Abstract
Metazoan cells rapidly exchange signals at tight cell–cell interfaces, including synapses and gap junctions. Advances in imaging recently exposed a third mode of intercellular cross-talk mediated by thin, actin-containing membrane extensions broadly known as “membrane” or “tunneling” nanotubes. An explosion of research suggests diverse functions for nanotubular superhighways, including cell–cell electrical coupling, calcium signaling, small-molecule exchange, and, remarkably, the transfer of bulky cargoes, including organelles or pathogenic agents. Despite great enthusiasm for all things nanotubular and their potential roles in cell signaling and pathogenesis, key questions remain regarding the mechanisms by which these structures regulate directional cell–cell exchange; how these linkages are formed and between which cells and, critically, whether nanotubes are as prevalent in vivo as they appear to be in the incubator.
INTRODUCTION
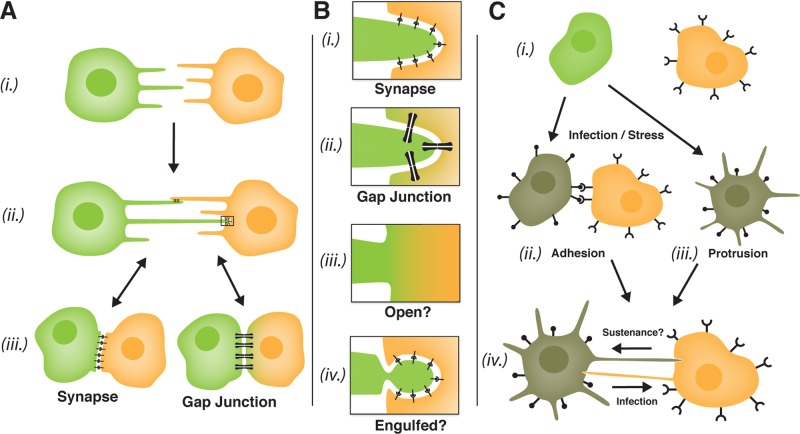
Actin-rich membrane protrusions such as filopodia and lamellipodia can guide the convergence of cells during the formation of complex signaling interfaces in vivo, for example, during synaptogenesis in the nervous system or the merging of epithelial cell sheets during development or wound healing (Mattila and Lappalainen, 2008). Alternatively, extended (>40 μm) parallel arrays of filopodia termed cytonemes (cell threads) observed in the Drosophila embryo wing imaginal disk may function as cellular “antennae” that connect cells over large distances to regulate the detection and transport of morphogen ligands that determine tissue patterning (Ramirez-Weber and Kornberg, 1999). In 2004, pioneering video microscopy studies by the Gerdes and Davis labs revealed a novel variation on this theme: intercellular nanotubular highways. Gerdes and colleagues described thread-like (∼50-nm diameter) connections linking cultured rat neuron–derived pheochromocytoma 12 (PC12) cells formed after the convergence of dynamic filopodia extending from neighboring cells (Rustom et al., 2004; Figure 1A, (i) and (ii)). They called these structures tunneling nanotubes (TNTs) based on their apparent capacity to create a continuous channel between two cell cytoplasms, in analogy to plasmodesmata in plant tissues. Remarkably, PC12 TNTs supported a directional flow of surface-bound molecules, cytoskeletal elements, and even endosomal membranes from cell to cell. Soon after, the Davis group described similar membrane nanotubes linking cultured primary lymphocytes, including natural killer cells, Epstein–Barr virus–transformed B cells, and macrophages. Unlike TNTs, these nanotubes represented residual membrane tethers formed after the disassembly of tight cell–cell contacts (Figure 1A, (iii) to (ii)) and were proposed to provide motile lymphocytes with a mechanism for maintaining immune signaling over long distances (Onfelt and Davis, 2004). Consistent with this notion, Watkins and Salter (2005) demonstrated “functional connectivity” between primary dendritic cells (DCs) in culture, showing that a calcium signal could be propagated through nanotubes among DCs separated by multiple cell lengths.
FIGURE 1:
Mechanisms of cell–cell nanotube formation, modes of transfer, and proposed roles in tissue homeostasis and the spread of infection. (A) Nanotubes can form by either of two mechanisms. Filopodial interplay ((i) to (ii)) involves the convergence of protruding filopodia from neighboring cells, followed by anchoring. Dislodgement ((iii) to (ii)) involves the formation of residual tethers after the disassembly of tight cell–cell contacts. Nanotubes may mediate long-distance signaling or in some instances be precursors to the formation of more complex cell–cell interfaces ((ii) to (iii)). (B) Possible modes of nanotube-mediated signaling and exchange. Nanotubular linkages can be synaptic in nature and signal through ligand–receptor interactions (i) or retain connectivity at a gap junction–like interface maintained by connexons (bow ties) made up of hexamers of connexin proteins. Gap junctions regulate a gated flow of ions or small molecules from cell to cell (ii). To exchange larger cargoes, nanotube connections must either be open and membrane continuous (iii) or use an alternative mechanism of membrane exchange such as membrane engulfment/phagocytosis (iv). (C) Mechanisms of induced nanotube connectivity. Infection and cell stress may signal the up-regulation of adhesive factors at the cell surface, for example, retroviral Env glycoproteins (i), that drive the formation of tight cell–cell contacts (e.g., virological synapses) or are extended to form nanotubes or filopodial bridges ((ii) and iv)). Alternatively (or possibly in addition to), cell signaling through the exocyst complex (e.g., in response to HIV-1 Nef expression or cell stress) can induce the extension of membrane protrusions that reach out to bind neighboring cells ((iii) and (iv)). Induced nanotubular superhighways may function to promote the rapid intercellular spread of infection but could also promote the transit of cell-sustaining signals or cargoes (iv).
A DILEMMA FOR THE INTERCELLULAR COMMUTER: BRIDGES OR TUNNELS?
Subsequently, TNTs and related structures have been described in diverse ex vivo cell culture systems and implicated in the cell–cell exchange of a wide variety of cargoes. Attempts have been made to categorize nanotubes based on structure: for example, slender, type I TNTs that are <100 nm in diameter and contain actin versus type II TNTs that are larger (up to 1-μm diameter) and contain both actin and microtubules (Onfelt et al., 2006). However, such categorization is now complicated by the broad heterogeneity of described TNT-like structures (for excellent reviews see Kimura et al., 2012; Marzo et al., 2012; Wang and Gerdes, 2012). We previously argued that an important distinction be made between structures that are “tunneling” (i.e., open ended and capable of transmitting an intracellular signal) and linkages that are closed ended and more bridge-like or synaptic in nature (Sherer and Mothes, 2008; see Figure 1B). Indeed, there is limited ultrastructural evidence that nanotubes are open ended, and a wide-open configuration would pose problems, such as how linked cells prevent cell–cell fusion or maintain a cytoskeletal polarity (cargo transit is frequently unidirectional). Additional work by the Gerdes group may resolve this issue, revealing that TNTs formed by dislodgement in cultures of normal rat kidney (NRK) cells are, indeed, not open ended but gated (Wang et al., 2010). These structures accumulated the gap junction (GJ) protein connexin 43 (Cx43) at their bases and, consistent with GJ function, supported the transmission of an electrical signal from cell to cell (Figure 1B, (ii)). By contrast, classic TNTs from PC12 cells formed by filopodial interplay did not contain Cx43 and did not support electrical coupling. Therefore neither NRK nor PC12 TNTs are constitutively open-ended structures, and it is important to consider that many (and perhaps the majority) of described TNT-like linkages represent extensions of either synapse or GJ biology (e.g., Figure 1B, (i) and (ii)).
If nanotubes are not open ended, then how do they support the transfer of bulky intracellular cargoes such as mitochondria and endosomes, as is now apparent in numerous studies (see Marzo et al., 2012)? Indeed, such a process challenges the basic notion of cell autonomy and, if confirmed in vivo, might alter our perception of how tissues are maintained and suggest opportunities to design new cell-based therapeutic strategies. Zhang and colleagues recently showed that oxidative stress or serum starvation can trigger selective nanotube formation between stressed and unstressed astrocytes, and they proposed that a nanotube-mediated directional flow of “healthy” intracellular cargoes from unstressed to stressed cells provided a cell-sustaining effect (Wang et al., 2011; Figure 1C, (iii) to (iv)). While compelling, it is not clear how organelles can successfully breach two apposed plasma membranes. Wang and Gerdes (2012) have suggested three potential scenarios: 1) a “tunnel and toll booth” mechanism in which large cargoes await a transient opening of a fusion pore, 2) a “carrier” mechanism in which vesicles are secreted at the nanotube tip and taken up by target cells, and 3) a “snatch and grab” mechanism in which a portion of the nanotube is engulfed and endocytosed. Mechanisms 1 and 2 would necessarily invoke novel modes of cell–cell fusion and vesicle formation, respectively. Mechanism 3 seems most likely, considering the fragile nature of nanotubes, as well as the propensity of cells to pull and tear at tightly apposed membranes (Figure 1B(iv)). However, a caveat is that endocytosed membrane would have to somehow accomplish “back-fusion” with the endosome to release its cargo into the cytoplasm. Careful live-cell imaging coupled to electron microscopy will be essential to defining the determinants, as well as the frequency and success, of these “organelle transplant” operations.
LESSONS LEARNED FROM VIRAL HIJACKERS
If nanotubular transport plays a role in tissue homeostasis, the corollary is that this mode of transfer can promote the spread of damaging or infectious cargoes, including prions, viruses, and bacteria. Intracellular pathogens often exploit existing cell–cell contacts or induce infected cells to form specialized structures in order to favor their cell–cell transmission (Figure 1C). For example, we demonstrated that retroviruses, including the murine leukemia virus (MLV) and the human immunodeficiency virus type 1 (HIV-1), drive the formation of nanotube-like filopodial bridges between infected and uninfected fibroblasts that provide for actin-dependent virion trafficking (surfing) of virions from cell to cell on the outer surface of membrane (Sherer et al., 2007). Stable bridge formation required specific, high-avidity interactions between viral envelope (Env) glycoproteins and receptor molecules on apposed cell surfaces. HIV-1 infects CD4+ T-cells and macrophages in vivo, and several reports have demonstrated nanotubes, related actin-rich structures, and “virological synapses” that link infected and uninfected lymphocytes and likely contribute to the rapid spread of infection (e.g., Sowinski et al., 2008; Rudnicka et al., 2009; reviewed in Sattentau, 2010). That Env expression is capable of promoting cell–cell connectivity in vivo was emphasized by recent striking intravital multiphoton fluorescence imaging of HIV-1–infected T-cells in humanized mice by Mempel and colleagues (Murooka et al., 2012). Infected, motile T-cells in lymph nodes formed dramatic Env-dependent membrane extensions connecting cells over distances sometimes >100 μm. Moreover, Env-dependent connectivity was also recently confirmed for MLV-infected B cells and T-cells in living mice (Sewald et al., 2012). Although the resolution of multiphoton microscopy did not discern nanotubes, viral surface glycoproteins are clearly capable of stimulating the formation of complex membrane networking in tissues. Therefore the up-regulation of surface adhesion properties may serve as a general mechanism for stimulating nanotube formation during infection or in response to other signaling (Figure 1C, (ii) to (iv)).
Alternatively, viral infection or cell stress may promote nanotube formation by directly stimulating the extension of filopodial protrusions. For example, HIV-1 infection induces the formation of filopodial or lamellipodial extensions in T-cells through the activity of the viral Nef accessory protein (Nobile et al., 2010; Figure 1C(iii)). Of interest, a recent proteomic analysis of Nef-associated proteins revealed that Nef interacts with several components of the exocyst complex, a cellular machinery that regulates both the secretory pathway and the extension of actin-rich cell surface protrusions (Mukerji et al., 2012). These findings have striking overlap to the recent identification of the cellular factor M-Sec, the first cellular factor demonstrated to induce the de novo formation of nanotubes when overexpressed in cultured cells (Hase et al., 2009). M-Sec activates the exocyst complex through Ral GTPase signaling and is highly expressed in both DCs and macrophages. Therefore it seems logical to determine whether HIV infection modulates M-Sec expression or activity in infected CD4+ lymphocytes, as well as to determine the general role of the exocyst pathway to HIV-1 replication in vitro and in vivo. Moreover, the extension of nascent filopodia by astrocytes in response to cell stress conditions (as discussed earlier) correlated with an up-regulation of M-Sec expression and also required signaling through the tumor suppressor p53 and the Akt/PI3K/mTor pathway. Thus M-Sec and the exocyst complex may be a common target of multiple prominent cell signaling pathways associated with infection, cell stress, and possibly cancer. Induced nanotubes in these scenarios could, in theory, serve to rapidly mediate the exchange of either beneficial or detrimental cell cargoes (Figure 1C(iv)).
IN VITRO VERITAS?
Stress-induced signaling and viral infection should continue to provide tractable systems for dissecting the molecular details underlying filopodial extension, the formation of nanotubular linkages, and how cargoes move from cell to cell. However, a core question remains: do nanotubes really play important roles in tissues in vivo? Nanotube-like structures were described in vivo for the first time relatively recently, originating from stromal DCs within the mouse cornea (Chinnery et al., 2008). These observations have now been reproduced with additional detail (Seyed-Razavi et al., 2013). In addition, observations of nanotubes connecting cells in solid tumor explants may support the notion that nanotubes can fuel cancer (Lou et al., 2012). Increased visual resolution in tissue preparations, as well as continuing progress in live-cell intravital systems, will be essential to achieving a realistic picture of nanotube structure and function in vivo. Also, the identification of cellular factors regulating nanotube formation may provide opportunities for animal studies, for example, in p53, M-Sec, or exocyst-deficient mice. Finally, it is important to consider that nanotubes may not be working alone either in vivo or in culture. For example, HIV-1 Nef expression also induces the secretion of Nef-containing vesicular structures, including exosomes and microvesicles, likely by stimulating alternative functions of the exocyst complex. Indeed, tissues are a sticky proposition; studies of nanotubular linkages have provided important new concepts toward the goal of understanding the complexity of cell–cell signaling in these dynamic environments.
Acknowledgments
I thank Jaye Gardiner and Walther Mothes for helpful discussions and Jolynne Roorda for contributing artwork. This work was supported by National Institutes of Health/National Institute of General Medical Sciences Grant P30 GM092385 and startup funds from the University of Wisconsin–Madison School of Medicine and Public Health, the University of Wisconsin–Madison Graduate School, and the University of Wisconsin–Madison Carbone Cancer Center.
Abbreviations used:
- akt
protein kinase B
- Cx43
connexin 43
- DC
dendritic cell
- Env
envelope
- GJ
gap junction
- HIV-1
human immunodeficiency virus type 1
- MLV
murine leukemia virus
- mTor
mammalian target of rapamycin
- Nef
negative factor
- NRK
normal rat kidney
- PC12
pheochromocytoma 12
- PI3K
phosphatidylinositide 3-kinase
- TNT
tunneling nanotube
Footnotes
REFERENCES
- Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K, et al. M-Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427–1432. doi: 10.1038/ncb1990. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hase K, Ohno H. Tunneling nanotubes: emerging view of their molecular components and formation mechanisms. Exp Cell Res. 2012;318:1699–1706. doi: 10.1016/j.yexcr.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Lou E, Fujisawa S, Morozov A, Barlas A, Romin Y, Dogan Y, Gholami S, Moreira AL, Manova-Todorova K, Moore MA. Tunneling nanotubes provide a unique conduit for intercellular transfer of cellular contents in human malignant pleural mesothelioma. PloS One. 2012;7:e33093. doi: 10.1371/journal.pone.0033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo L, Gousset K, Zurzolo C. Multifaceted roles of tunneling nanotubes in intercellular communication. Front Physiol. 2012;3:72. doi: 10.3389/fphys.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev. Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mukerji J, Olivieri KC, Misra V, Agopian KA, Gabuzda D. Proteomic analysis of HIV-1 Nef cellular binding partners reveals a role for exocyst complex proteins in mediating enhancement of intercellular nanotube formation. Retrovirology. 2012;9:33. doi: 10.1186/1742-4690-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka TT, Deruaz M, Marangoni F, Vrbanac VD, Seung E, von Andrian UH, Tager AM, Luster AD, Mempel TR. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile C, et al. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J Virol. 2010;84:2282–2293. doi: 10.1128/JVI.02230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onfelt B, Davis DM. Can membrane nanotubes facilitate communication between immune cells. Biochem Society Trans. 2004;32:676–678. doi: 10.1042/BST0320676. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- Sattentau QJ. Cell-to-cell spread of retroviruses. Viruses. 2010;2:1306–1321. doi: 10.3390/v2061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewald X, Gonzalez DG, Haberman AM, Mothes W. In vivo imaging of virological synapses. Nat Commun. 2012;3:1320. doi: 10.1038/ncomms2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed-Razavi Y, Hickey MJ, Kuffova L, McMenamin PG, Chinnery HR. Membrane nanotubes in myeloid cells in the adult mouse cornea represent a novel mode of immune cell interaction. Immunol Cell Biol. 2013;91:89–95. doi: 10.1038/icb.2012.52. [DOI] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends Cell Biol. 2008;18:414–420. doi: 10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowinski S, et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Wang X, Gerdes HH. Long-distance electrical coupling via tunneling nanotubes. Biochim Biophys Acta. 2012;1818:2082–2086. doi: 10.1016/j.bbamem.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci USA. 2010;107:17194–17199. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui J, Sun X, Zhang Y. Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ. 2011;18:732–742. doi: 10.1038/cdd.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]