Abstract
Objectives
Blood culture contamination is a common problem in the emergency department (ED) that leads to unnecessary patient morbidity and health care costs. The study objective was to develop and evaluate the effectiveness of a quality improvement (QI) intervention for reducing blood culture contamination in an ED.
Methods
The authors developed a QI intervention to reduce blood culture contamination in the ED and then evaluated its effectiveness in a prospective interrupted times series study. The QI intervention involved changing the technique of blood culture specimen collection from the traditional clean procedure, to a new sterile procedure, with standardized use of sterile gloves and a new materials kit containing a 2% chlorhexidine skin antisepsis device, a sterile fenestrated drape, a sterile needle, and a procedural checklist. The intervention was implemented in a university-affiliated ED and its effect on blood culture contamination evaluated by comparing the biweekly percentages of blood cultures contaminated during a 48-week baseline period (clean technique), and 48-week intervention period (sterile technique), using segmented regression analysis with adjustment for secular trends and first-order autocorrelation. The goal was to achieve and maintain a contamination rate below 3%.
Results
During the baseline period, 321 out of 7,389 (4.3%) cultures were contaminated, compared to 111 of 6,590 (1.7%) during the intervention period (p < 0.001). In the segmented regression model, the intervention was associated with an immediate 2.9% (95% CI = 2.2% to 3.2%) absolute reduction in contamination. The contamination rate was maintained below 3% during each biweekly interval throughout the intervention period.
Conclusions
A QI assessment of ED blood culture contamination led to development of a targeted intervention to convert the process of blood culture collection from a clean to a fully sterile procedure. Implementation of this intervention led to an immediate and sustained reduction of contamination in an ED with a high baseline contamination rate.
INTRODUCTION
The blood culture is an essential tool for diagnosing bloodstream infections and guiding antibiotic therapy.1 However, false positive blood cultures due to specimen contamination with skin bacteria are a common problem that leads to unnecessary patient morbidity, increased hospital costs, and health care system inefficiencies.2–8 Patients who have contaminated cultures undergo unneeded emergency department (ED) visits, hospitalizations, antibiotic treatments, laboratory tests, and invasive procedures.2–8 On average, each contaminated culture results in several thousands of dollars in extra hospital charges that would have been avoided if cultures had not been contaminated.2–4,6 The Clinical and Laboratory Standards Institute recommends each health care institution maintain a contamination rate less than 3%.9
Emergency departments are systems particularly susceptible to a high burden of contaminated blood cultures due to high staff turnover, the need to collect cultures in critically ill patients prior to resuscitation, and the time pressure of obtaining cultures before the first dose of antibiotics.10–13
Historically, blood culture contamination was a significant problem in our university-affiliated, tertiary-care adult hospital, with contamination rates consistently higher than the 3% benchmark. Therefore, in August 2009, we developed an interdisciplinary task force with the aim of reducing the blood culture contamination rate in our ED to less than 3%, and sustaining this low rate. The task force included an emergency physician (EP) champion, an ED nurse champion, the chief hospital epidemiologist, ED administrators, ED nursing leadership, microbiology laboratory personnel, and infection control personnel. The objectives of this study were to describe the quality improvement (QI) intervention developed by the task force and evaluate its effectiveness for reducing blood culture contamination in our ED.
METHODS
Study Design
This study included two components: 1) development and implementation of a QI intervention to reduce blood culture contamination in the ED, and 2) evaluation of the effectiveness of this intervention. The intervention was developed through QI methodologies, including process mapping, fishbone diagraming, and plan-do-study-act (PDSA) cycles.14,15 Intervention effectiveness was evaluated using an interrupted time series analysis comparing contamination rates prior to and after implementation of the intervention.16,17 The local institutional review board approved this study with waiver of informed consent. Research funding was provided by the Emergency Medicine Patient Safety Foundation, and the Veterans Affairs Quality Scholars Program; these funding sources had no role in conduct of the study or interpretation of its results.
Study Setting and Population
The study was conducted in a single ED at a university-affiliated, tertiary care adult hospital in the United States with approximately 55,000 patient visits per year.
Study Protocol
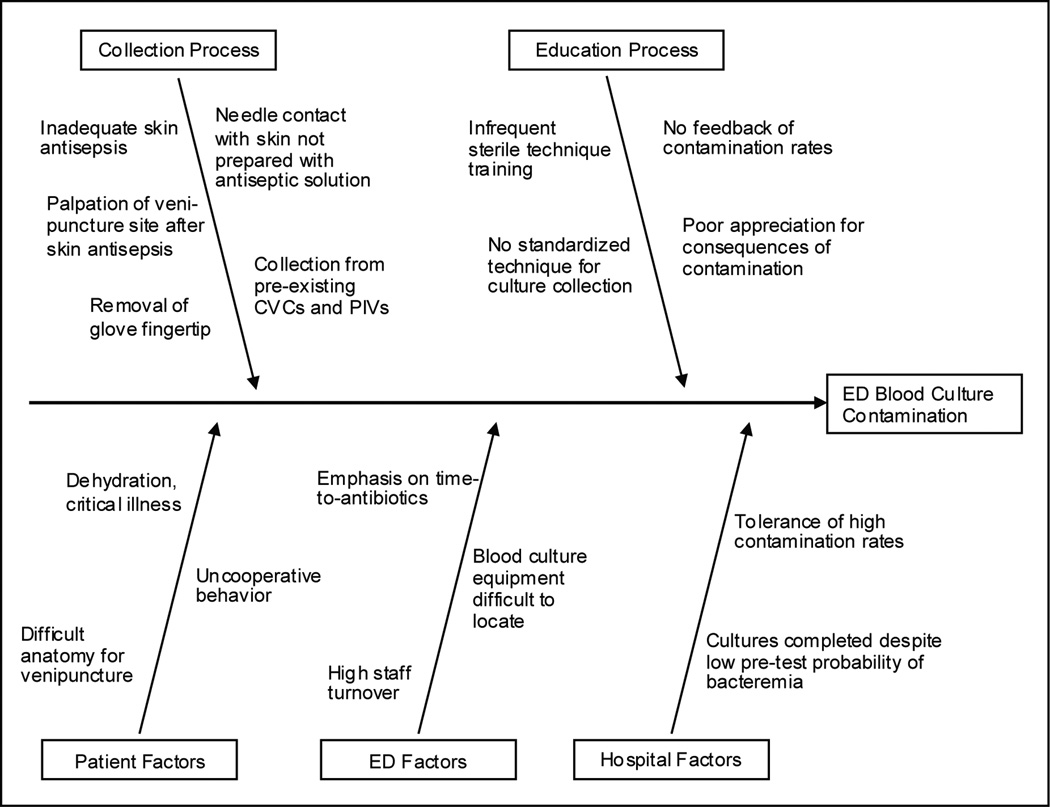
During August 2009 through December 2009, the task force studied the process of blood culture collection in our ED through structured interviews, surveys, and direct observation. We found that many factors contributed to the high contamination rate (Figure 1), and identified the technique used to collect blood from a patient’s vein as the key step to target for improvement efforts. All of the cultures were collected by ED nurses and paramedics. No standardized technique for blood culture collection had been instituted, and there was significant variation in practice. Many staff members stated that they collected cultures using a method their preceptors taught them during orientation in the first weeks after joining the ED staff. Fully sterile technique was not used by any of the nurses or paramedics when collecting cultures. Instead, the traditional clean technique was employed, which included use of nonsterile gloves, a 0.67 ml solution of 2% chlorhexidine gluconate/70% isopropyl alcohol for skin antisepsis, and no well-defined sterile fields. As outlined in our process map (Figure 2a), this clean technique frequently led to breaches in sterility with the potential to introduce contamination.
Figure 1.
Fishbone diagram outlining factors the task force identified as contributors to a high emergency department blood culture contamination rate.
CVC: central venous catheter; PIV: peripheral intravenous catheter.
Figure 2.
Process maps of blood culture collection methods during (A) the baseline period prior to implementation of the sterile blood culture intervention, and (B) the intervention period after implementing the sterile blood culture intervention. Blood culture collection technique was not standardized during the baseline period; the method mapped represents the most common technique used.
CVC: central venous catheter; PIV: peripheral intravenous catheter; g: gauge; ml: milliliter
Guided by these findings, we developed the sterile blood culture intervention to convert blood culture collection from a clean to a sterile procedure. The intervention consisted of three components: 1) the sterile blood culture kit—a new materials kit containing the equipment needed to collect a culture using strict sterile technique, 2) a checklist designed for use at the time of culture collection that outlined the optimal technique for using the sterile kits, and 3) a policy change to standardize blood culture collection technique to a sterile procedure with use of the sterile kits.
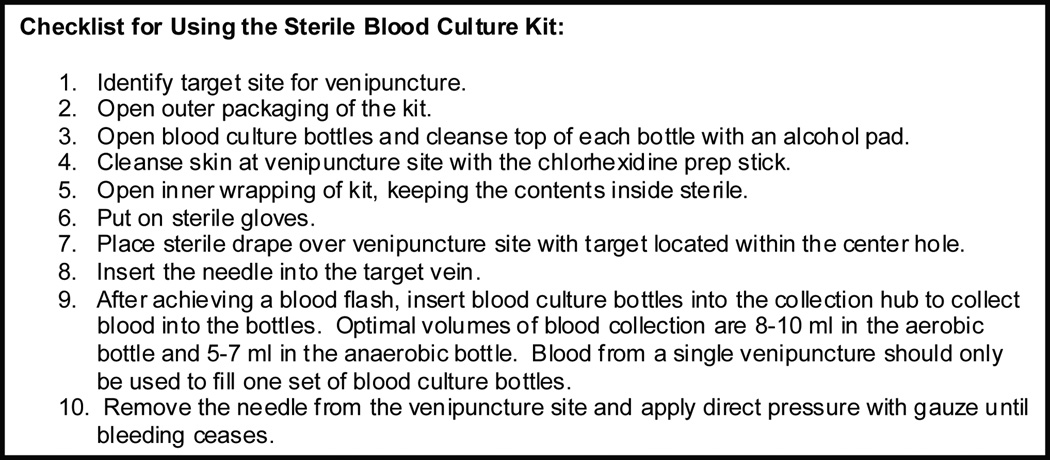
The sterile blood culture kit was modeled after a typical lumbar puncture kit and designed to promote sterility during culture collection by consolidating necessary materials into a single, easy-to-use packet. In an iterative process, task force members demonstrated kit prototypes to ED nurses and paramedics and modified its contents based on feedback. For example, the kit was designed to be used with sterile gloves, but sterile gloves were not included in the final kit because ED staff indicated they preferred to use individually sized gloves available at the bedside rather than “one-size-fits-all” gloves packaged inside the kit. In December 2009, further demonstrations of the modified kit did not result in significant suggestions for change and the contents were finalized: 3 ml 2% chlorhexidine gluconate/70% isopropyl alcohol (Chloraprep) skin antiseptic device (CareFusion; San Diego, CA), 18 inch × 26 inch fenestrated drape, 21 gauge “butterfly” needle with a closed tubing system, gauze, 10 ml syringe for initiating blood flow through the tubing when needed, and two 70% isopropyl alcohol pads for cleansing the tops of blood culture bottles (Kendall; Deerfield Beach, FL). We designed a standardized process to use the sterile blood culture kit to collect a blood sample (Figure 2b). Each kit contained a checklist outlining this process for optimal kit use (Figure 3). We introduced the kit to Medical Action Industries (Brentwood, NY) for packaging and distribution.
Figure 3.
Checklist outlining optimal technique for using the sterile blood culture kit to collect a blood culture specimen. Each kit contained a checklist.
Intervention Implementation
In December 2009 and January 2010, task force members, with the assistance of ED nursing educators, trained the ED staff how to use the sterile blood culture kit with hands-on educational sessions, which were standardized by using an internet training video and a scripted curriculum. Additionally, we presented data to the staff demonstrating the negative consequences of contaminated blood cultures and emphasized the departmental goal of maintaining a contamination rate less than 3%.
The ED adopted a policy stating that the standard first attempt for collecting blood cultures was by dedicated peripheral venipuncture using the kit. This policy was endorsed by departmental physician and nursing leadership. If a blood culture specimen could not be obtained by dedicated venipuncture using a kit, alternative methods for collection could be attempted based on the bedside nurse’s judgment.
On January 31, 2010, the intervention was introduced; kits were stocked in each ED treatment room, and the new blood culture collection policy was instituted. The four weeks following introduction of the intervention, January 31, 2010 to February 27, 2010, was considered an implementation phase for the sterile collection technique to be incorporated into daily practice. During this implementation phase, task force members regularly met with the ED staff at shift-change meetings to re-enforce sterile technique, elicit feedback on the program, and review contamination rates. On February 28, 2010, the intervention was considered fully implemented, and the sterile blood culture kits were stocked in ED treatment rooms as a component of routine ED re-supplying.
Intervention Effectiveness
A blood culture was classified as contaminated if one or more of the following organisms grew in only one culture of a series of blood cultures collected within 24 hours: Aerococcus species, α-hemolytic Streptococcus, Bacillus species except anthracis, coagulase negative Staphylococcus species except lugdunensis, Corynebacterium species, Micrococcus species, and Propionibacterium species.1,18 All positive cultures not classified as contaminated were considered true positives.
We measured the effectiveness of the sterile blood culture intervention to reduce contamination using an interrupted time series design16,17 and segmented linear regression analysis.19 This is a robust technique to evaluate the immediate and sustained effect of a change in health care delivery, such as a policy change or quality improvement intervention.19 The percentage of blood cultures contaminated during a 48-week baseline period prior to implementation (March 1, 2009 to January 30, 2010) was compared to a 48-week intervention period after implementation (February 28, 2010 to January 29, 2011). The four-week implementation phase (January 31, 2010 to February 27, 2010) was considered a transition period and not included in comparisons of the baseline and intervention periods. In a before-after comparison, the percentage of cultures contaminated during the baseline and intervention periods were compared using the chi-square test.
Next, we built a segmented linear regression model by dividing the study period into two-week intervals, with the baseline and intervention periods each containing 24 time points. The dependent variable for the model was the log-transformed percentage of cultures contaminated during biweekly intervals; these data were log-transformed to stabilize variances. Independent variables included terms for intervention implementation and secular trends during the baseline and intervention periods. Due to the time series nature of the data and the potential for autocorrelation (the tendency for error terms of data points to be correlated due to their temporal relationships), an autoregressive integrated moving average (ARIMA) model with correction for first order autocorrelation was fit to the data.20 Robust standard errors were used to generate 95% confidence intervals (CIs) for independent variables, while allowing individual patients to contribute more than one blood culture specimen. Additional details about this model are available in Data Supplement S1, available as an online supplement.
Using time series forecasting,20 we projected the baseline period secular trend into the intervention period to estimate expected contamination rates if the intervention had not been implemented. By comparing these forecasted estimates to the observed data after the intervention, we evaluated the impact of the intervention on the percentage of cultures contaminated. We quantified this impact during the first (February 28, 2010 to March 13, 2010) and last (January16, 2011 to January 29, 2011) biweekly time intervals in the intervention period to measure the immediate effect of the intervention and its sustainability after one year, respectively.
The 48-week duration for the intervention period was chosen to test for sustained effectiveness of the intervention for a year after implementation (4 week implementation phase plus 48 week intervention period). Two-week intervals were chosen for the time series analysis to allow for a sufficient number of data points for robust linear regression while maintaining an acceptable level of variability for the estimate at each time point; we followed the commonly used recommendation of at least 12 data points in each segment and at least 100 observations per data point.19 For power calculations, we used historical data in our ED to estimate that 150 blood cultures per week would be collected and 4% would be contaminated during the baseline period. Using a type I error of 5% and the chi-square test, baseline and intervention periods of 48 weeks had 80% power to detect a 25% relative change in the percentage of blood cultures contaminated in the intervention period compared to the baseline period.
Unintended Adverse Consequences
During development of the intervention, task force members and ED leaders noted that if additional time were required to collect cultures using the new sterile technique, unintended delays in ED care could be caused by the intervention. Therefore, we measured the time delay between order entry for intravenous (IV) antibiotics and delivery of those antibiotics among patients who had blood cultures collected (“time-to-antibiotics”). We used time-to-antibiotics as a measure for the timeliness of ED care. Using electronic timestamps embedded within the hospital electronic medical records system, we calculated time-to-antibiotics by subtracting the time of computerized physician order entry for an IV antibiotic from the time an ED nurse electronically acknowledged the antibiotic began infusing. Using a segmented linear regression model similar to that described above, we compared time-to-antibiotics during the baseline and intervention periods. The dependent variable for this model was the median time-to-antibiotics during biweekly intervals.
Data Analysis
Statistical analyses were performed with Stata/IC 11.1 (College Station, TX). Two-sided p values less than 0.05 were considered statistically significant.
RESULTS
During the 100-week study period, 14,475 blood cultures were collected from 5,681 unique patients. During the baseline period, 321 out of 7,389 (4.3%) cultures were contaminated, compared to 111 out of 6,590 (1.7%) during the intervention period (p < 0.001) (Table 1).
Table 1.
Characteristics of the baseline period, implementation phase, and intervention period.
| Characteristics | Baseline Period (3/1/09 – 1/30/10) |
Implementation Phase (1/31/10 – 2/27/10) |
Intervention Period (2/28/10 – 1/29/11) |
|---|---|---|---|
| ED patient visits | |||
| Total visits | 49,638 | 4,045 | 50,801 |
| Visits with ≥ 1 blood culture collected | 3,737 (7.5%) | 257 (6.4%) | 3,379 (6.6%) |
| Visits with IV antibiotics after blood culture | 2,480 (5.0%) | 204 (5.0%) | 3,265 (6.4%) |
| Blood cultures | |||
| Total blood cultures | 7,389 | 496 | 6,590 |
| Contaminated cultures | 321 (4.3%) | 6 (1.2%) | 111 (1.7%) |
| True positive cultures | 598 (8.1%) | 35 (7.1%) | 546 (8.3%) |
| Time-to-antibiotics | |||
| Median (IQR) time-to-antibiotics (mins) | 49 (27–81) | 46 (29–85) | 42 (24–72) |
IV: intravenous; mins: minutes; IQR: interquartile range
Values are n (%) unless otherwise noted
In the blood culture contamination segmented regression model, the baseline period did not have a significant secular trend (β-coefficient: −0.005, 95% CI = −0.017 to 0.007; p = 0.43), while the intervention period had a slight trend toward higher contamination (β-coefficient: 0.025, 95% CI = 0.003 to 0.046; p = 0.024) (Figure 4). After adjusting for baseline and intervention period secular trends, implementation of the sterile blood culture intervention was associated with an immediate absolute reduction in contamination of 2.9% (95% CI = 2.2% to 3.2%), representing a relative reduction of 71.7% (95% CI = 56.5% to 81.6%) compared to expected values. To evaluate the sustainability of the intervention effects, we first calculated expected contamination rates during the intervention period assuming no intervention was implemented; this is displayed in Figure 4 on the dotted line projecting the baseline period trend into the intervention period. Compared to this expected rate, the intervention was associated with a 1.6% (95% CI = 0.3% to 2.3%) absolute and 44.0% (95% CI = 9.5% to 65.3%) relative reduction in contamination during the last time interval of the intervention period (January 16, 2011 to January 29, 2011). The goal of maintaining < 3% contamination during each biweekly interval in the intervention period was achieved (Figure 4).
Figure 4.
Segmented regression analysis displaying the percentage of blood cultures contaminated during two-week intervals, March 1, 2009 – January 29, 2011. The sterile blood culture intervention was implemented on January 31, 2010, followed by a four week implementation phase that was not included in trend line calculations. Baseline period and intervention period trend lines were generated with an autoregressive integrated moving average (ARIMA) model (solid lines). The baseline period trend line was projected into the intervention period to display expected estimates of contamination assuming the intervention had not been implemented (dashed line).
During the baseline period, there were 2,480 occurrences of a patient receiving IV antibiotics in the ED after a blood culture was collected; the median time-to-antibiotics was 49 minutes (interquartile range [IQR]: 27 minutes to 81 minutes) (Table 1). During the intervention period, there were 3,265 occurrences of a patient receiving IV antibiotics after a blood culture, with a median time-to-antibiotics of 42 minutes (IQR: 24 minutes to 72 minutes). In the time-to-antibiotics segmented regression model, the baseline period did not have a significant trend (β-coefficient: 0.13, 95% CI = −0.11 to 0.37; p = 0.30), while the intervention period exhibited a trend toward shorter time-to-antibiotics (β-coefficient: −0.33, 95% CI = −0.54 to −0.11; p = 0.003). After adjusting for secular trends, implementation of the sterile blood culture intervention was not associated with an immediate change in time-to-antibiotics (−2.9 minutes; 95% CI = −7.0 to 1.3 minutes). Due to the downward trend in time-to-antibiotics during the intervention period, observed times-to-antibiotics were significantly shorter than expected assuming no intervention implementation during the later weeks of the intervention period. For example, during the last time interval of the intervention period, the intervention was associated with a −13.4 minute (95% CI = −21.8 to −5.0 minute) change in time-to-antibiotics.
DISCUSSION
We identified a high contamination rate among blood cultures collected in the adult ED at our hospital. We studied the process of blood culture collection and found inconsistent methods for culture collection with frequent breaches in aseptic technique. Therefore, we developed the sterile blood culture intervention to facilitate a standardized, sterile process for culture collection using chlorhexidine skin antisepsis, sterile gloves, sterile drapes, and checklists. Implementing the new process resulted in an immediate 2.9% absolute reduction in the percentage of cultures contaminated. Significant improvement was also sustained for a year following implementation. Furthermore, the new process of culture collection was not associated with delays in ED care, as measured by the time to delivery of IV antibiotics.
The sterile blood culture intervention was developed to overcome specific problems we faced in the process of blood culture collection. Our first improvement strategy involved spreading awareness about our local contamination problem, and motivating the ED staff for change. At the beginning of the project, most of the ED staff were not aware of our serious contamination problem. Additionally, the ED staff was largely shielded from the negative consequences of contaminated blood cultures, such as increased hospital length of stay and unnecessary antibiotics, because these typically become apparent only after admission. We held regular meetings with the staff to discuss our contamination problem and emphasize the morbidity experienced by patients due to contaminated cultures. Stories of individual patients who the staff remembered and who suffered morbidity due to a contaminated culture seemed to have a large effect on motivating the staff for change. Our next strategy involved identifying specific causes of contamination in our setting. Again, the frontline nurses and paramedics were directly involved in this process and identified most of these problems while actively drawing a culture. We summarized these factors in a fishbone diagram (Figure 1) and process map (Figure 2a) and classified each as “modifiable” (i.e. palpation of venipuncture site after skin antisepsis) or “not modifiable” (i.e. dehydrated, critically ill patient). Then, we brainstormed with nurses and infection control experts about potential methods to intervene on the modifiable contributors to contamination.
With a high turn-over in staff and variability in how staff members originally learned to perform blood culture collection, implementing standardized education was essential. We created a ten minute internet video that reviewed the negative consequences of contaminated cultures and how to use the sterile blood culture kit to prevent contamination. At the time of implementation, each ED nurse and paramedic viewed this video and looked through a kit in a hands-on educational session. As new personnel joined the staff, they were trained on the blood culture technique by an ED nurse educator with the same materials.
Our nurses and paramedics found themselves frequently needing to re-palpate venipuncture sites after applying antiseptic solution in order to locate veins and successfully draw blood. When evaluating the process map for culture collection (Figure 2a), this step was repeatedly identified as a likely culprit for introducing contamination. We quickly found that discouraging re-palpation was not a viable strategy, because our staff found this essential for routinely having success in obtaining blood samples. Therefore, we developed a technique that allowed for re-palpation of veins, and even simultaneous palpation and venipuncture, without introducing contamination. This involved using a large volume of chlorhexidine solution (3 ml) for skin antisepsis, placement of a sterile fenestrated drape over the procedure site, and use of sterile gloves.21–23 This allowed nurses and paramedics to widely palpate throughout their procedural fields to find an optimal location for venipuncture without introducing contaminants from the patient’s adjacent skin, their own fingers, or venous catheters.24 From a human factors perspective,25 we believe the sterile blood culture kit was successful in reducing contamination by providing the optimal equipment for nurses and paramedics to use the most natural technique for finding a venipuncture site—feeling the target vein with the index finger of the non-dominant hand while inserting a needle into the vein with the dominant hand (Figure 2b).
A key aspect of the sterile blood culture intervention was its sustained effectiveness over time. Several factors were incorporated into its design to facilitate sustainability. End-users of the intervention, including ED nurses and paramedics who collected blood cultures, were integrally involved in each step of its development and deployment. This likely not only led to a user-friendly intervention, but also greater investment in its success among its users, manifested by higher compliance with the new technique.14 The intervention was carefully inserted into the normal flow of ED processes with the intention of not creating “extra work” for the nurses and paramedics. Training activities for the intervention were incorporated into existing educational time slots for the staff. The sterile blood culture kits were stocked in bedside cabinets in each ED room next to the blood culture bottles. Several staff members commented that picking up one kit from the bedside cabinet was more efficient than gathering several separate materials from different locations they had previously used to collect a culture; this may have contributed to the decreasing time-to-antibiotics observed during the intervention period. Finally, a paper checklist outlining the sterile collection technique was placed in each kit. These checklists served as ongoing reminders at the time of culture collection for the importance of maintaining sterility and how to use the kit.26,27
The combined cost of a sterile blood culture kit and pair of sterile gloves was $4.88, compared to approximately $2.00 for materials used to collect a culture by usual care during the baseline period. We estimated that the intervention led to approximately $19,000 in additional material expenses and 172 fewer contaminated cultures during the 48-week intervention period. Several previous studies have estimated increased hospital charges associated with a contaminated blood culture to be $4,000–$9,000.2–4,6 Based on these estimates, we believe collecting blood cultures by sterile technique has the potential to significantly reduce net costs by reducing the number of contaminated cultures.
LIMITATIONS
We implemented the sterile blood culture intervention in an ED with a high baseline contamination rate compared to national medians and benchmarks and with strong administrative support for the improvement effort.9,18 The success of the intervention may not be generalizable to units with lower baseline contamination rates, or those without supportive leadership. We found no evidence to suggest that the intervention resulted in delays in the administration of antibiotics. However, other unmeasured adverse consequences were possible; these potentially included patient dissatisfaction with a greater number of venipunctures due to cultures not being obtained through pre-existing vascular catheters. The intervention involved simultaneously changing several aspects of the blood culture collection process. Using this design, only the net effect resulting from all the changes could be evaluated; independent contributions of individual components of the bundle were not measured. During the intervention period, if multiple attempts at dedicated venipuncture using a kit failed to obtain blood, nurses and paramedics had the option of using an alternative collection method, such as sampling from an existing catheter. We did not measure the frequency with which alternative methods were needed. We used a series definition of contamination, with cultures classified as contaminated if only one culture within a 24 hour period grew the same skin contaminant organism. This definition is commonly used for calculating contamination rates,18 but may overestimate or underestimate true rates. The same definition of contamination was consistently used throughout the baseline and intervention periods, minimizing the risk that the definition of contamination affected the change in contamination rates observed.
CONCLUSIONS
Using a quality improvement approach, we developed a new process for blood culture collection emphasizing strict attention to sterile technique with standardized use of chlorhexidine skin antisepsis, sterile needle, sterile gloves, sterile fields, and a procedural checklist. Implementing this intervention in a university-affiliated adult ED with a high baseline contamination rate resulted in an immediate and sustained reduction in blood culture contamination below the 3% benchmark. If these results are replicated in other EDs, sterile technique for blood culture collection using a standardized kit could be a key strategy to reducing the burden of blood culture contamination nationally. Future work will investigate the effectiveness of this intervention in other settings, including pediatric units, community hospitals, and sites with lower baseline contamination rates.
Supplementary Material
Acknowledgements
We would like to sincerely thank the nurses and paramedics working in our emergency department, whose enthusiastic dedication to improving patient care made this project a success.
Funding: 1) Supported by a grant from the Emergency Medicine Patient Safety Foundation. 2) Supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program with resources and the use of facilities at VA Tennessee Valley Healthcare System, Nashville, TN. 3) Supported by the Vanderbilt University Clinical and Translational Science Award (CTSA), National Institutes of Health grant 1 UL1 RR024975.
Disclosures: WHS reports receiving research funding from CareFusion, the makers of Chloraprep, for a subsequent research study that began in August 2011. CareFusion did not fund or participate in the current study.
Footnotes
Presented at: Society for Academic Emergency Medicine Annual Meeting, Boston MA, June 2011.
References
- 1.Weinstein MP, Towns ML, Quartey SM, Mirrett S, Reimer LG, Parmigiani G. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24:584–602. doi: 10.1093/clind/24.4.584. [DOI] [PubMed] [Google Scholar]
- 2.Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization: the true consequences of false-positive results. JAMA. 1991;265:365–369. [PubMed] [Google Scholar]
- 3.Little JR, Murray PR, Traynor PS, Spitznagel E. A randomized trial of povidone-iodine compared with iodine tincture for venipuncture site disinfection: effects on rates of blood culture contamination. Am J Med. 1999;107:119–125. doi: 10.1016/s0002-9343(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 4.Gander RM, Byrd L, DeCrescenzo M, Hirany S, Bowen M, Baughman J. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47:1021–1024. doi: 10.1128/JCM.02162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souvenir D, Anderson DE, Palpant S, et al. Blood cultures positive for coagulase-negative staphylococci: antisepsis, pseudobacteremia, and therapy of patients. J Clin Microb. 1998;36:1923–1926. doi: 10.1128/jcm.36.7.1923-1926.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwang O, Albert RK. Analysis of strategies to improve cost effectiveness of blood cultures. J Hosp Med. 2006;1:272–276. doi: 10.1002/jhm.115. [DOI] [PubMed] [Google Scholar]
- 7.van der Heijden YF, Miller G, Wright PW, Shepherd BE, Daniels TL, Talbot TR. Clinical impact of blood cultures contaminated with coagulase-negative staphylococci at an academic medical center. Infect Control Hosp Epidemiol. 2011;32:623–625. doi: 10.1086/660096. [DOI] [PubMed] [Google Scholar]
- 8.Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19:788–802. doi: 10.1128/CMR.00062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Principles and procedures for blood cultures: approved guideline. Wayne PA: Clinical and Laboratory Standards Institute; 2007. CLSI document M47-A. [Google Scholar]
- 10.Norberg A, Christopher NC, Ramundo ML, Bower JR, Berman SA. Contamination rates of blood cultures obtained by dedicated phlebotomy vs intravenous catheter. JAMA. 2003;289:726–729. doi: 10.1001/jama.289.6.726. [DOI] [PubMed] [Google Scholar]
- 11.Archibald LK, Pallangyo K, Kazembe P, Reller LB. Blood culture contamination in Tanzania, Malawi, and the United States: a microbiological tale of three cities. J Clin Microbiol. 2006;44:4425–4429. doi: 10.1128/JCM.01215-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qamruddin A, Khanna N, Orr D. Peripheral blood culture contamination in adults and venepuncture technique: prospective cohort study. J Clin Pathol. 2008;61:509–513. doi: 10.1136/jcp.2007.047647. [DOI] [PubMed] [Google Scholar]
- 13.Madeo M, Jackson T, Williams C. Simple measures to reduce the rate of contamination of blood cultures in accident and emergency. Emer Med J. 2005;22:810–811. doi: 10.1136/emj.2005.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson EC, Batalden PB, Godfrey MM. Quality by design a clinical microsystems approach. San Francisco, CA: Jossey-Bass; 2007. [Google Scholar]
- 15.Varkey P, Reller MK, Resar RK. Basics of quality improvement in health care. Mayo Clin Proc. 2007;82:735–739. doi: 10.4065/82.6.735. [DOI] [PubMed] [Google Scholar]
- 16.Speroff T, O’Connor GT. Study designs for PDSA quality improvement research. Q Manage Health Care. 2004;13:17–32. doi: 10.1097/00019514-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Eccles M, Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care. 2003;12:47–52. doi: 10.1136/qhc.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekeris LG, Tworek JA, Walsh MK, Valenstein PN. Trends in blood culture contamination: a College of American Pathologists Q-Tracks study of 356 institutions. Arch Pathol Lab Med. 2005;129:1222–1225. doi: 10.5858/2005-129-1222-TIBCCA. [DOI] [PubMed] [Google Scholar]
- 19.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 20.Box GEP, Jenkins GM, Reinsel GC. Time series analysis: Forecasting and control. Fourth Edition. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 21.Mimoz O, Karim A, Mercat A, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture: a randomized controlled trial. Ann Intern Med. 1999;131:834–837. doi: 10.7326/0003-4819-131-11-199912070-00006. [DOI] [PubMed] [Google Scholar]
- 22.Madeo M, Barlow G. Reducing blood-culture contamination rates by the use of a 2% chlorhexidine solution applicator in acute admission units. J Hosp Infect. 2008;69:307–309. doi: 10.1016/j.jhin.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Kim NH, Kim M, Lee S, et al. Effect of routine sterile gloving on contamination rates in blood culture: A cluster randomized trial. Ann Intern Med. 2011;154:145–152. doi: 10.7326/0003-4819-154-3-201102010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Self WH, Speroff T, McNaughton CD, et al. Blood culture collection through peripheral intravenous catheters increases the risk of specimen contamination among adult emergency department patients. Infect Control Hosp Epidemiol. 2012;33:524–526. doi: 10.1086/665319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gawron VJ, Drury CG, Fairbanks RJ, Berger RC. Medical error and human factors engineering: where are we now? Am J Med Qual. 2006;21:57–67. doi: 10.1177/1062860605283932. [DOI] [PubMed] [Google Scholar]
- 26.Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14:295–302. doi: 10.1136/qshc.2004.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.