Abstract
Objectives/Hypothesis
To review the presentation and management of improper electrode array placement, and to help guide clinical decision-making.
Study Design
Retrospective case series.
Methods
Pediatric and adult cochlear implant patients managed from January 2001 to present whose electrode arrays were not placed properly within the cochlea or extended beyond the cochlea into the internal auditory canal or adjacent structures.
Results
Four patients, three pediatric and one adult, were identified from over 824 cases (< 1%) managed over the study duration. All cases had normal cochlear anatomy. These cases were initially identified due to poor auditory skill development or absent behavioral responses following implantation, which prompted imaging. Two patients presented several years after surgery. Sites of improper placement included the eustachian tube, vestibule, internal carotid artery canal, and internal auditory canal (IAC). Intraoperative findings and management are reviewed.
Conclusions
Electrode array malpositioning is a rare, but serious and correctable complication in cochlear implant surgery. A multidisciplinary approach, including prompt audiologic evaluation and imaging, is important, particularly when benefit from the implant is limited or absent. Management of electrode arrays in the IAC may be more challenging.
Keywords: Extracochlear electrode array misplacement, cochlear implant explantation and reimplantation, complications
INTRODUCTION
Cochlear implantation is now an established means of rehabilitating severe-to-profound sensorineural hearing loss in patients for whom traditional amplification provides limited benefit. Outcomes are overall highly successful with low complication rates. The three most likely reasons that have been cited for revision surgery with reimplantation are wound infection or device extrusion, device failure, and electrode misplacement.1 Complication rates are low overall, with flap-related complications reported to occur in 0.26 to 2.09% of cases, while electrode array-related problems occur in 0.17 to 2.12%.2
Cochlear implant electrode array misplacement has been reported only rarely, and predominately in individual case report and case series formats. Consequences include failure to provide benefit, and injury to important adjacent neurovascular structures that are within millimeters from the cochlea, such as the vestibular system and neural structures within the internal auditory canal, facial nerve, and major vessels ( including jugular vein and carotid artery). Furthermore, proper electrode position is critical to optimize interface with and stimulation of neural elements within the cochlea. At the current time, cochleostomy placement primarily is dependent on the surgeon’s intraoperative assessment of landmarks, typically the round window and related anatomic relationships. Final electrode position is confirmed by a combination of electrophysiologic measures (electrical impedance and neural response telemetry) and imaging. There is no universally agreed upon protocol for intraoperative monitoring during cochlear implantation to ensure proper electrode array positioning within the cochlea.
The purpose of this study is to report both the incidence of and clinical findings in cases of electrode array misplacement, as well as to report management of array misplacement. In addition, a literature review was performed to compile similar, previously reported cases, and to report their incidence, presentation, and management.
MATERIALS AND METHODS
In total, four patients were identified from 824 cochlear implantation procedures performed in pediatric and adult patients between January 2001 and December 2011, at the authors’ institution. This study as performed with approval of Institutional Review Board at Baylor College of Medicine and affiliated hospitals. Patients included in the study were those undergoing cochlear implantation with normal cochlear and inner ear anatomy, in both pediatric and adult populations. Cases with partial insertions, dysplasia, or congenital inner ear malformation; cochlear ossification from meningitis; or temporal bone fracture were excluded. A literature review was also performed to identify previously reported cases.
RESULTS
Case 1 (Eustachian Tube)
A 2-year-old male with congenital severe-profound sensorineural hearing loss and limited benefit from amplification underwent cochlear implantation after preoperative workup was completed. This included audiometric testing and imaging studies (CT and MRI), and formal speech and language evaluation. Surgery was performed via standard mastoidectomy and facial recess approach to the round window. Considerable mucosal edema in the mastoid and middle ear were observed intraoperatively. Thickened mucosa was removed from the promontory surface to aid visualization of the round window. A cochleostomy was created anterior to the round window niche, but the basal turn was not opened. Hence, drilling was directed more anteriorly and superiorly toward the oval window, allowing entry into the scala vestibuli of the cochlea. Slight resistance during electrode insertion was documented in the operative report. Normal impedance measurement was obtained immediately after implantation. Auditory nerve response telemetry (NRT) was unavailable at the time of surgery in 2004. No intraoperative imaging was obtained.
Postoperatively, initial implant activation indicated very low impedance values on several electrodes. Despite increasing the stimulation levels for those electrodes, the patient failed to demonstrate any behavioral responses to stimulation. A CT scan showed the electrode extending into the eustachian tube with the distal tip in the posterior aspect of the nasopharynx (Fig. 1). Explantation and reimplantation was performed. The cochleostomy was enlarged, and full electrode insertion was achieved. Intraoperative impedance measurement was normal, and intraoperative plain film verified the correct placement of the electrode array within the cochlea.
Fig. 1.
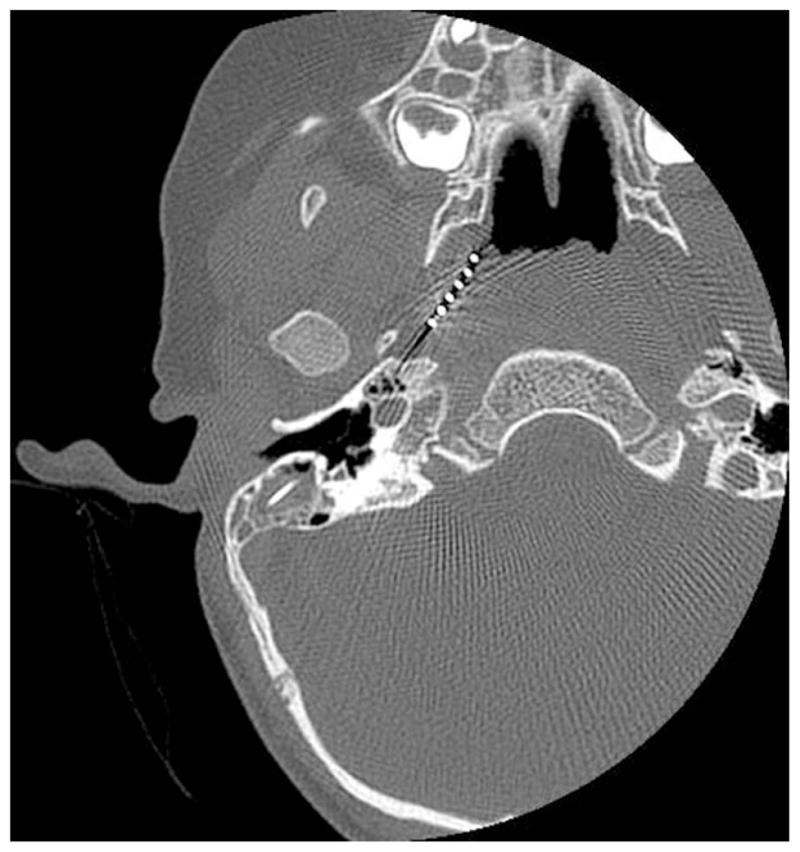
Electrode array extending into left eustachian tube with distal tip in nasopharynx.
Case 2 (Internal Carotid Artery)
A 14-year-old female with Down syndrome, history of cleft palate and lip repair, and bilateral sensorineural hearing loss presented 8 years after cochlear implantation for evaluation of her left cochlear implant. She had undergone left-sided cochlear implantation at an outside facility when she was 6 years old. She was still aided in the contralateral ear and used some signing for communication. Reportedly, the patient had some responses to the implant initially; however, the device had not worked well for many years, and she was not responding to auditory stimuli. Recent programming and increasing stimulation through five of the electrode sites elicited unusual responses, such as repeated swallowing and facial twitching, which are suggestive of nonauditory stimulation. A CT scan demonstrated the electrode array to be situated in the hypotympanum and extending into the carotid canal. The tip of the electrode could be seen extending about a third of the distance into the carotid canal at the level of the first turn (Fig. 2).
Fig. 2.
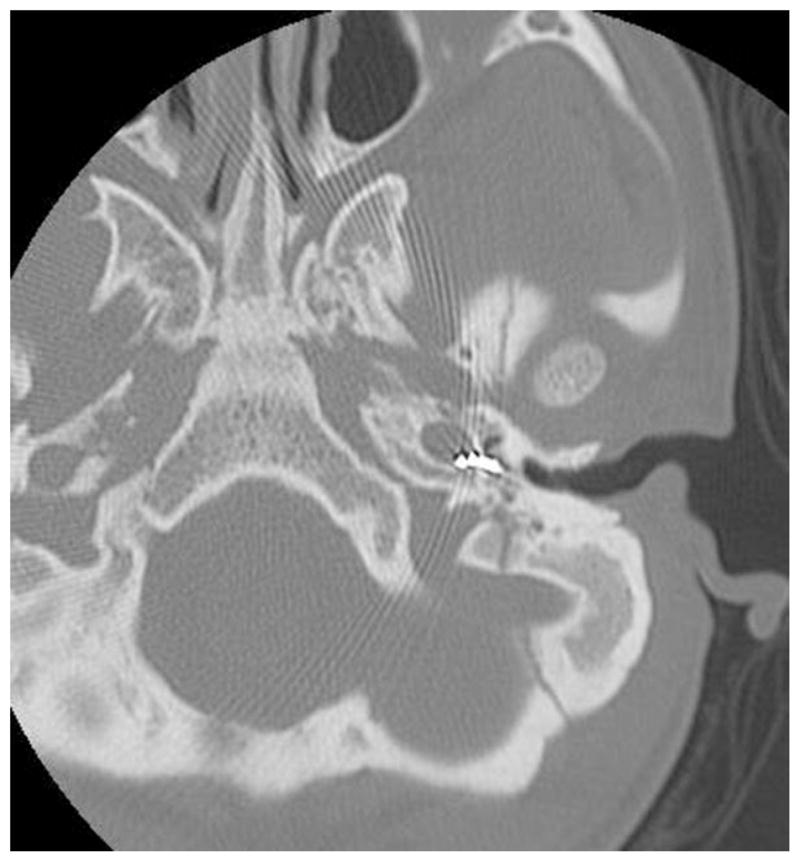
Electrode array tip extending into the carotid canal at level of first turn.
Management options were discussed with the family. Before considering surgical intervention to remove the implant, an angiogram was recommended to assess the integrity of the internal carotid artery. Available imaging studies could not delineate the status of the carotid artery. The recommended intervention was to perform a staged procedure to remove the old implant with possible endovascular intervention, if necessary, and then reimplant a new device at a later date. The parents ultimately sought another opinion, and the patient was lost to follow-up.
Case 3 (Internal Auditory Canal)
A 4-year-old male with a history of congenital profound bilateral sensorineural hearing loss presented for evaluation of a right cochlear implant placed at another facility 2 years earlier. Parents reported no benefit in speech or language development since the implantation. Audiologic testing demonstrated normal impedance, but irregular telemetry mapping. Despite several adjustments, there was no significant improvement in patient’s auditory perception or behavioral response. CT scan of temporal bones demonstrated the electrode array to be positioned within the internal auditory canal (Fig. 3). After multidisciplinary discussion and planning, the patient underwent a modified translabyrinthine approach to visualize the malpositioned electrode within the internal auditory canal as it was being removed, and simultaneous reimplantation of a new cochlear implant into the cochlea via a new cochleostomy. Postoperative CSF rhinorrhea was managed with eustachian tube plugging and middle ear obliteration, and subsequently resolved.
Fig. 3.
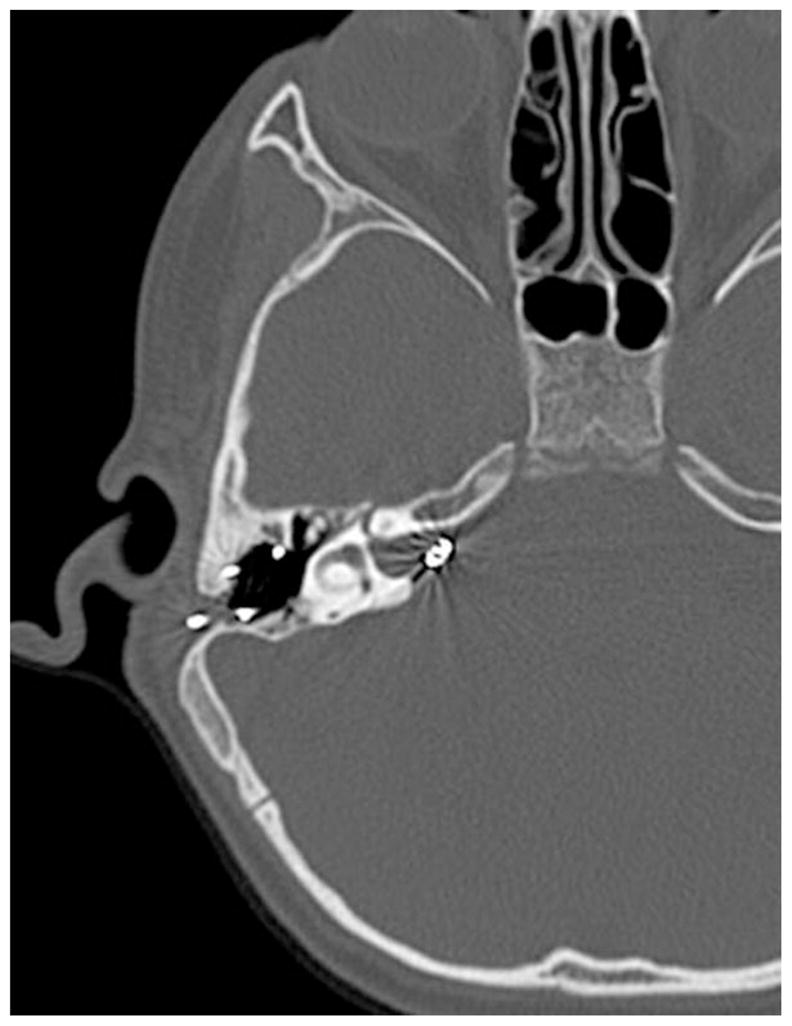
Electrode array position within the internal auditory canal.
Unfortunately, the patient did not receive any benefit from reimplantation of a new right cochlear implant, despite integrity testing that showed the implant to be functioning normally. A CT scan of temporal bone demonstrated correct electrode placement in the right cochlea. An MRI was also done to confirm the presence and integrity of the auditory nerves. The parents ultimately decided to undergo a left-sided cochlear implantation. Intraoperative measures including impedance and telemetry were normal. Subsequently, the patient has developed awareness of sound and auditory cues, and some speech and language development using the left cochlear implant.
Case 4 (Vestibule)
A 69-year-old male was referred for cochlear implant evaluation because of progressive bilateral profound sensorineural hearing loss since the late 1970s. Hearing aids worn for the past 15 years were no longer helpful. Preoperative workup included audiometric testing and a normal CT of the temporal bones, and the patient was thought to be an appropriate candidate for cochlear implantation. Surgery was performed via standard mastoidectomy and facial recess approach to the round window. However, the round window was poorly visualized because it appeared ossified. A cochleostomy was performed superior to the opacified round window and full electrode insertion of an implant was achieved. Intraoperative impedance testing was normal. However, neural response telemetry (NRT) was measured only in the basal electrodes and absent in the apical electrodes. No intraoperative imaging study was obtained.
Postoperatively, the patient became acutely vertiginous overnight with grade II nystagmus to the right. CT temporal bone demonstrated the electrode to have pierced the thin bone above the round window and coursed superiorly and posteriorly, extending into the vestibule, immediately anteromedial to the lateral semicircular canal (Fig. 4). The patient underwent explantation and reimplantation of a second device in the left ear the following day. In the revision procedure, the bony round window niche was drilled away to visualize the round window membrane. Electrode insertion through the round window was performed. Intraoperative impedance and NRT measurements were normal. In addition, intraoperative fluoroscopic imaging confirmed electrode placement within the cochlea.
Fig. 4.
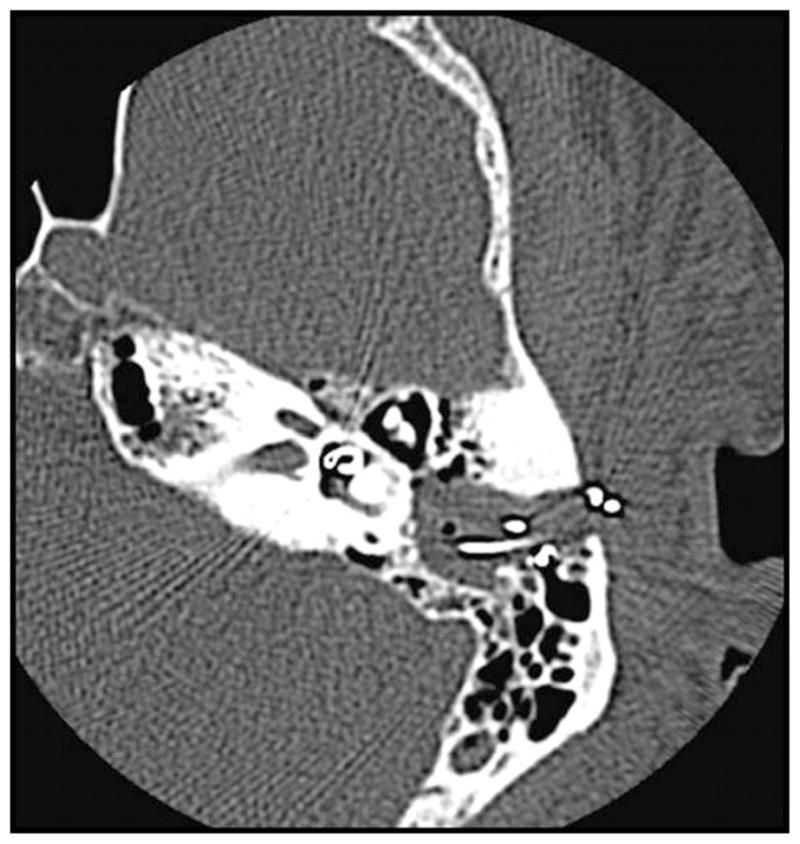
Electrode array extending into the vestibule.
DISCUSSION
The standard location for insertion of the cochlear implant electrode array is into the scala tympani of the cochlea. Failure to insert the electrode array into the scala tympani has been documented in the literature (Table I). Misplacement of the electrode array, considered to be a major complication since proper placement of the array is essential to successful hearing rehabilitation, is a rare complication in cochlear implant surgery. According to complication databases from implant manufacturers, misplacement of the cochlear electrode has been reported in 64 cases.3 Furthermore, electrode malposition has been cited as the cause of cochlear implant revision in up to 13% to 16% of cases.4,5 The majority of those cases represent intracochlear malpositioning in dysplastic cochleae or cochlear ossification.
TABLE I.
Literature Search for Cochlear Implantation Electrode Array Misplacement and Revision.
| Authors | Year Published | Study | Revision Rate
|
Incidence (electrode array misplacement)
|
Location | Impedance | Intraoperative Data
|
Post-Op
|
Management | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Revison #/Total CI # | E Misplaced #/Total CI # | % Electrode Misplacement | tNRT | Imaging | Imaging (indication) | ||||||
| Muzzi et al. (21) | 2012 | CR | Vestibule | + | Absent | X-ray:normal | CT | Reimplantation | |||
| Cosetti et al. (34) | 2012 | CS | 34/277 | 5/277 | 1.80 | Tip rollover | First run: 4 electrodes open; Second run: 1 electrode open | + | X-ray | Reimplantation | |
| Tip rollover | + | + | X-ray | Reimplantation | |||||||
| Tip rollover | + | + | X-ray | Reimplantation | |||||||
| Tip rollover | + | + | X-ray | Reimplantation | |||||||
| 1/277 | 0.36 | Superior SCC | + | ¾, present, NR on E10 | X-ray | Reimplantation | |||||
| Mouzali et al. (8) | 2011 | CR | Hyrtl’s fissure | n/a | Absent | Not obtained | CT | Reimplantation | |||
| Nevoux et al. (25) | 2010 | CR | Petrous ICA | Absent | Absent | Not obtained | CT | Reimplantation | |||
| Viccaro et al. (17) | 2009 | CR | Superior SCC | n/a | Neural potential only in two apical electrodes | X-ray | Reimplantation | ||||
| Superior SCC | n/a | Higher (400 CU) neural potential in three electrodes | Static fluoroscopy | Reimplantation | |||||||
| Sorrentino et al. (7) | 2009 | CS | 20/487 | 2/487 | 0.41 | Vestibule and Superior SCC | n/a | Not tested | X-ray:normal | CT (absence of auditory response) | NS |
| Not tested | X-ray:normal | CT (dizziness and absence of auditory response) | NS | ||||||||
| Ramalingam et al. (18) | 2008 | CR | Superior SCC | n/a | Absent | X-ray | Reimplantation | ||||
| Venail et al. (36) | 2008 | CS | 51/500 | 1/500 | 0.2 | Vestibule | n/a | n/a | n/a | X-ray | Reimplantation |
| Son et al. (24) | 2007 | CR | Petrous ICA | n/a | n/a | n/a | X-ray (ipsilateral facial paresis) | Reimplantation | |||
| Marlowe et al. (5) | 2007 | CS | 62/482 | 10/482 | 2.07 | 7 partially inserted* | n/a | n/a | n/a | Reimplantation | |
| 2/482 | 0.41 | 2 extracochlear: NS | n/a | n/a | n/a | Reimplantation | |||||
| 1: NS | n/a | n/a | n/a | Reimplantation | |||||||
| Tange et al. (2) | 2006 | CR | Horizontal SCC | + | + | CT (intermittent vertigo) | Reimplantation | ||||
| Pau et al. (19) | 2005 | CR | Vestibule | Implant-evoked EABR: only E14-22 responsive | Satisfactory full array insertion | Not obtained | CT (vertigo with inconsistent mapping response) | Reimplantation | |||
| Donatelli Lassig et al. (37) | 2005 | CS | 58/>900 | 8/900 | 0.89 | Partial insertion due to ossification (6 cases) | n/a | n/a | n/a | Reimplantation | |
| 2/900 | 0.22 | Extracochlearpetrous ICA | n/a | n/a | n/a | Reimplantation | |||||
| Extracochlear: NS | n/a | n/a | n/a | Reimplantation | |||||||
| Gastman et al. (23) | 2002 | CR | Petrous ICA | Normal: except electrodes 12 and 18: low | Not tested | Not obtained | X-ray (absence of auditory response) | Reimplantation | |||
| Rich et al. (9) | 2002 | CR | Hyrtl’s fissure | n/a | n/a | n/a | CT (absence of auditory response) | NS | |||
| Woolford et al. (22) | 1995 | CS | 3/76 | 1/76 | 1.32 | Eustachian tube | Normal: 5/6 electrodes | n/a | X-ray | Reimplantation | |
| Ito et al. (38) | 1993 | CS | 2/21 | 1/21 | 4.76 | Hypotympanic cell | n/a | n/a | X-ray | Reimplantation | |
| Windmill et al. (32) | 1990 | CS | 2/17 | 1/17 | 5.88 | Hypotympanic cell | + | Electric stimulus thresholds higher than normal | X-ray | Reimplantation | |
| Cohen et al. (16) | 1988 | CS | 18/459 | 4/459 | 0.87 | 3: NS | n/a | n/a | n/a | X-ray | Reimplantation |
| 1/459 | 0.21 | Hypotympanic cell | n/a | n/a | n/a | Reimplantation | |||||
| Total Combined Cases (Extracochlear) | 12/3219 | 0.37 | |||||||||
CR = Case Report; CS = Case Series; SCC = Semicircular Canal; ICA = Internal Carotid Artery; + = Normal impedance on all electrodes, no open or short; Normal telemetry; n/a = Information not available/reported; Revision rate = Revision of cochlear implants due to all causes (hard failure, soft failure, etc.);
= array inserted but migrated at later date;
NS = not specified; NR = no response.
The true incidence of electrode array misplacement into extracochlear sites is unknown. Furthermore, available manufacturer and FDA-maintained databases such as the Manufacturer User Facility and Distributor Experience (MAUDE) do not capture these cases routinely. There are significant limitations to the utility of the current MAUDE database for analyzing cochlear implant device complications, including electrode array misplacement, as expressed by other groups.6 Upon review of available published case series on cochlear implant complications that include data specifically on any electrode array misplacement, the published literature reports an incidence rate ranging from 0.2% to 5.8%, with an average of 1.02% (Table I). This figure includes intracochlear misplacements such as tip rollover and partial insertion. If the subset of extracochlear electrode array misplacement is compiled, the average incidence is lower (0.37%). However, these figures likely underestimate the true incidence as cochlear implant nonusers, device failures, and other implant-related problems may be underreported or unrecognized to be electrode array misplacement.
Possible Causes
Electrode array misplacement may be due to unidentified inner ear malformations, including the possibility of anatomic variation of the basal turn of the cochlea. Preoperative radiographic examination should help to avoid such complications. Yet, a normal preoperative CT scan does not exclude inner ear malformation that could lead to misplacement of the electrode array, such as malformation of the osseous spiral lamina.7 In addition, incomplete ossification of the tympano meningeal fissure (Hyrtl’s fissure) that usually occurs by the 24th week in utero causes permanent patency that can be another potential space for extracochlear misplacement of the electrode array. There are two reported cases in the literature involving pediatric patients.8,9 In the case presented by Mouzali et al.,8 the authors acknowledged that knowing about the abnormality prior to surgery would have suggested the possibility of misdirection of the electrode array, and cautious consideration of the axis of insertion.
Besides congenital inner ear findings, review of preoperative CT scans should alert the surgeon to possible anatomic limitations such as temporal bone fracture, otosclerosis, or labyrinthitis ossificans. Malpositioning of electrodes has been described in otosclerotic patients undergoing implantation.10 In these four cases, cochlear anatomy was normal; therefore, other factors were likely causative.
The most frequent error is inadvertent implantation of a hypotympanic air cell, which is more likely to occur if the round window niche is not clearly identified. This may occur even in experienced hands if there is fibrous or bony obliteration of the niche. Therefore, reliance on other landmarks (i.e., oval window position) after opening the facial recess is important. The surgeon must be able to identify the round window niche and promontory, and not be misled by hypotympanic air cells.11
Electrode insertion into an aberrantly placed cochleostomy can also lead to improper electrode positioning. Jain and Mukherji reported that the electrode array may be misplaced into the middle ear cavity, mastoid bowl, cochlear aqueduct, petrous carotid canal, or eustachian tube, or may be only partially inserted into the cochlea.12 The electrode may also be inserted into the vestibular system, most commonly the superior or lateral semicircular canal.2,13,14 Therefore, vestibular symptoms that are associated with cochlear implantation should arouse suspicion of electrode array misplacement. In addition, electrode array malposition should be considered in all cases when no benefit is achieved, and should be evaluated both by device-integrity testing and CT imaging, even in the setting of late presentation after implant surgery. In this study, two of four patients presented in delayed fashion due to lack of benefit from the implanted device.
The possibility of electrode extrusion from the cochlea may also explain some cases of array malposition. Electrode migration has been described in a review of implant complications in the American, Melbourne, and Hannover series.15,16 The effects of scar tissue around the extracochlear component of array has been proposed to account for electrode extrusion or migration.
Possible Locations
In the four cases presented here, electrode array misplacement was identified in the eustachian tube, internal carotid artery, internal auditory canal, and vestibule. A review of the literature was performed to identify other reports of similar anatomic sites of electrode misplacement and discussion of management (see Table I). Superior semicircular canal, followed by vestibule, are the two most common anatomic sites reported in the literature for electrode array misplacement.
Superior semicircular canal
There are six published cases of cochlear implant electrode array into the superior semicircular canal with normal anatomy. Intraoperative imaging study demonstrated the electrode array entering the vestibule and then taking a superior course into the superior semicircular canal. In one case, there was concern about the direction and angle of electrode array during insertion. In two other cases, cochleostomy position may have lead to malpositioning of the electrode array in the superior semicircular canal. The authors reported that possible contributing factors were the improper inclination of the patient’s head on the operative table with respect to the surgeon, allowing for adequate visualization of the round window membrane, and the small dimension for the cochleostomy, which did not allow adequate visualization of the direction of the electrode array into the labyrinth.
In all cases there was either absent or abnormal intraoperative neural response telemetry that lead to intraoperative imaging. In all revision cases, the cochleostomy was widened antero-inferiorly to allow correct insertion of the electrode array.13,17,18
Horizontal semicircular canal
The study identified only one case of misplaced cochlear electrode into the horizontal semicircular canal.2 A mastoid-saving surgical approach was used such that a very small suprameatal canal was drilled out and a tight-fitting cochleostomy (< 1.2 mm) was created. The insertion of the electrode was hampered because of the narrow anatomical situation in the middle ear. Nonetheless, intraoperative measurements showed impedance, and NRT measurements were indicative of appropriate position and function of the implant. Postoperative imaging demonstrated the tip of electrode to be situated in the horizontal semicircular canal after traversing through the vestibule. In the reimplantation surgery, the cochleostomy was enlarged and reshaped.
Vestibule
In one case, a cochlear implant device was inserted fully via the standard mastoidectomy approach. The immediate intraoperative implant-evoked ABR indicated that only electrodes 14–22 elicited evoked responses, whereas NRT indicated satisfactory full insertion of the array. During initial mapping, the patient experienced severe vertigo and there were inconsistent responses to all electrical stimulation. Postoperative CT scan showed the middle electrodes in the vestibule. The authors proposed a possible mechanism for misplaced electrode was damage of the basilar membrane during cochleostomy, allowing part of the array to herniate through the weakened site into the vestibule.19
Another case report highlighting the interesting management of cochlear implant electrode array misplacement into the vestibule involves a retro-facial approach similar to that described by Beltrame et al.,20 which created an awkward insertion angle for the electrode array.21 During reimplantation surgery via the same retro-facial approach, cerebrospinal fluid leakage was observed. The authors proposed that reshaping of the original cochleostomy may have violated the lateral aspect of internal auditory canal. Furthermore, several attempts to insert an electrode array into the cochlea invariably entered the internal auditory canal. A canal wall down mastoidectomy was performed in order to gain better visualization of the round window for a second cochleostomy, and for full insertion of the electrode array. The mastoid and tympanic cavities were obliterated with abdominal fat grafts in order to control CSF leakage.
Eustachian tube
Of 76 patients successfully implanted at the Cochlear Implant Programme in Manchester Royal Infirmary since its inception in 1988, there were three patients who underwent revision surgery reported in a 1995 article from that institution.22 In one case, the postoperative radiograph showed the cochlear implant to be incorrectly positioned, running across the promontory toward the eustachian tube orifice. This was reimplanted with satisfactory outcome.
Carotid canal
The apparent lack of adherence of the carotid artery to its canal laterally may explain why a potential space could be created by an electrode. There are three published cases of cochlear misplacement into the carotid canal. Two cases involved pediatric patients, and one case in an adult patient. In all three cases, reimplantation was performed without any further complication.
The first description of a misplaced cochlear implant in the carotid canal was reported in 2002 in a 64- year-old-male.23 No complications were noted intraoperatively, and normal impedance values were noted for all electrodes when stimulated with the exception of electrodes 12 and 18. Neither neural response telemetry nor postoperative radiograph was obtained. When no response was identified during cochlear implant stimulation, it prompted an imaging study that confirmed the electrode was within the carotid canal along the inferior aspect of the basal turn of the cochlea.
During revision surgery, the original cochleostomy was noted to be inferior to the round window, and a new cochleostomy was made anterior to the round window and superior to the first cochleostomy. The electrode was removed without incident and a new electrode array was fully inserted. Intraoperative imaging, impedance, and NRT measurements were all normal.
The first pediatric case involved a 34-month-old boy after right-ear cochlear implantation.24 No intraoperative neural response telemetry was performed. The patient developed ipsilateral facial weakness, which prompted a CT scan showing that the electrode was inserted into the petrous carotid canal. Findings during revision surgery suggested an inadequate facial recess opening, which may have misled the surgeon to mistake the hypotympanic air cell for the round window niche. The electrode was removed without any arterial injury or bleeding. A new cochleostomy was made anterior and slightly inferior to the round window, and superior to the previous opening. The authors emphasized widening of the facial recess to allow an adequate visual field of the round window niche for proper cochleostomy placement.
The other pediatric case report occurred in a 10-month-old boy with normal inner ear morphology.25 Intraoperatively, there was polypoid mucosa in the middle ear, making it challenging to identify anatomical landmarks. The round window was partially visible. No perilymph egressed after cochleostomy was performed, and insertion of the electrode was straightforward. However, no intraoperative neural telemetry responses were obtained.
Postoperative CT showed the electrode inside the carotid canal outside the vessel lumen, and placed below the basal turn of the cochlea. In revision surgery, a second cochleostomy was performed posterior and superior to the first one. The electrode was removed without bleeding, and the site was obliterated with a muscle graft. The new electrode was correctly inserted into the second cochleostomy without difficulty.
These three published cases, plus the one in the current study, highlight the importance of assessing on preoperative CT scan the close anatomic relationship between the cochlea and carotid. Since the internal carotid artery lacks surrounding soft tissue when passing in the carotid canal, the risk of injury with electrode insertion is theoretically high. The bony separation between the two structures has been reported to be 0.2 to 6.2 mm thick.26,27 The proximity between cochlea and carotid canal has been shown to be as small as 1.05 mm, and mostly in relation to basal turn.28 Another study based on the analysis of temporal bone CT scans established mean measurements of the cochlear-carotid interval at 1.2 mm.29 These dimensions were not influenced by age. These dimensions are even more critical in drill-out procedures in cases of labyrinthitis ossificans.
Whenever there is potential injury to the carotid artery by a misplaced electrode array, proper preparation must be made in case of potential hemorrhage. This should include possible endovascular management, a head and neck vascular surgeon on standby for transcervical control of the carotid artery, and blood product availability. Fortunately, no serious complications associated with CI revision involving carotid artery have been published.
Internal Auditory Canal
Interestingly, there are no published reports of electrode array misplacement into the internal auditory canal, as illustrated in case 3. Single case report by Muzzi et al.21 described revision cochleostomy with inadvertent opening of the lateral internal auditory canal. Cerebrospinal fluid leakage was noted, and repetitive electrode array insertion into the internal auditory canal occurred before conversion to a canal wall down procedure with mastoid obliteration. This is different from our case report 3, where the cochleostomy was planned too far superiorly. Preoperative imaging studies and intraoperative finding did not reveal any anatomic bony defect between the lateral end of the internal auditory canal and the basal turn of the cochlea, such as that described in a particular form of X-linked deafness that would allow the introduction of electrode array into the internal canal at the time of implantation from an obvious communication between the two structures.
Though not reported in the literature, different management options are available for the misplaced electrode array in the internal auditory canal. In the single case (#3) in this study, prompted by parental wishes, a modified translabyrinthine approach was used to allow direct visualization of the entire array during removal. With modiolar-hugging electrode designs, there is a theoretical risk of neural or vascular injury, and exposure of the contents of the IAC is useful. While the revision surgery was uneventful, it remained unclear why the patient did not receive any auditory benefit. It is possible there could have been traumatic injury to the cochlear nerve or its vascular supply at the initial electrode insertion that cannot be detected on CT imaging or during intraoperative inspection. Other management options include the careful removal of the misplaced array by simple traction, without visualizing the contents of the internal auditory canal. Intraoperative fluoroscopy may be useful in this setting. A third option for management might be amputation of the array, and to allow it to remain within the IAC, but untoward or delayed effects on neural structures or a properly placed cochlear implant electrode are unknown.
Important Considerations
Surgical approach to maintain the correct axis in creating the cochleostomy is important. Some authors have argued that the most important landmark is the stapes tendon, which makes it possible to assess the correct axis and location for the cochleostomy. Drilling for the cochleostomy must be done in a vertical line to avoid the natural tendency to shift anteriorly toward the carotid canal.25 The presence of soft tissue in the lumen of the cochleostomy should alert the surgeon to a possible misplaced cochleostomy.
Intraoperative Monitoring
Electrophysiological and radiological intraoperative monitoring tests during cochlear implant surgery play different roles. Measurement of electrode impedance and electrically evoked compound action potential can evaluate the integrity of implant electrodes and the status of the interface between the electrode and neural responsiveness of the auditory nerve. Radiological imaging is useful to evaluate correct positioning of the array, depth of insertion, and proximity to modiolus, and the presence of bending or kinking of the array. Although electrical stimulation and the response of all stimulated channels can be useful for the surgeon to indirectly determine electrode positioning, they cannot actually confirm correct positioning of the array.
Intraoperative neural response telemetry recordings may suggest cochlear implant failure or nonfunction. The action potentials recorded on NRT are usually generated by the cochlear nerve. However, neural response telemetry results cannot determine whether the electrode array is placed within the cochlea rather than in the vestibule, because cochlear and vestibular action potentials may be similar.19 In the described case of electrode insertion into the vestibule, the NRT recordings obtained were theoretically arising from the vestibular nerve, as the amplitudes of the vestibular action potentials were larger than those of the cochlear action potentials. Therefore, the authors conclude that it is not currently possible to use NRT to confirm that the array is within the cochlea and has not migrated into the vestibule.
In another two cases presented by Viccaro et al.,17 where the electrode array was misplaced into the superior semicircular canal, stimulated channels gave a response with normal morphology and a growing amplitude at increasing stimulation intensity. It was hypothesized that the near-normal neural responses obtained were probably due to a spread of the current secondary to the high stimulation intensity, similar to the response observed when the electrode array is placed in the cochlea. In contrast, the absence of a detectable intraoperative neural response telemetry threshold has been observed in some patients even with a functional device in the correct location.30 Therefore, it does not necessarily indicate device failure or correlate to postoperative performance.
If the surgeon suspects inappropriate positioning of the electrode array, intraoperative plain film, or fluoroscopic imaging should be used to clarify placement. One study showed that even when the surgeon thought the procedure went without difficulty, there was a 7% chance of an abnormal postoperative radiograph.31 The experience of hypotympanic placement of electrode array has prompted some authors to use intraoperative x-rays to ensure proper positioning of the implant.32 Although the operating room time is extended slightly, the opportunity to correct this condition is immediate, thereby avoiding the need for revision surgery.
However, there is still debate about the routine use of intraoperative imaging. In Copeland and colleagues’ prospective analysis, intraoperative plain radiographs appeared to be of negligible value in assessing correct electrode array placement. The authors reported that intraoperative plain radiographs changed intraoperative management in only one out of 79 cases, despite multiple x-ray examinations in 23% of cases.33 In contrast, at NYU’s tertiary cochlear implant center, using three routine modalities of intraoperative testing (individual electrode impedance measurements, neural response telemetry levels for selected four electrodes, and plain film radiograph assessment of electrode position), only the radiographic results impacted intraoperative surgical decision making and led to the use of the backup device.34
Tip Rollover
In the recent paper by Cosetti et al.,34 describing the algorithm for intraoperative monitoring during cochlear implantation, the authors routinely used Stenver’s view plain film radiography to assess electrode position. In the series of 277 cochlear implantation surgeries, there were five patients with malposition of electrodes discovered on intraoperative radiograph. Four electrodes had tip rollovers and one electrode was misplaced into the superior semicircular canal. While the authors classified both as electrode array malpositioning, there is a distinction between electrode insertion into the cochlea with tip rollover versus extracochlear electrode placement. It has been suggested that tip rollover can be detected by intraoperative spread of excitation measurements as it provides information regarding the selectivity of neural excitation fields around each electrode. 35 At this time, there are very few data on the effects of tip rollover on clinical performance. As more studies are performed, electrode array malpositioning can be further classified by insertion into the cochlear versus extracochlear insertion since clinical functional outcome would be expected to be quite different.
Limitations of this study include its retrospective nature; small case number; and in two cases the initial surgery was done elsewhere, making intraoperative details unavailable for review. There are also few large cochlear implant case series that describe electrode misplacement, and all data are retrospective. Additional studies will be needed to answer the difference between extracochlear malpositioning as presented in our cases and intracochlear malposition (i.e., tip rollover), which have often being combined in previous case series, and its unique impact on auditory rehabilitation and speech development. Future studies may also incorporate the use of newer technologies, including “real-time” impedance and NRT measurement as the electrode array is actually being inserted into the cochlea; tactile feedback sensors on the electrode array to guide optimal positioning; and intraoperative CT imaging, which provides immediate feedback and better anatomic correlation than plain x-ray. The goal of each is to optimize electrode array positioning in the cochlea and clinical outcome.
CONCLUSION
In situations of poor cochlear implant performance, the evaluation should include CT imaging, device integrity testing, audiometric, and possible cognitive evaluation. Electrode array misplacement should be included in the differential diagnosis in cases where patients present in delayed fashion, even several years after implantation with little or no auditory benefit, and should be evaluated both with device integrity testing and CT imaging. Extracochlear electrode array misplacement is an infrequent complication with a published incidence rate between 0.2% and 5.8%, and an average of 0.37% in the literature. However, this range is likely underreported and the true incidence may remain unknown until there is a central database for universal recording of such events. Intraoperative electrophysiologic testing (NRT), and radiographic examination of the electrode array position can also be helpful, but not necessarily definitive. Explantation and reimplantation is a successful management option in most reported cases of misplacement in the literature, and in the current study. However, there is controversy and lack of consensus surrounding management of electrodes misplaced into the internal auditory canal. Modiolar-hugging electrodes should be removed under direct visualization to avoid neurovascular injury, but other management options may be considered.
Footnotes
Presentated at the Triological Society Annual COSM meeting, San Diego, U.S.A., April 20, 2012.
Level of Evidence: 4.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Webb RL, Lehnhardt E, Clark GM, Laszig R, Pyman BC, Franz BK-HG. Surgical complications with the cochlear multiple-channel intracochlear implant: experience at Hannover and Melbourne. Ann Otol Rhinol Laryngol. 1991;100:131–6. doi: 10.1177/000348949110000208. [DOI] [PubMed] [Google Scholar]
- 2.Tange RA, Grolman W, Maat A. Intracochlear misdirected implantation of a cochlear implant. Acta Otolaryngologica. 2006;126:650–652. doi: 10.1080/00016480500445206. [DOI] [PubMed] [Google Scholar]
- 3.Roland JT. Complications of cochlear implant surgery. In: Waltzman SB, Cohen N, editors. Cochlear Implants. New York: Thieme; 2000. pp. 170–7. [Google Scholar]
- 4.Lassig AA, Zwolan TA, Telian SA. Cochlear implant failures and revision. Otol Neurotol. 2005;26:624–34. doi: 10.1097/01.mao.0000178123.35988.96. [DOI] [PubMed] [Google Scholar]
- 5.Marlowe Al, Chinnici JE, Rivas A, et al. Revision cochlear implant surgery in children: The Johns Hopkins experience. Otol Neurotol. 2010;31:74–82. doi: 10.1097/MAO.0b013e3181c29fad. [DOI] [PubMed] [Google Scholar]
- 6.Tambyraja R, Gutman MA, Megrian CA. Cochlear implant complications. Arch Oto-Head Neck Surg. 2005;131:245–250. doi: 10.1001/archotol.131.3.245. [DOI] [PubMed] [Google Scholar]
- 7.Sorrentino T, Cote M, Eter E, Laborde ML, Cochard N, Olivier D, Fraysse B. Cochlear reimplantations: technical and surgical failures. Acta Oto--Laryngologica. 2009;129:380–384. doi: 10.1080/00016480802552576. [DOI] [PubMed] [Google Scholar]
- 8.Mouzali A, Ouennoughi K, Haraoubia MS, Zemirli O, Triglia JM. Cochlear implant electrode array misplaced in Hyrtl’s fissure. Int J Pediatr Otorhinolaryngol. 2011;74:1459–1462. doi: 10.1016/j.ijporl.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Rich PM, Graham J, Phelps PD. Hyrtl’s fissure. Otol Neuroto. 2002;23:476–482. doi: 10.1097/00129492-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Rottevee LJ, Proops DW, Ramsden RT, Saeed SR, Van Olphen AF, Mylanus EA. Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25:943–952. doi: 10.1097/00129492-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Woolford TJ, Saeed SR, Boyd P, Hartley C, Ramsden RT. Cochlear reimplantation. Ann Otol Rhinol Laryngol Suppl. 1995;166:449–53. [PubMed] [Google Scholar]
- 12.Jain R, Mukherji SK. Cochlear implant failure: imaging evaluation of the electrode course. Clin Radiol. 2003;58:288–93. doi: 10.1016/s0009-9260(02)00523-8. [DOI] [PubMed] [Google Scholar]
- 13.Mecca AM, Wagle W, Lupinetti A, Parnes S. Complication of cochlear implantation surgery. AJNR Am J Neuroradiol. 2003;24:2089–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Sorrentino T, Cote M, Eter E, et al. Cochlear reimplantations: technical and surgical failures. Acta Otolaryngol. 2009;129:380–4. doi: 10.1080/00016480802552576. [DOI] [PubMed] [Google Scholar]
- 15.Webb RL, Lehnhardt E, Clark GM, Laszig R, Pyman BC, Franz BK-HG. Surgical complications with the cochlear multiple-channel intracochlear implant: experience at Hannover and Melbourne. Ann Otol Rhinol Laryngol. 1991;100:131–6. doi: 10.1177/000348949110000208. [DOI] [PubMed] [Google Scholar]
- 16.Cohen NL, Hoffman RA, Stroschein M. Medical or surgical complications related to the nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol. 1988;97:8–13. doi: 10.1177/00034894880975s202. [DOI] [PubMed] [Google Scholar]
- 17.Viccaro M, Covelli E, De Seta E, Balsamo G, Filipo R. The importance of intra-operative imaging during cochlear implant surgery. Cochlear Implants Int. 2009;10:198–202. doi: 10.1179/cim.2009.10.4.198. [DOI] [PubMed] [Google Scholar]
- 18.Ramalingam R, Ramalingam KK, Padmaja HS. An unusual occurrence in cochlear implantation surgery: misplaced electrode. J Laryngol Otol. 2009;123:e4. doi: 10.1017/S0022215108004064. [DOI] [PubMed] [Google Scholar]
- 19.Pau H, Parker A, Sanli H, Gibson WPR. Displacement of electrodes of a cochlear implant into vestibular system: intra-and postoperative electrophysiological analyses. Acta Oto-Laryngologica. 2005;125:1116–1118. doi: 10.1080/00016480510038554. [DOI] [PubMed] [Google Scholar]
- 20.Beltrame MA, Frau GN, Shanks M, Robinson P, Anderson I. Double posterior labyrinthotomy technique: results in three Med-El patients with common cavity. Otol Neurotol. 2005;26:177–82. doi: 10.1097/00129492-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Muzzi E, Boscolo-Rizzo P, Santarelli R, Beltrame MA. J Laryngol Otol. 2012;126:414–417. doi: 10.1017/S0022215112000059. [DOI] [PubMed] [Google Scholar]
- 22.Woolford TJ, Saeed SR, Boyd P, Hartley C, Ramsden RT. Cochlear reimplantation. Ann Otol Rhinol Laryngol Suppl. 1995;166:449–53. [PubMed] [Google Scholar]
- 23.Gastman BR, Hirsch BE, Sando I, Fukui MB, Wargo ML. The potential risk of carotid injury in cochlear implant surgery. Laryngoscope. 2002;112:262–266. doi: 10.1097/00005537-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Son EJ, Kim SC, Choi JY. Cochlear implant electrode misplaced in the carotid canal. Arch Otolaryngol Head Neck Surg. 2007;133:827–829. doi: 10.1001/archotol.133.8.827. [DOI] [PubMed] [Google Scholar]
- 25.Nevoux J, Loundon N, Leboulanger N, Roger G, Ducou Le Pointe H, Garabedian EN. Cochlear implant in the carotid canal. Case report and literature review. Int J of Pediatric Otorhinolaryngol. 2010;74:701–703. doi: 10.1016/j.ijporl.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Muren C, Wadin K, Wilbrand HF. The cochlea and the carotid canal. Acta Radiol. 1990;31:33–35. [PubMed] [Google Scholar]
- 27.Wysocki J, Skarzynski H. Distances between the cochlea and adjacent structures related to cochlear implant surgery. Surg Radio Anat. 1998;20:267–271. doi: 10.1007/BF01628488. [DOI] [PubMed] [Google Scholar]
- 28.de Penido NO, Borin A, Fukuda Y, Lion CN. Microscopic anatomy of the carotid canal and its relations with cochlea and middle ear. Braz J Otorhinolaryngol. 2005;71:410–414. doi: 10.1016/S1808-8694(15)31191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RJ, Shatzkes DR, Babb JS, Lalwani AK. The cochlear-carotid interval: anatomic variation and potential clinical implications. AJNR Am J Neuroradiol. 2006;27:1486–1490. [PMC free article] [PubMed] [Google Scholar]
- 30.Cosetti MK, Shapiro WH, Green JE, et al. Intraoperative neural response telemetry as a predictor of performance. Otol Neurotol. 2010;31:1095–9. doi: 10.1097/MAO.0b013e3181ec1b8c. [DOI] [PubMed] [Google Scholar]
- 31.Proops DW, Stoddart RL, Donaldson I. Medical, surgical and audiological complications of the first 100 adult cochlear implant patients in Birmingham. J Laryngol Otol Suppl. 1999;24:14–17. doi: 10.1017/s002221510014602x. [DOI] [PubMed] [Google Scholar]
- 32.Windmill IM, Martinez SA, Nolph MB, Eisenmenger BA. Surgical and nonsurgical complications associated with cochlear prosthesis implantation. Am J of Otol. 1990;11:415–420. [PubMed] [Google Scholar]
- 33.Copeland BJ, Pillsbury HC, Buchman CA. Prospective evaluation of intraoperative cochlear implant radiographs. Otol Neurotol. 2004;25:295–7. doi: 10.1097/00129492-200405000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Cosetti MK, Troob SH, Latzman JM, Shapiro WH, Roland JT, Waltzman SB. An evidence-based algorithm for intraoperative monitoring during cochlear implantation. Otol Neurotol. 2012;33:169–176. doi: 10.1097/MAO.0b013e3182423175. [DOI] [PubMed] [Google Scholar]
- 35.Grolman W, Maat A, Verdam F, et al. Spread of excitation measurements for the detection of electrode array foldovers: a prospective study comparing 3-dimenstional rotational x-ray and intraoperative spread of excitation measurements. Otol Neurotol. 2009;30:27–33. doi: 10.1097/mao.0b013e31818f57ab. [DOI] [PubMed] [Google Scholar]


