Abstract
Objectives/Hypothesis
To examine the characteristics of pediatric cochlear implant channel malfunction preceding device failure.
Study Design
Retrospective review.
Methods
All pediatric patients who underwent cochlear implantation at a tertiary academic medical center were reviewed regarding device type, reason for replacement, time to replacement, and timing and pattern of channel faults in failed versus nonfailed devices.
Results
Between 1993 and 2008, 264 pediatric cochlear implantations were performed. With an average 894-day follow-up, the replacement rate was 9.5% (25/264). Reasons for replacement were device failure (6.4%), medical/surgical failure (2.3%), and obsolescence (0.8%). Replacement rates were comparable among Advanced Bionics (13.3%), Cochlear Corporation (6.3%), and MED-EL (10.3%) devices. Fifty-two cochlear implants developed at least one channel fault, and 13 eventually progressed to failure requiring replacement. MED-EL devices comprised 12 of these 13 failures. At the 12-month follow-up interval, one, three, and five channel faults predicted 40%, 75%, and 100% probabilities of eventual electrode failure, respectively. Channels destined to fail demonstrated small, yet statistically significant, impedance elevations 12 months before failure and large elevations 3 months before failure.
Conclusions
Replacement of cochlear implants in pediatric patients is common and is due to device malfunction about one half of the time. Earlier initial channel fault, earlier subsequent channel faults, adjacent channel faults, and a greater total number of channel faults were associated with the need for replacement surgery. Elevations in a channel’s impedance should raise the concern for an impending failure. These predictors can help the cochlear implant team when considering surgery to replace the device.
Keywords: Cochlear implant, hearing loss, pediatric, prosthesis, cochlea, revision
INTRODUCTION
Cochlear implantation has become conventional therapy for children with severe to profound sensorineural hearing loss. Replacement surgery comprises approximately 5% to 11% of cochlear implant (CI) procedures, and in general, device failure rates and overall replacement rates are higher in children versus adults.1–6 A recent review by Cullen et al.7 of 952 pediatric CI procedures reported a pediatric replacement surgery rate of 11.2%. Most of the literature regarding CI replacement surgery has focused on descriptive reviews of the causes for CI failure. Reasons for CI replacement have been classified into several categories: 1) hard failures, 2) soft failures, 3) medical/surgical failures, and 4) technology upgrade. Hard failures are the most common cause for replacement CI surgery.5–8 Aside from the recognition that the repeated episodes of minor head trauma associated with a normal, active childhood may predispose pediatric CI patients to device failure, no other factors have been identified that might predict CI failure.7,9
However, it is a common clinical scenario for single CI channels to fail over time, requiring that they be removed from the stimulation program map. If enough channels are lost, a performance decline will likely ensue, requiring replacement of the device.10 We hypothesized that early trends in channel failures might predict long-term CI failure. To study this, we examined the function of CI electrodes on a channel-by-channel basis by reviewing data collected during each routine programming session on every patient in our pediatric CI population. A pediatric population was used because of the critical nature of early diagnosis of a CI failure. Delay in diagnosis of a CI failure would in essence be failure to provide timely and adequate intervention to a deaf child, who has only a short window of opportunity to acquire optimal speech and language skills.11–13
MATERIALS AND METHODS
Institutional review board approval was obtained for this study (H-24980). We performed a retrospective review of all pediatric patients who had undergone cochlear implantation from 1993 to 2008 and were still being followed at our tertiary care children’s hospital.14 Implantation procedures were performed by three surgeons. After implantation surgery, patients were seen by audiology on a monthly basis for the first 6 postoperative months, then bimonthly for the next 6 months, then quarterly thereafter. Using telemetry, impedance values were obtained at each follow-up visit and entered into a custom database designed in Access (Microsoft Corp., Redmond, WA). Other data included the etiology of hearing loss, inner ear anomalies, implant model and manufacturer, implant surgeon, date and reason for any replacement surgeries, and the patient age and date at the time of the initial implantation. Statistical analyses were performed using Excel (Microsoft) using a P < .05 level of significance.
The following definitions were used in this study. Each CI electrode has multiple channels (the electrical contact points). MED-EL (Innsbruck, Austria) electrodes have 12 channels, Advanced Bionics (Sylmar, CA) electrodes have 16 channels, and Cochlear Corporation (Lane Cove, Australia) electrodes have 22 channels. A channel failure occurred when a channel was identified by diagnostic telemetry to have either a short circuit or a high impedance measurement. When multiple channel failures accumulated, particularly in patients with deteriorating clinical performance, we asked the manufacturer to evaluate the electrode and make recommendations regarding whether replacement surgery is warranted. An electrode failure can be due to device failure—defined as a malfunction of the receiver/ stimulator (i.e., a defect in the body of the cochlear implant, such as case fracture or loss of hermeticity) or the channels (i.e., multiple channel failures leading to inadequate auditory stimulation)—or other causes, such as medical/surgical complications or device obsolescence. Failed electrodes for any reason required replacement surgery. In making this decision, we also assessed for declines in the child’s performance noted by caregivers, teachers, therapists, and serial speech and language testing.
RESULTS
Over the 15-year study period, 217 pediatric patients underwent 264 primary cochlear implantations (i.e., 47 patients had bilateral implantation). Causes of hearing loss included 158 nonsyndromic cases (33 connexin mutations, 21 inner ear anomalies, 104 unknown genetic etiologies), 22 syndromic cases (eight with inner ear anomalies), and 70 nongenetic cases (prematurity, congenital infections, meningitis, and aminoglycoside toxicity). All patients remained with our program for follow-up; 107 were male and 110 were female. A total of 30 Advanced Bionics, 79 Cochlear Corporation, and 155 MED-EL devices were implanted. The age at initial implantation was 3.6 ± 0.2 years (range, 0.5–19.6 years), and the length of follow-up was 2.5 ± 0.1 years (range, 0.1–14.1 years). Although the length of follow-up time did not differ significantly among manufacturers, the average age at initial implantation was significantly older in children receiving Advanced Bionics devices compared to children receiving Cochlear Corporation or MED-EL devices (5.4 vs. 3.3 or 3.0 years, respectively, P < .003).
Replacement Surgery
There were 17 patients who underwent a total of 25 replacement procedures comprising 9.5% of all pediatric CI operations (Table I). No significant differences were found in the distribution of electrodes requiring replacement among manufacturers or within manufacturer models, although for the latter, statistical analysis was not performed due to the small numbers of electrode failures. Causes for hearing loss, existence of inner ear anomalies, and implant surgeon all had no statistical effect on electrode replacement rate. Replacement procedures did not occur at a significantly higher rate in any particular time period of the study, suggesting that the surgeons’ learning curve did not contribute significantly to observed rates of replacement.
TABLE I.
Electrode and Channel Failures Stratified by Manufacturer and Model.
| Electrodes | Electrodes Requiring Replacement (% Total) | Electrodes With Channel Failure (% Total) | |
| Advanced Bionics | |||
| CII | 4 | 0 | 0 |
| HiRes90K | 26 | 4 | 1 |
| Total | 30 | 4 (13.3) | 1 (3.3) |
| Cochlear Corporation | |||
| Nucleus24 | 32 | 0 | 5 |
| NucleusFreedom | 47 | 5 | 7 |
| Total | 79 | 5 (6.3) | 12 (15.2)* |
| MED-EL | |||
| Combi40+ | 66 | 3 | 14 |
| Pulsar100 | 82 | 13 | 24 |
| Sonata | 7 | 0 | 1 |
| Total | 155 | 16 (10.3) | 39 (25.2)† |
| Grand Total | 264 | 25 (9.5) | 52 (19.7) |
P = 04 versus Advanced Bionics.
P = .003 versus Advanced Bionics; P = .04 versus Cochlear Corporation.
We categorized the reasons for replacement into device failures versus other reasons (Table II). Device failures were responsible for 17/25 (68%) of the replacement surgeries. Receiver/stimulator failure was the cause of all of the device failures in Advanced Bionics implants. In contrast, 12 of 13 device failures in MED-EL implants were due to multiple channel failures. Other reasons for replacement surgery accounted for only 8/25 (32%) of the cases.
TABLE II.
Reasons for Replacement Stratified by Manufacturer.
| Device Failure
|
Other Reasons
|
||||
|---|---|---|---|---|---|
| Manufacturer | Total Replacements | Channel Failure | Receiver/Stimulator Failure | Medical/Surgical Failure | Obsolescence |
| Advanced Bionics | 4 | 0 | 3 | 0 | 1 |
| Cochlear Corporation | 5 | 1 | 0 | 3* | 1 |
| MED-EL | 16 | 12 | 1 | 3† | 0 |
| Total | 25 | 13 | 4 | 6 | 2 |
All three failures secondary to chronic otitis media.
Two failures due to implant infection; one failure due to electrode migration.
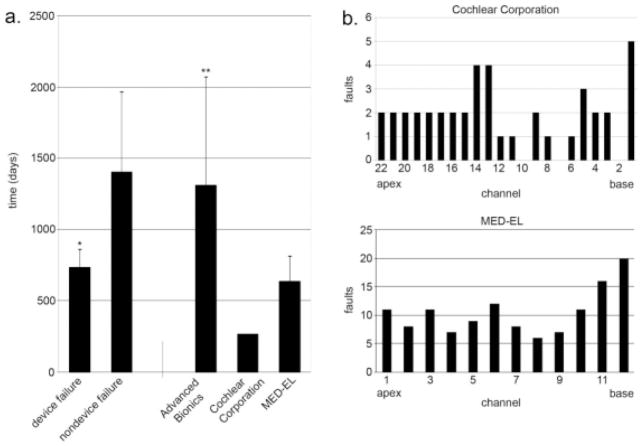
The average time after original implantation before replacement surgery was required was 912 days (Fig. 1a). Replacement surgery for device failure was required sooner for MED-EL implants than for Advanced Bionics implants (635 days vs. 1312 days, respectively, P =.02). The single Cochlear Corporation device failure was replaced at 266 days after initial implantation. The time to replacement surgery for other reasons besides device failure was not obviously different among the manufacturers, but low numbers precluded statistical analysis. Importantly though, when all of the manufacturers were combined together, the time to replacement surgery for device failure was significantly less than for other reasons.
Fig. 1.
(a) Time to cochlear implant replacement stratified by reason for failure in all manufacturers combined (left), and by manufacturer for device failures only (right). *Versus nondevice failures; P <.03. **Versus MED-EL device failures only; P = .004. (b) Distribution of failed channels in Cochlear Corporation and MED-EL devices (all devices are included, not just those requiring replacement).
Device failure was the most common reason for replacement surgery, and it occurred earlier than failure for other reasons. We therefore focused on trying to understand the basis for device failure and on trying to identify clinically practical predictors. Postexplant analysis by the implant manufacturer identified the basis for the receiver/stimulator failure in our patients with Advanced Bionics implants as loss of hermeticity (i.e., the Supplier B recall). According to the manufacturer and the US Food and Drug Administration, this problem has been addressed.15 The progressive accumulation of multiple channel faults, however, was the most common reason for eventual device replacement, and this occurred in one Cochlear and 12 MED-EL implants. Excepting two electrodes that sustained injury due to bony growth at the cochleostomy site that penetrated the Silastic, postexplant analyses of these devices yielded no other clues as to the cause of channel faults. In order to describe and, hopefully, comprehend this poorly understood yet common problem, we then investigated data from all of our patients, including those who did not require replacement surgery.
Channel Failure
Of the 264 devices studied, 52 had at least one channel failure (Table I). Importantly, at least one channel failure was found in 25% of MED-EL electrodes, but only in 3.3% of Advanced Bionics, and 15.2% of Cochlear Corporation electrodes. Both Cochlear Corporation and MED-EL electrodes demonstrated similar patterns of increased channel failures at the basal end and along the apical half of the electrode array (Fig. 1b). Inner ear anomaly, etiology of hearing loss, and implant surgeon did not statistically predispose an electrode to developing a channel fault. Rate of channel faults did not change significantly from year to year over the course of the study.
Channel Failure in MED-EL Implants
Thirteen of the 52 implants with at least one faulty channel ultimately failed and required replacement surgery. Since 12 of these were manufactured by MED-EL, we decided to perform detailed channel failure analyses only on this brand. Thus, all presented data from this point forward reflect only MED-EL implants. The average follow-up times for failed and nonfailed implants were 489 and 1,031 days, respectively. The average age at time of implantation for failed and nonfailed implants differed significantly (2.1 vs. 3.6 years, respectively, P =.03).
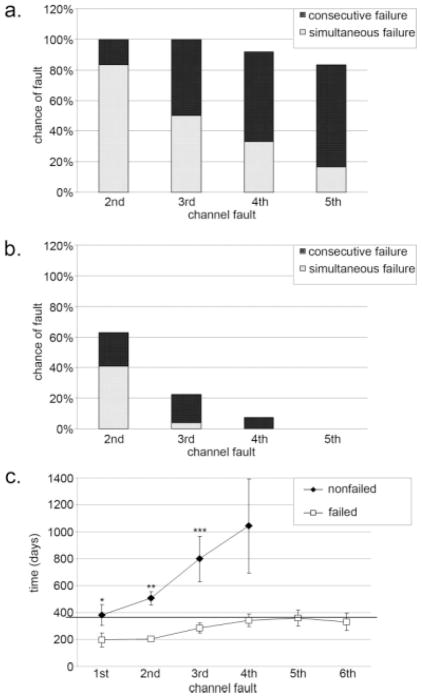
As expected, the average number of channel failures in implants that required replacement was greater than in those that did not require replacement (6.4 channels vs. 2.4 channels, respectively, P < .001). Consecutive channel failure always occurred more frequently in failed versus nonfailed electrodes (compare Figure 2a and 2b). Furthermore, simultaneous initial channel failures also occurred more frequently in failed versus nonfailed electrodes. It appeared that four to five consecutive channel failures, or three to four simultaneous initial channel failures, were highly associated with ultimate electrode failure.
Fig. 2.
(a) Profile of consecutive and simultaneous channel failures in failed electrodes. The top of the black bar shows the chance of each channel failure occurring. The top of the white bar shows the chance that each channel failure will occur simultaneously with the first channel failure. (b) Profile of consecutive and simultaneous channel failures in functioning electrodes. (c) Time course of consecutive channel failures in failed versus nonfailed electrodes. The dotted line marks 1 year. *Versus failed electrodes; P = .03. **Versus failed electrodes; P =.02. ***Versus failed electrodes; P =.05.
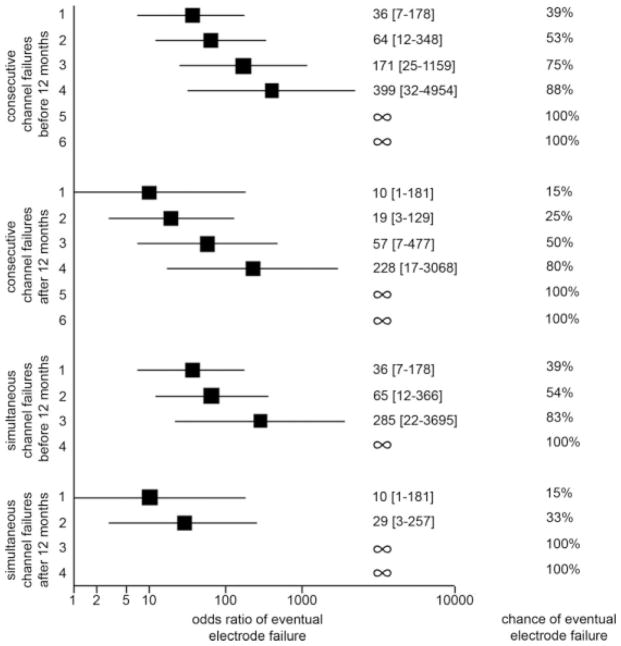
Cochlear implants that required replacement developed their first channel failure sooner than those that did not require replacement (Fig. 2c; 196 days vs. 381 days, respectively; P = .03). Additional channel failures also occurred earlier for failed versus nonfailed electrodes. Channel failures that occurred within 1 year of device activation predicted higher odds of requiring replacement surgery than an equivalent number of channel failures that did not occur within the first year (Fig. 3). Multiple simultaneous channel failures did not further increase the odds of electrode failure for small numbers of channel failures, but did lower the threshold number of channel failures that predicted 100% chance of eventual electrode failure compared with consecutive channel failures. Thus, the pattern of channel failures was progressive in nature, and the rate of progression was higher in those electrodes that ultimately required replacement. Simultaneous channel failures represent the maximum limit of rate of progression and predict the highest risk of requiring replacement surgery whether or not they occur within the first year.
Fig. 3.
Chances and odds ratios of electrode failure stratified by number, timing, and patterns of channel failures.
Implants with more than one channel failure were analyzed to determine how frequently channel failures occurred in adjacent channels. Adjacent channel faults were found 67% (8/12) of the time in electrodes that required replacement, but only 26% (7/27) of the time in those that did not require replacement (the chance of a random adjacent channel fault was 16.6%). Odds ratio (OR) analysis demonstrated a significant increase in odds of electrode failure in the presence of adjacent channel faults (OR = 5.7; 95% confidence interval, 1.3–25.0).
Impedance Measurements of MED-EL Channels
The impedance of non-faulty channels remained stable over time. Over a time period of 3 years, channel impedances stayed within a tight range at around 7 kΩ (Fig. 4). On the other hand, impedance variance in faulty channels extended beyond the 95% confidence interval beginning approximately 1 year prior to channel fault. Channels destined to fail began to demonstrate small statistically significant impedance elevations around 12 months before failure, but large clinically suspicious elevations in the impedance were found only in the 3 months just before failure.
Fig. 4.
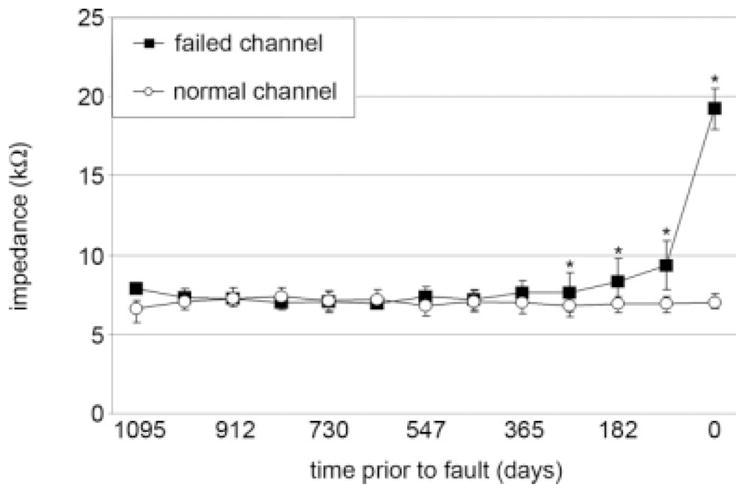
Time course of impedance values of failed channels versus normal channels prior to channel fault. *Versus normal channels; P <.001.
DISCUSSION
Herein, we show that a common mode of device failure in MED-EL implants is the accumulation of progressive channel faults, and that this mechanism of device failure is not as prevalent in other brands. It is critical to note, however, that we did not find marked differences in the overall rate of repeat surgery among the three device manufacturers or in the rate of surgery to replace a failed device. Furthermore, our overall pediatric cochlear implant replacement surgery rate of 9.5%, and overall time to replacement surgery for any failed electrode of 948 days, fall within the parameters reported in the literature.1–8 Thus, our cochlear implant program continues to offer all three devices, and we thoroughly discuss the known risks, benefits, and other differences of each device with our patients. Unless medical issues require the selection of one device over another, we firmly believe that the final decision of device selection should rest with the patients and their families.
When a cochlear implant appears to be trending on a path toward eventual failure, the question of when to replace the device arises. In adults, one can measure speech discrimination ability quite easily and also question the patient about their auditory sensation. In children, assessing the ability of a cochlear implant to appropriately stimulate the brain may be limited by inconsistent behavioral responses. Yet the consequence of a delay in diagnosis of a cochlear implant failure is dire and greatly impacts a child’s auditory development, and consequently, his or her speech and language development.11–13,16–18 Our finding that failed implants occur in a significantly younger population of children emphasizes the importance of diagnosing implant failure as the average age of cochlear implant candidacy continues to decrease.
Assuming that our findings are transferrable to other pediatric populations, these data will be helpful to the clinician and audiologist when counseling parents of children with MED-EL devices that have channel faults. A larger number of channel faults, the sooner they happen, adjacent channel faults, and the occurrence of multiple simultaneous faults all increase the risk of needing to replace the device. Thus, in a child with one or more of these predictors, earlier rather than later device replacement may be warranted in order to minimize the risk of delays in language acquisition.
A caveat to this section of the study is that one of the independent variables (number of channel faults) is not independent from the outcome measure (device failure). This is because MED-EL recommends (appropriately) that if greater than five electrodes are faulty, the implant should be replaced. Nevertheless, the focus of this study’s results is not on the predictive value of the number of channel faults per se, but rather the accumulation of these channel faults within a given timeframe. The limited length of the follow-up period is also a concern, particularly in the patients with functioning electrodes. Vigilance must be maintained for detection of failure in all cochlear implants, even if they do not display the characteristics identified in this study.
The discussion about channel failures may be relatively more important in MED-EL implants compared to Advanced Bionics or Cochlear Corporation implants because they have fewer channels. Therefore, each channel within the MED-EL electrode represents a larger proportion of the total number of channels, and losing channels in a MED-EL electrode may produce a larger clinical impact than losing the same number of channels in another brand of electrode. This difference emphasizes the possible benefit of larger numbers of channels within an electrode, which may allow some channels to serve a role as backup channels.
In MED-EL implants, we noted statistically significant changes in the channel impedance characteristics prior to channel failure. Furthermore, the average time to first channel failure is about 6 months. This suggests that some type of progressive degradative process is ongoing prior to fault, rather than a major traumatic event (i.e., trauma to the implant directly during surgery or indirectly during head bumps that all children routinely experience) that would be expected to cause an immediate loss of function. No clear cause for channel failure could be identified from postexplant manufacturer evaluation of the electrodes. However, during replacement surgery, two implants were found to have bony growth at the cochleostomy site that had penetrated the Silastic. Such a mechanism for insidious, progressive electrode damage would be consistent with the timing of channel failures. MED-EL electrodes may be softer and more flexible than the other two company’s electrodes, which may preferentially predispose them to damage from bony ingrowth. Further investigation of this mechanism for electrode injury is warranted.
CONCLUSION
Although all three brands of CIs have similar rates of replacement surgery, a common mode of device failure in MED-EL implants in particular is the accumulation of progressive channel failures. Associated with ultimate device failure were: 1) the number of total channel failures, 2) earlier occurrence of these channel failures, 3) the presence of adjacent channel failures, and 4) simultaneous occurrences of multiple channel failures. Elevations in a channel’s impedance suggest impending channel failure. These characteristics of channel failures can facilitate the earlier diagnosis of device failure, which is crucial in the appropriate management of pediatric patients hearing through a CI.
Acknowledgments
This work was supported by the Hearing Center at Texas Children’s Hospital. No support of any kind was received from any cochlear implant manufacturer.
BIBLIOGRAPHY
- 1.Parisier SC, Chute PM, Weiss MH, et al. Results of cochlear implant reinsertion. Laryngoscope. 1991;101:1013–1015. doi: 10.1288/00005537-199109000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Fayad JN, Baino T, Parisier SC. Revision cochlear implant surgery: causes and outcome. Otolaryngol Head Neck Surg. 2004;131:429–432. doi: 10.1016/j.otohns.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto RT, Svirsky MA, Myres WA, et al. Cochlear implant reimplantation. Am J Otol. 1997;18:S60–S61. [PubMed] [Google Scholar]
- 4.Balkany TJ, Hodges AV, Gomez-Marin O, et al. Cochlear reimplantation. Laryngoscope. 1999;109:351–355. doi: 10.1097/00005537-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lassig AA, Zwolan TA, Telian SA. Cochlear implant failures and revision. Otol Neurotol. 2005;26:624–634. doi: 10.1097/01.mao.0000178123.35988.96. [DOI] [PubMed] [Google Scholar]
- 6.Cote M, Ferron P, Bergeron F, et al. Cochlear reimplantation: causes of failure, outcomes, and audiologic performance. Laryngoscope. 2007;117:1225–1235. doi: 10.1097/MLG.0b013e31805c9a06. [DOI] [PubMed] [Google Scholar]
- 7.Cullen RD, Fayad JN, Luxford WM, et al. Revision cochlear implant surgery in children. Otol Neurotol. 2008;29:214–220. doi: 10.1097/MAO.0b013e3181635e9a. [DOI] [PubMed] [Google Scholar]
- 8.Parisier SC, Chute PM, Popp AL, et al. Cochlear implant mechanical failures. Am J Otol. 1996;17:730–734. [PubMed] [Google Scholar]
- 9.Weise JB, Muller-Deile J, Brandemann G, et al. Impact to the head increases cochlear implant reimplantation rate in children. Auris Nasus Larynx. 2005;32:339–343. doi: 10.1016/j.anl.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Zeitler DM, Lalwani AK, Roland JT, Jr, et al. The effects of cochlear implant electrode deactivation on speech perception and in predicting device failure. Otol Neurotol. 2009;30:7–13. doi: 10.1097/MAO.0b013e31818a08ba. [DOI] [PubMed] [Google Scholar]
- 11.McConkey RA, Koch DB, Osberger MJ, et al. Effect of age at cochlear implantation on auditory sill development in infants and toddlers. Arch Otolaryngol Head Neck Surg. 2004;130:570–574. doi: 10.1001/archotol.130.5.570. [DOI] [PubMed] [Google Scholar]
- 12.Connor CM, Craig HK, Raudenbush SW, et al. The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: is there an added value for early implantation? Ear Hear. 2006;27:628–644. doi: 10.1097/01.aud.0000240640.59205.42. [DOI] [PubMed] [Google Scholar]
- 13.Geers AE. Speech, language and reading skills after early cochlear implantation. Arch Otolaryngol Head Neck Surg. 2004;130:634–638. doi: 10.1001/archotol.130.5.634. [DOI] [PubMed] [Google Scholar]
- 14.Oghalai JS, Tonini R, Rasmus J, et al. Intra-operative monitoring of cochlear function during cochlear implantation. Cochlear Implants Int. 2009;10:1–18. doi: 10.1002/cii.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auditory reliability report. [Accessed November 2, 2008];Advanced Bionics Web site. Available at: http://www.bionicear.com/UserFiles/File/ReliabilityRev4.pdf.
- 16.Tibussek D, Meister H, Walger M, et al. Hearing loss in early infancy affects maturation of the auditory pathway. Dev Med Child Neurol. 2002;44:123–129. doi: 10.1017/s0012162201001785. [DOI] [PubMed] [Google Scholar]
- 17.Kral A, Hartman R, Tillein J, et al. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurotol. 2001;6:346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Gilley PM, Dorman MF, et al. Deprivation-induced cortical reorganization in children with cochlear implants. Int J Audiol. 2007;46:494–499. doi: 10.1080/14992020701524836. [DOI] [PubMed] [Google Scholar]