B) were decreased dramatically in SCP-derived ILN compared to normal ILN. In contrast, in DED draining CLN, the CEC and SCP groups exhibited increased IL-17+CD4+ T-cell frequencies (Fig. 4C) as well IL-17 levels (Fig. 4D) compared to the normal group. Furthermore, IL-17+CD4+ T-cell frequency and IL-17 level were higher in the CEC group than the SCP group (Figs. 4C, 4D).
Figure 4.
Th17 cell response evaluation. Distant ILN (A, B) and DED draining CLN (C, D) were harvested for Th17 frequency analysis and signature cytokine level quantification. The experiment was repeated twice with 6 animals in each group. (A, C) Representative flow cytometry dot plots from one of the experiments are located on the left. The indicated percentages of Th17 cells as a proportion of total CD4+ T-cells were measured in normal, CEC-challenged, and SCP-challenged mice. The fold changes (mean ± SD) of Th17 cell frequencies in CEC and SCP groups relative to normal group are summarized for all the repeated experiments, and presented as a bar graph located on the right. (B, D) IL-17 protein levels in each group were measured with ELISA. Data represent the mean ± SD of 6 mice from one representative experiment. *P < 0.05 when compared to normal group. †P < 0.05 between CEC and SCP groups.
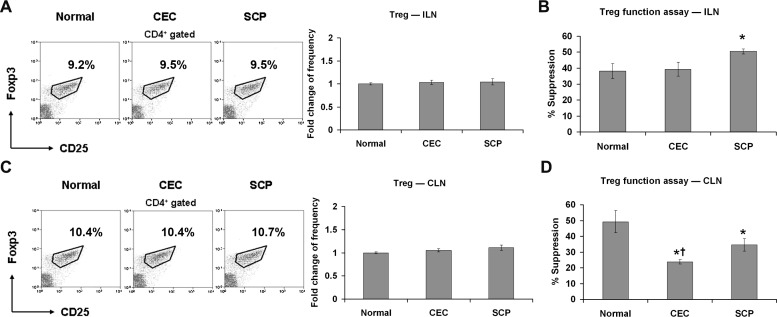
SCP Promotes the Suppressive Function of Tregs
Given the recently recognized importance of Treg function in the pathogenesis of DED,6 the effects of SCP on this immunity-restricted T-cell population were assessed. First, the frequencies of CD4+CD25+Foxp3+ Tregs in ILN were evaluated, but there were no significant differences between groups (Fig. 5A). Next, using an in vitro coculture suppression assay, the potential of purified Tregs to suppress CD4+ T-cell proliferation was compared between CEC- and SCP-treated mice. CEC-derived ILN Tregs suppressed equivalently to normal Tregs; however, SCP-derived ILN Tregs demonstrated significantly increased suppressive activity (Fig. 5B), suggesting that a functional imbalance between Tregs and effector Th (especially Th17) cells was caused by SCP. The same analysis on CLN revealed that Tregs from the CEC and SCP groups exhibited defects in quality of the Treg response (suppression efficiency, Fig. 5D), but not in the quantity of Tregs (cell frequency, Fig. 5C), and that Treg dysfunction was greatest in the CEC-induced group (Fig. 5D).
Figure 5.
Treg response evaluation. Distant ILN (A, B) and DED draining CLN (C, D) were harvested for Treg frequency and functional analysis. The experiment was repeated twice with 6 animals in each group. (A, C) Representative flow cytometry dot plots (Treg percentage of total CD4+ T-cells) from one of the experiments are displayed on the left. The fold changes (mean ± SD) of Treg cell frequencies in CEC and SCP groups relative to normal group are summarized for all the repeated experiments and presented as a bar graph located on the right. (B, D) Suppression of T-cell proliferation by Tregs was assessed using an in vitro suppression assay as shown on the right. Data are presented as the mean ± SD of 6 mice and each sample was measured in triplicates. *P < 0.05 when compared to normal group. †P < 0.05 between CEC and SCP groups.
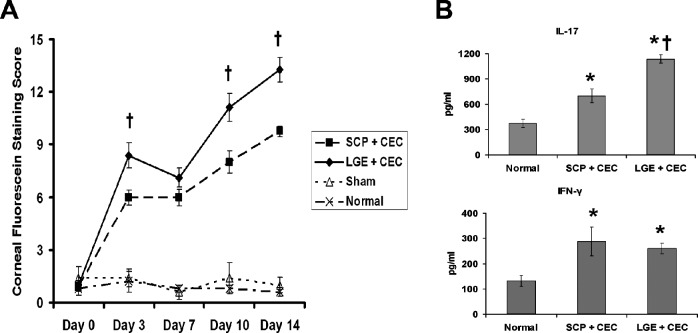
LGE Induces More Severe DED Than SCP
To confirm further the in vivo effect of SCP on CD4+ T-cells and DED severity, LGE was performed to induce lacrimal hyposecretion without affecting the systemic immune response. Based on CFS scoring, LGE combined with CEC (LGE + CEC) induced more severe disease than SCP combined with CEC (SCP + CEC, Fig. 6A). At the end of the disease induction (day 14), DED draining CLN from both groups were harvested, and analyzed for IFN-γ and IL-17 levels. Both groups showed significantly increased IFN-γ and IL-17 levels compared to normal controls. There was no significant difference in IFN-γ levels between the LGE + CEC and SCP + CEC groups; however, the LGE + CEC group demonstrated a significantly higher level of IL-17 than SCP + CEC group (Fig. 6B).
Figure 6.
Disease severity and pathogenic T-cell responses in DED induced by physical removal versus pharmacologic blockade of lacrimal gland. SCP and LGE groups were placed in the CEC for 14 days, and DED draining CLN were collected for examination of Th1 and Th17 responses. (A) Clinical disease severity was evaluated with CFS. Data are the mean ± SEM of five mice at each time point. (B) IL-17 and IFN-γ levels in CLN detected by ELISA. Data are presented as the mean ± SD of five mice. *P < 0.05 when compared to normal group. †P < 0.05 between SCP and LGE groups.
Discussion
In our study, we showed that the in vivo inhibition of mAChRs variably affects CD4+ T-cell subsets. Furthermore, by comparing various models of experimental DED, we demonstrated that desiccating environmental stress and systemic mAChR blockade induce DED through different primary pathogenic mechanisms.
Historically, DED was thought to be the result of inadequate tear film quantity or quality alone. However, the definition of DED since has been modified to recognize the importance of tear film hyperosmolarity and ocular surface inflammation in the pathogenesis of DED.23 These changes came about in part due to clinical and experimental studies that implicated immunity in the pathogenesis of DED. A majority of the experimental studies investigating the immunopathogenesis of DED have used a combination of desiccating environmental stress (e.g., CEC) and pharmacologic tear inhibition (e.g., SCP) to induce murine DED.4–9 A previous study showed that SCP not only reduced lacrimal secretion, but induced lacrimal gland inflammation.24 Given the potential effects of SCP on immune responses, it is vital to determine the effects of mAChR blockade on pathogenic immune responses in this model of experimental DED. To elucidate this, we investigated CD4+ T-cell responses in disease-related draining CLNs and disease-independent distant ILNs.
Our data demonstrated that there are no significant changes in Th1, Th2, Th17, or Treg responses in the distant ILNs of CEC-induced DED mice, indicating that distant ILNs are not involved actively in the localized DED immune response. Therefore, in DED induced by systemic mAChR blockade, changes in immune activity observed in the ILN reflect the direct pharmacologic effects of mAChR on the immune system. Specifically, systemic administration of SCP leads to upregulated Th2 (IL-4) and Treg (suppressive activity) responses, downregulated Th17 (IL-17) responses, and unchanged Th1 (IFN-γ) responses in the ILN. These results differed somewhat from the findings of Qian et al. regarding the muscarinic cholinergic regulation of CD4+ T-cell differentiation.25 They reported that in vitro stimulation of CD4+ T-cells with the mAChR agonist muscarine increases IL-17 production and decreases IFN-γ secretion without affecting IL-4 levels, and these effects are abolished with the administration of the mAChR antagonist atropine. These in vitro results and our in vivo results demonstrate that mAChR blockade leads to Th17 inhibition; however, the findings regarding Th1 and Th2 responses are not consistent between the two studies. In another in vitro experiment, the mAChR agonist pilocarpine has been shown to decrease IFN-γ synthesis by human mononuclear leukocytes.19 However, in the combined M1 and M5 mAChR knockout mice, there is no significant change in IFN-γ secretion, but there is decreased IL-6 production.20 This is in accordance with our demonstration that SCP does not affect Th1 response. Since IL-6 is a critical differentiation factor for the generation of Th17 cells,26 the downregulated Th17 response by SCP administration may be due to the decreased expression of IL-6. In addition, our study has revealed that mAChR blockade enhances the suppressive capacity of Tregs, suggesting that Th17 inhibition also can result from the restriction imposed by Tregs.27 In summary, our findings indicated that mAChR blockade facilitates the development of Th2 and Treg responses, and inhibits Th17 polarization. These findings are similar to the reported effects of nicotine, a nicotinic AChR agonist, on immune response, including suppression of the Th1 and Th17 lineages, and promotion of the Th2 lineage,28 as well as increased suppressive capacity of Tregs.29
In the DED draining CLN, CEC- and SCP-induced models exhibit increased Th1 and Th17 responses, accompanied by deficient Treg suppression. These findings are consistent with previous studies that combined desiccating stress (e.g., CEC) and SCP-induced DED.5–7 However, immune responses in the draining CLN of CEC and SCP-induced models differ (Fig. 7). SCP-induced DED demonstrated significantly lower Th17 responses and higher Treg activity than CEC-induced DED, and these alterations are attributable to the pharmacologic effects of SCP on CD4+ T-cells. Therefore, the immune responses that occur in the CLN of SCP-induced DED mice can be considered the result of the ocular disease and drug effects. The increased Th2 responses observed in the CLN of SCP-induced DED are attributable to the pharmacologic effects of SCP, and are thought to be unrelated to the immunopathogenesis of DED. Corresponding to the relatively lower effector T-cell responses in the disease-draining lymph nodes, SCP-induced DED exhibits fewer CD11b+ and T-cell infiltration in the ocular surface than CEC-induced DED, which can be due to the lower numbers of effector T-cells generated in the CLN, as well as the suppressive effect of mAChR blockade on monocytes and T-cell migration.18 Although SCP induces weaker disease-promoting inflammatory immune responses, it generates comparable levels of clinical severity as the CEC, as demonstrated by comparable CFS scores. CFS is the recommended method of measuring ocular surface epithelial damage and the resultant barrier disruption,21 which is the characteristic pathology of DED at the cellular level.30 This most likely is due to SCP-induced lacrimal hyposecretion, which is an important factor in the pathogenesis of DED (Fig. 7). To confirm the role of SCP further in the immunopathogenesis of DED, mice were subjected to LGE to mimic the aqueous tear deficiency induced by systemic administration of SCP. LGE has been reported to induce dry eye in rats by reducing tear secretion.31 In our study, LGE and SCP caused tear excretory dysfunction; however, in comparison with SCP, LGE caused a more robust localized immune response without affecting systemic immunity. As a result, LGE + CEC induces more severe disease than SCP + CEC, as correlated by the higher Th17 responses measured using the LGE + CEC model (Fig. 6).
Figure 7.
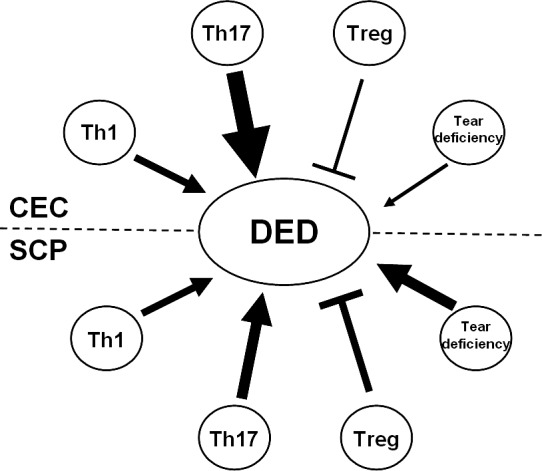
Pathogenic mechanisms compared between CEC- and SCP-induced DED. CEC induces clinically evident DED as effectively as SCP. Regarding disease-promoting factors, CEC and SCP led to comparable Th1 responses; however, CEC induced a much stronger Th17 response. Additionally, the CEC did not lead to significant tear deficiency. In contrast, SCP suppressed exocrine function and resulted in notable tear deficiency. Regarding disease inhibiting factors, the CEC impairs Treg functioned dramatically; while SCP-induced DED showed much less damage to Treg function. →, promotion; ⊣, inhibition. Symbol thickness indicates intensity.
In summary, we presented novel findings regarding the in vivo effects of mAChR blockade on various CD4+ T-cell subsets. Although desiccating environmental stress and mAChR blockade induced similar levels of clinical disease, they did so through different primary pathogenic mechanisms. Systemic mAChR blockade promotes pathogenic aqueous tear deficiency, while simultaneously inhibiting pathogenic immune responses. These findings reinforce the idea that DED models are not necessarily interchangeable, and care should be taken in choosing the model that will yield the most meaningful study results.
Acknowledgments
Supported by NIH Grant EY20889 and Allergan, Inc.
Disclosure: Y. Chen, None; S.K. Chauhan, None; H.S. Lee, None; W. Stevenson, None; C.S. Schaumburg, Allergan (E); Z. Sadrai, None; D.R. Saban, None; S. Kodati, None; M.E. Stern, Allergan (E); R. Dana, Allergan (F)
References
- 1. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003; 136: 318–326 [DOI] [PubMed] [Google Scholar]
- 2. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians' Health Studies. Arch Ophthalmol. 2009; 127: 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjögren's and nonSjögren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002; 43: 2609–2614 [PubMed] [Google Scholar]
- 4. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T-cell-mediated Sjögren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176: 3950–3957 [DOI] [PubMed] [Google Scholar]
- 5. El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T-cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009; 50: 3802–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009; 182: 1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009; 2: 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002; 43: 632–638 [PubMed] [Google Scholar]
- 9. Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008; 126: 219–225 [DOI] [PubMed] [Google Scholar]
- 10. Barabino S, Shen L, Chen L, Rashid S, Rolando M, Dana MR. The controlled-environment chamber: a new mouse model of dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2766–2771 [DOI] [PubMed] [Google Scholar]
- 11. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009; 28: 155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005; 27: 655–665 [DOI] [PubMed] [Google Scholar]
- 13. Dawson L, Tobin A, Smith P, Gordon T. Antimuscarinic antibodies in Sjögren's syndrome: where are we, and where are we going? Arthritis Rheum. 2005; 52: 2984–2995 [DOI] [PubMed] [Google Scholar]
- 14. Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of nonneuronal acetylcholine: expression of an independent, nonneuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008; 106: 186–192 [DOI] [PubMed] [Google Scholar]
- 15. Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003; 74: 675–696 [DOI] [PubMed] [Google Scholar]
- 16. Fujii T, Kawashima K. Ca2+ oscillation and c-fos gene expression induced via muscarinic acetylcholine receptor in human leukemic T- and B-cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2000; 362: 14–21 [DOI] [PubMed] [Google Scholar]
- 17. Nomura J, Hosoi T, Okuma Y, Nomura Y. The presence and functions of muscarinic receptors in human T-cells: the involvement in IL-2 and IL-2 receptor system. Life Sci. 2003; 72: 2121–2126 [DOI] [PubMed] [Google Scholar]
- 18. Razani-Boroujerdi S, Behl M, Hahn FF, Pena-Philippides JC, Hutt J, Sopori M. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J Neuroimmunol. 2008; 194: 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arzt ES, Fernández-Castelo S, Diaz A, Finkielman S, Nahmod VE. The muscarinic agonist pilocarpine inhibits DNA and interferon-gamma synthesis in peripheral blood mononuclear cells. Int J Immunopharmacol. 1989; 11: 275–281 [DOI] [PubMed] [Google Scholar]
- 20. Fujii YX, Tashiro A, Arimoto K, et al. Diminished antigen-specific IgG1 and interleukin-6 production and acetylcholinesterase expression in combined M1 and M5 muscarinic acetylcholine receptor knockout mice. J Neuroimmunol. 2007; 188: 80–85 [DOI] [PubMed] [Google Scholar]
- 21. Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995; 21: 221–232 [PubMed] [Google Scholar]
- 22. Hicks DG, Kushner L, McCarthy K. Breast cancer predictive factor testing: the challenges and importance of standardizing tissue handling. J Natl Cancer Inst Monogr. 2011; 42: 43–45 [DOI] [PubMed] [Google Scholar]
- 23. Subcommittee of the International Dry Eye Workshop The definition and classification of dry eye disease: report of the Definition and Classification. Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 24. Pitcher JD 3rd, De Paiva CS, Pelegrino FS., et al. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2011; 52: 3221–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian J, Galitovskiy V, Chernyavsky AI, Marchenko S, Grando SA. Plasticity of the murine spleen T-cell cholinergic receptors and their role in in vitro differentiation of naïve CD4 T-cells toward the Th1, Th2 and Th17 lineages. Genes Immun. 2011; 12: 222–230 [DOI] [PubMed] [Google Scholar]
- 26. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T-cells. Nature. 2006; 441: 235–238 [DOI] [PubMed] [Google Scholar]
- 27. Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T-cells control TH17 responses in a Stat3-dependent manner. Science. 2009; 326: 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nizri E, Irony-Tur-Sinai M, Lory O, Orr-Urtreger A, Lavi E, Brenner T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J Immunol. 2009; 183: 6681–6688 [DOI] [PubMed] [Google Scholar]
- 29. Wang DW, Zhou RB, Yao YM, et al. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T-cells in mice in vitro. J Pharmacol Exp Ther. 2010; 335: 553–561 [DOI] [PubMed] [Google Scholar]
- 30. Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009; 2: 375–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nemet A, Belkin M, Rosner M. Transplantation of newborn lacrimal gland cells in a rat model of reduced tear secretion. Isr Med Assoc J. 2007; 9: 94–98 [PubMed] [Google Scholar]