Reconstitution of GLUT4 vesicle fusion in a defined fusion system shows that the C2-domain factor Doc2b activates the SNARE-dependent fusion reaction by a calcium- and membrane bending–dependent mechanism. Of importance, certain features of Doc2b function appear to be distinct from how synaptotagmin-1 promotes synaptic release.
Abstract
The glucose transporter GLUT4 plays a central role in maintaining body glucose homeostasis. On insulin stimulation, GLUT4-containing vesicles fuse with the plasma membrane, relocating GLUT4 from intracellular reservoirs to the cell surface to uptake excess blood glucose. The GLUT4 vesicle fusion reaction requires soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs) as the core fusion engine and a group of regulatory proteins. In particular, the soluble C2-domain factor Doc2b plays a key role in GLUT4 vesicle fusion, but its molecular mechanism has been unclear. Here we reconstituted the SNARE-dependent GLUT4 vesicle fusion in a defined proteoliposome fusion system. We observed that Doc2b binds to GLUT4 exocytic SNAREs and potently accelerates the fusion kinetics in the presence of Ca2+. The stimulatory activity of Doc2b requires intact Ca2+-binding sites on both the C2A and C2B domains. Using electron microscopy, we observed that Doc2b strongly bends the membrane bilayer, and this membrane-bending activity is essential to the stimulatory function of Doc2b in fusion. These results demonstrate that Doc2b promotes GLUT4 exocytosis by accelerating the SNARE-dependent fusion reaction by a Ca2+- and membrane bending–dependent mechanism. Of importance, certain features of Doc2b function appear to be distinct from how synaptotagmin-1 promotes synaptic neurotransmitter release, suggesting that exocytic Ca2+ sensors may possess divergent mechanisms in regulating vesicle fusion.
INTRODUCTION
Regulated exocytosis is the basis of a wide range of fundamental biological processes, including neurotransmitter release, hormone secretion, and inside–outside distributions of surface transporters and receptors (Schekman and Novick, 2004; Sudhof and Rothman, 2009). One prominent example of regulated exocytosis is the insulin-regulated trafficking of the glucose transporter GLUT4, which plays a central role in maintaining blood glucose homeostasis (Birnbaum, 1989; Charron et al., 1989; James et al., 1989). GLUT4 is normally sequestered in intracellular vesicles in adipocytes and skeletal muscles. In response to elevated levels of blood glucose, insulin binds to cell surface receptors and activates a complex signaling cascade, ultimately leading to the exocytosis of GLUT4-containing vesicles. Once on the cell surface, GLUT4 facilitates the uptake of excess blood glucose into the cell for disposal (Lavan and Lienhard, 1994; Bryant et al., 2002; Watson and Pessin, 2006; Huang and Czech, 2007; Blot and McGraw, 2008; Vassilopoulos et al., 2009; Jewell et al., 2010). Imbalances in GLUT4 exocytosis disrupt body glucose balance and ultimately give rise to insulin resistance and type 2 diabetes (Kewalramani et al., 2010; Hoffman and Elmendorf, 2011).
Although the physiological and medical importance of the GLUT4 exocytic pathway is well established, we are still at the beginning of understanding the underlying molecular mechanisms. GLUT4 exocytosis is mediated by the fusion of GLUT4-containing vesicles with the plasma membrane. Membrane fusion—the merging of two separate lipid bilayers into one—involves substantial lipid rearrangements and imposes a high energy barrier that must be overcome by specialized membrane fusion proteins (Sudhof and Rothman, 2009). The core engine of intracellular vesicle fusion is the soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs; Wickner and Schekman, 2008; Sudhof and Rothman, 2009; Rizo and Sudhof, 2012; Jahn and Fasshauer, 2012). SNAREs are membrane-associated proteins localized to both the vesicle (v-SNAREs or R-SNAREs) and the target membrane (t-SNAREs or Q-SNAREs; Sutton et al., 1998; Weber et al., 1998; Katz and Brennwald, 2000; Burgoyne and Morgan, 2007; Schwartz and Merz, 2009). Fusion is initiated when the v-SNARE pairs with the t-SNAREs to form a four-helix trans-SNARE complex. N- to C-terminal zippering of the trans-SNARE complex brings the two membranes into close proximity to fuse (Sollner et al., 1993; Melia et al., 2002; Pobbati et al., 2006). In GLUT4 exocytosis, the fusion reaction requires syntaxin-4 and SNAP-23 as the t-SNAREs, and VAMP2/synaptobrevin as the primary v-SNARE (Latham et al., 2006; Vicogne et al., 2006; D'Andrea-Merrins et al., 2007; Brandie et al., 2008).
Although unlikely a direct fusion trigger, the Ca2+ ion is critical to insulin-regulated GLUT4 exocytosis (Chou et al., 1998; Brozinick et al., 1999; Whitehead et al., 2001). Ca2+ promotes exocytosis primarily through exocytic Ca2+ sensors possessing C2 domains— autonomously folded modules that bind to both Ca2+ and phospholipids (Mackler et al., 2002; Sudhof, 2004; Chapman, 2008; Martens and McMahon, 2008; Graham and Kozlov, 2010). In GLUT4 exocytosis, the fusion reaction is regulated by the C2 domain–containing factor Doc2b (Doc2β; Fukuda et al., 2009; Ramalingam et al., 2012), a ubiquitously expressed member of the Doc2 protein family (Orita et al., 1995; Hori et al., 1999; Ke et al., 2007; Higashio et al., 2008). Similar to the well-characterized synaptic Ca2+ sensor synaptotagmin-1, Doc2b possesses two homologous C2 domains—C2A and C2B. However, Doc2b is a cytoplasmic protein lacking a transmembrane domain (Martens and McMahon, 2008). In Doc2b-deficient cells, insulin-triggered GLUT4 exocytosis is abrogated (Fukuda et al., 2009; Ramalingam et al., 2012). However, it remains to be determined how Doc2b regulates the SNARE-dependent GLUT4 vesicle fusion. Doc2 family proteins are also implicated in neurotransmitter release at the chemical synapse (Groffen et al., 2010; Pang et al., 2011; Yao et al., 2011), but attempts to define their functional roles are impeded by the coexistence of other synaptic C2-domain factors that may play redundant/compensatory roles in the fusion reaction (Martens and McMahon, 2008). As such, the functions of Doc2 family proteins in synaptic vesicle fusion are still being debated (Groffen et al., 2010; Pang et al., 2011; Yao et al., 2011), providing little insight into the mechanism of Doc2b in GLUT4 exocytosis.
Here we sought to unravel the molecular mechanism of Doc2b by reconstituting it into a defined fusion reaction containing GLUT4 exocytic SNAREs. We observed that Doc2b interacts with GLUT4 exocytic SNAREs and strongly accelerates the fusion kinetics in the presence of Ca2+ ions. The stimulatory activity of Doc2b is compatible with multiple v-SNARE isoforms, including VAMP2, VAMP3, and VAMP8, in agreement with the abilities of these v-SNAREs to support GLUT4 exocytosis in vivo (Zhao et al., 2009). Doc2b also binds to syntaxin-4 monomer, but the Doc2b–syntaxin-4 heterodimer does not block the assembly of the t-SNARE complex. The stimulation of fusion by Doc2b requires intact Ca2+-binding sites on both the C2A and C2B domains, distinct from the activity of synaptotagmin-1 in synaptic vesicle fusion (Bhalla et al., 2005; Stein et al., 2007). Using electron microscopy, we show that Doc2b strongly bends lipid bilayers in the presence of Ca2+. This curvature-inducing activity is critical to the stimulation of fusion by Doc2b. Because synaptotagmin-1 also requires membrane bending to regulate fusion (Martens et al., 2007; Lynch et al., 2008; Hui et al., 2009), these data suggest that membrane curvature induction likely constitutes a general mechanism of exocytic Ca2+ sensors.
RESULTS
Doc2b accelerates the SNARE-dependent fusion reaction reconstituted with GLUT4 exocytic SNAREs
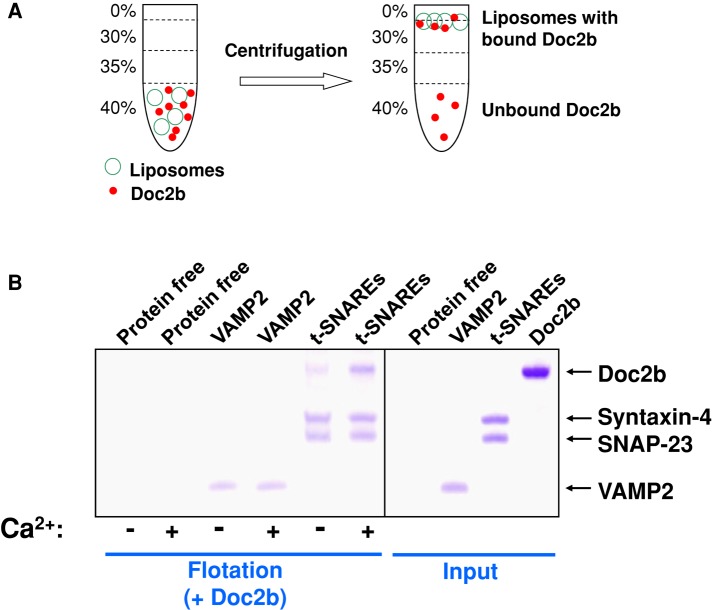
Doc2b can directly bind to membrane bilayers in the presence of Ca2+ and phosphatidylserine (PS; Supplemental Figure S7; Groffen et al., 2010; Yao et al., 2011). To examine the association of Doc2b with GLUT4 exocytic SNAREs, we prepared SNARE liposomes using the neutral lipid phosphatidylcholine (PC; Figure 1A). In a liposome coflotation assay (Figure 1A), we observed that Doc2b bound to GLUT4 exocytic t-SNAREs (heterodimer of syntaxin-4 and SNAP-23) but not to the v-SNARE VAMP2 (Figure 1B). Doc2b associated with GLUT4 exocytic t-SNAREs even in the absence of Ca2+ ions, but the binding was enhanced by Ca2+ (Figure 1B). Doc2b did not bind to protein-free liposomes or proteoliposomes reconstituted with yeast exocytic t-SNAREs (Figures 1B and Supplemental Figure S1), indicating that the interaction between Doc2b and GLUT4 exocytic SNAREs was specific. We also performed a dose-dependence test of Doc2b-SNARE binding in the liposome coflotation assay by varying the amount of Doc2b protein. We found that maximum binding was achieved when Doc2b was added at the same molar concentration as the t-SNAREs (Supplemental Figure S3). Although unable to bind the v-SNARE monomer (Figure 1B), Doc2b interacted with the ternary SNARE complex assembled from the v- and t-SNAREs (Supplemental Figure S4). These data demonstrate that Doc2b interacts stoichiometrically with the GLUT4 exocytic SNAREs.
FIGURE 1:
Doc2b binds to the GLUT4 exocytic SNAREs. (A) Diagram of the liposome coflotation assay used to probe Doc2b-SNARE interactions. (B) Coomassie blue–stained SDS–PAGE gels showing the binding of Doc2b to protein-free or SNARE liposomes in the presence of 1 mM EGTA or CaCl2. The liposomes were prepared with the PC lipid. Because Doc2b comigrates with syntaxin-4 on SDS–PAGE, a SUMO-tagged Doc2b protein, which exhibits the same biological activity as untagged Doc2b (Supplemental Figure S2), was used in the liposome flotation assays. Each binding reaction contained 5 μM v- or t-SNAREs. Soluble factors were added to a final concentration of 5 μM.
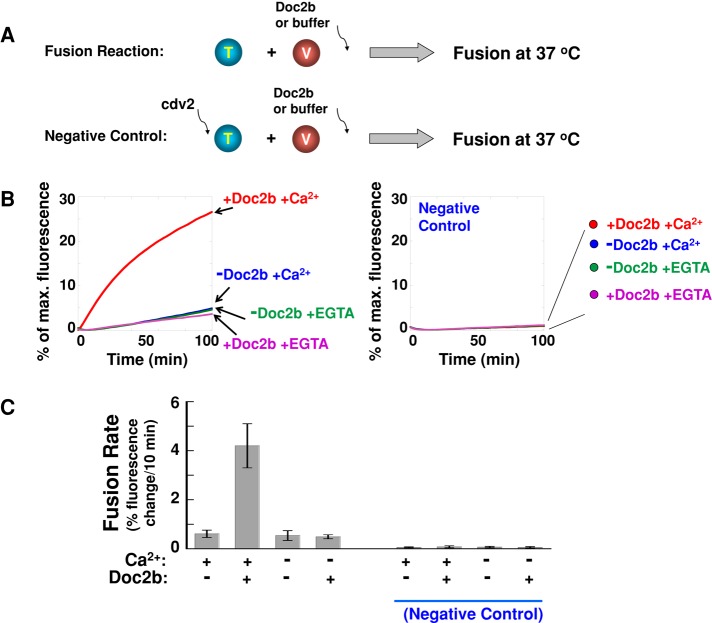
Next we examined how Doc2b regulates the SNARE-dependent GLUT4 vesicle fusion. GLUT4 exocytic SNAREs were reconstituted into a defined fusion system in which the v- and t-SNAREs were anchored in separate populations of proteoliposomes (Figure 2A). The fusion of v- and t-SNARE liposomes was monitored through lipid mixing using fluorescence resonance energy transfer (FRET). GLUT4 exocytic SNAREs alone drove a (slow) basal level of membrane fusion (Figure 2, B and C). Doc2b strongly accelerated the kinetics of this SNARE-dependent fusion reaction in the presence of Ca2+, with an increase in the initial fusion rate of approximately sevenfold (Figure 2, B and C). The stimulatory activity of Doc2b in the fusion reaction was completely blocked by coincubation with the cytoplasmic domain of VAMP2 (VAMP2 CD; Figure 2, B and C), a dominant-negative inhibitor of trans-SNARE assembly (Weber et al., 1998; Scott et al., 2003). Hence Doc2b acts by facilitating the SNARE-mediated fusion pathway rather than by causing fusion via an alternative mechanism (e.g., nonspecific aggregation of the liposomes).
FIGURE 2:
Doc2b strongly accelerates the kinetics of the SNARE-dependent membrane fusion. (A) Illustrations of the liposome fusion procedures. The t-SNARE liposomes were reconstituted with syntaxin-4 and SNAP-23, and the v-SNARE liposomes contained VAMP2. (B) Fusion of the reconstituted proteoliposomes in the absence or presence of 5 μM Doc2b. The fusion reactions included 1 mM EGTA or CaCl2. Negative controls: 20 μM of the dominant-negative inhibitor VAMP2 CD (cdv2) was added at the beginning of the fusion reactions. (C) Initial rates of the fusion reactions in B. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min. Error bars, SD.
Next we tested whether the observed lipid mixing signals resulted from hemifusion or full fusion. We used dithionite to selectively abolish the nitrobenzoxadiazole (NBD) fluorophores on the outer leaflets of the liposomes, such that only the inner-leaflet fluorescence was detected. The fluorescence signal was decreased by 40–60% throughout the fusion reactions (Supplemental Figure S5), indicating equal lipid mixing in both the inner and outer leaflets of the liposomes. Therefore full fusion occurred in the reconstituted liposome fusion reactions. We also examined the dose dependence of Doc2b activity in the reconstituted SNARE-dependent fusion reaction. The maximum stimulation of fusion was reached with 5 μM Doc2b, similar to the concentration of t-SNARE proteins present on the liposomes (Figure 3). These data are in agreement with the liposome coflotation results (Supplemental Figure S3) and suggest that Doc2b interacts stoichiometrically with GLUT4 exocytic SNAREs during fusion.
FIGURE 3:
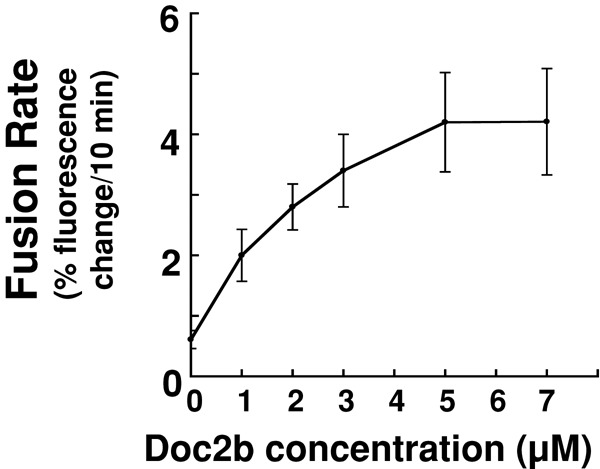
Dose dependence of Doc2b activity in the SNARE-dependent fusion reaction. Doc2b was added to the reconstituted fusion reaction at the indicated concentrations in the presence of 1 mM CaCl2. Initial fusion rates of the fusion reactions are shown. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min. Error bars, SD.
The stimulatory activity of Doc2b in the fusion reaction is compatible with multiple v-SNAREs
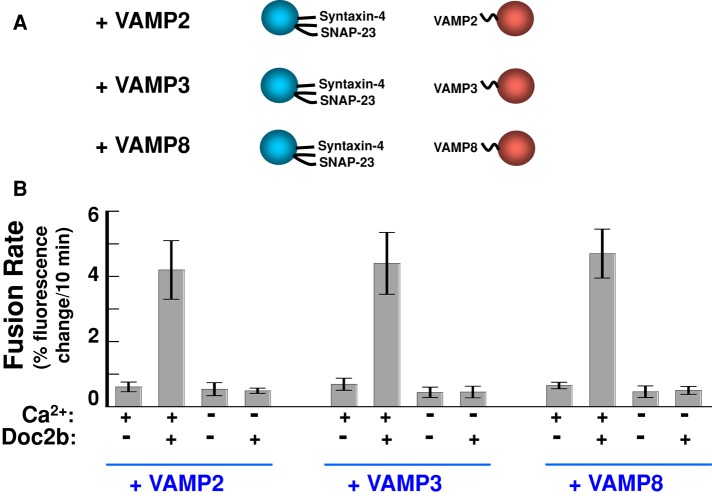
Whereas VAMP2 constitutes the primary v-SNARE in GLUT4 vesicle fusion, VAMP3 and VAMP8 can serve as alternative/compensatory v-SNAREs (Zhao et al., 2009). Next we examined whether Doc2b could stimulate the fusion reactions in which VAMP2 was substituted with VAMP3 or VAMP8. Proteoliposomes bearing GLUT4 exocytic t-SNAREs (syntaxin-4 and SNAP-23) were directed to fuse with liposomes reconstituted with VAMP2, VAMP3, or VAMP8 (Figure 4A). We found that Doc2b strongly accelerated all of these fusion reactions in the presence of Ca2+ (Figure 4B). Therefore the stimulatory function of Doc2b in the SNARE-dependent fusion reaction is compatible with VAMP2, VAMP3, and VAMP8, in agreement with the abilities of these v-SNAREs to support GLUT4 exocytosis in vivo (Zhao et al., 2009).
FIGURE 4:
The stimulation of fusion by Doc2b is compatible with multiple v-SNAREs. (A) Illustrations of the liposome fusion pairs. The v-SNAREs were reconstituted into proteoliposomes at similar surface densities (Shen et al., 2007; Rathore et al., 2010). (B) Initial rates of the indicated fusion reactions in the presence of 1 mM EGTA or CaCl2. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min (Rathore et al., 2010). Error bars, SD.
The Doc2b–syntaxin-4 heterodimer does not inhibit the assembly of the GLUT4 t-SNARE complex
In the foregoing reconstituted fusion reactions, the t-SNARE complexes were preassembled to examine the membrane-fusing activity of the trans-SNARE fusion engine (Figure 2A). The upstream t-SNARE assembly, however, is a key regulatory step in exocytosis (Jahn and Fasshauer, 2012; Rizo and Sudhof, 2012). Multiple factors have been shown to bind syntaxin monomers and block the assembly of t-SNARE complexes, thereby clamping the fusion reaction at a basal state (Rowland et al., 2011; Jahn and Fasshauer, 2012). Doc2b binds to syntaxin-4 in solution (Fukuda et al., 2009; Ramalingam et al., 2012), but the functional role of this binding is unknown. Here we examined the Doc2b–syntaxin-4 interaction in a membrane environment by reconstituting the syntaxin-4 monomer into proteoliposomes. SNAP-23, the other subunit of the t-SNARE complex, was subsequently added as soluble proteins. In a liposome coflotation assay, we observed that the membrane-anchored syntaxin-4 monomer interacted with Doc2b to form a heterodimer (Supplemental Figure S6). However, Doc2b binding did not block the assembly of syntaxin-4 with SNAP-23 to form the t-SNARE complex (Supplemental Figure S6), indicating that the Doc2b–syntaxin-4 heterodimer does not inhibit t-SNARE formation.
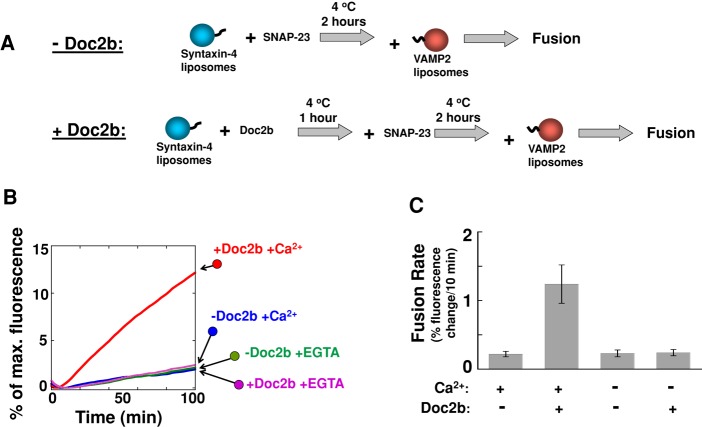
Next we examined the role of the Doc2b–syntaxin-4 binding in the dynamic fusion reaction (Figure 5A). In agreement with the liposome-binding results, the interaction of Doc2b with syntaxin-4 did not inhibit the SNARE-dependent fusion reaction (Figure 5, B and C). Instead, Doc2b strongly accelerated the fusion rate in the presence of Ca2+ (Figure 5C), similar to its regulatory activity in the fusion reactions containing preassembled t-SNAREs (Figure 2). These data demonstrate that, unlike other syntaxin-binding factors, Doc2b does not inhibit the assembly of the GLUT4 t-SNARE complex.
FIGURE 5:
The Doc2b-syntaxin-4 heterodimer does not inhibit the assembly of the GLUT4 t-SNARE complex. (A) Diagram illustrating the experimental procedures for the reconstituted fusion reactions. (B) The binding of Doc2b to syntaxin-4 did not inhibit the SNARE-dependent fusion reaction. Syntaxin-4 liposomes were incubated with or without Doc2b at 4°C for 1 h before SNAP-23 was added. After 2 h at 4°C, VAMP2 liposomes were introduced to initiate fusion. The fusion reactions were carried out in the presence of 1 mM CaCl2 or EGTA. (C) Initial rates of the indicated fusion reactions in B. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min. Error bars, SD.
The promotion of fusion by Doc2b requires intact Ca2+-binding sites on both the C2A and C2B domains
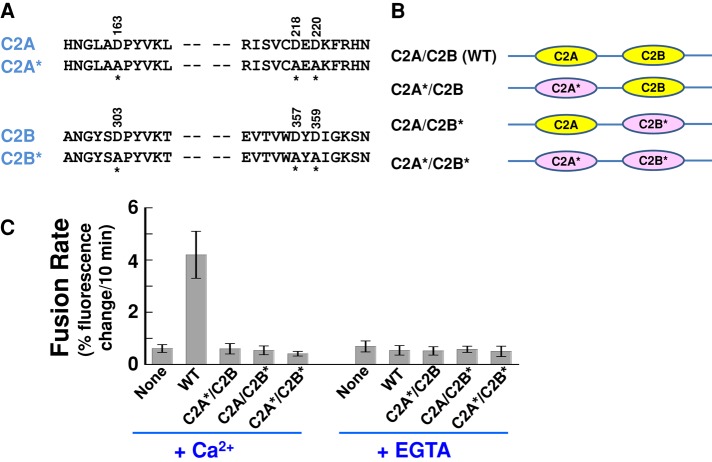
The reconstitution of Doc2b into the defined fusion system enabled us to further dissect its molecular mechanism of action in GLUT4 vesicle fusion. Doc2b accelerates the SNARE-dependent fusion reaction in a Ca2+-dependent manner (Figure 2), suggesting that Ca2+ binding is essential to its stimulatory function. To define which C2 domain(s) of Doc2b confers the Ca2+ sensitivity, we tested Doc2b mutants bearing substitutions in the conserved Ca2+-binding sites of the C2 domains. The binding of Ca2+ to C2 domains is coordinated by a series of aspartate residues lining the pockets on one end of the C2 domain (Chapman, 2008; Pang et al., 2011). Although the crystal structure of Doc2b is unavailable, its Ca2+-binding sites can be modeled based on the structures of the synaptic Ca2+ sensor synaptotagmin-1 (Figure 6, A and B; Shao et al., 1998; Fernandez et al., 2001). A triple substitution D163A/D218A/D220A abolishes the Ca2+ binding to the C2A domain of Doc2b, whereas the D303A/D357A/D359A mutations disrupt the Ca2+-binding sites on the C2B domain (Figure 6A; Pang et al., 2011). Of importance, these point mutations do not interfere with the folding of the C2 domains based on circular dichroism (CD) measurements (Pang et al., 2011).
FIGURE 6:
The stimulatory activity of Doc2b in the fusion reaction requires intact Ca2+ sites on both the C2A and C2B domains. (A) Sequences showing the aspartic acid residues in the Doc2b C2A and C2B domains that coordinate Ca2+ binding. Mutated residues are indicated with amino acid numbers on the top and asterisks at the bottom. (B) Illustrations of WT and mutant Doc2b proteins used in the fusion reactions. C2 domains with Ca2+-binding sites mutated are marked with asterisks. WT C2 domains are shown in yellow, and mutant C2 domains are shown in pink and marked with asterisks. CD spectroscopic analysis demonstrated that these Ca2+-binding mutations do not affect the folding of the C2 domains (Pang et al., 2011). (C) Initial rates of the indicated fusion reactions in the presence of 1 mM EGTA or CaCl2. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min. Error bars, SD.
Next we reconstituted the Ca2+-binding–defective Doc2b mutants into the FRET-based, SNARE-dependent fusion reaction to examine their regulatory activities. We found that the stimulation of fusion by Doc2b was abolished when the Ca2+-binding sites of both C2A and C2B were mutated (Figure 6C). Remarkably, the stimulatory activity of Doc2b was also abrogated when either the C2A or C2B domain was mutated (Figure 6C). Thus Doc2b requires intact Ca2+-binding sites on both the C2A and C2B domains to accelerate the fusion reaction. Of interest, the synaptic Ca2+ sensor synaptotagmin-1 appears to use only one of its C2 domains to sense Ca2+, whereas Ca2+ binding to the other C2 domain is largely dispensable (Bhalla et al., 2005; Stein et al., 2007). Hence, despite the overall similarities in their domain organization and stimulatory functions, Doc2b and synaptotagmin-1 likely differ in coupling Ca2+ binding to fusion regulation.
The stimulatory function of Doc2b in the SNARE-dependent fusion reaction involves membrane curvature induction
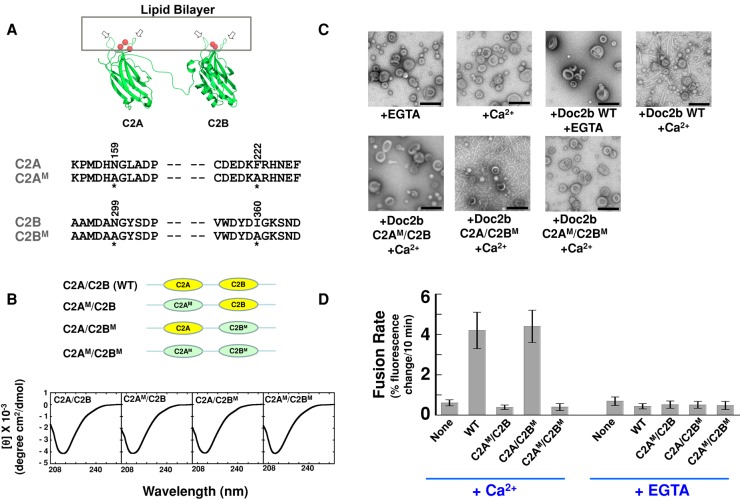
Binding to Ca2+ ions enables the hydrophobic loops of a C2 domain to penetrate into the outer leaflet of a lipid bilayer (Figure 7A; Chapman, 2008; Martens and McMahon, 2008). In synaptic vesicle fusion, this Ca2+-dependent membrane penetration allows synaptotagmin-1 to locally induce membrane curvature, which is critical to its stimulatory activity in fusion (Martens et al., 2007; Lynch et al., 2008; Hui et al., 2009). However, it remains unclear whether membrane bending constitutes a common mechanism of exocytic Ca2+ sensors. Using electron microscopy (EM), we observed that Doc2b strongly bent liposome membranes in the presence of Ca2+ (Figure 7C), consistent with a previous report (Groffen et al., 2010). To examine the functional role of this membrane-bending activity, we mutated the bilayer-penetrating hydrophobic residues of Doc2b. Structural modeling suggests that the hydrophobic residues N159/F222 (C2A) and N299/I360 (C2B) are located at the tips of the membrane-binding loops that insert into the surface of membrane bilayers (Figure 7A). We mutated these hydrophobic residues into alanines (Figure 7A) and then used EM to test how the mutations affect the membrane-bending activity of Doc2b. We found that the Doc2b mutant bearing mutations in the C2A domain lost the ability to induce membrane curvature (Figure 7C). By contrast, mutation of the C2B domain had little effect on the membrane-bending function of Doc2b (Figure 7C). As expected, mutations of both the C2A and C2B domains abrogated the membrane-bending activity of Doc2b (Figure 7C). These data demonstrate that the hydrophobic residues N159/F222 in C2A are critical to the membrane-bending function of Doc2b. Using a liposome coflotation assay, we found that the membrane-bending–defective Doc2b mutants failed to interact with the membrane bilayer (Supplemental Figure S7). By contrast, none of these mutations affected the association of Doc2b with the GLUT4 exocytic SNAREs (Supplemental Figure S8), indicating that the mutations specifically interfere with the curvature-inducing function of Doc2b. Of importance, CD spectrum measurements demonstrate that mutations of the hydrophobic residues do not affect the overall folding of the Doc2b proteins (Figure 7B).
FIGURE 7:
The stimulatory function of Doc2b involves membrane curvature induction. (A) Top, model depicting the binding of Doc2b to the membrane. The two hydrophobic loops (arrows) of the C2 domain insert into the lipid bilayer in the presence of Ca2+ ions (red balls). Modeled from the crystal structure of synaptotagmin-1 C2A domain (PDB: 1BYN) and C2B domain (PDB: 1K5W; Shao et al., 1998; Fernandez et al., 2001). Bottom, sequences of hydrophobic residues in the C2A and C2B domains of Doc2b that are predicted to embed in the lipid bilayer. Mutated residues are indicated with amino acid numbers on the top and asterisks at the bottom. (B) Top, illustrations of WT and mutant Doc2b proteins used in the fusion reactions. WT C2 domains are shown in yellow, and mutant C2 domains are shown in green and labeled with M. Bottom, CD spectroscopic analysis of WT and mutant Doc2b proteins. (C) Electron micrographs showing the bending of Folch liposomes by Doc2b in the presence of 1 mM EGTA or CaCl2. (D) Initial rates of the indicated fusion reactions in the presence of 1 mM EGTA or CaCl2. Each fusion reaction contained 5 μM t-SNAREs and 1.5 μM v-SNARE. Data are presented as percentage of fluorescence change per 10 min. Error bars, SD.
Next we examined how these Doc2b mutants regulate the SNARE-dependent fusion reaction. We observed that the Doc2b mutant bearing substitutions in the C2A domain failed to accelerate the fusion reaction, whereas the stimulatory activity of the C2B domain mutant was comparable to that of wild-type (WT) Doc2b (Figure 7D). As expected, the Doc2b mutant bearing substitutions in both of the C2 domains was defective in accelerating the fusion reaction (Figure 7D). We also mutated the hydrophobic N159/F222 residues of the C2A domain into the bulky tryptophans (Supplemental Figure S9A). We found that the Doc2b tryptophan mutant bound to the membrane bilayer and strongly induced membrane curvature in a Ca2+-dependent manner (Supplemental Figure S9, B and C). The Doc2b tryptophan mutant strongly accelerated SNARE-mediated membrane fusion in the presence of Ca2+ (Supplemental Figure S9D). Therefore the stimulatory functions of Doc2b mutants correlate with their abilities to bend lipid bilayers (Figure 7C), indicating that the stimulation of fusion by Doc2b involves membrane curvature induction. Taken together with previous findings of synaptotagmin-1 (Martens et al., 2007; Lynch et al., 2008; Hui et al., 2009), our data suggest that membrane curvature induction likely represents a common mechanism of exocytic Ca2+ sensors in regulating vesicle fusion.
DISCUSSION
Decades of genetic and physiological research have firmly established the essential role of insulin-controlled GLUT4 exocytosis in maintaining blood glucose homeostasis. However, the molecular basis of the GLUT4 exocytic pathway remains poorly understood. It poses significant challenges to delineate complex membrane trafficking pathways that require the dynamic assembly of multiple layers of functional units at membrane–cytosol interfaces. Fusion regulators often operate at similar or overlapping steps of the fusion reaction such that they might exhibit identical loss-of-function phenotypes in vivo, precluding further mechanistic insights (Sudhof, 2004). Traditional biochemical assays (e.g., pull-down and immunoprecipitation), on the other hand, mostly use detergent-solubilized or truncated proteins, which are trapped in dead-end conformations and often behave differently from the native proteins (Jahn, 2004).
We sought to dissect the GLUT4 exocytic pathway from a novel angle by reconstituting GLUT4 vesicle fusion in vitro using purified components. This SNARE-dependent defined fusion system is well suited for addressing the fundamental questions of regulated exocytosis because the protein composition and topology can be precisely controlled. Regulatory factors can be individually added or perturbed without the complications of other molecules naturally present in the cell, allowing their kinetic effects on fusion to be causally established.
In this study, we characterized the soluble Ca2+ sensor Doc2b in GLUT4 exocytosis. Doc2b is critical to insulin-triggered GLUT4 exocytosis, but its molecular mechanism of action has been unclear. When reconstituted into a FRET-based proteoliposome fusion system, GLUT4 exocytic SNAREs—syntaxin-4, SNAP-23, and VAMP2—mediate a basal level of membrane fusion. We discovered that Doc2b binds to the GLUT4 exocytic SNAREs and strongly accelerates the fusion kinetics in the presence of Ca2+ ions. Dose-dependence analysis suggests that Doc2b may bind stoichiometrically to GLUT4 exocytic SNAREs during the fusion reaction. The stimulatory activity of Doc2b is compatible with multiple v-SNARE isoforms, including VAMP2, VAMP3 and VAMP8, in agreement with the physiological observations that these v-SNAREs support GLUT4 exocytosis in vivo (Zhao et al., 2009). Doc2b also binds to syntaxin-4 monomer, but the Doc2b-syntaxin-4 heterodimer does not inhibit t-SNARE assembly or the fusion kinetics, suggesting that Doc2b does not regulate t-SNARE complex assembly. Of interest, the Sec1/Munc18 (SM) protein Munc18c appears to act at similar stages of the SNARE pathway as Doc2b in GLUT4 exocytosis (Jewell et al., 2008; Ramalingam et al., 2012). It is possible that Doc2b binds to the t-SNAREs and induces a local curvature on the target membrane, whereas Munc18c binds to the ternary SNARE complex and promotes the zippering of the trans-SNARE complex. Together Munc18c and Doc2b may form a supracomplex that positively regulates the SNARE-mediated vesicle fusion. It is also possible that Doc2b plays an additional role in fusion by regulating the interaction between Munc18c and syntaxin-4 monomer.
The stimulatory function of Doc2b in the reconstituted SNARE-dependent fusion reaction is strictly dependent on the presence of Ca2+ ions, in agreement with the proposed Ca2+-sensing role of Doc2b in GLUT4 exocytosis (Fukuda et al., 2009). We discovered that Doc2b uses both the C2A and C2B domains to sense Ca2+. Of interest, the synaptic Ca2+ sensor synaptotagmin-1 requires active Ca2+-binding sites only in one of its C2 domains, whereas Ca2+ binding to the other C2 domain is largely dispensable (Bhalla et al., 2005; Stein et al., 2007). Thus the C2 domain requirement of Doc2b in GLUT4 exocytosis is distinct from that of synaptotagmin-1 in synaptic vesicle fusion. Another notable difference between Doc2b and synaptotagmin-1 is the topological restriction of their regulatory functions. Like Doc2b, synaptotagmin-1 binds to both SNAREs and membrane bilayers (Chapman, 2008; Martens and McMahon, 2008). However, synaptotagmin-1 is a transmembrane vesicular protein that promotes synaptic vesicle fusion only when localized to vesicles (on the same membrane as the v-SNARE; Mahal et al., 2002; Wang et al., 2011). By contrast, Doc2b is a soluble protein that is recruited to the membrane through binding to SNAREs and phospholipids, intrinsically lacking the topological restriction of synaptotagmin-1. Hence, despite their analogous functions in exocytic Ca2+ sensing, Doc2b and synaptotagmin-1 likely differ in certain features of their regulatory activities.
Doc2 family proteins are also implicated in synaptic neurotransmitter release. It was suggested that Doc2 promotes synaptic vesicle fusion in a Ca2+-dependent manner (Groffen et al., 2010; Yao et al., 2011). However, in another study, Doc2b was found to regulate synaptic release as a Ca2+-independent adaptor (Pang et al., 2011). Attempts to solve this discrepancy are impeded by the coexistence of multiple double–C2 domain factors at synaptic terminals, including synaptotagmin-1, 2, and 9, as well as Doc2 proteins (Martens and McMahon, 2008; Walter et al., 2011). By contrast, Doc2b is the only double–C2 domain factor involved in GLUT4 exocytosis, presenting a unique opportunity for dissecting its molecular function in regulated exocytosis. Our findings provide the molecular explanation of the Ca2+-dependent stimulatory function of Doc2b observed in GLUT4 exocytosis (Fukuda et al., 2009; Ramalingam et al., 2012). It would be interesting to determine whether Doc2b also harbors a second, Ca2+-independent function in GLUT4 exocytosis.
The final step of the membrane fusion reaction is to merge the two separate lipid bilayers into one (Jahn and Scheller, 2006; Martens and McMahon, 2008). Bilayer merging, however, is opposed by powerful hydrophobic forces, and, for fusion to occur, a high energy barrier must be overcome (Hanson et al., 1997; Martens and McMahon, 2008; van der Bliek, 2009). A major way to overcome this energy barrier is believed to involve the induction of extreme membrane curvature (membrane bending; Chernomordik and Kozlov, 2005; Kozlov et al., 2010; McMahon et al., 2010). Membrane bending results in a curved “dimple” structure that points toward the apposed bilayer. The tip of the dimple is under strong curvature stress, which reduces the energy barrier for membrane fusion and therefore promotes fusion kinetics (Chernomordik and Kozlov, 2005; Graham and Kozlov, 2010). In synaptic vesicle fusion, synaptotagmin-1 has been shown to promote the fusion reaction by bending the lipid bilayer (Martens et al., 2007; Lynch et al., 2008; Hui et al., 2009). However, it is unclear whether this membrane-bending function constitutes a general mechanism for exocytic Ca2+ sensors or represents a specialized function of synaptotagmin-1 in synaptic release. To address this question, it is necessary to dissect a distinct Ca2+ sensor in a nonsynaptic exocytic pathway. Our functional studies of Doc2b indicate that its stimulatory function in the SNARE-dependent vesicle fusion depends on its membrane-bending activity. Doc2b mutants defective in membrane curvature induction can still bind SNAREs but fail to stimulate the fusion kinetics. These data suggest that membrane curvature induction likely represents a common mechanism for the regulatory functions of exocytic Ca2+ sensors in vesicle fusion.
Taken together, these findings establish the molecular mechanism by which Doc2b promotes the SNARE-dependent GLUT4 vesicle fusion. Furthermore, our findings suggest that exocytic Ca2+ sensors possess both common and specialized mechanisms in regulating vesicle fusion. This study will also serve as a paradigm for dissecting how protein–protein networks mediate and regulate GLUT4 exocytosis in body glucose homeostasis.
MATERIALS AND METHODS
Protein expression and purification
All recombinant proteins were expressed in Escherichia coli and purified by nickel affinity chromatography. GLUT4 exocytic t-SNAREs were composed of an untagged syntaxin-4 subunit and the SNAP-23 protein with an N-terminal hexahistidine tag. The v-SNAREs VAMP3 and VAMP8 were expressed in a similar way as VAMP2 (Shen et al., 2010) and had no extra residues left after the tags were proteolytically removed. SNAREs were stored in a buffer containing 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.4), 400 mM KCl, 1% n-octyl-β-d-glucoside, 10% glycerol, and 0.5 mM Tris(2-carboxyethyl)phosphine. Recombinant rat Doc2b (amino acids 125–412) was expressed and purified from E. coli in a similar way as described for the synaptic factor Munc18-1 (Shen et al., 2007, 2010). Doc2b mutants were generated by site-directed mutagenesis and expressed following the same procedure as for the wild-type protein.
Proteoliposome reconstitution
Proteoliposomes were prepared using an improved reconstitution procedure known to yield homogeneous populations of proteoliposomes that exhibit similar fusion properties as native membranes (Takamori et al., 2006; Holt et al., 2008; Rathore et al., 2010; Shen et al., 2010). All lipids were obtained from Avanti Polar Lipids (Alabaster, AL). For t-SNARE reconstitution, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS), and cholesterol were mixed in a molar ratio of 60:20:10:10. For v-SNARE reconstitution, POPC, POPE, POPS, cholesterol, (N-(7-nitro-2,1,3-benzoxadiazole-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine, and N-(Lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidylethanolamine) were mixed at a molar ratio of 60:17:10:10:1.5:1.5. SNARE proteoliposomes were prepared by detergent dilution and isolated on a Nycodenz (Axis-Shield, Oslo, Norway) density gradient flotation (Shen et al., 2010). Complete detergent removal was achieved by overnight dialysis of the samples in Novagen (Gibbstown, NJ) dialysis tubes against the reconstitution buffer (25 mM HEPES, pH 7.4, 100 mM KCl, 10% glycerol, and 1 mM dithiothreitol [DTT]). On SNARE liposomes, the protein:lipid ratio was 1:200 for v-SNAREs and 1:500 for t-SNARE liposomes. Reconstituted liposomes were routinely monitored by electron microscopy with negative staining and dynamic light scattering.
Liposome fusion assay
Fusion reactions and data analysis were performed as previously described (Shen et al., 2010). A standard fusion reaction contained 45 μl of unlabeled t-SNARE liposomes and 5 μl of labeled v-SNARE liposomes and was conducted in a 96-well Nunc plate (Nunc/Thermo Fisher Scientific, Rochester, NY) at 37°C. The fusion reaction was carried out in a reaction buffer (25 mM HEPES, pH 7.4, 50 mM KCl and 1 mM DTT). Fusion was followed by measuring the increase in NBD fluorescence at 538 nm (excitation 460 nm) every 2 min in a BioTek Synergy HT microplate reader (BioTek, Winooski, VT). At the end of the reaction, 10 μl of 2.5% dodecylmaltoside was added to the liposomes. Fusion data were presented as the percentage of maximum fluorescence change. To assess the regulatory activity of Doc2b, we incubated v- and t-SNARE liposomes with or without 5 μM Doc2b at 37°C. The maximum fusion rate within the first 10 min of liposome fusion was used to represent the initial rate of a fusion reaction. In fusion reactions with decreases in initial fluorescence (due to temperature change), the phase of fluorescence decrease was omitted from the calculation. Full accounting of statistical significance was included for each figure based on at least three independent experiments.
Liposome coflotation assay
Binding of Doc2b to liposomes was carried out using a coflotation assay essentially in the same manner as previously described (Shen et al., 2007). Doc2b was incubated with liposomes at 4°C with gentle agitation in the presence of 1 mM ethylene glycol tetraacetic acid or CaCl2. After 1 h, an equal volume of 80% Nycodenz (wt/vol) in reconstitution buffer was added and transferred to 5 × 41 mm centrifuge tubes. The liposomes were overlaid with 200 μl each of 35 and 30% Nycodenz and then with 20 μl of reconstitution buffer on the top. The gradients were centrifuged for 4 h at 52,000 rpm in a Beckman SW55 rotor (Beckman Coulter, Brea, CA). Samples were collected from the 0/30% Nycodenz interface (2 × 20 μl) and analyzed by SDS–PAGE.
Circular dichroism spectroscopy
CD spectra were measured using a Jasco J-815 spectropolarimeter (Jasco, Tokyo, Japan) equipped with a 1-mm quartz cell. The readings were made at 0.1-nm intervals, and each data point represented the average of six scans at a speed of 50 nm/min over the wavelength range 200–260 nm. The data were converted into mean-residue-weighted molar ellipticity using the equation [θ]MRW = 100θ/Cnl, where C is the protein concentration (mM), θ is the measured ellipticity (mdeg), n is the number of residues, and l is the path length (cm).
Electron microscopy
EM imaging of liposomes was carried out at the Boulder Laboratory for 3-D Electron Microscopy of Cells (Boulder, CO). Brain lipid extract (Folch fraction I; Sigma-Aldrich, St. Louis, MO) was dried and resuspended at 1 mg/ml in HEPES-buffered saline (50 mM HEPES, pH 7.4, and 100 mM NaCl). The resuspended lipid bilayers were then sonicated for 10 min to generate Folch liposomes. Folch liposomes (0.3 mg/ml) were incubated with WT or mutant Doc2b proteins (10 μM) in the presence of 1 mM EDTA or CaCl2 for 10 h at room temperature. Subsequently the samples were stained with 2% uranyl acetate and observed under a Philips CM100 transmission electron microscope (Philips, Amsterdam, Netherlands).
Supplementary Material
Acknowledgments
We thank Tom Giddings (University of Colorado at Boulder) for assistance with electron microscopy, Brooke Hirsch (University of Colorado School of Medicine, Aurora, CO) for assistance with circular dichroism measurements, and Thomas Südhof (Stanford University, Stanford, CA) for providing plasmids encoding Doc2b mutants defective in Ca2+ binding. This work was supported by National Institutes of Health Pathway to Independence Award DK080080 and National Institutes of Health Grant DK095367 to J.S. J.S. is a Pew Scholar in the Biomedical Sciences.
Abbreviations used:
- Doc2
double C2-like domains
- FRET
Förster (fluorescence) resonance energy transfer
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- VAMP
vesicle-associated membrane protein
- WT
wide type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-11-0810) on February 20, 2013.
REFERENCES
- Bhalla A, Tucker WC, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol Biol Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Blot V, McGraw TE. Molecular mechanisms controlling GLUT4 intracellular retention. Mol Biol Cell. 2008;19:3477–3487. doi: 10.1091/mbc.E08-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandie FM, Aran V, Verma A, McNew JA, Bryant NJ, Gould GW. Negative regulation of syntaxin4/SNAP-23/VAMP2-mediated membrane fusion by Munc18c in vitro. PLoS One. 2008;3:e4074. doi: 10.1371/journal.pone.0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozinick JT, Jr, Reynolds TH, Dean D, Cartee G, Cushman SW. 1-[N, O-bis-(5-isoquinolinesulphonyl)-N-methyl-L-tyrosyl]-4- phenylpiperazine (KN-62), an inhibitor of calcium-dependent camodulin protein kinase II, inhibits both insulin- and hypoxia-stimulated glucose transport in skeletal muscle. Biochem J. 1999;339:533–540. [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Membrane trafficking: three steps to fusion. Curr Biol. 2007;17:R255–R258. doi: 10.1016/j.cub.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Chapman ER. How does synaptotagmin trigger neurotransmitter release. Annu Rev Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- Charron MJ, Brosius FC, 3rd, Alper SL, Lodish HF. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci USA. 1989;86:2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- D'Andrea-Merrins M, Chang L, Lam AD, Ernst SA, Stuenkel EL. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J Biol Chem. 2007;282:16553–16566. doi: 10.1074/jbc.M610818200. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson RG, Sudhof TC, Rizo J. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32:1057–1069. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Emoto M, Nakamori Y, Taguchi A, Miyamoto S, Uraki S, Oka Y, Tanizawa Y. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes. 2009;58:377–384. doi: 10.2337/db08-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TR, Kozlov MM. Interplay of proteins and lipids in generating membrane curvature. Curr Opin Cell Biol. 2010;22:430–436. doi: 10.1016/j.ceb.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen AJ, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Heuser JE, Jahn R. Neurotransmitter release—four years of SNARE complexes. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Higashio H, Nishimura N, Ishizaki H, Miyoshi J, Orita S, Sakane A, Sasaki T. Doc2 alpha and Munc13-4 regulate Ca(2+)-dependent secretory lysosome exocytosis in mast cells. J Immunol. 2008;180:4774–4784. doi: 10.4049/jimmunol.180.7.4774. [DOI] [PubMed] [Google Scholar]
- Hoffman NJ, Elmendorf JS. Signaling, cytoskeletal and membrane mechanisms regulating GLUT4 exocytosis. Trends Endocrinol Metab. 2011;22:110–116. doi: 10.1016/j.tem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Riedel D, Stein A, Schuette C, Jahn R. Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+ Curr Biol. 2008;18:715–722. doi: 10.1016/j.cub.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Takai Y, Takahashi T. Presynaptic mechanism for phorbol ester-induced synaptic potentiation. J Neurosci. 1999;19:7262–7267. doi: 10.1523/JNEUROSCI.19-17-07262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. Principles of exocytosis and membrane fusion. Ann NY Acad Sci. 2004;1014:170–178. doi: 10.1196/annals.1294.018. [DOI] [PubMed] [Google Scholar]
- Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- James DE, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Jewell JL, Oh E, Bennett SM, Meroueh SO, Thurmond DC. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J Biol Chem. 2008;283:21734–21746. doi: 10.1074/jbc.M710445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Oh E, Thurmond DC. Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4. Am J Physiol Regul Integr Comp Physiol. 2010;298:R517–R531. doi: 10.1152/ajpregu.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L, Brennwald P. Testing the 3Q:1R “rule”: mutational analysis of the ionic “zero” layer in the yeast exocytic SNARE complex reveals no requirement for arginine. Mol Biol Cell. 2000;11:3849–3858. doi: 10.1091/mbc.11.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B, Oh E, Thurmond DC. Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J Biol Chem. 2007;282:21786–21797. doi: 10.1074/jbc.M701661200. [DOI] [PubMed] [Google Scholar]
- Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci. 2010;35:699–706. doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham CF, et al. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Lavan BE, Lienhard GE. Insulin signalling and the stimulation of glucose transport. Biochem Soc Trans. 1994;22:676–680. doi: 10.1042/bst0220676. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Gerona RR, Kielar DM, Martens S, McMahon HT, Martin TF. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol Biol Cell. 2008;19:5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- Mahal LK, Sequeira SM, Gureasko JM, Sollner TH. Calcium-independent stimulation of membrane fusion and SNAREpin formation by synaptotagmin I. J Cell Biol. 2002;158:273–282. doi: 10.1083/jcb.200203135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Kozlov MM, Martens S. Membrane curvature in synaptic vesicle fusion and beyond. Cell. 2010;140:601–605. doi: 10.1016/j.cell.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Melia TJ, Weber T, McNew JA, Fisher LE, Johnston RJ, Parlati F, Mahal LK, Sollner TH, Rothman JE. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J Cell Biol. 2002;158:929–940. doi: 10.1083/jcb.200112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita S, Sasaki T, Naito A, Komuro R, Ohtsuka T, Maeda M, Suzuki H, Igarashi H, Takai Y. Doc2: a novel brain protein having two repeated C2-like domains. Biochem Biophys Res Commun. 1995;206:439–448. doi: 10.1006/bbrc.1995.1062. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Sudhof TC. Doc2 supports spontaneous synaptic transmission by a ca(2+)-independent mechanism. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- Ramalingam L, Oh E, Yoder SM, Brozinick JT, Kalwat MA, Groffen AJ, Verhage M, Thurmond DC. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes. 2012;61:2424–2432. doi: 10.2337/db11-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore SS, Bend EG, Yu H, Hammarlund M, Jorgensen EM, Shen J. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc Natl Acad Sci USA. 2010;107:22399–22406. doi: 10.1073/pnas.1012997108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, their accomplices—guilty as charged. Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- Rowland AF, Fazakerley DJ, James DE. Mapping insulin/GLUT4 circuitry. Traffic. 2011;12:672–681. doi: 10.1111/j.1600-0854.2011.01178.x. [DOI] [PubMed] [Google Scholar]
- Schekman R, Novick P. 23 genes, 23 years later. Cell. 2004;116:S13–S15. doi: 10.1016/s0092-8674(03)00972-3. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Merz AJ. Capture and release of partially zipped trans-SNARE complexes on intact organelles. J Cell Biol. 2009;185:535–549. doi: 10.1083/jcb.200811082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Scott BL, Komen JS, Liu S, Weber T, Melia TJ, McNew JA. Liposome fusion assay to monitor intracellular membrane fusion machines. Methods Enzymol. 2003;372:274–300. doi: 10.1016/S0076-6879(03)72016-3. [DOI] [PubMed] [Google Scholar]
- Shao X, Fernandez I, Sudhof TC, Rizo J. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change. Biochemistry. 1998;37:16106–16115. doi: 10.1021/bi981789h. [DOI] [PubMed] [Google Scholar]
- Shen J, Rathore S, Khandan L, Rothman JE. SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J Cell Biol. 2010;190:55–63. doi: 10.1083/jcb.201003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat Struct Mol Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM. Fussy mitochondria fuse in response to stress. EMBO J. 2009;28:1533–1534. doi: 10.1038/emboj.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos S, Esk C, Hoshino S, Funke BH, Chen CY, Plocik AM, Wright WE, Kucherlapati R, Brodsky FM. A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science. 2009;324:1192–1196. doi: 10.1126/science.1171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci USA. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter AM, Groffen AJ, Sorensen JB, Verhage M. Multiple Ca(2+) sensors in secretion: teammates, competitors or autocrats. Trends Neurosci. 2011;34:487–497. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu H, Gu Y, Chapman ER. Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J Cell Biol. 2011;195:1159–1170. doi: 10.1083/jcb.201104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci. 2006;31:215–222. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem. 2001;276:27816–27824. doi: 10.1074/jbc.M011590200. [DOI] [PubMed] [Google Scholar]
- Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147:666–677. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Yang L, Lopez JA, Fan J, Burchfield JG, Bai L, Hong W, Xu T, James DE. Variations in the requirement for v-SNAREs in GLUT4 trafficking in adipocytes. J Cell Sci. 2009;122:3472–3480. doi: 10.1242/jcs.047449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.