Abstract
This study is the first systematic review of risk factors for stroke in China and supports the importance of current public health initiatives to manage the risk factors appropriately to reduce risk of stroke in high risk patients. Additionally, this study has been co-authored by prominent Chinese and US physicians and researchers with expertise in cardiovascular disease, neurologic disorders, epidemiology, and real world data. While there have been several systematic reviews of real world associations of risk factors for coronary artery disease, none focus specifically on the population of China, where there is growing evidence that such risk factors are poorly treated or uncontrolled, especially in rural areas.
Background
To better understand the impact of traditional cardiovascular risk factors on risk of coronary artery disease (CAD) in China, a systematic review of all Chinese observational studies published in either English or Chinese in MEDLINE and EMBASE over the last 5 years was performed and the association between any of 5 traditional risk factors (ie, hypertension, diabetes, elevated lipid levels, obesity, and smoking) and the risk of CAD was studied.
Methods and results
The study found a consistent relationship between lipid levels and CAD. Higher low-density lipoprotein cholesterol values were associated with greater risk of CAD, with an odds ratio as high as 3.31. Other factors found to be significant contributors to the risk of CAD included hypertension (crude odds ratio range of 1.40–5.11), diabetes (1.50–5.97), and smoking (1.37–5.19). An association between obesity and CAD in China was observed, but the evidence supporting this was considered weak due to the paucity of studies found as part of this review.
Conclusions
This review provides a systematic summary of CAD risk factors in China and demonstrates the important differences that exist in CAD risk factors between countries and regions. Approaches to reduce CAD globally must take into account the unique risk factors that drive CAD in each country and region as is demonstrated by these findings.
Keywords: coronary disease, risk factors, smoking, diabetes, hypertension, dyslipidemia
Introduction
Worldwide, over 7 million people each year die from coronary artery disease (CAD),1 a condition where plaque builds up in the blood vessels supplying the heart. Evidence supports an association between CAD trends with major cardiovascular risk factors.2 Major modifiable risk factors include high blood pressure, diabetes, obesity, metabolic syndrome, abnormal lipids, tobacco use, and physical inactivity.1 The prevention and control of major risk factors of CAD among the developed nations has contributed to a significant reduction in CAD mortality rates.3
Contrary to trends in developed nations, China has experienced a considerable increase in the prevalence of CAD over the past several decades.4 CAD has climbed from the fifth most common heart disease in 1948–1957 to the most common in 1980–1989, where it has remained to this day.4 CAD is reported as one of the leading causes of death in China, where it contributes to 51.4% of the mortality attributed to cardiovascular disease (CVD) in urban areas and 32.8% in rural areas. It is projected that from 1990 to 2020, CAD is likely to have reached 72.7 million men and 72.1 million women5 in the general Chinese population, with CVD mortality likely to increase by 108% in men and 79% in women.5
China is also not experiencing a decrease in these risk factors, especially in diabetes and smoking. Hypertension,6–11 diabetes,7,9,11,12 obesity,6,7,9–11,13 dyslipidemia,11 and hypercholesterolemia10 are rising rapidly in the Chinese population and an estimated 28.1% of adults and 52.9% of males were current smokers in 2010, contributing to China as the world’s largest consumer and producer of tobacco products. Hypertension, diabetes, abnormal lipid conditions, obesity, and smoking are all major risk factors of CAD.
Given the potential economic, social, and public health burden of CAD on China’s large population, effective primary and secondary prevention strategies for risk factors can greatly reduce the risk of CAD. However, the impact of each of these risk factors in the Chinese population is unknown, and most models linking these traditional risk factors to CAD have been derived from studies on largely Caucasian populations including whether there might be geographic variation of the impact of these traditional risk factors. Regarded as a leading developing economy with an estimated population of 1.3 billion people,14 a careful examination of this epidemic increase in China will benefit the future prevention of the disease worldwide and contribute to a stronger understanding of the relationship between cardiovascular risk factors and CAD within the Chinese population. We performed a systematic review of the literature to assess the impact of hypertension, diabetes, abnormal lipid conditions, obesity, and smoking on the risk of CAD in China.
Methods
The literature search was performed in MEDLINE (via PubMed) and EMBASE for all observational studies published in either English or Chinese in the last 5 years (2006–2011) on the association between any of the 5 risk factors (ie, hypertension, diabetes, abnormal lipid conditions, obesity, and smoking) and the risk of CAD in the general population of China. Chinese literature databases Wanfang Data and Chinese National Knowledge Infrastructure (CNKI) were also searched but with no significant yield. The main search terms used were as follows: “coronary artery disease” [Mesh], “China,” “incidence” [MeSH Terms], “prevalence” [MeSH Terms], “epidemiology” [MeSH Terms], “observational,” “community-based,” and “cross-section.” Procedures for the review followed established methods used in the science of systematic review research.15,16
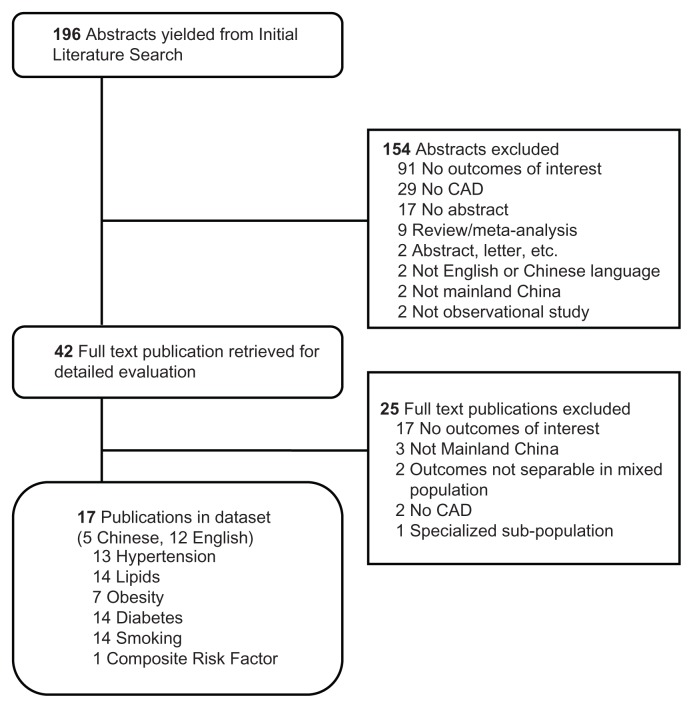
The initial search yielded 196 abstracts. We manually reviewed the abstracts of the articles to exclude study types such as abstracts, case reports, letters, commentaries, editorials, reviews, meta-analyses and clinical trials, studies not on population from mainland China, and studies with no apparent outcomes of interest. If an article did not have an abstract, the article was still retrieved if the title suggested the full text would include the outcomes of interest. Forty-two articles were selected for detailed evaluation. The full text was reviewed to identify only observational studies reporting the association of any of the 5 risk factors and risk of CAD. Seventeen publications met these criteria and were selected for review (Table 1). The article attrition diagram lists the number of articles excluded at each step and the reason for exclusion.
Table 1.
Systematic literature review of risk factors of coronary artery disease (CAD) in China.
| Author, year | Citation | Publication language | Region | Patient population | Sample size | Risk factors | Study design | Study length | Risk of CAD |
|---|---|---|---|---|---|---|---|---|---|
| Su G, 2011 | Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovascular Diabetology. 2011;10:19. | English | Beijing | Consecutive T2DM patients with chest pain referred to coronary angiography | 344 (CAD: 252; non CAD: 92) | Hypertension, lipids, obesity, diabetes, smoking | Prospective cohort | NR | |
| Sai XY, 2007 | Sai XY, He Y, Men K, et al. All-cause mortality and risk factors in a cohort of retired military male veterans, Xi’an, China: an 18-year follow up study. BMC Public Health. 2007;7:290. | English | Xi’an | Retired military men aged 55 or older from 22 military retirement centers in Xi’an | 1268 | Smoking | Survey | 1987–2005/ 18 years | CHD adjusted mortality rates: 421 per 100,000 person years |
| Hu DY, 2006 | Hu DY, Pan CY, Yu JM. The relationship between coronary artery disease and abnormal glucose regulation in China: The China Heart Survey. European Heart Journal. 2006;27(21):2573–9. | English | Seven cities | Patients admitted to hospital cardiovascular wards, T1DM excluded | 3513 | Smoking, obesity, hypertension, hyperlipidemia, diabetes | Prospective cohort | Jun–Aug 2005 | |
| Chen ZW, 2011 | Chen ZW, Qian JY, Jian Y, et al. Prevalence and severity of coronary artery disease in diabetic patients with aortic valve calcification. Acta Cardiol. 2011;66(1):15–20. | English | Shanghai | Consecutive patients with chest pain or chest distress referred for coronary angiography | 325 (CAD: 222; non-CAD: 103) | Diabetes, Hypertension | Prospective cohort | Jun–Dec 2007 | |
| Zhang K, 2010 | Zhang K, Wang YY, Liu QJ, et al. Two single nucleotide polymorphisms in ALOX15 are associated with risk of coronary artery disease in a Chinese Han population. Heart Vessels. 2010;25(5):368–3. | English | Shandong province | Subjects consecutively recruited from hospital inpatients who underwent coronary angiography. History of other diseases were excluded | 1127 (CAD: 519; control 608) | Hypertension, lipids, obesity, diabetes, smoking | Case control | 2006–2008 | |
| Han Y, 2010 | Han Y, Xu W, Zhang W, Liu N, Ji Y. T-786C polymorphism in the endothelial nitric oxide synthase gene is associated with increased risk of coronary artery disease in a Chinese population. Pharmacology. 2010;85(4):211–6. | English | Jianshu province | Chinese Han subjects, CAD confirmed by angiography and healthy controls | 622 (CAD 312; control 310) | Hypertension, lipids, smoking | Case-control | NR | |
| Xu H, 2008 | Xu H, Hou X, Wang N, et al. Genderspecific effect of estrogen receptor-1 gene polymorphisms in coronary artery disease and its angiographic severity in Chinese population. Clin Chim Acta. 2008;395(1–2):130–3. | English | Nanjing | Angiographically defined CAD patients and controls in hospital | 384 (CAD 210; control 174) | Hypertension, lipids, BMI, diabetes, smoking | Case control | NR | |
| Tang NP, 2008 | Tang NP, Wang LS, Yang L, et al. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 2008;395(1–2):89–93. | English | Jiangsu province | Consecutive CAD inpatients admitted for angina pectoris or other symptoms/signs of cardiovascular diseases and controls | 530 (CAD 265; control 265) | Hypertension, lipids, obesity, diabetes, smoking | Case control | NR | |
| Cui, 2007 | Cui HB, Wang SH, Wang DQ, et al. Modified classic risk factors for coronary artery disease in Chinese Han population. Chin Med Sci J. 2007;22(4):216–23. | English | Xi’an, Shanxi, Lanzhou, Ningbo, Shiyan | Angiographic assessed consecutive subjects from Chinese coronary collaborative group presenting at five hospitals with coronary angiography | 762 (CAD 423; control 339) | Hypertension, lipids, diabetes, smoking | Prospective cohort | NR | |
| Ni M, 2007 | Ni M, Zhang XH, Jiang SL, Zhang Y. Homocysteinemia as an independent risk factor in the Chinese population at a high risk of coronary artery disease. Am J Cardiol. 2007;100(3):455–8. | English | Shandong province | Consecutive patients undergoing coronary angiography | 237 (CAD:138; control 99) | Hypertension, lipids, obesity, smoking, diabetes | Prospective cohort | NR | |
| Han Y, 2007 | Han Y, Yang Y, Zhang X, Yan C, Xi S, Kang J. Relationship of the CAG repeat polymorphism of the MEF2A gene and coronary artery disease in a Chinese population. Clin Chem Lab Med. 2007;45(8):987–92. | English | Shenyang | Coronary angiography patients and healthy controls, Han Chinese | 726 (CAD 378; control 348) | Hypertension, diabetes, hyperlipidemia, smoking | Case control | 2003–2006 | |
| Jin Z, 2006 | Jin Z, Zhang Y, Chen J, et al. Study of the correlation between blood lipid levels and the severity of coronary atherosclerosis in a Chinese population sample. Acta Cardiol. 2006;61(6):603–6. | English | Zhejiang | Patients with coronary artery atherosclerosis verified by coronary angiography | 363 | Lipids | Prospective cohort | Jan–Dec 2004 | |
| Liu, 2008 | Liu J, Zhao D, Liu Q, et al. Study on the prevalence of diabetes mellitus among acute coronary syndrome inpatients in a multiprovincial study in China. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2008;29(6):526–9. | Chinese | 64 hospitals representative of China | Inpatients diagnosed with acute coronary syndrome (ACS) | 3223 | Diabetes | Survey | March 2006–May 2006 | |
| Wang, 2007 | Wang Y, Huang JY, Cao YF, et al. Risk factors for type 2 diabetes mellitus in middle-aged and elderly populations of Shanghai rural areas: A nested case-control study. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007;11(52):10433–6. | Chinese | Shanghai | Diabetes patients and control | 597 (type 2 diabetes: 199 non diabetes 398) | Diabetes | Case control | 2003 and 2005 | |
| Li, 2007 | Li BL, Li L, Hou XL, et al. Prevalence of coronary artery disease in patients with rheumatic heart disease in China. National Medical Journal of China. 2007;87(47):3313–6. | Chinese | Shanghai | Patients with rheumatic heart disease aged > 40 who were scheduled for valve surgery | 651: CAD 71 non CAD 580 | Diabetes mellitus, hypertension, smoking, dyslipidemia | Retrospective cohort | Sep 2001–Apr 2006 | 71 (10.91%) Male: 17.94%, Female 4.86% (P < 0.01) Age: 40–59, 6.39% 60–69 21.47% (P < 0.01) ≥ 70 22.22% (P < 0.01) |
| Wang, 2006 | Wang W, Zhao D, Sun JY, et al. Risk factors comparison in Chinese patients developing acute coronary syndrome, ischemic or hemorrhagic stroke: a multi-provincial cohort study. Zhonghua Xin Xue Guan Bing Za Zhi [Chinese Journal of Cardiovascular Diseases]. 2006;34(12):1133–7. | Chinese | 11 provinces | Chinese population aged 35–64 | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Hypertension, smoking, diabetes, high TC, low HDL-C, obesity | Survey | 1992–2003/ 6.6 years | Overall: 114 per 100,000 person-year 35–44: 53 per 100,000 person-year 45–54: 106 per 100,000 person-year 55–64: 249 per 100,000 person-year (3.7 folds compared to 35–44) |
| Li, 2006 | Li X, Gao X, Zhang B, Gu Q, Ren LM, Gao J. Glucose metabolism status and angiographic features of coronary artery in patients undergoing their first coronary angiography: study of 553 cases. Zhonghua Yi Xue Za Zhi. 2006;86(24):1689–92. | Chinese | NR | Inpatients with suspected or confirmed CAD | 553: CAD 388 non CAD 165 | Hypertension, smoking, TC, TG, HDL-C, LDL-C, diabetes | Prospective cohort | Aug 2004–Oct 2005 |
Both study-level and patient-level information from each article were reviewed. Study-level information included publication language, patient population characteristics, geographic region of China, study design (prospective/retrospective cohort, survey or case control), and characteristics such as study period and length of follow-up. Information on distinctive sample characteristics, sample size, baseline demographics, and comorbidities was reviewed for each cohort patient population if reported, such as CAD patients versus non-CAD patients. Main outcomes of interest included any reported association between the 5 risk factors (hypertension, abnormal lipid conditions, obesity, diabetes, and smoking) and CAD, such as odds ratios (OR) or relative risks (RR). Only half of the articles reported adjusted hazard ratios or relative risks. For those that did not, we calculated crude odds ratios from the counts available.
Review was performed by 1 investigator and checked by another who reviewed the extracted data for consistency and accuracy. Data discrepancies were resolved by consensus of the 2 investigators. Articles published in Chinese were translated into English by a native Chinese speaker with fluency in English. Translation was validated by a second native speaker. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.17
Results
Of the 17 articles identified and reviewed for reported association between any of the 5 risk factors and risk of CAD, 12 were published in English and 5 in Chinese. Most studies (70.6%) reported on multiple risk factors. The review included 8 cohort studies (7 prospective, 1 retrospective), most of which examined consecutive patients referred for coronary angiography with suspected or confirmed CAD. Sample sizes ranged from 237 to 3,513 patients (mean: 843). There were 6 case-control studies with sample sizes ranging from 384 to 1,127 (mean: 664). Three of the larger studies were survey studies. One specifically targeted retired military men over age 55 and had a sample size of 1,268. Another, Liu et al,18 included 3,223 inpatients diagnosed with acute coronary syndrome (ACS) from 64 hospitals representative of China. The third, Wang et al,19 surveyed 30,378 individuals among the general Chinese population across 11 provinces. With regard to geographic coverage, only 1 study18 was found to be representative of both urban and rural China. The other 16 focused on major cities (Beijing, Shanghai, Nanjing, Shenyang, and Xi’an) or provincial areas (Shandong, Jiangshu, and Zhejiang provinces).
Association between hypertension and CAD was reported in 13 studies (Table 2A). Association between abnormal lipid conditions and CAD was reported in 12 studies (Table 2B). Seven studies reported the association between obesity and CAD (Table 2C). Associations between CAD and diabetes, and between CAD and smoking were reported each in 14 studies (Tables 2D–E). One study reported association with a composite risk factor (Table 2F).
Table 2A.
Association between hypertension and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Han Y, 2007 | Case-control | 726 (CAD 378; control 348) | Mean: 57.2 (10.5) Range: 29–89 CAD: mean: 57.7 (10.7) Control: 55.6 (10.4) |
492:234 CAD: 284:94 Control: 210:138 |
Being treated for hypertension | 5.11 (P = 0.002) | OR (95% CI): 2.47 (2.20–2.78) (P = 0.00) |
Logistic: age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking | |
| Cui, 2007 | Prospective cohort | 762 (CAD 423; control 339) | Mean: 60 (10) Range 17–81 |
481:281 CAD: 261:162 Control: 220:119 |
100%: lipid-lowering agents | According to Joint National Committee (JNC) VI guideline |
Women: 95% significant association with hypertension Men: No significant association with hypertension |
||
| Han Y, 2010 | Case-control | 622 (CAD 312; control 310) | Mean: 61.96 (10.71) CAD: 61.96 (10.71) Control: 60.54 (10.18) P = 0.09 |
209:103 CAD: 209:103 Control: 184:126 P = 0.056 |
NR | 1.94 (P < 0.01) | |||
| Ni M, 2007 | Case-control | 237 (CAD:138; control 99) | Mean: 54.18 (9.25) Range 35–70 CAD: 55.28 (9.03) Control: 52.65 (9.39) P = 0.031 |
163:74 CAD: 108:30 Control: 55:44 P < 0.01 |
Systolic and diastolic BP ≥ 140/90 mmHg or use of antihypertensive treatment | 4.69 (P < 0.01) | |||
| Su G, 2011 | Prospective cohort | 344 (CAD: 252; non CAD: 92) | CAD: mean: 65 (9) Non CAD: 61 (9) |
CAD: 165:87 Non CAD: 48:44 |
Oral anti-hyperglycemic CAD: 45.7% Non-CAD: 44.0% Not significant Insulin CAD: 35.9% Non-CAD: 40.9% Not significant Statins CAD: 61.9% Non CAD: 69.4% Not significant |
Systolic blood pressure ≥ 140 mmHg and/ or diastolic blood pressure ≥ 90 mmHg or treatment with oral anti-hypertension drugs | 1.52 (NS) | OR: 1.857 (95% CI: 0.969, 3.557, P = 0.062) | Logistic: smoking, male, older age, MAGE (mean amplitude of glycemic excursions), hs-CRP, hyperlipidemia, hypertension, renal insufficiency |
| Tang NP, 2008 | Case-control | 530 (CAD 265; control 265) | CAD: 64 (56–71) Control: 64 (55–71) NS |
CAD: 194:71 Non CAD: 194:71 NS |
Resting systolic blood pressure N140 mmHg and/or diastolic blood pressure N90 mmHg or in the presence of active treatment with antihypertensive agents | 3.46 (P < 0.001) | |||
| Xu H, 2008 | Case-control | 384 (CAD 210; control 174) | CAD: 56 (7.3) Non CAD: 55 (8.6) | 201:183 CAD: 116:94 Control: 85:89 | NR | OR: 1.676 (95% CI: 1.165–2.788); P = 0.014 | Logistic: diabetes, hypertension, high LDL levels and genotype | ||
| Zhang K, 2010 | Case-control | 1127 (CAD: 519; control 608) | CAD: 61.285 (10.755) Control: 60.3777 (10.730) P = 0.16 |
CAD: 362:157 Control: 401:207 |
NR | Health control cohort had zero patients with hypertension. (OR can’t be calculated.) % of patients with hypertension: 63% in the CAD vs. 0% in the non CAD | |||
| Chen ZW, 2011 | Prospective cohort | 325 (CAD: 222; non-CAD: 103) | 63.4 (9.7) Diabetic: 66.2 (8.1) Non diabetic: 62.2 (10.2) P < 0.01 |
218:107 Diabetic 61:43 Non diabetic: 157:64 P = 0.027 |
Systolic pressure >> 140 mmHg or diastolic pressure > 90 mmHg or being treated with antihypertensive medication | Diabetic: OR: 1.846 (P = 0.389) Non diabetic: OR: 1.280 (P = 0.638) |
Logistic regression: aortic valve calcification (AVC), sex, age, hypertension, smoking, serum level of fibrinogen, total cholesterol, triglyceride, highdensity lipoprotein cholesterol, low-density lipoprotein cholesterol, apoprotein | ||
| Hu DY, 2006 | Prospective cohort | 3513 (CAD: 3513) | 69 (65–77) | 2341:1172 | Systolic blood pressure 140 mmHg, and/or diastolic blood pressure 90 mmHg, or current antihypertensive treatment | ||||
| Li, 2007 | Retrospective cohort | 651: CAD 71 non CAD 580 |
Mean: 56 (8) Range: 42–75 CAD: 63 (9) Non-CAD: 54 (9) |
301:350 CAD: 54:17 Non-CAD: 247:333 |
NR | 1.74 (P = 0.05) | |||
| Li, 2006 | Prospective cohort | 553: CAD 388: non-CAD 165 | Mean: 60.1 (9.7) CAD: 61.4 (9.7) Non CAD: 57.2 (8.8) P = 0.00 |
CAD: 82.6% Non-CAD: 63.9% P = 0.00 |
NR | 1.40 (P = 0.08) | |||
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non-CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
BP ≥ 140/90 mmHg or on antihypertension medication | % of patients with hypertension: ACS 49.8% vs. non CVD 26.0% | RR: 1.914 | Cox regression: age, gender, blood pressure, TC, smoking, low HDL-C, diabetes, obesity |
Table 2B.
Association between lipids and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Han Y, 2007 | Case-control | 726 (CAD 378; control 348) | Mean: 57.2 (10.5) Range: 29–89 CAD: mean: 57.7 (10.7) Control: 55.6 (10.4) |
492:234 CAD: 284:94 Control: 210:138 |
Hyperlipidemia | 2.77 (P < 0.01) | OR (95% CI): 2.63 (2.32–2.99) (P = 0.00) |
Logistic: age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking | |
| Cui, 2007 | Prospective cohort | 762 (CAD 423; control 339) | Mean: 60 (10) Range: 17–81 |
481:281 CAD: 261:162 Control: 220:119 |
100%: lipid-lowering agents | Low HDL-C, LDL, TC, TG, LDL/HDL | Low HDL-C (men): RR = 2.8 (95% CI: 1.5–4.2) LDL, TC, TG (men): 95% significant association LDL/HDL: 95% significant association |
||
| Han Y, 2010 | Case-control | 622 (CAD 312; control 310) | Mean: 61.96 (10.71) CAD: 61.96 (10.71) Control: 60.54 (10.18) P = 0.09 |
209:103 CAD: 209:103 Control: 184:126 P = 0.056 |
TC, TG, HDL, LDL values | TC (mmol/l): CAD 4.38 (1.19) vs. non CAD 4.84 (1.09) P < 0.01 TG (mmol/l): CAD 1.76 (1.23) vs. non CAD 1.72 (0.95) P = 0.657 HDL (mmol/l): CAD 1.05 (0.27) vs. non CAD 1.25 (0.31) P < 0.01 LDL (mmol/l): CAD 2.50 (0.94) vs. non CAD 2.82 (0.85) P < 0.01 |
|||
| Jin Z, 2006 | Prospective cohort | 363 | NR | NR | TC, TG, HDL-C, LDL-C, non-HDL-C values | TG (mmol/l): Group I 1.91 (1.20) vs. group II 1.73 (0.88) vs. group III 1.86 (1.40) vs. group IV 1.48 (0.60) TC (mmol/l): Group I 4.38 (0.93) vs. group II 4.73 (0.99) (P < 0.05) vs. group III 4.87 (1.50) (P < 0.01) vs. group IV 4.78 (0.82) HDL-C (mmol/l): Group I 1.21 (0.39) vs. group II 1.30 (0.34) vs. group III 1.28 (0.38) vs. group IV 1.20 (0.27) LDL-C (mmol/l): Group I 2.30 (0.77) vs. group II 2.64 (0.84) (P < 0.01) vs. group III 2.74 (1.23) (P < 0.01) vs. group IV 2.91 (0.68) (P < 0.01) Non-HDL-C (mmol/l): Group I 3.17 (0.91) vs. group II 3.43 (0.94) (P < 0.05) vs. group III 3.59 (1.41) (P < 0.05) vs. group IV 3.58 (0.75) (P < 0.05) *Group according to the number of coronary arteries diseased |
|||
| Ni M, 2007 | Case-control | 237 (CAD:138; control 99) | Mean: 54.18 (9.25) Range: 35–70 CAD: 55.28 (9.03) Control: 52.65 (9.39) P = 0.031 |
163:74 CAD: 108:30 Control: 55:44 P < 0.01 |
Dyslipidemia: Total cholesterol level 5.2 mmol/L (200 mg/dL), LDL cholesterol level 3.4 mmol/L (130 mg/dL), triglyceride level 1.7 mmol/L (150 mg/dL), or HDL cholesterol level 1.03 mmol/L (40 mg/dL) | 3.71 (P < 0.001) | |||
| Su G, 2011 | Prospective cohort | 344 (CAD: 252; non CAD: 92) | CAD: mean: 65 (9) Non CAD: 61 (9) |
CAD: 165:87 Non CAD: 48:44 |
Oral anti-hyperglycemic CAD: 45.7% Non CAD: 44.0% Not significant Insulin CAD: 35.9% Non CAD: 40.9% Not significant Statins CAD: 61.9% Non CAD: 69.4% Not significant |
Hyperlipidemia: diagnosed according to guideline of the National Cholesterol Education Program (ATP III). TC, LDL-C, HDL-C, TG values | Hyperlipidemia: 1.44 (NS) TC (mmol/l): CAD 4.8 (1.2) vs. non CAD 4.6 (1.1) (NS) LDL-C (mmol/l): CAD 2.9 (1.0) vs. non CAD 2.7 (0.8) (NS) HDL-C (mmol/l): CAD 1.1 (0.3) vs. Non CAD 1.0 (0.2) (NS) TG (mmol/l): CAD 2.2 (1.6) vs. non CAD 2.1 (1.2) (NS) |
Hyperlipidemia: OR: 1.425 (95% CI: 0.817, 2.486, P = 0.212) |
Logistic: smoking, male, older age, MAGE (mean amplitude of glycemic excursions), hs-CRP, hyperlipidemia, hypertension, renal insufficiency |
| Tang NP, 2008 | Case-control | 530 (CAD 265; control 265) | CAD: 64 (56–71) Control: 64 (55–71) |
CAD: 194:71 Non CAD: 194:71 NS |
Dyslipidemia: total cholesterol level of 6.2 mmol/l or on drugs TC, TG, HDL-C, LDL-C values | Dyslipidemia: 2.76 (P < 0.01) TC (mmol/l): CAD 4.11 (3.48–4.71) vs. non CAD 3.95 (3.31–4.54) (P = 0.036) TG (mmol/l): CAD 1.46 (1.05–2.14) vs. non CAD 1.14 (0.79–1.62) (P < 0.001) HDL-C (mmol/l): CAD 0.98 (0.84–1.14) non CAD 1.08 (0.90–1.29) (P < 0.001) LDL-C (mmol/l): 2.36 (1.83–2.82) vs. Non CAD 2.14 (1.66–2.63) (P = 0.005) |
|||
| Xu H, 2008 | Case-control | 384 (CAD 210; control 174) | CAD: 56 (7.3) Non CAD: 55 (8.6) |
201:183 CAD: 116:94 Control: 85:89 |
TC, TG, HDL-C, LDL values | TC (mg/dL): CAD 194 (8.6) vs. non CAD 186 (10.2) (NS) TG (mg/dL): CAD 4.7 (1.4) vs. non CAD 4.5 (1.9) (NS) HDL-C (mg/dL): CAD 39.2 (11.4) vs. non CAD 44.6 (10.3) (P = 0.057) LDL (mg/dL): CAD 119 (17.7) vs. non CAD 99.2 (16.4) (P = 0.003) |
LDL: OR: 3.314 (95% CI: 1.565–7.174); P = 0.002 |
Logistic: diabetes, hypertension, high LDL levels and genotype | |
| Zhang K, 2010 | Case-control | 1127 (CAD: 519; control 608) | CAD: 61.285 (10.755) Control: 60.3777 (10.730) P = 0.16 |
CAD: 362:157 Control: 401:207 |
TC, TG, HDL-C, LDL values | TC (mmol/l): CAD 4.71 (1.06) vs. non CAD 4.78 (0.67) (P = 0.066) TG (mmol/l): CAD 2.02 (1.37) vs. non CAD 1.04 (0.33) (P < 0.001) HDL-C (mmol/l): CAD 1.12 (0.34) vs. non CAD 1.43 (0.30) (P < 0.001) LDL (mmol/l): CAD 2.98 (0.91) vs. non CAD 2.67 (0.49) (P = 0.001) |
|||
| Chen ZW, 2011 | Prospective cohort | 325 (CAD: 222; non-CAD: 103) | 63.4 (9.7) Diabetic: 66.2 (8.1) Non diabetic: 62.2 (10.2) P < 0.01 |
218:107 Diabetic: 61:43 Non diabetic: 157:64 P = 0.027 |
TC, TG, HDL-C, LDL-C | TC (mmol/l) Diabetic: OR: 2.543 (P = 0.504) Non diabetic: OR: 0.172 (P = 0.043) TG (mmol/l) Diabetic: OR: 0.780 (P = 0.651) Non diabetic: OR: 1.345 (P = 0.334) HDL-C (mmol/l) Diabetic: OR: 0.008 (P = 0.053) Non diabetic: OR: 0.866 (P = 0.891) LDL-C (mmol/L) Diabetic: OR: 1.131 (P = 0.941) Non diabetic: OR: 3.588 (P = 0.082) |
Logistic regression: aortic valve calcification (AVC), sex, age, hypertension, smoking, serum level of fibrinogen, total cholesterol, triglyceride, high-density lipoprotein Cholesterol, low-density lipoprotein cholesterol, apoprotein |
||
| Li, 2006 | Prospective cohort | 553: CAD 388 non CAD 165 |
Mean: 60.1 (9.7) CAD: 61.4 (9.7) Non CAD: 57.2 (8.8) P = 0.00 |
CAD: 82.6% Non CAD: 63.9% P = 0.00 |
TC, TG, HDL-C, LDL values | TC (mmol/l): CAD 4.5 (1.1) vs. non CAD 4.4 (0.9) (P = 0.09) TG (mmol/l): CAD 1.9 (1.7) vs. non CAD 2.0 (1.6) (P = 0.64) HDL-C (mmol/l): CAD 1.0 (0.3) vs. non CAD 1.1 (0.3) (P = 0.00) LDL (mmol/l): CAD 2.6 (0.9) vs. non CAD 2.4 (0.8) (P = 0.01) |
|||
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
High TC: TC ≥ 240 mg/dL Low HDL-C: HDL-C < 40 mg/dL |
High TC 1.48 (P = 0.116) Low HDL-C 1.75 (P < 0.001) |
High TC RR: 1.732 Low HDL-C RR: 1.387 |
Cox regression: age, gender, Blood pressure, TC, smoking, low HDL-C, diabetes, obesity |
Table 2C.
Association between obesity and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Ni M, 2007 | Case-control | 237 (CAD:138; control 99) | Mean: 54.18 (9.25) Range: 35–70 CAD: 55.28(9.03) Control: 52.65 (9.39) P = 0.031 |
163:74 CAD: 108:30 Control: 55:44 P < 0.01 |
BMI ≥ 30 km/m2 | 2. 05 (P = 0.032) | |||
| Su G, 2011 | Prospective cohort | 344 (CAD: 252; non CAD: 92) | CAD: mean: 65 (9) Non CAD: 61(9) |
CAD: 165:87 Non CAD: 48:44 |
Oral anti-hyperglycemic CAD: 45.7% Non CAD: 44.0% Not significant Insulin CAD: 35.9% Non CAD: 40.9% Not significant Statins CAD: 61.9% Non CAD: 69.4% Not significant |
BMI not statistically difference between CAD and non-CAD groups | |||
| Tang NP, 2008 | Case-control | 530 (CAD 265; control 265) | CAD: 64 (56–71) Control: 64 (55–71) |
CAD: 194:71 non CAD: 194:71 NS |
BMI | BMI: CAD 25.1 (3.3) Non CAD 23.8 (3.6) (P < 0.001) |
|||
| Xu H, 2008 | Case-control | 384 (CAD 210; control 174) | CAD: 56 (7.3) Non CAD: 55 (8.6) |
201:183 CAD: 116:94 Control: 85:89 |
BMI: CAD 24.6 (4.2) Non CAD 23.6 (6.1) (P = 0.056) |
||||
| Zhang K, 2010 | Case-control | 1127 (CAD: 519; control 608) | CAD: 61.285 (10.755) Control: 60.3777 (10.730) P = 0.16 |
CAD: 362:157 Control: 401:207 |
BMI (kg/m^2) | BMI: CAD 26.0 (13.6) vs. non CAD 24.3 (13.3) (P < 0.001) | |||
| Hu DY, 2006 | Prospective cohort | 3513 (CAD: 3513) | 69 (65–77) | 2341:1172 | BMI (kg/m^2) | BMI: CAD Median: 24.2 (quartiles: 22.1–26.4) |
|||
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
BMI ≥ 28 kg/m2 | 1.68 (P < 0.001) | RR: 1.290 | Cox regression: age, gender, Blood pressure, TC, smoking, low HDL-C, diabetes, obesity |
Table 2D.
Association between diabetes and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Han Y, 2007 | Case-control | 726 (CAD 378; control 348) | Mean: 57.2 (10.5) Range: 29–89 CAD: mean: 57.7 (10.7) Control: 55.6 (10.4) |
492:234 CAD: 284:94 Control: 210:138 |
Type 1 and type 2 | 3.83 (P < 0.01) | OR (95% CI): 3.28 (2.60–4.14) (P = 0.00) |
Logistic: age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking | |
| Cui, 2007 | Prospective cohort | 762 (CAD 423; control 339) | Mean: 60 (10); Range: 17–81 |
481:281 CAD: 261:162 Control: 220:119 |
100%: lipid-lowering agents | Self-reported or oral glucose tolerance and insulin level assayed | 95% significant association with diabetes | ||
| Ni M, 2007 | Case-control | 237 (CAD:138; control 99) | Mean: 54.18 (9.25) Range: 35–70 CAD: 55.28 (9.03) Control: 52.65 (9.39) P = 0.031 |
163:74 CAD: 108:30 Control: 55:44 P < 0.01 |
NR | 3.02 (P = 0.005) | |||
| Su G, 2011 | Prospective cohort | 344 (CAD: 252; non CAD: 92) | CAD: mean: 65 (9) Non CAD: 61 (9) |
CAD: 165:87 Non CAD: 48:44 |
Oral anti-hyperglycemic CAD: 45.7% Non CAD: 44.0% Not significant Insulin CAD: 35.9% Non-CAD: 40.9% Not significant Statins CAD: 61.9% Non CAD: 69.4% Not significant |
Diagnosed according to the American Diabetes Association criteria | Duration of diabetes (months): CAD: 78 (77) No CAD: 58 (68) P = 0.022 | ||
| Tang NP, 2008 | Case-control | 530 (CAD 265; control 265) | CAD: 64 (56–71) Control: 64 (55–71) |
CAD: 194:71 non CAD: 194:71 NS |
Fasting blood glucose N7.8 mmol/l or a diagnosis of diabetes needing diet or antidiabetic drug therapy | 1.47 (NS) | |||
| Xu H, 2008 | Case-control | 384 (CAD 210; control 174) | CAD: 56 (7.3) Non CAD: 55 (8.6) |
201:183 CAD: 116:94 Control: 85:89 |
NR | 1.50 (P = 0.02) | OR: 4.381 (95% CI: 2.536–7.764); P < 0.001 | Logistic: diabetes, hypertension, high LDL levels and genotype | |
| Zhang K, 2010 | Case-control | 1127 (CAD: 519; control 608) | CAD: 61.285 (10.755) Control: 60.3777 (10.730) P = 0.16 |
CAD: 362:157 Control: 401:207 |
22% of CAD patients had diabetes, 0% in non CAD patients. (P = 1.00) | ||||
| Chen ZW, 2011 | Prospective cohort | 325 (CAD: 222; non-CAD: 103) | 63.4 (9.7) Diabetic: 66.2 (8.1) Non diabetic: 62.2 (10.2) P < 0.01 |
218:107 Diabetic: 61:43 Non diabetic: 157:64 P = 0.027 |
1999 WHO diagnostic criteria | 1.50 (P = 0.31) | |||
| Hu DY, 2006 | Prospective cohort | 3513 (CAD: 3513) | 69 (65–77) | 2341:1172 | Type 2 diabetes only: ≥ 7.0 or ≥11.1 mmol/L on FPG test | 52.9% CAD patients had diabetes 5.97 (P < 0.01) |
|||
| Li, 2007 | Retrospective cohort | 651: CAD 71 non CAD 580 |
Mean: 56 (8) Range: 42–75 CAD: 63 (9) Non CAD: 54 (9) |
301:350 CAD: 54:17 Non CAD: 247:333 |
|||||
| Li, 2006 | Prospective cohort | 553: CAD 388 Non CAD 165 |
Mean: 60.1 (9.7) CAD: 61.4 (9.7) Non CAD: 57.2 (8.8) P = 0.00 |
CAD: 82.6% Non CAD: 63.9% P = 0.00 |
History of DM and Newly diagnosed | 2.97 (P < 0.05) | |||
| Wang, 2007 | Survey | 597 (Type 2 Diabetes: 199 non diabetes 398) | Range: 40–85 | Diabetes: 76:123 Non diabetes: 152:246 |
1999 WHO and International Diabetes Association criteria | 2.08 (1.16–3.74) (P < 0.01) | |||
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
Fasting blood glucose ≥ 7 mmol/L or previous diagnosis by physicians | 1.53 (P < 0.001) | RR: 1.191 | Cox regression: age, gender, blood pressure, TC, smoking, low HDL-C, diabetes, obesity | |
| Liu, 2008 | Survey | 3223 (ACS history 27.1%) | 65 (11) | 2183:1040 | History of DM or newly diagnosed | 22.6% By gender Female: 26.3% Male: 20.8% P < 0.01 By age <45 10.4% 45 ~ 20.2% 55 ~ 23.2% 65 ~ 23.8% ≥75 25.2% |
Table 2E.
Association between smoking and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Han Y, 2007 | Case-control | 726 (CAD 378; control 348) | Mean: 57.2 (10.5) Range: 29–89 CAD: mean: 57.7 (10.7) Control: 55.6 (10.4) |
492:234 CAD: 284:94 Control: 210:138 |
Past and present (former smoker+ current smoker) | 1.42 (P < 0.01) | OR (95% CI): 1.23 (1.09–1.39) (P = 0.00) | Logistic: age, gender, hypertension, diabetes mellitus, hyperlipidemia, smoking | |
| Cui, 2007 | Prospective cohort | 762 (CAD 423; control 339) | Mean: 60 (10); range 17–81 | 481:281 CAD: 261:162 Control: 220:119 |
100%: lipid-lowering agents | Smoked at least one cigarette per day in at least one year | Men: RR=2.4 (95% CI: 1.6–3.3) |
||
| Han Y, 2010 | Case-control | 622 (CAD 312; control 310) | Mean: 61.96 (10.71) CAD: 61.96 (10.71) Control: 60.54 (10.18) P = 0.09 |
209:103 CAD: 209:103 Control: 184:126 P = 0.056 |
NR | 0.41 (P < 0.01) | |||
| Ni M, 2007 | Case-control | 237 (CAD: 138; control 99) | Mean: 54.18 (9.25) Range: 35–70 CAD: 55.28 (9.03) Control: 52.65 (9.39) P = 0.031 |
163:74 CAD: 108:30 Control: 55:44 P < 0.01 |
Current smoker | 3.06 (P < 0.001) | OR (95% CI): 3.83 (1.08–13.68) P = 0.038 | Logistic: age, male, gender, CAD family history, smoking, obesity, dyslipidemia, diabetes mellitus, hypertension, systolic BP, diastolic BP, fasting glucose, total cholesterol, triglycerides, LDL-C, HDL-C, hs—CRP, homocysteine | |
| Sai XY, 2007 | Cross-sectional survey | 1268 | Mean: 62.95 (5.18) Never smoker: 62.52 (5.20) Ever smoker: 63.13 (5.16) |
1268:0 | Ever vs. never (ever-smoker: one who had smoked at least one cigarette daily for one year or more) Former smoker: those who had stopped for at least 2 years. Current smoker: ever-smokers who were smoking at baseline |
Ever smoker: 1.37 | CHD mortality: former smoker HR 0.681 (95% CI: 0.376–1.233) Current smoker HR 1.805 (95% CI: 1.022–3.188) |
||
| Su G, 2011 | Prospective cohort | 344 (CAD: 252; non CAD: 92) | CAD: mean: 65 (9) Non CAD: 61 (9) |
CAD: 165:87 Non CAD: 48:44 |
Oral anti-hyperglycemic CAD: 45.7% Non CAD: 44.0% Not significant Insulin CAD: 35.9% Non CAD: 40.9% Not significant Statins CAD: 61.9% Non CAD: 69.4% Not significant |
2.17 (P = 0.007) | OR: 2.492 (95% CI: 1.315, 4.720, P = 0.005) | Logistic: smoking, male, older age, MAGE (mean amplitude of glycemic excursions), hs-CRP, hyperlipidemia, hypertension, renal insufficiency | |
| Tang NP, 2008 | Case-control | 530 (CAD 265; control 265) | CAD: 64 (56–71) Control: 64 (55–71) |
CAD: 194:71 Non CAD: 194:71 NS | ≥ 10 cigarettes/d | 2.02 (P < 0.001) | |||
| Xu H, 2008 | Case-control | 384 (CAD 210; control 174) | CAD: 56 (7.3) Non CAD: 55 (8.6) |
201:183 CAD: 116:94 Control: 85:89 |
NR | 1.53 (NS) | |||
| Zhang K, 2010 | Case-control | 1127 (CAD: 519; control 608) | CAD: 61.285 (10.755) Control: 60.3777 (10.730) P = 0.16 |
CAD: 362:157 Control: 401:207 |
5.19 (P < 0.001) | ||||
| Chen ZW, 2011 | Prospective cohort | 325 (CAD: 222; non-CAD: 103) | 63.4 (9.7) Diabetic: 66.2 (8.1) Non diabetic 62.2 (10.2) P < 0.01 |
218:107 Diabetic: 61:43 Non diabetic: 157:64 P = 0.027 |
NR | Diabetic: OR: 2.941 (P=0.199) Non diabetic: OR: 1.603 (P=0.256) |
Logistic regression: aortic valve calcification (AVC), sex, age, hypertension, smoking, serum level of fibrinogen, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, apoprotein | ||
| Hu DY, 2006 | Prospective cohort | 3513 (CAD: 3513) | 69 (65–77) | 2341:1172 | 50% of CAD were never smokers, 30% former smokers, 20% current smokers | ||||
| Li, 2007 | Retrospective cohort | 651: CAD 71 non CAD 580 | Mean: 56 (8) Range: 42–75 CAD: 63 (9) Non CAD: 54 (9) |
301:350 CAD: 54:17 Non CAD: 247:333 |
3.89 (P < 0.01) | ||||
| Li, 2006 | Prospective cohort | 553: CAD 388 non CAD 165 | Mean: 60.1 (9.7) CAD: 61.4 (9.7) Non CAD: 57.2 (8.8) P = 0.00 |
CAD: 82.6% Non CAD: 63.9% P = 0.00 |
3.30 (P = 0.00) | ||||
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
Currently smoking and ≥ 1 cigarette per day | 1.69 (P < 0.001) | RR: 1.750 | Cox regression: age, gender, blood pressure, TC, smoking, low HDL-C, diabetes, obesity |
Table 2F.
Association between composite risk factor and risk of CAD.
| Author, year | Study design | Number of patients | Age | Gender (M:F) | Treatment history | Definition of risk factor | Crude OR | Adjusted association | Adjusted model configuration |
|---|---|---|---|---|---|---|---|---|---|
| Wang, 2006 | Survey | 30,378 (ACS 227 stroke 582 non CVD 29,569) | Mean: 46.89 ASC: 52.4 (7.9) Non CVD: 46.7 (8.0) |
ASC: male 70.5% Non CVD: male 53.2% |
Composite 1 Any one of the list: hypertension, smoking, high TC, low HDL-C, diabetes, obesity Composite 2 Any two of the list: hypertension, smoking, high TC, low HDL-C, diabetes, obesity Composite 3 Any three of the list: hypertension, smoking, high TC, low HDL-C, diabetes, obesity |
Composite 1: 2.80 (P < 0.001) Composite 2: 2.68 (P < 0.001) Composite 3: 3.21 (P < 0.001) |
Hypertension and risk of CAD
Thirteen studies were selected for review on the association between hypertension and CAD. The association ranged between 1.40 and 5.11 for crude ORs and between 1.68 and 2.47 for adjusted relative ratios. Seven studies had crude ORs derived from the available prevalence of hypertension in the CAD group versus non-CAD group. Four studies reported crude ORs between 1.40 and 1.94, while 3 others20–22 reported much higher crude ratios of 5.11, 4.69, and 3.46. Adjusted RR ratios ranged between 1.28 and 2.47. Cui et al23 did not report the magnitude of the association, but found the risk association among women to be significant while that among men to be insignificant. Chen et al24 reported differential ratios among diabetic and non-diabetic populations. The adjusted ORs among diabetics were found to be higher than that among non-diabetics (1.85 vs. 1.28).
There were 6 case-control studies, 6 cohort studies, and 1 survey. The case-control studies generally reported higher ORs compared to the cohort studies (mean 3.8 vs. 1.55).
The largest crude ORs and adjusted RR ratios were both found in Han et al.25 The study was a case-control study that recruited 378 CAD patients and 348 healthy controls from Northern Hospital in Shenyang.
Lipid profile and risk of CAD
Twelve studies were selected for review on the association between lipid conditions and risk of CAD. Three studies reported the association between hyperlipidemia and risk of CAD, 2 between dyslipidemia and risk of CAD, and 10 between values on total cholesterol (TC), triglyceride (TG), LDL cholesterol (LDL-C) and HDL cholesterol levels (HDL-C), and risk of CAD.
For the association between hyperlipidemia and the risk of CAD, significant crude and adjusted odds ratios were reported in only 1 case-control study conducted in Shenyang,21 where the crude OR was reported as 2.77 and adjusted OR as 2.63 (95% confidence interval [CI]: 2.32–2.99). The criteria for defining hyperlipidemia were not provided in this study. For the association between dyslipidemia and the risk of CAD, significance was found in 2 of 3 studies reviewed. Ni et al20 reported the crude ORs to be 3.71 for a case-control study conducted in Shandong province. The study population consisted of 138 CAD patients and 99 controls, where the CAD patient cohort was significantly older (55.3 vs. 52.7, P = 0.03) and had a higher percentage of male patients (78.3% vs. 55.6%, P < 0.01) than the control cohort. Dyslipidemia was defined as total cholesterol ≥ 5.2 mmol/L, LDL ≥ 3.4 mmol/L, triglycerides ≥ 1.7 mmol/L or HDL ≥ 1.03 mmol/L. Tang et al22 reported the crude OR to be 2.76. This study had a similar study design as Ni et al,20 where 265 patients were selected each for a CAD cohort and healthy control cohort. However, Tang et al22 found no significant difference in baseline patient characteristics. Dyslipidemia in this study was defined as total cholesterol ≥ 6.2 mmol/L or on drugs.
For the association between total cholesterol (TC), triglycerides (TG), HDL-C, LDL-C, and risk of CAD, 7 studies compared values of lipoprotein profile between CAD patients and the healthy controls. Su et al26 found the difference in the lipid conditions reported between the 2 cohorts to be generally insignificant. Two studies25,27 found CAD patients had slightly lower TC values than healthy controls (P < 0.01); 2 studies22,28 found CAD patients had significant higher values; and 1 study29 found the difference to be insignificant. For reported TG values, 2 studies22,27 found CAD patients to have significant higher values than their comparative healthy controls, although 3 studies25,28,29 found the difference to be insignificant. Comparison on HDL-C and LDL-C values is more consistent between studies. All 5 studies22,25,27–29 reported significantly lower HDL-C values than non-CAD patients. Wang et al19 reported a crude OR of 1.75 and adjusted RR of 1.39 for low HDL-C among CAD patients, and Cui et al23 reported the adjusted risk among men to be higher at 2.80 (95% CI: 1.50–4.20). For LDL-C values, 4 studies22,27–29 found significantly higher levels among CAD patients, but 1 study25 found the opposite. This is consistent with the adjusted RR reported in Xu et al,29 where LDL-C is associated with an OR of 3.31 for risk of CAD.
Jin et al30 reported the association between the severity of CAD and lipoprotein profiles. Severity was positively correlated with the number of coronary arteries diseased. Among the lipid conditions examined, which include TG, TC, HDL-C, LDL-C, and non-HDL-C, only LDL-C was found to be consistently related to the progression of the disease. Patients in more severe disease conditions were found to have significantly higher LDL-C values. Chen et al24 reported differential adjusted association among diabetic and non-diabetic patients. Among diabetic patients, the association between TC, TG, and LDL-C and risk of CAD were statistically insignificant, while among non-diabetic patients, the association between TG, HDL-C, and risk of CAD were not significant. The remaining significant ORs were relatively small except for LDL-C, where non-diabetic patients had an OR of 3.59.
Diabetes and risk of CAD
Fourteen studies were reviewed for the association between diabetes and risk of CAD. While for 2 studies22,24 the association was found to be insignificant, the rest of the reviewed studies reported relatively high association between diabetes and risk of CAD. For crude OR, the association ranged between 1.50 and 5.97. Two studies19,29 reported similar crude ORs of 1.50 and 1.53, respectively. However, study designs differed considerably between the two. Xu et al29 employed a case-control setting in Nanjing where 210 CAD patients and 174 controls were enrolled in the hospital; Wang et al19 employed data from the Chinese Multi-Provincial Cohort Study (CMCS), where 227 acute coronary syndrome patients and 29,569 non- CAD patients were surveyed across 11 provinces in China. Three studies20,28,31 reported the crude ORs between 2.08 and 3.02. Li et al28 and Ni et al20 performed similar studies where consecutive patients were enrolled as comparative cohorts and patients characteristics differed in terms of age and gender distribution. Han et al25 reported a crude OR of 3.83, and the definition of diabetes used included both type 1 and type 2 diabetes. The upper bound of crude OR at 5.97 was reported in Li et al,9 which was a retrospective cohort study conducted in Shanghai Second Military Medical University Hospital. The study enrolled 71 CAD patients and 580 non-CAD patients, and patient characteristics seemed to have different mean of age and gender distribution, but no statistical significance on the difference was reported.
Four studies reported adjusted RR ratios of diabetes on risk of CAD. Wang et al,19 a multi-provincial cohort study, reported a risk ratio of 1.19; Han et al,25 which included both type 1 and type 2 diabetes, reported the ratio to be 3.28 (95% CI: 2.60–4.14); and Xu et al,29 a case-control study in Nanjing reported the ratio to be 4.38 (95% CI: 2.54–7.76). Cui et al,23 a prospective cohort study conducted in 5 cities, reported significant association between diabetes and risk of CAD, but the risk ratio was not represented. Both crude and adjusted ORs indicated that diabetes is a significant contributor to the risk of CAD.
Obesity and risk of CAD
Seven studies were reviewed for the association between obesity or body mass index (BMI) and risk of CAD. Two studies19,20 defined obesity as ≥28 kg/m2 and ≥30 kg/m2 and reported statistically significant crude ORs to be 2.05 and 1.68, respectively. Only 1 study19 reported adjusted RR of 1.29. Four studies22,26,27,29 used BMI as a surrogate for obesity and reported the difference in BMI between CAD patients and non-CAD patients. Three of these studies22,27,29 found CAD patients had significantly higher levels of BMI, while 1 study26 found the difference in BMI between CAD and non-CAD groups to be statistically insignificant.
Smoking and risk of CAD
Fourteen studies were reviewed for the association between smoking and risk of CAD. The definition of smoking applied varied between studies, which included former smokers, current smokers, or ever smokers. Ever smokers included former smokers and current smokers. Thus reported crude ORs ranged widely, between 1.37 and 5.19. For the studies that provided the categories of smokers included, current smokers had crude ORs reported of 3.06,20 2.02,22 and 1.6919; while ever smokers had crude ORs reported of 1.4221 and 1.37.21 Seven studies did not refine the definition of smoking status used in the study. However, except for 1 study,29 the rest found smoking to be a significant factor to the risk of CAD. The highest crude association was reported in Zhang et al27 at 5.19, which had 519 CAD patients and 608 controls comparable in demographic characteristics at baseline.
Adjusted risk ratios ranged between 1.23 and 3.83. Current smokers had adjusted OR of 3.83 (95% CI: 1.08–13.68)20 and 1.75,19 while men had a RR of 2.40 (95% CI: 1.60–3.30).23 Ever smokers had adjusted OR of 1.23 (95% CI: 1.09–1.39). One study21 reported the risk ratio for CAD mortality. Former smokers had a risk ratio of 0.68 for CAD when compared to never smokers, while current smokers had a risk ratio of 1.81. When stratified by diabetic and nondiabetic populations, the adjusted RR ratios were not significant.24
Composite risk factor and CAD
One study, in addition to reporting on the association of the risk factors of interest, also reported an association between composite risk factors and risk of CAD.19 The composite risk factor was defined as having any 1, 2, or 3 of the conditions, which include hypertension, smoking, high TC, low HDL-C, diabetes, and obesity. Among CAD patients, 83.7% had at least 1 risk factor, 47.6% had at least 2, and 18.5% had at least 3 risk factors. Among patients with no CAD, only 64.7%, 25.3%, and 6.6% had at least 1, 2, or 3 risk factors, respectively, and these were each statistically different from the results for CAD patients. Of all patients with at least 1 risk factor, CAD patients had more additional risk factors than non CAD patients by a factor of more than 2 to 1. Additionally, each of the individual risk factors were significant contributors to the risk of CAD (P < 0.001).
Discussion
This study provides the first large systematic review of the available evidence on the association between the traditional risk factors—hypertension, abnormal lipid levels (hyperlipidemia, dyslipidemia, TC, TG, HDL-C, LDL-C), diabetes, obesity, and smoking— and risk of CAD within the last 5 years in China. Of the 5 risk factors examined, lipid conditions, especially high LDL-C values, were found consistently to be associated with high risk of CAD. The adjusted RR rate was as high as 3.31 and consistent with findings in Western populations, which report adjusted RR rates of 1.74 in men and 0.68 in women.32 As in other countries, such as the United States,33 diabetes was also a significant contributor to the risk of CAD, with an adjusted RR rate as high as 1.191 and adjusted ORs of 3.28 and 4.381 (P < 0.001). Hypertension and smoking were found to be significant contributors to the risk of CAD, with adjusted RR rates as high as 1.91 and 1.75 and crude ORs as high as 5.11 and 5.19, respectively. While data on the association between obesity and CAD was suggestive of a positive association (adjusted RR rate of 1.91), we consider the evidence to be weak due to the small number of studies examining that particular risk factor.
Northeast China had the highest CAD mortality rate (28.0%), while Southern China had the lowest rate (17.1%).21 A study conducted in the North21 reported the highest crude ORs and adjusted RR ratios for hypertension, diabetes, smoking, and hyperlipidemia in CAD patients, suggesting that the population of Northern China may be at greater risk for stroke than the Chinese population as a whole. Such geographic variation was also found in the United States, and this was largely suspected to be because areas with higher CAD prevalence were frequently characterized as rural and poor.34 However, while differences in measurement were in part based on geography, studies were mostly regional in scope and lacked comparison across different areas of the country. Well-designed epidemiological studies are needed to better estimate the impact of individual and overall risk factors on reduction and prevention of CAD in China.
Gender and age differences in CAD mortality and prevalence were widely reported in the articles included in this review, and gender played a significant role in the differentiation of prevention and reduction of CAD. CAD mortality rates increased by 50% in men and 21% in women when adjusted for age. This systematic review also confirmed previous findings9 that in China, hypertension among women was found to be significant, while hypertension among men was found not to be significant.9 Prevalence also seemed to differ by age group with the likelihood of having diabetes among CAD patients increasing with age. This observation is supported by a meta-analyses of 41 cohort studies conducted from Asia, Australia, and New Zealand that found higher CAD risk among stratified age groups, particularly amongst women.33
When looking at other countries such as the United States35,36 and France,37 CAD has declined significantly due to treatments and changes in diet,38 the addition of health and nutrition programs, and promoting healthy eating and physical activity via marketing.19 A study conducted in Singapore38 suggested changes in diet to address incidence of CAD. Additionally, Wang et al11 advised that preparations be made in the health care infrastructure to accommodate the growing need for treatment of CAD and related chronic disease. Additionally, antihypertensives and statins have proved an effective treatment for preventing coronary events and death from coronary heart disease, especially for preventing secondary coronary events. Three large trials in the United States39,40 and Scandinavia41 demonstrated that statin treatment reduced coronary events by 23%–34% and reduced CAD mortality by 20%–42%. Antihypertensive medication use in China is only 28.2% even among those aware of their hypertensive condition. Increasing antihypertensive medication use, therefore, has the potential to greatly impact hypertension-related CAD mortality.
Meta-analyses were considered, but differences represented in these studies in terms of study design (eg, case-control vs. cohort, blinding vs. non-blinding) patient population, and outcome measurement can have large effects on results.42 These problems were exacerbated in the current dataset by heterogeneity in the statistical methods employed when examining relationships between comorbidities and outcomes. For instance, in the 23 hypertension studies, there were:
Differences in definition of hypertension (eg, ≥140/90 vs. ≥160/95)
Differences in the type of stroke (ischemic vs. total vs. hemorrhagic vs. stroke mortality, with several different types of ischemic stroke)
Differences in patient population (eg, some studies are on the general population, some on only diabetics, some only on patients with atrial fibrillation (AF), some only on elderly patients)
Differences in how relationships are measured (some analyses given a crude OR/RR, others adjust for multiple factors)
Differences in factors controlled for in multivariate analyses (some control for age and duration of diabetes, while some control for 10 or more variables such as familial stroke history, ie, variables which may be endogenous to risk, thus resulting in a lower than expected hazard ratio between the variables of interest
Differences in study design (prospective cohort vs. retrospective cohort vs. survey vs. case-control)
Given this host of differences, we concluded that while meta-analysis was statistically viable, the results would not be interpretable without digging down into very small subsets of studies.
Another weakness of the data extracted is that fewer than half of the articles reported adjusted hazard ratios or RRs. Much of the discussion relies on crude ORs extracted from the reported frequencies. This dependence on crude ORs ignores the role of other important risk factors for CAD such as age and gender. Though a strong association was found in this review between lipid levels and risk of CAD, evidence relating these 5 risk factors to CAD has largely gone unexamined in the Chinese population. As noted earlier, the lack of quality data makes difficult comparisons between the different regions of China, both geographic and economic. More large epidemiologic studies relating CAD to its risk factors, especially nationwide studies, would go a long way towards clarifying the effects these 5 conditions have on incidence of CAD in China. The need for large, regional studies is made more important by the fact that China has experienced a considerable increase in the prevalence of CAD in recent decades, and understanding the contribution of the leading 5 risk factors to CAD in China is a critical first step toward future prevention of this disease.
Regardless of the quantity or quality of research in this area, this review found that all 5 of the risk factors examined—hypertension, smoking, diabetes, obesity, and, in particular, low LDL-C levels— were associated with CAD in China. Hypertension, diabetes, and smoking were associated with CAD, with crude ORs ranging from 1.37 to 5.97. While few ORs or RR ratios were calculated, high LDL-C levels were consistently associated with CAD, and to a somewhat lesser extent, so were low HDL-C levels. While the connection between obesity and CAD deserves additional study due to a paucity of existing research within the subject population, studies in this review did find that obesity was positively associated with CAD in China, and this matches results of similar studies conducted in Western populations. Addressing these 5 risk factors through drug treatment as well as diet and lifestyle changes has led to reduced risk of CAD in countries such as the United States. Given that the prevalence of these risk factors in China, especially smoking, is comparatively greater than these other populations, we suspect that treating the risk factors discussed in this review will lead to dramatic and positive effects in the risk of CAD in China. Therefore, we recommend that the Chinese health care system accommodate the increased need for treatment of CAD and its related chronic diseases, in particular the risk factors hypertension, smoking, diabetes, obesity, and elevated lipid levels.
Figure 1.
Attrition diagram.
Notes: Rounded corners = Accepted articles at each stage. Squared corners = Rejected articles.
Acknowledgements
Medical writing and editorial support to prepare this manuscript under the direction of the authors was provided by Dylan Boyd of United BioSource Corporation and funded by Pfizer Inc.
Footnotes
Author Contributions
Conceived and designed the experiments: JF, HJM, SS. Analyzed the data: DB, SS. Wrote the first draft of the manuscript: DB, SS. Contributed to the writing of the manuscript: SS. Jointly developed the structure and arguments for the paper: Made critical revisions and approved final version: JF, YH, LJ, DZ, DH. All authors reviewed and approved of the final manuscript.
Competing Interests
Prof. Yong Huo, Prof. Linong Ji, Prof. Dong Zhao, and Prof. Dayi Hu have no conflicts of interest. Dr. JoAnne Foody is a Pfizer Inc, consultant. Dylan Boyd is an employee of United BioSource Corporation. Dr. Hai Jin Meng is an employee of Pfizer, Ltd, Dr. Susan Shiff is a Pfizer Inc, medical employee and is also an owner of Pfizer stock.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
This study was sponsored by Pfizer Inc, Emerging Markets Outcomes Research and Epidemiology. The principal investigators and co-investigators had full access to the data and were responsible for the study protocol, study progress, analysis, study reporting, and decision to publish the paper. Pfizer Inc, had the opportunity to comment on the manuscript before submission.
References
- 1.Machay J, Mensah G. The Atlas of Heart Disease and Stroke. 1st ed. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Critchley J, Liu J, Zhao D, Wei W, Capewell S. Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation. 2004 Sep 7;110(10):1236–44. doi: 10.1161/01.CIR.0000140668.91896.AE. [DOI] [PubMed] [Google Scholar]
- 3.Sytkowski PA, Kannel WB, D’Agostino RB. Changes in risk factors and the decline in mortality from cardiovascular disease. The Framingham Heart Study. N Engl J Med. 1990 Jun 7;322(23):1635–41. doi: 10.1056/NEJM199006073222304. [DOI] [PubMed] [Google Scholar]
- 4.Cheng TO. The current state of cardiology in China. Int J Cardiol. 2004 Sep;96(3):425–39. doi: 10.1016/j.ijcard.2003.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004 Jul;148(1):7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 6.Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008 Mar;9(Suppl 1):14–21. doi: 10.1111/j.1467-789X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 7.Huxley R, Barzi F, Stolk R, et al. Ethnic comparisons of obesity in the Asia-Pacific region: protocol for a collaborative overview of cross-sectional studies. Obes Rev. 2005 Aug;6(3):193–8. doi: 10.1111/j.1467-789X.2005.00189.x. [DOI] [PubMed] [Google Scholar]
- 8.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005 Jan 15–21;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 9.Li BL, Li L, Hou XL, et al. Prevalence of coronary artery disease in patients with rheumatic heart disease in China. National Medical Journal of China. 2007;87(47):3313–6. [PubMed] [Google Scholar]
- 10.Liu L, Ikeda K, Chen M, et al. Obesity, emerging risk in China: trend of increasing prevalence of obesity and its association with hypertension and hypercholesterolaemia among the Chinese. Clin Exp Pharmacol Physiol. 2004 Dec;31(Suppl 2):S8–10. doi: 10.1111/j.1440-1681.2004.04105.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007 Jan;31(1):177–88. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010 Mar 25;362(12):1090–101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YX, Wang SR. Distribution of body mass index and the prevalence changes of overweight and obesity among adolescents in Shandong, China from 1985 to 2005. Ann Hum Biol. 2008 Sep-Oct;35(5):547–55. doi: 10.1080/03014460802334239. [DOI] [PubMed] [Google Scholar]
- 14.The World Bank, editor. World Development Indicators. 1st ed. Washington, DC: World Bank Publications; 2011. [Google Scholar]
- 15.Alderson P, Green S, Higgins J, editors. Cochrane Collaboration Handbook. Vol. 421. Chichester, UK: John Wiley & Sons, Ltd; 2004. [Google Scholar]
- 16.Cook DJ, Guyatt GH, Laupacis A, Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1992 Oct;102(Suppl 4):305S–11. [PubMed] [Google Scholar]
- 17.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the international journal of cardiology. Int J Cardiol. 2011 Dec 15;153(3):239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Zhao D, Liu Q, et al. Study on the prevalence of diabetes mellitus among acute coronary syndrome inpatients in a multiprovincial study in China. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2008;29(6):526–9. [PubMed] [Google Scholar]
- 19.Wang W, Zhao D, Sun JY, et al. Risk factors comparison in Chinese patients developing acute coronary syndrome, ischemic or hemorrhagic stroke: a multi-provincial cohort study. Chinese Journal of Cardiovascular DiseasesZhonghua Xin Xue Guan Bing Za Zhi. 2006;34(12):1133–7. [PubMed] [Google Scholar]
- 20.Ni M, Zhang XH, Jiang SL, Zhang Y. Homocysteinemia as an independent risk factor in the Chinese population at a high risk of coronary artery disease. Am J Cardiol. 2007 Aug 1;100(3):455–8. doi: 10.1016/j.amjcard.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 21.Sai XY, He Y, Men K, et al. All-cause mortality and risk factors in a cohort of retired military male veterans, Xi’an, China: an 18-year follow up study. BMC Public Health. 2007;7:290. doi: 10.1186/1471-2458-7-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang NP, Wang LS, Yang L, et al. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 2008 Sep;395(1–2):89–93. doi: 10.1016/j.cca.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Cui HB, Wang SH, Wang DQ, et al. Modified classic risk factors for coronary artery disease in Chinese Han population. Chin Med Sci J. 2007 Dec;22(4):216–23. [PubMed] [Google Scholar]
- 24.Chen ZW, Qian JY, Jian Y, et al. Prevalence and severity of coronary artery disease in diabetic patients with aortic valve calcification. Acta Cardiol. 2011 Feb;66(1):15–20. doi: 10.1080/ac.66.1.2064962. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Xu W, Zhang W, Liu N, Ji Y. T-786C polymorphism in the endothelial nitric oxide synthase gene is associated with increased risk of coronary artery disease in a Chinese population. Pharmacology. 2010;85(4):211–6. doi: 10.1159/000275135. [DOI] [PubMed] [Google Scholar]
- 26.Su G, Mi S, Tao H, et al. Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovascular Diabetology. 2011;10:19. doi: 10.1186/1475-2840-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Wang YY, Liu QJ, et al. Two single nucleotide polymorphisms in ALOX15 are associated with risk of coronary artery disease in a Chinese Han population. Heart Vessels. 2010 Sep;25(5):368–73. doi: 10.1007/s00380-009-1223-5. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Gao X, Zhang B, Gu Q, Ren LM, Gao J. Glucose metabolism status and angiographic features of coronary artery in patients undergoing their first coronary angiography: study of 553 cases. Zhonghua Yi Xue Za Zhi. 2006 Jun 27;86(24):1689–92. [PubMed] [Google Scholar]
- 29.Xu H, Hou X, Wang N, et al. Gender-specific effect of estrogen receptor-1 gene polymorphisms in coronary artery disease and its angiographic severity in Chinese population. Clin Chim Acta. 2008 Sep;395(1–2):130–3. doi: 10.1016/j.cca.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Jin Z, Zhang Y, Chen J, et al. Study of the correlation between blood lipid levels and the severity of coronary atherosclerosis in a Chinese population sample. Acta Cardiol. 2006 Dec;61(6):603–6. doi: 10.2143/AC.61.6.2017958. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Huang JY, Cao YF, et al. Risk factors for type 2 diabetes mellitus in middle-aged and elderly populations of Shanghai rural areas: A nested case-control study. Journal of Clinical Rehabilitative Tissue Engineering Research. 2007;11(52):10433–6. [Google Scholar]
- 32.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998 May 12;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 33.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006 Jan 14;332(7533):73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper R, Cutler J, Desvigne-Nickens P, et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000 Dec 19;102(25):3137–47. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 35.Ford ES. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the US: findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care. 2011 Jun;34(6):1337–43. doi: 10.2337/dc10-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford K, Sankey J, Crisp J. Development of children’s assent documents using a child-centred approach. J Child Health Care. 2007 Mar;11(1):19–28. doi: 10.1177/1367493507073058. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AK, Haas B, Ruidavets JB. Trends in coronary heart disease morbidity and mortality in France from 2000 to 2007: Data from the three French coronary heart disease population-based registries. European Heart Journal. 2011;32:54. [Google Scholar]
- 38.Dwyer T, Emmanuel SC, Janus ED, Wu Z, Hynes KL, Zhang C. The emergence of coronary heart disease in populations of Chinese descent. Atherosclerosis. 2003 Apr;167(2):303–10. doi: 10.1016/s0021-9150(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 39.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998 Nov 5;339(19):1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 40.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996 Oct 3;335(14):1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 41.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994 Nov 19;344(8934):1383–9. [PubMed] [Google Scholar]
- 42.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998 Jan 10;316(7125):140–4. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]