Abstract
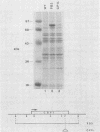
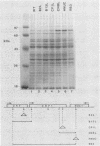
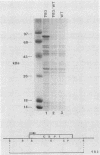
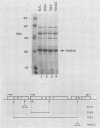
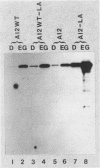
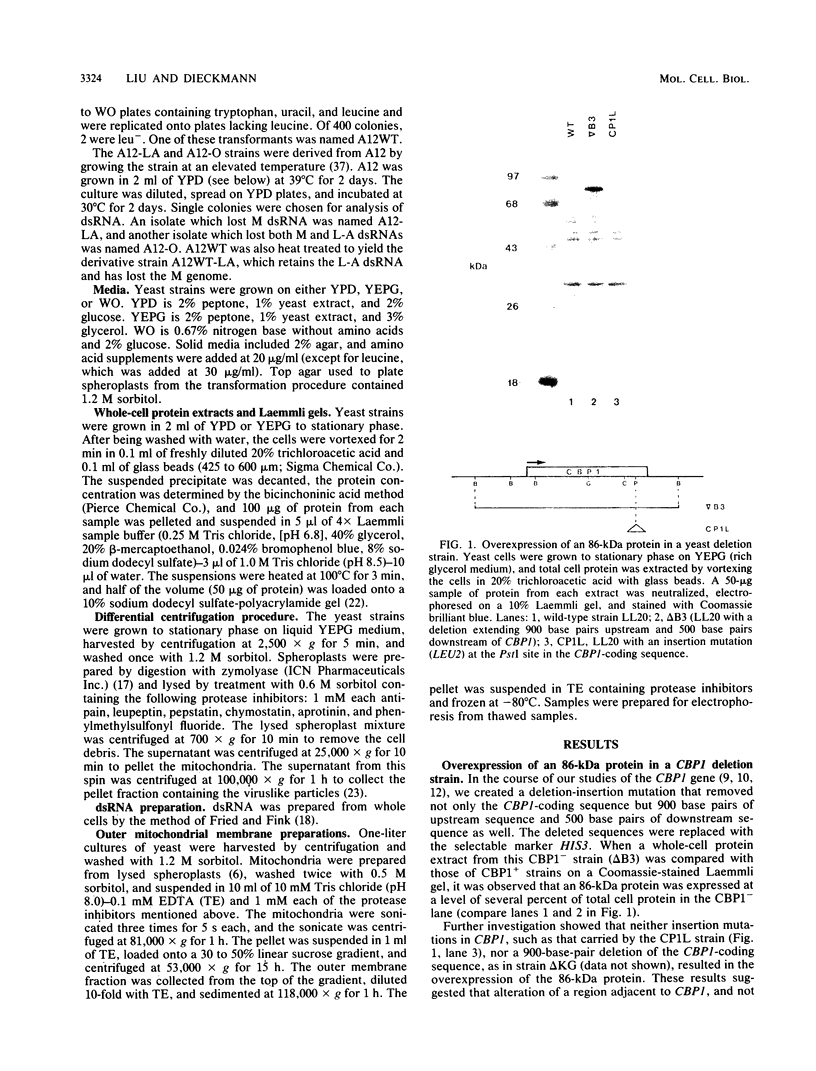
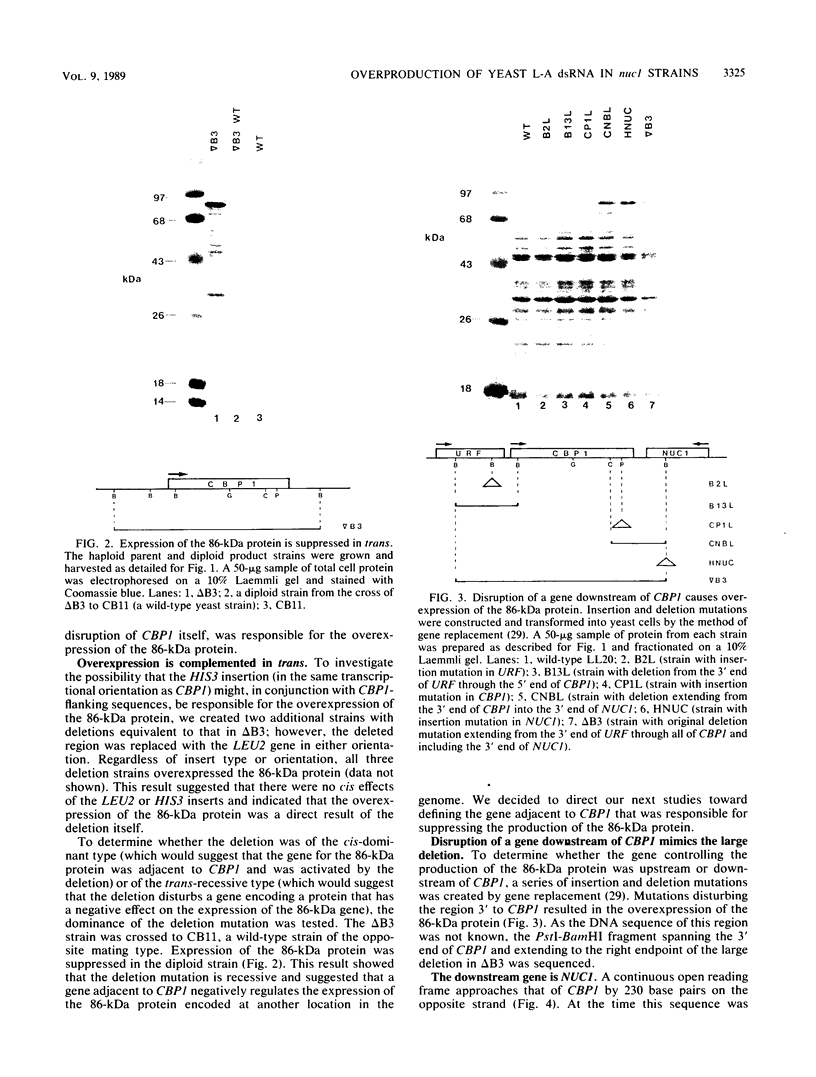
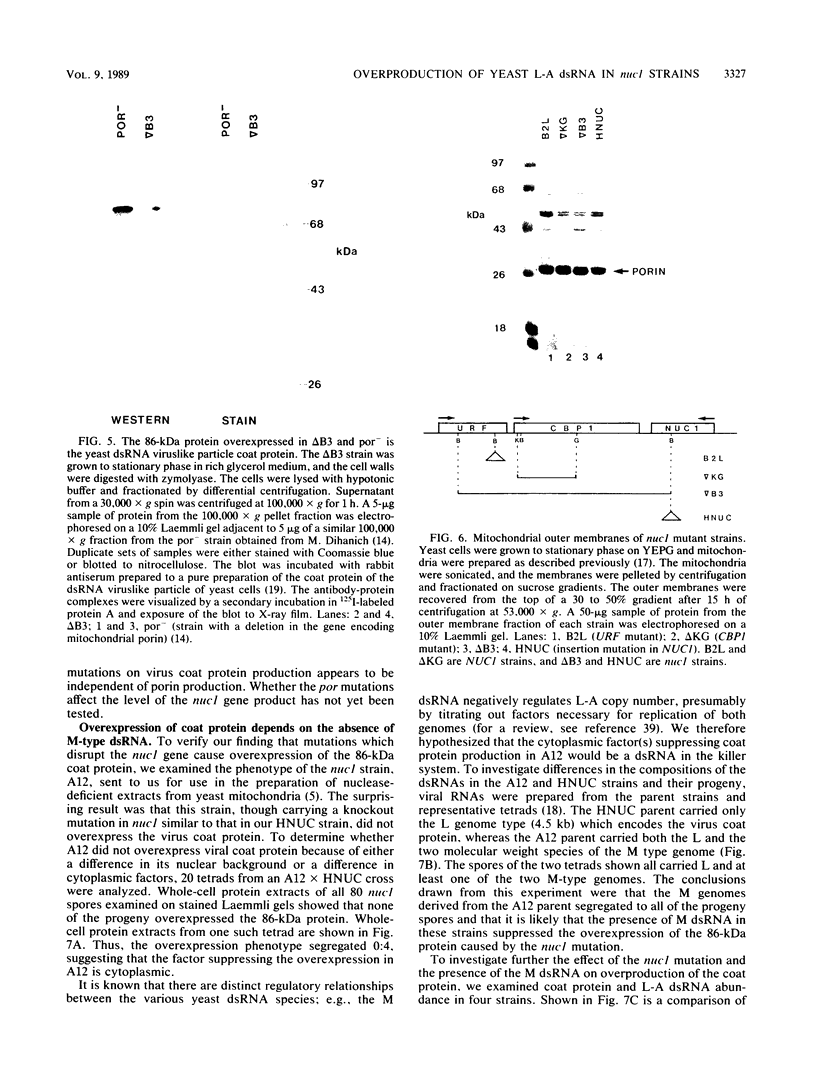
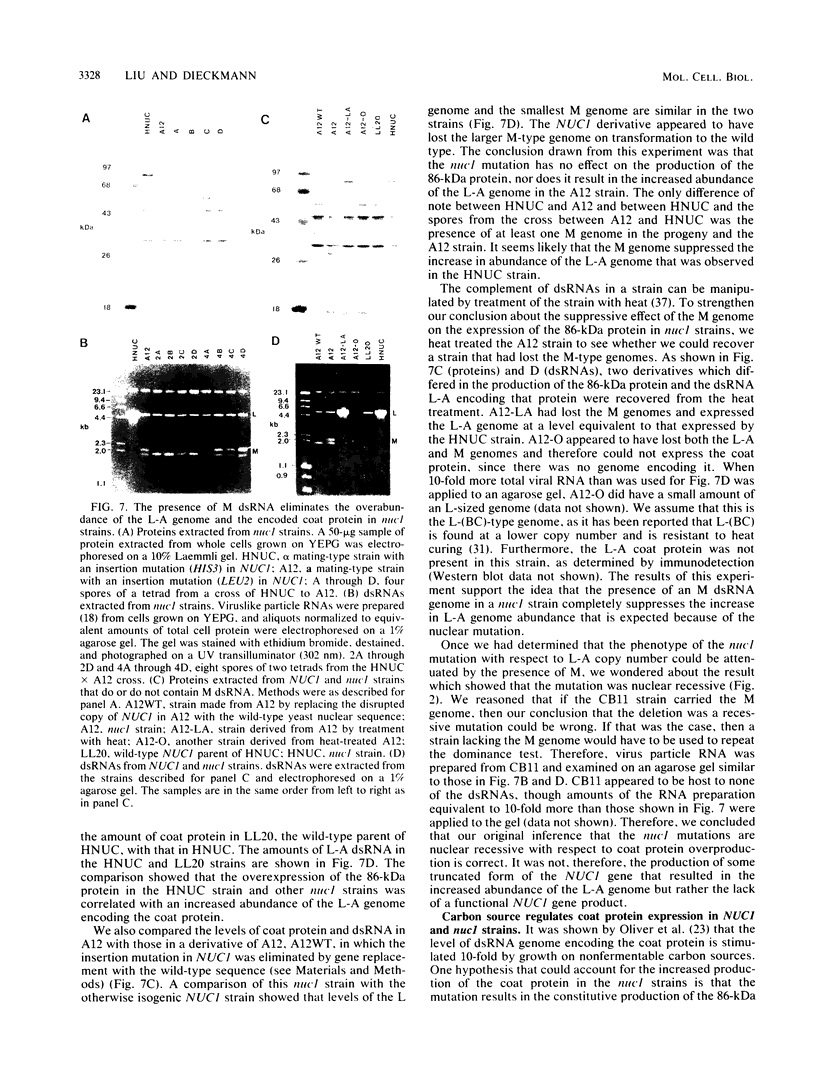
Saccharomyces cerevisiae strains are often host to several types of cytoplasmic double-stranded RNA (dsRNA) genomes, some of which are encapsidated by the L-A dsRNA product, an 86,000-dalton coat protein. Here we present the finding that nuclear recessive mutations in the NUC1 gene, which encodes the major nonspecific nuclease of yeast mitochondria, resulted in at least a 10-fold increase in amounts of the L-A dsRNA and its encoded coat protein. The effect of nuc1 mutations on L-A abundance was completely suppressed in strains that also hosted the killer-toxin-encoding M dsRNA. Both NUC1 and nuc1 strains containing the L-A genome exhibited an increase in coat protein abundance and a concomitant increase in L-A dsRNA when the cells were grown on a nonfermentable carbon source rather than on glucose, an effect independent of the increase in coat protein due to nuc1 mutations or to the absence of M. The increase in L-A expression in nuc1 strains was similar to that observed in strains with mutations in the nuclear gene encoding the most abundant outer mitochondrial membrane protein, porin. nuc1 mutations did not affect the level of porin in the mitochondrial outer membrane. Since the effect of mutations in nuc1 was to alter the copy number of the L-A coat protein genome rather than to change the level of the M toxin genome (as do mak and ski mutations), these mutations define a new class of nuclear genes affecting yeast dsRNA abundance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S. G., Tirtiaux C., Wickner R. B. Genetic Control of L-a and L-(Bc) Dsrna Copy Number in Killer Systems of SACCHAROMYCES CEREVISIAE. Genetics. 1984 Jun;107(2):199–217. doi: 10.1093/genetics/107.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Sequence of the preprotoxin dsRNA gene of type I killer yeast: multiple processing events produce a two-component toxin. Cell. 1984 Mar;36(3):741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Dake E., Hofmann T. J., McIntire S., Hudson A., Zassenhaus H. P. Purification and properties of the major nuclease from mitochondria of Saccharomyces cerevisiae. J Biol Chem. 1988 Jun 5;263(16):7691–7702. [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Deutsch J., Dujon B., Netter P., Petrochilo E., Slonimski P. P., Bolotin-Fukuhara M., Coen D. Mitochondrial genetics. VI. The petite mutation in Saccharomyces cerevisiae: interrelations between the loss of the p+ factor and the loss of the drug resistance mitochondrial genetic markers. Genetics. 1974 Feb;76(2):195–219. doi: 10.1093/genetics/76.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Gandy B. Preferential recombination between GC clusters in yeast mitochondrial DNA. EMBO J. 1987 Dec 20;6(13):4197–4203. doi: 10.1002/j.1460-2075.1987.tb02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Homison G., Tzagoloff A. Assembly of the mitochondrial membrane system. Nucleotide sequence of a yeast nuclear gene (CBP1) involved in 5' end processing of cytochrome b pre-mRNA. J Biol Chem. 1984 Apr 25;259(8):4732–4738. [PubMed] [Google Scholar]
- Dieckmann C. L., Koerner T. J., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP1, a yeast nuclear gene involved in 5' end processing of cytochrome b pre-mRNA. J Biol Chem. 1984 Apr 25;259(8):4722–4731. [PubMed] [Google Scholar]
- Dieckmann C. L., Mittelmeier T. M. Nuclearly-encoded CBP1 interacts with the 5' end of mitochondrial cytochrome b pre-mRNA. Curr Genet. 1987;12(6):391–397. doi: 10.1007/BF00434815. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Pape L. K., Tzagoloff A. Identification and cloning of a yeast nuclear gene (CBP1) involved in expression of mitochondrial cytochrome b. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1805–1809. doi: 10.1073/pnas.79.6.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Dihanich M., Suda K., Schatz G. A yeast mutant lacking mitochondrial porin is respiratory-deficient, but can recover respiration with simultaneous accumulation of an 86-kd extramitochondrial protein. EMBO J. 1987 Mar;6(3):723–728. doi: 10.1002/j.1460-2075.1987.tb04813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich M., van Tuinen E., Lambris J. D., Marshallsay B. Accumulation of viruslike particles in a yeast mutant lacking a mitochondrial pore protein. Mol Cell Biol. 1989 Mar;9(3):1100–1108. doi: 10.1128/mcb.9.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Site-specific binding of viral plus single-stranded RNA to replicase-containing open virus-like particles of yeast. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4411–4415. doi: 10.1073/pnas.85.12.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye G., Kujawa C., Fukuhara H. Physical and genetic organization of petite and grande yeast mitochondrial DNA. IV. In vivo transcription products of mitochondrial DNA and localization of 23 S ribosomal RNA in petite mutants of saccharomyces cerevisiae. J Mol Biol. 1974 Sep 5;88(1):185–203. doi: 10.1016/0022-2836(74)90304-0. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Gene overlap results in a viral protein having an RNA binding domain and a major coat protein domain. Cell. 1988 Nov 18;55(4):663–671. doi: 10.1016/0092-8674(88)90225-5. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Douglas M. G. Selection and characterization of nuclear genes coding mitochondrial proteins: genetic complementation of yeast pet mutants. Methods Enzymol. 1983;97:344–355. doi: 10.1016/0076-6879(83)97147-1. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Gould J., Kim S., Kane M. Y., Hereford L. Identification of sequences in a yeast histone promoter involved in periodic transcription. Cell. 1986 May 23;45(4):537–544. doi: 10.1016/0092-8674(86)90285-0. [DOI] [PubMed] [Google Scholar]
- Ridley S. P., Sommer S. S., Wickner R. B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984 Apr;4(4):761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hay R., Gasser S., Daum G., Schneider G., Witte C., Schatz G. The outer membrane of yeast mitochondria: isolation of outside-out sealed vesicles. EMBO J. 1983;2(7):1105–1111. doi: 10.1002/j.1460-2075.1983.tb01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R. D., Hofmann T. J., Zassenhaus H. P. Sequence and expression of NUC1, the gene encoding the mitochondrial nuclease in Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Apr 25;16(8):3297–3312. doi: 10.1093/nar/16.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Wickner R. B. Two new double-stranded RNA molecules showing non-mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):181–187. doi: 10.1128/mcb.4.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. "Killer character" of Saccharomyces cerevisiae: curing by growth at elevated temperature. J Bacteriol. 1974 Mar;117(3):1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Deletion of mitochondrial DNA bypassing a chromosomal gene needed for maintenance of the killer plasmid of yeast. Genetics. 1977 Nov;87(3):441–452. doi: 10.1093/genetics/87.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA replication in yeast: the killer system. Annu Rev Biochem. 1986;55:373–395. doi: 10.1146/annurev.bi.55.070186.002105. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Chromosomal genes essential for replication of a double-stranded RNA plasmid of Saccharomyces cerevisiae: the killer character of yeast. J Mol Biol. 1976 Aug 15;105(3):427–443. doi: 10.1016/0022-2836(76)90102-9. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Hofmann T. J., Uthayashanker R., Vincent R. D., Zona M. Construction of a yeast mutant lacking the mitochondrial nuclease. Nucleic Acids Res. 1988 Apr 25;16(8):3283–3296. doi: 10.1093/nar/16.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge A. M., Zoutewelle G., Needleman R. B. Regulation of maltose fermentation in Saccharomyces carlsbergensis. 3. Constitutive mutations at the MAL6-locus and suppressors changing a constitutive phenotype into a maltose negative phenotype. Mol Gen Genet. 1974;131(2):113–121. doi: 10.1007/BF00266147. [DOI] [PubMed] [Google Scholar]