Abstract
Comorbid conditions play a pivotal role in rheumatoid arthritis management and outcomes. We estimated the percentage of comorbid illness among rheumatoid arthritis patients and explored the relationship between this comorbidity and different prescriptions. A cross-sectional study of patients with rheumatoid arthritis in three centers in Saudi Arabia was carried out. Comorbidity and antirheumatoid medication regimens prescribed were recorded on a specially designed Performa. The association between comorbidity and different drugs was analyzed. A total of 340 patients were included. The most comorbidities were hypertension 122 (35.9%), diabetes 105 (30.9%), osteoporosis 88 (25.8%), and dyslipidemia in 66 (19.4). The most common drug prescribed was prednisolone in 275 (80.8%) patients followed by methotrexate in 253 (74.4%) and biological therapy in 142 (41.5%) patients. Glucocorticoids were prescribed considerably more frequently in hypertensive and diabetic patients as well as in patients with osteoporosis and dyslipidemia. Most patients with rheumatoid arthritis suffered from comorbid diseases.
Keywords: rheumatoid arthritis, comorbidity, hypertension, diabetes, RA, NSAIDs, glucocorticoids
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory multisystemic disease targeting the synovium.1 The condition exists worldwide and affects all ethnic groups at a rate of approximately 0.5% to 1% of the adult population.2 The etiology of RA is unknown;3–5 however, it may be triggered by reactions to infections in susceptible people.6 Female gender, monozygotic twins, and cigarette smoking appear to be risk factors for RA.4,7 Diagnosis of RA is based on patient history, thorough examination, and laboratory testing.4,11 The characteristic presentation of RA involves a gradual onset of symmetrical arthralgia and synovitis of small joints of the hands, feet, and wrists, as well as morning stiffness.1,4,12
Among drug therapy, single drugs or combinations of drugs such as non-steroidal antiinflammatory drugs (NSAIDs), corticosteroids, and disease-modifying antirheumatic drugs (DMARDs) are used for treating RA.1,4 Currently, methotrexate and sulfasalazine are accepted as first-choice DMARDs. Biological agents such as tumor necrosis factor (TNF) inhibitors are newer agents used in RA treatment.1,16
It is increasingly recognized that comorbid conditions play a pivotal role in RA outcomes. Studies reveal that patients suffering from RA carry two or more comorbid conditions.17 Cardiovascular, osteoporosis, and other associated conditions increase mortality in RA patients.18 These comorbid conditions also affect the choice of RA treatment. In a previous study, Linde et al22 demonstrated a 73% incidence of comorbidities associated with RA.
The aim of this study was to estimate the percentage of comorbid illnesses among RA patients and to determine the relationship between this comorbidity and varying prescriptions given by different rheumatologists.
Materials and Methods
This cross-sectional study was carried out at three hospitals in the Kingdom of Saudi Arabia, including Al-Hada Hospital, National Guard Hospital, and King Abdul-Aziz Hospital. The duration of the study was 1 year from October 2011 to October 2012. Using a non-probability judgment sampling technique, a total of 340 patients diagnosed with RA based on the 1987 American College of Rheumatology criteria were included in the study; however, pregnant and lactating women were excluded from the study. Among the 340 patients, 265 (77.9%) were female and 75 (22.1%) were male. A special Performa was designed that included patient data based on files, reviews, a detailed history, and examination, as well as the latest laboratory test results.
Antirheumatoid medication regimens prescribed by rheumatologists and comorbidities such as hypertension, diabetes, osteoporosis, dyslipidemia, liver, and cardiac diseases were recorded. The association between comorbidity and different drug prescriptions was analyzed using the Chi-squared test with SPSS version 17 (IBM Corporation, Armonky, NY, USA). The results were displayed in tabulated form showing comparisons and frequencies of variables.
Results
In our study, the total number of patients with diagnosed RA from the three centers was 340. The prevalence of the disease was greater in females [265 (78%)] than in males [75 (22%)]. The male to female ratio is shown in Figure 1. Rheumatoid factor was found to be positive in 259 (76%) patients and 11 (3%) patients complained of associated allergies. The duration of RA was also noted. A total of 113 (33%) patients suffered from RA for the past 0–5 years, 11 (35%) for 6–10 years, 63 (19%) for 11–15 years, 25 (7%) for 16–20 years, and 21 (6%) for more than 20 years. A demographic profile of 340 patients with RA is shown in Table 1.
Figure 1.
Male to female ratio of RA patients.
Table 1.
Demographic profile of 340 patients with RA.
| Variable | Frequency n (%) |
|---|---|
| Age “years” | 53.3 ± 11.3 |
| Gender male:female (1:3.5) | |
| Male | 75 (22.1%) |
| Female | 265 (77.9%) |
| Rheumatoid factor, no. (%) | 259 (76.1) |
| Duration of the disease “years” | |
| 0–5 | 113 (33.2%) |
| 6–10 | 118 (34.7%) |
| 11–15 | 63 (18.5%) |
| 16–20 | 25 (7.4%) |
| >20 | 21 (6.2%) |
| History of allergy | 11 (3.2%) |
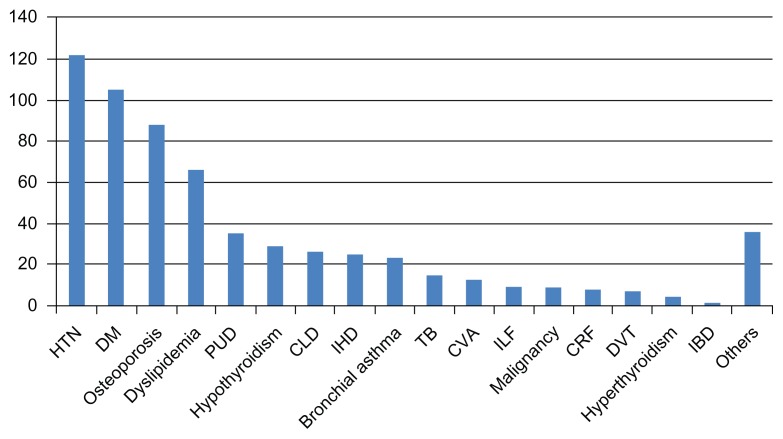
In this study, 225 (66%) patients of the 340 investigated were found to suffer from at least one comorbidity. The number of patients with various comorbidities is shown in Figure 2. The most common comorbidity was hypertension (HTN). Of the 340 patients suffering from RA, 122 (36%) were hypertensive. The second-most common debilitating condition was diabetes mellitus (DM). A total of 105 (31%) patients suffered from DM. As a comorbidity of RA, osteoporosis was third in our study. Eighty-eight (26%) of the 340 patients exhibited decreased bone mineral density. Sixty-six patients had abnormal serum levels of lipids (dyslipidemia). Thirty-five (10%) patients were diagnosed with peptic ulcer disease and 29 (9%) had hypothyroidism. Some RA patients were also found to have hepatic and cardiac problems. Twenty-six (8%) patients had chronic liver disease, while 25 (7%) had ischemic heart disease (IHD). Similarly, bronchial asthma, tuberculosis (TB), cerebrovascular accident (CVA), intestinal lung fibrosis, malignancy, chronic renal failure, deep venous thrombosis, hyperthyroidism, inflammatory bowel disease, and others were also observed in the patients. Comorbidities in RA patients are shown in Table 2 and Figure 3.
Figure 2.
Patients and number of comorbid conditions.
Table 2.
Comorbidity of patients.
| Co-morbidity | n (%) |
|---|---|
| Hypertension (HTN) | 122 (35.9) |
| Diabetes mellitus (DM) | 105 (30.9) |
| Osteoporosis | 88 (25.8) |
| Dyslipidemia | 66 (19.4) |
| Peptic ulcer disease (PUD) | 35 (10.3) |
| Hypothyroidism | 29 (8.5) |
| Chronic liver disease (CLD) | 26 (7.6) |
| Ischemic heart disease (IHD) | 25 (7.4) |
| Bronchial asthma | 23 (6.8) |
| Tuberculosis (TB) | 15 (4.4) |
| Cardiovascular accidents (CVA) | 13 (3.3) |
| Interstitial lung fibrosis (ILF) | 9 (2.7) |
| Malignancy | 9 (2.7) |
| Chronic renal failure (CRF) | 8 (2.4) |
| Deep venous thrombosis | 7 (2.1) |
| Hyperthyroidism | 4 (1.2) |
| Inflammatory bowel disease (IBD) | 1 (0.3) |
| Other | 36 (0.0) |
Figure 3.
Comorbid conditions.
In our study, 275 (81%) patients were taking prednisolone. Additionally, 120 (35%) patients were on NSAIDs. A total of 86% of patients were taking one or more DMARDs (eg, methotrexate, sulfasalazine, antimalarial agents). A total of 253 patients were taking methotrexate, 120 were on sulfasalazine, and 110 were using antimalarial drugs. Among other drugs, biological agents were used by 141 patients; thus, most patients were on DMARDs and corticosteroids. Drugs prescribed to the 340 patients are shown in Table 3.
Table 3.
Total drug prescriptions for the 340 patients.
| Drugs | n (%) | Current dose mg |
|---|---|---|
| Prednisolone | 275 (80.8) | 9.4 ± 8.0 |
| Methotrexate | 253 (74.4) | 14.8 ± 4.3 |
| Sulfasalzine | 120 (35.3) | 1800 ± 165 |
| NSAIDs | 120 (35.2) | |
| Antimalarial | 110 (32.3) | 280 ± 92 |
| Biological therapy | 142 (41.5) | |
| Rituximab | 57 (16.8) | |
| Adalimumab | 38 (11.2) | |
| Etanercept | 26 (7.6) | |
| Infliximab | 20 (5.8) | |
| DMARDs | ||
| 0 DMARD | 47 | |
| 1 DMARD | 172 | |
| 2 DMARD | 97 | |
| 3 DMARD | 24 | |
The pattern of drug prescription in patients suffering from RA with comorbid conditions is shown in Table 4. A significant association between steroids use and risks of IHD was observed, including DM, HTN, and dyslipidemia. Prednisolone was prescribed in approximately 90% patients with HTN, DM, or dyslipidemia. Additionally, steroids were prescribed primarily in patients with osteoporosis (89.7%). Overall, more than 60% of patients with any comorbid condition were taking prednisolone. Approximately 72% of cases with hypothyroidism were receiving biological therapy.
Table 4.
Presentation of different drugs in patients with co-morbid diseases suffering from RA.
| Comorbid disease (total patients) | Number of patients | Prescription of drug in patients with specific comorbid conditions | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Glucocorticoids | Methotrexate | NSAIDs | Antimalarial drugs | Sulphasalazine | Biological agents | ||
| Hypertension | 122 | 107 (87.7)* | 93 (76.2) | 50 (41.0) | 36 (29.5) | 31 (25.4) | 62 (50.8) |
| Diabetes mellitus | 105 | 100 (95.2)* | 82 (78) | 42 (40.0) | 29 (27.6) | 25 (23.8) | 52 (49.2) |
| Osteoporosis | 88 | 79 (89.7)* | 65 (72.7) | 37 (42.0) | 35 (39.8) | 17 (19.3) | 38 (43.2) |
| Dyslipidemia | 66 | 63 (95.4)* | 47 (72.3) | 30 (44.5) | 18 (27.3) | 13 (19.7) | 30 (45.5) |
| Peptic ulcer disease | 35 | 32 (91.4) | 30 (85.7) | 15 (42.9) | 10 (28.6) | 11 (31.4) | 17 (48.6) |
| Hypothyroidism | 29 | 24 (82.7) | 19 (65.5) | 16 (55.2) | 12 (41.4) | 11 (37.9) | 21 (72.4)* |
| Chronic hepatitis | 26 | 18 (69.2) | 17 (65.3) | 8 (30.8) | 18 (69.2)* | 7 (26.9) | 7 (26.9) |
| Ischemic heart disease | 25 | 20 (80) | 16 (64) | 9 (36.0) | 6 (24.0) | 8 (32.0) | 11 (44.0) |
| Bronchial asthma | 23 | 19 (82.6) | 18 (78.3) | 10 (43.3) | 5 (21.7) | 8 (35.8) | 10 (43.5) |
| Treated tuberculosis | 15 | 12 (80.0) | 12 (80.0%) | 10 (66.7)* | 8 (53.3) | 7 (46.7) | 11 (73.3) |
| CVA | 13 | 11 (87.5) | 13 (87.5) | 9 (62.5) | 11 (87.5) | 1.6 (12.5) | 7 (54) |
Note:
Significant P < 0.05, obtained by Chi-square testing where the drug is more prescribed in the comorbid illness.
Abbreviation: CVA, Cerebrovascular diseases.
Chronic hepatitis was observed in a total of 26 (7.6%) patients. Hepatitis B was found in 18 (5.2%) patients and hepatitis C was present in eight (2.3%) patients. None of the patients with hepatitis B received biologic therapy. Seven patients had hepatitis C with Child–Pugh scores of A–B. All were treated with etanercept. In patients with chronic viral hepatitis, 17 (65.3%) used MTX. Fifteen (88.2%) patients had Child–Pugh scores of A–B and two (11.8%) had advanced liver disease. We observed a significant increase in the use of antimalarial drugs among patients with chronic hepatitis (18; 69.2%).
IHD was found in 25 patients (7.4%). All 11 patients with IHD who used biologic therapy were NYHA class 1–2.
Thirty-five (10%) patients had peptic ulcer disease. Fifteen (42.9%) were taking NSAIDs. Inappropriate use of NSAIDs was found in two patients with high risk and in four patients with moderate risk.
Discussion
Our study demonstrates that the most patients with RA suffer from a number of associated cormobodities. Major risks factors for cardiovascular disease such as HTN, diabetes, and dyslipidemia account for most of these comorbidities. Corticosteroids are the most commonly prescribed medication.
This cross-sectional study examining 340 patients showed similar results to previous studies. Similar to previous studies, our study showed that more women (78%) than men (22%) suffer from RA.1,4 Ikuyama et al23 conducted a study involving 296 patients with RA. In their study, the prevalence of women was significantly higher (84% women, 16% men). In another study, Islander et al24 also found RA more frequently in women than in men. Similarly, Kuo et al25 carried out a nationwide study to examine the incidence and mortality of RA in Taiwan and found that both the incidence and mortality of RA was higher in women than that in men.
In our study, the average patient age was 53.3 ± 11.3 years, which is similar to the average age of patients with RA. Similarly, in a recent study by Sineglazova et al,26 the average age of patients with RA was 49 ± 7.4 years. In another study, McCoy et al27 found that the average age of patients with RA was 55.5 years; thus, RA typically occurs in older patients. Previous studies support our results of average age. In our study, RA factor was found to be positive in 76% of patients. In a study by Suresh,12 RA factor was positive in 70% of patients suffering from RA; hence, the presence of RA factor aids in the diagnosis of RA.
The primary purpose of this study was to identify comorbidities associated with RA and their impacts on drug prescription. In our study, 66% patients had at least one comorbidity. The five most common associated comorbidities were HTN (36%), DM (31%), osteoporosis (26%), dyslipidemia (19%), and peptic ulcer disease (10%). Patients with different comorbidities were prescribed different drugs. More than 60% of patients with comorbidities of HTN, DM, osteoporosis, and dyslipidemia were prescribed prednisolone and DMARDs; however, biological agents were prescribed in more than 50% of patients with HTN, DM, and treated TB. A total of 88% patients with HTN received glucocorticoids (GC). Long-term GC therapy is associated with a very high prevalence of HTN.28 It is clear that patients with RA and HTN who receive GC therapy should be closely followed-up. GC-induced HTN can exacerbate the condition of these patients.
The finding that fewer patients (41%) with HTN were prescribed NSAIDs was a positive result. NSAIDs are thought to exacerbate preexisting HTN.29 In this regard, NSAIDs are thought to cause HTN through different mechanisms (eg, sodium and water retention, renin–angiotensin–aldosterone system activation, inhibition of renal vasodilator prostaglandins).30–33 Thus, these agents should be avoided in patients with HTN. However, NSAIDs with nitric oxide-promoting properties can help RA patients with HTN. In the same manner, in patients at a high risk of bleeding (eg, those with peptic ulcer, gastrointestinal risk factors), alternative therapy should be considered. If NSAID therapy is necessary, cyclooxygenase (COX)-2 inhibitors with misoprostol or proton pump inhibitors (PPI) can be used.34
GCs (eg, prednisolone) cause hyperglycemia;35 thus, GCs should be avoided in patients with DM along with RA. In our study, more than 95% RA patients with diabetes were on prednisolone therapy; this indicates that special guidelines to physicians treating RA and adequate education of patients with DM are required. Patients suffering from RA are prone to develop insulin resistance;36 however, in some patients, the use of GC is unavoidable since it is the only agent that will control pain for a short period of time. Studies show that the use of anti-TNFα in patients with RA reduces the risk of developing DM.37 Thus, controlling inflammation reduces insulin resistance.38 In our study, approximately half of the patients (49.2%) received biological agents. The cost of the biologic therapy remains the main obstacle to using other medication, and alternative medications may have a high rate of side effects such as those observed with long-term GC use.37
Similarly, 90% of RA patients with osteoporosis received GC therapy. GC therapy is associated with an increased incidence of osteoporosis.39,40 Our study involved a large number of patients who were at risk for exacerbation of osteoporosis. Thus, physicians must carefully consider prescribed drugs for RA patients with osteoporosis, conduct bone density measurements, and employ primary prevention techniques and secondary treatments. Similarly, GC-induced dyslipidemia has been extensively examined;41,42 a total of 95% patients with dyslipidemia were on GC therapy in our study. Again, physicians should clearly consider comorbidities while prescribing drugs for RA patients.
The number of patients with hypothyroidism and hyperthyroidism were 29 (8.5%) and four (1.2%), respectively. A literature review disclosed a geographical variation of thyroid disease in RA ranging from 0.5% to 27% .43 Interestingly, the use of biologic therapy was significantly higher, as it was observed in 21 hypothyroid patients (72.4%). It is unknown whether these conditions can be used to predict the severity of RA.
In the present study, a very large number of patients were taking methotrexate. In this regard, patients starting methotrexate must undergo clinical as well as a laboratory assessments for liver function, blood and circulation, lipid profile, blood sugar level, and pregnancy.44 Obesity, diabetes, and viral or alcoholic hepatitis can deteriorate the condition of patients taking methotrexate.45 According to the recommendations of the American College of Rheumatology, patients with RA and hepatitis B and C can be treated using etanercept.46 In our study, seven patients had hepatitis C with Child–Pugh scores of A–B; all were treated with etanercept, although there appears to be no benefit of using a biological agent during the early stages of hepatitis. Similarly, 11 patients with IHD were treated with biological therapy as they had New York Heart Association class 1–2.
The statement “Treat the patient, not the disease” compels physicians to consider all aspects of a patients’ condition, including disease severity, associated conditions, and socioeconomic aspects, among other factors. RA is associated with a significant number of comorbidities that must be addressed to achieve a beneficial outcome. The present study found that many comorbidities of RA are overlooked. A limitation of our study is the retrospective nature, since some data may be overlooked; additionally, data regarding the impact of comorbidities on disease activity and quality of health were not available. Further prospective studies and follow-up of patients over a period of 10 years, particularly for those undergoing more aggressive treatment, should be conducted to determine whether comorbidities are preventable; an RA patient registry would be useful for answering these questions.
Conclusion
In this study, most RA patients suffered from comorbid conditions, including HTN, diabetes, osteoporosis, and dyslipedemia. These comorbid conditions may impact treatment regimens of RA, or the prescribed drugs may worsen the comorbidity. Additionally, in many instances, physicians may be forced to prescribe RA medications that exacerbate the comorbid conditions. Therefore, to successfully manage RA, comorbidities should be carefully considered and they should be treated in addition to prescribing antirheumatoid medication regimens.
Footnotes
Author Contributions
Conceived and designed the experiments: JA. Analyzed the data: JA. Wrote the first draft of the manuscript: JA. Contributed to the writing of the manuscript: JA, SMA, NB, YA-N, HQ, SA-H, SS. Agree with manuscript results and conclusions: JA, SMA, NB, YA-N, HQ, SA-H, SS. Jointly developed the structure and arguments for the paper: JA, SMA, NB, YA-N, HQ, SA-H, SS. Made critical revisions and approved final version: JA, SMA, NB. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the author has provided signed confirmation of compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
Funding
Author(s) disclose no funding sources.
References
- 1.Fischer C, Faselis C, Levy J. USMLE Step 2 CK Lecture notes, Internal Medicine. Vol. 9. New York, NY: Kaplan Medical; 2008. pp. 46–50. [Google Scholar]
- 2.Miyasaka N for CHANGE Study Investigators. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18(3):252–62. doi: 10.1007/s10165-008-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turesson C, Jacobsson LTH, Matteson EL. Cardiovascular co-morbidity in rheumatic diseases. Vas Health Risk Manag. 2008;4(3):605–14. doi: 10.2147/vhrm.s2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon NA, Colledge NR, Walker BR, Hunter JAA. Davidson’s Principles & Practice of Medicine. 20th ed. Philadelphia, PA: Churchill Livingstone; 2009. pp. 1101–6. [Google Scholar]
- 5.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular manifestations in rheumatoid arthritis. Maedica. 2010;5(4):286–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Ebringer A, Rashid T. Rheumatoid arthritis is caused by Proteus: the molecular mimicry theory and Karl Popper. Front Biosci (Elite Ed) 2009;1:577–86. doi: 10.2741/e56. [DOI] [PubMed] [Google Scholar]
- 7.Kvien TK, Uhlig T, Ødegård S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212–22. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- 8.Svendsen AJ, Holm NV, Kyvik K, Petersen PH, Junker P. Relative importance of genetic effects in rheumatoid arthritis: historical cohort study of Danish nationwide twin population. BMJ. 2002;324(7332):264–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Wohlgethan JR, Stilmant MM, Harris JM, 3rd, Smith HR. Acute non-infectious arthritis of the hip in rheumatoid arthritis: synovial membrane findings. Ann Rheum Dis. 1989;48(3):250–3. doi: 10.1136/ard.48.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute of Arthritis and Musculoskeletal and Skin Diseases. Handout on health: rheumatoid arthritis [webpage on the Internet] Bethesda, MD: AMS Circle; 2009. [Accessed Dec 17, 2009]. Available from: http://www.niams.nih.gov/health_info/Rheumatic_Disease/default.asp. [Google Scholar]
- 11.Castrejón I, McCollum L, Tanriover MD, Pincus T. Importance of patient history and physical examination in rheumatoid arthritis compared to other chronic diseases: results of a physician survey. Arthritis Care Res (Hoboken) 2012;64(8):1250–5. doi: 10.1002/acr.21650. [DOI] [PubMed] [Google Scholar]
- 12.Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med. 2004;97(9):421–4. doi: 10.1258/jrsm.97.9.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazici Y, Pincus T, Kautiainen H, Sokka T. Morning stiffness in patients with early rheumatoid arthritis is associated more strongly with functional disability than with joint swelling and erythrocyte sedimentation rate. J Rheumatol. 2004;31(9):1723–6. [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1987;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Cuccurullo S, editor. Physical Medicine and Rehabilitation Board Review. New York, NY: Demos Medical Publishing; 2004. [Google Scholar]
- 16.National Health Service (NHS) Rheumatoid arthritis—treatment [webpage on the Internet] London, UK: National Health Service; 2012. [Accessed Dec 18, 2012]. Available from: http://www.nhs.uk/Conditions/Rheumatoid-arthritis/Pages/Treatment.aspx. [Google Scholar]
- 17.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21(5):885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469–83. doi: 10.1016/j.berh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Keyser FD. Choice of biologic therapy for patients with rheumatoid arthritis: the infection perspective. Curr Rheumatol Rev. 2011;7(1):77–87. doi: 10.2174/157339711794474620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh JA, Cameron DR. Summary of AHRQ’s comparative effectiveness review of drug therapy for rheumatoid arthritis (RA) in adults—an update. J Manag Care Pharm. 2012;18(4 Suppl C):S1–18. doi: 10.18553/jmcp.2012.18.s4-c.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linde L, Sørensen J, Østergaard M, Hetland ML. Health-related quality of life of patients with rheumatoid arthritis. Which factors are of significance? Ugeskr Laeger. 2008;170(10):855–8. [PubMed] [Google Scholar]
- 22.Tuominen U, Blom M, Hirvonen J, et al. The effect of co-morbidities on health-related quality of life in patients placed on the waiting list for total joint replacement. Health Qual Life Outcomes. 2007;5:16. doi: 10.1186/1477-7525-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikuyama S, Imamura-Takase E, Tokunaga S, Oribe M, Nishimura J. Sixty percent of patients with rheumatoid arthritis in Japan have used dietary supplements or health foods. Mod Rheumatol. 2009;19(3):253–9. doi: 10.1007/s10165-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 24.Islander U, Jochems C, Lagerquist MK, Forsblad-d’Elia H, Carlsten H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol Cell Endocrinol. 2011;335(1):14–29. doi: 10.1016/j.mce.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Kuo CF, Luo SF, See LC, Chou IJ, Chang HC, Yu KH. Rheumatoid arthritis prevalence, incidence, and mortality rates: a nationwide population study in Taiwan. Rheumatol Int. 2012;33(2):355–60. doi: 10.1007/s00296-012-2411-7. [DOI] [PubMed] [Google Scholar]
- 26.Sineglazova AV, Kalev OF, Trushin IV, Rostovtsev MV. Special features of coronary artery involvement in women with rheumatoid arthritis. Kardiologiia. 2012;52(8):44–7. [PubMed] [Google Scholar]
- 27.McCoy SR, Warren RF, Bade HA, 3rd, Ranawat CS, Inglis AE. Total shoulder arthroplasty in rheumatoid arthritis. J Arthroplasty. 1989;4(2):105–13. doi: 10.1016/s0883-5403(89)80062-2. [DOI] [PubMed] [Google Scholar]
- 28.Panoulas VF, Douglas KM, Stavropoulos-Kalinoglou A, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47(1):72–5. doi: 10.1093/rheumatology/kem311. [DOI] [PubMed] [Google Scholar]
- 29.Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hyptertens. 2011;2011:236239. doi: 10.4061/2011/236239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2(11):879–90. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 31.Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011;9:38. doi: 10.1186/1741-7015-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galesić K, Jelaković B. Nonsteroidal antirheumatics and hypertension. Lijec Vjesn. 2011;133(3–4):101–6. [PubMed] [Google Scholar]
- 33.Francois H, Coffman TM. Prostanoids and blood pressure: which way is up? J Clin Invest. 2004;114(6):757–9. doi: 10.1172/JCI22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanza FL, Chan FK, Quigley EM for Practice Parameters Committee of the American College of Gastroenterology. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104(3):728–38. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Yoo CG, Lee CT, et al. Incidence and risk factors of steroid-induced diabetes in patients with respiratory disease. J Korean Med Sci. 2011;26(2):264–7. doi: 10.3346/jkms.2011.26.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wasko MC, Kay J, Hsia EC, Rahman MU. Diabetes mellitus and insulin resistance in patients with rheumatoid arthritis: risk reduction in a chronic inflammatory disease. Arthritis Care Res (Hoboken) 2011;63(4):512–21. doi: 10.1002/acr.20414. [DOI] [PubMed] [Google Scholar]
- 37.Antohe JL, Bili A, Sartorius JA, et al. Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken) 2012;64(2):215–21. doi: 10.1002/acr.20657. [DOI] [PubMed] [Google Scholar]
- 38.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ledwich LJ, Clarke K. Screening and treatment of glucocorticoid-induced osteoporosis in rheumatoid arthritis patients in an urban multispecialty practice. J Clin Rheumatol. 2009;15(2):61–4. doi: 10.1097/RHU.0b013e31819b65bd. [DOI] [PubMed] [Google Scholar]
- 40.Heberlein I, Demary W, Bloching H, et al. Diagnosis and treatment of osteoporosis and rheumatoid arthritis in accordance with German guidelines. Results of a survey of patients, primary care physicians and rheumatologists. Z Rheumatol. 2011;70(7):592–601. doi: 10.1007/s00393-011-0821-7. German. [DOI] [PubMed] [Google Scholar]
- 41.Situnayake RD, Kitas G. Dyslipidemia and rheumatoid arthritis. Ann Rheum Dis. 1997;56(6):341–2. doi: 10.1136/ard.56.6.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ettinger WH, Klinefelter HF, Kwiterovitch PO. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis. 1987;63(2–3):167–72. doi: 10.1016/0021-9150(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 43.Cárdenas Roldán J, Amaya-Amaya J, Castellanos-de la Hoz J, et al. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis. 2012;2012:864907. doi: 10.1155/2012/864907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Visser K, van der Heijde DM. Risk and management of liver toxicity during methotrexate treatment in rheumatoid and psoriatic arthritis: a systematic review of the literature. Clin Exp Rheumatol. 2009;27(6):1017–25. [PubMed] [Google Scholar]
- 45.Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68(7):1086–93. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]