Abstract
The objective of this report is to present a case of Graves’ thyrotoxicosis-induced cardiomyopathy. This is a case of a 26 year old woman that presented with severe symptomatic congestive heart failure and was subsequently diagnosed with dilated cardiomyopathy secondary to Graves’ disease. Despite an initial left ventricular systolic ejection fraction of 20% on echocardiography, treatment with anti-thyroid agents led to rapid improvement of her clinical status and normalization of her ejection fraction. The proposed mechanisms underlying the development of systolic dysfunction in thyrotoxicosis are discussed and the literature on similar cases previously reported is highlighted. Cardiomyopathy should be considered even in young patients with Graves’ thyrotoxicosis.
Keywords: Graves’ disease, thyrotoxicosis, cardiomyopathy, congestive heart failure
Introduction
Graves’ thyrotoxicosis has profound cardiovascular effects; however, it rarely causes heart failure in otherwise healthy patients. Cardiomyopathy is the initial clinical presentation in approximately 6% of patients with hyperthyroidism,1 but less than 1% develop dilated cardiomyopathy with impaired left ventricular systolic function.2 Conventional treatment for hyperthyroidism usually reverses these cardiac complications.3 In this case study we describe a case of Graves’ hyperthyroidism-induced transient cardiomyopathy.
The Case
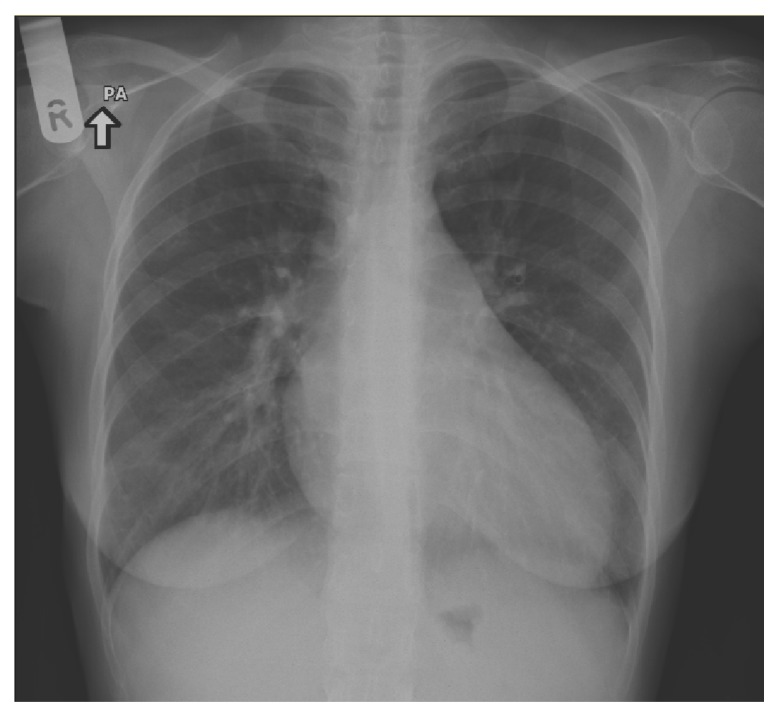
A 26 year old, previously healthy, post partum patient presented with two weeks history of dyspnea (grade 4) with progressive course, orthopnea, paroxysmal nocturnal dyspnea, and palpitation. She noted heat intolerance, sweating, and tremor. She had a 2 month old baby boy who was breast feeding. There was no previous history of cardio-respiratory diseases, diabetes mellitus, or hypertension. On examination of the patient, temperature was afebrile (37 °C), respiratory rate was 28 per minute, and blood pressure was 135/70 mmHg with a regular pulse of 125 beats per minute. She had a staring look with Graves’s orbitopathy—moderate exophthalmus and lid retraction but no lid lag. The thyroid gland weighed 40 grams. The hand was moist and hot with tremor. The cardiovascular examination was remarkable for a jugular venous pressure of 10 cm above the sternal angle, a displaced apex beat, S3, and bilateral pitting edema. Bilateral crackles were heard on chest auscultation. Chest radiograph showed cardiomegaly (Fig. 1). Electrocardiogram showed sinus tachycardia and non specific T wave changes. A trans thoracic echocardiogram showed a moderately dilated left ventricle with severe global systolic dysfunction (Ejection Fraction: 20%–25%). There was severe diastolic dysfunction, moderate RV systolic dysfunction, and moderate pulmonary hypertension. Laboratory investigation (September 2009) showed the following: TSH < 0.005 m IU/L (0.27–4.2), Free T4 > 100 pmL/L (12–22), Free T3 > 50 pmol/L (2.8–7.1), TSH receptor antibody was elevated 32 iu/L (normal < 1), anti-TPO > 2000 iu/mL (1–16) and anti-thyroglobline > 5000 iu/mL (5–100). She had normal blood count, electrolytes, liver, and cardiac enzymes. Thyroid technetium scan showed diffuse toxic goiter secondary to Graves’s disease. The diagnosis of dilated cardiomyopathy—probably secondary to Graves’ disease—was made and the patient was treated with diuretics, digoxin, B-blocker, and ACE inhibitor. After the results of her thyroid function tests and her scan were obtained, she was started on anti-thyroid drug, Carbimazole. The patient significantly improved. Six weeks later she was clinically and biochemically euthyroid. Her heart failure medication was discontinued but she was maintained on a small dose of Carbimazole. A repeat echocardiogram (November 2009) showed normal LV and RV systolic function with ejection fraction of 55%. After completion of her breast feeding, carbimazole was stopped for 5 days and thyroid Technetium scan demonstrated persistent diffuse toxic goiter secondary to Graves’ disease. Subsequently, she received radioactive iodine treatment (June 2010) with steroid coverage, after which she became hypothyroid and was started on levothyroxin replacement. As of this study she is on thyroxin replacement and all of other medications were stopped. Reversible cardiomyopathy without arrhythmia secondary to Graves’s thyrotoxicosis was demonstrated in this case.
Figure 1.
Chest X-ray, PA view showing cardiomegaly with left ventricular configuration and pulmonary congestion.
Discussion
In hyperthyroidism, heart failure may occur in the absence of underlying heart disease as reported in children by Cavallo et al.4 Reduced left ventricular contractile reserve may impair the ability to raise cardiac output to match the increase of peripheral metabolic demand. Left ventricular hypertrophy may result in impaired left ventricular filling, in particular when associated with accelerated heart rate. Atrial fibrillation may further compromise left ventricular filling because of loss of the atrial contribution and a rapid ventricular response rate. In addition, increased myocardial oxygen demand may ensue myocardial ischemia, particularly in the presence of coronary artery disease or spasm, and may contribute to the occurrence of heart failure.5 Adverse cardiovascular effects of hyperthyroidism are well documented in literature.6–8 In most cases, dilated cardiomyopathy becomes an unusual manifestation and clinicians should be aware because it is reversible.8 High output biventricular heart failure with normal or decreased systemic and pulmonary vascular resistance is the expected cardiovascular complication of hyperthyroidism.9,10
Isolated right heart failure, variable degrees of tricuspid regurgitation, pulmonary hypertension, or different combinations of the three in patients with thyrotoxicosis have been in frequently reported.8 Different studies have also demonstrated that thyroid hormones have a direct effect on myocardial contractility and left ventricle diastolic function. Hyperthyroidism also has its potential effects on the peripheral circulation, with documented increases in blood volume, decreases in peripheral resistance, increases in mean blood pressure, and proven increases in atrial natriuretic factor.10 Chronic tachycardia and arrhythmia (eg, atrial fibrillation) have been reported as causes of cardiomyopathy.12 Few reports documented clinical cases of thyrotoxicosis that presented as low cardiac output.13 There was only one report of four cases of thyrotoxicosis associated with irreversible cardiomyopathy.14 The vast majority of studies in patients with subclinical hyperthyroidism showed an increased left ventricular mass, which was sometimes accompanied by impaired ventricular relaxation. 15,16 Thyrotoxicosis has also been shown to prevent the rise of left ventricular ejection fraction during exercise.10 In a study of a series of seven patients with hyperthyroidism and congestive heart failure, the mean left ventricular systolic ejection fraction increased from 28% to 55% after treatment for thyrotoxicosis. The ejection fraction normalized in 5 patients, and an improvement from severe to mild systolic dysfunction was noted in the other two patients.17 Two case reports of middle-aged patients with heart failure and with an ejection fraction around 35% nearly normalized after treatment of the patients’ hyperthyroidism.18,19 In general, heart failure is reversible and responds dramatically to hyperthyroidism treatment.
Conclusion
The diagnosis of cardiomyopathy secondary to Graves’ hyperthyroidism should be considered in any patient—regardless of age—with clinical manifestations of cardiomyopathy of unknown etiology. An assessment of thyroid hormone status in patients with heart failure might permit the identification of patients with dilated cardiomyopathy and thyrotoxicosis who are likely to have reversible cardiac failure.
Footnotes
Consent
Written informed consent was obtained from the patient for the publication of this case and accompanying images.
Author Contributions
Conceived and designed the experiments: ASG, NA. Analysed the data: ASG, NA. Wrote the first draft of the manuscript: ASG, NA. Contributed to the writing of the manuscript: ASG, NA. Agree with manuscript results and conclusions: ASG, NA. Jointly developed the structure and arguments for the paper: ASG, NA. Made critical revisions and approved final version: ASG, NA. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Funding
Author(s) disclose no funding sources.
References
- 1.Siu CW, Yeung CY, Lau CP, Kung AWC, Tse HF. Incidence, clinical characteristics and outcome of congestive heart failure as the initial presentation in patients with primary hyperthyroidism. Heart. 2007;93:483–7. doi: 10.1136/hrt.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl P, Danzi S, Klein I. Thyrotoxic cardiac disease. Curr Heart Fail Rep. 2008:5170–6. doi: 10.1007/s11897-008-0026-9. [DOI] [PubMed] [Google Scholar]
- 3.Merce J, Ferras S, Oltra C, Sanz E, Vendrell J, Simon I, et al. Cardiovascular abnormalities in hyperthyroidism: a prospective Doppler echocardiographic study. Am J Med. 2005;118:126–31. doi: 10.1016/j.amjmed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo A, Joseph CJ, Casta A. Cardiac complications in juvenile hyperthyroidism. Am J Dis Child. 1984;138:479. doi: 10.1001/archpedi.1984.02140430055014. [DOI] [PubMed] [Google Scholar]
- 5.Featherstone HJ, Stewart DK. Angina in thyrotoxicosis: Thyroidrelated coronary artery spasm. Arch Int Med. 1983;143:554. doi: 10.1001/archinte.143.3.554. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MR, McGregor AM, Jewitt DE. Left ventricle filling abnormalities prior to and following treatment of thyrotoxicosis—is diastolic dysfunction implicated in thyrotoxic cardiomyopathy? Eur Heart J. 1993;14:662–8. doi: 10.1093/eurheartj/14.5.662. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz de Murua JA, Del Canizo FJ, Del Campo F, Avila MC, Villafranca JL. Some considerations on reversible dilated cardiomyopathy due to thyrotoxicosis. Am J Cardiol. 1993;71:501. doi: 10.1016/0002-9149(93)90490-4. [DOI] [PubMed] [Google Scholar]
- 8.Boccalandro C, Boccalandro F, Orlander P, Wei CF. Severe reversible dilated cardiomyopathy and hyperthyroidism: case report and review of the literature. Endocr Pract. 2003;9:140–6. doi: 10.4158/EP.9.2.140. [DOI] [PubMed] [Google Scholar]
- 9.Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94–8. doi: 10.1056/NEJM199207093270206. [DOI] [PubMed] [Google Scholar]
- 10.Forfar JC, Muir AL, Sawers SA, Toft AD. Abnormal left ventricular function in hyperthyroidism: evidence for a possible reversible cardiomyopathy. N Engl J Med. 1982;307:1165–70. doi: 10.1056/NEJM198211043071901. [DOI] [PubMed] [Google Scholar]
- 11.Paran Y, Nimrod A, Goldin Y, Justo D. Pulmonary hypertension and predominant right heart failure in thyrotoxicosis. Resuscitation. 2006;69:339–41. doi: 10.1016/j.resuscitation.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Packer DI, Brady GH, Worley SJ, et al. Tachycardia induced cardiomyopathy: a reversible from of left ventricular dysfunction. Am J Cardiology. 1986;75:563–70. doi: 10.1016/0002-9149(86)90836-2. [DOI] [PubMed] [Google Scholar]
- 13.Bauerlein EJ, Chakko CS, Kessler KM. Reversible dilated cardiomyopathy due to thyrotoxicosis. Am J Cardiology. 1992;70:132. doi: 10.1016/0002-9149(92)91412-w. [DOI] [PubMed] [Google Scholar]
- 14.Ebisawa K, Ikeda U, Murata M, Sekiguchi H, Nagai R, et al. Irreversible cardiomyopathy due to thyrotoxicosis. Cardiology. 1994;84:274–7. doi: 10.1159/000176411. [DOI] [PubMed] [Google Scholar]
- 15.Fazio S, Biondi B, Carella C, Sabatini D, Cittadini A, Panza N, et al. Diastolic dysfunction in patients on thyroid-stimulating hormone suppressive therapy with levothyroxine: beneficial effect of beta-blockade. J Clin Endocrinol Metab. 1995;80:2222–6. doi: 10.1210/jcem.80.7.7608283. [DOI] [PubMed] [Google Scholar]
- 16.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Challapalli S, Patterson C. Congestive heart failure due to reversible cardiomyopathy in patients with hyperthyroidism. Am J Med Sci. 1995;310:99–102. doi: 10.1097/00000441-199531030-00003. [DOI] [PubMed] [Google Scholar]
- 18.Goldman LE, Sahlas DJ, Sami M. A case of thyrotoxicosis and reversible systolic cardiac dysfunction. Can J Cardiol. 1999;15:811–4. [PubMed] [Google Scholar]
- 19.Kantharia BK, Richards HB, Battaglia J. Reversible dilated cardiomyopathy: an unusual case of thyrotoxicosis. Am Heart J. 1995;129:1030–2. doi: 10.1016/0002-8703(95)90128-0. [DOI] [PubMed] [Google Scholar]