Abstract
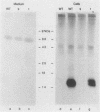
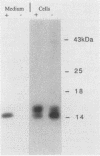
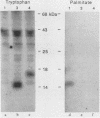
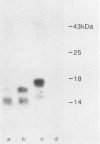
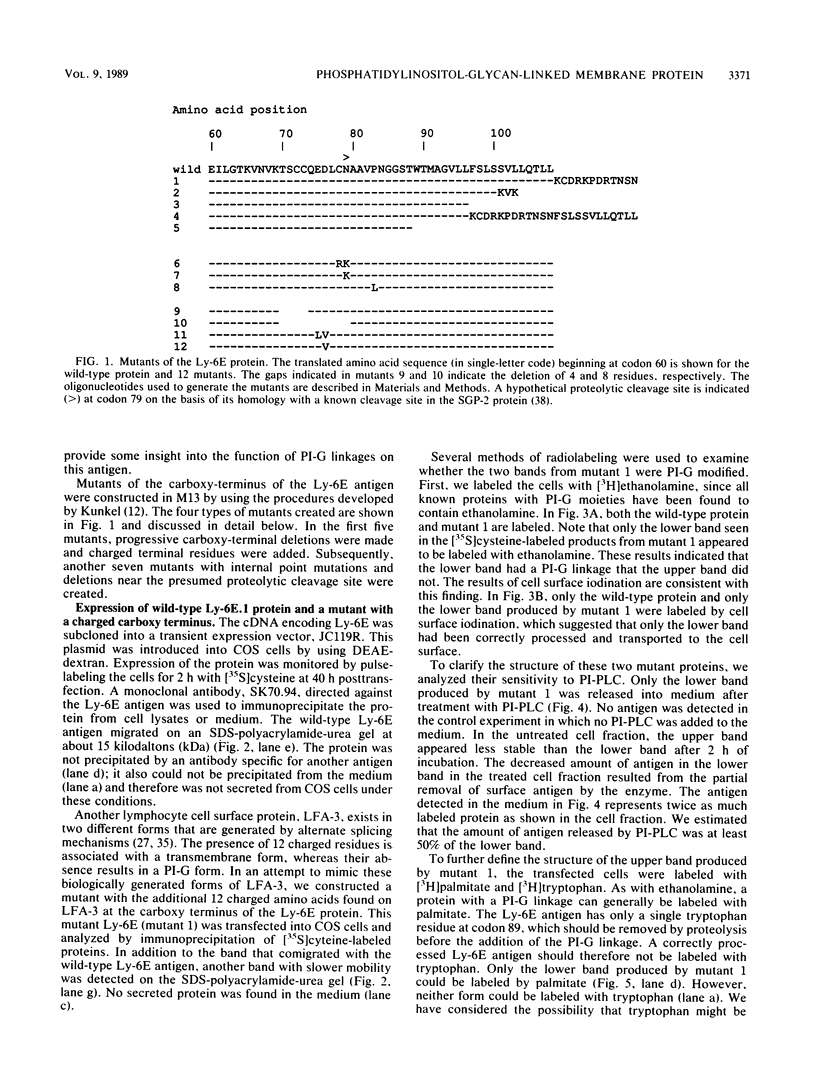
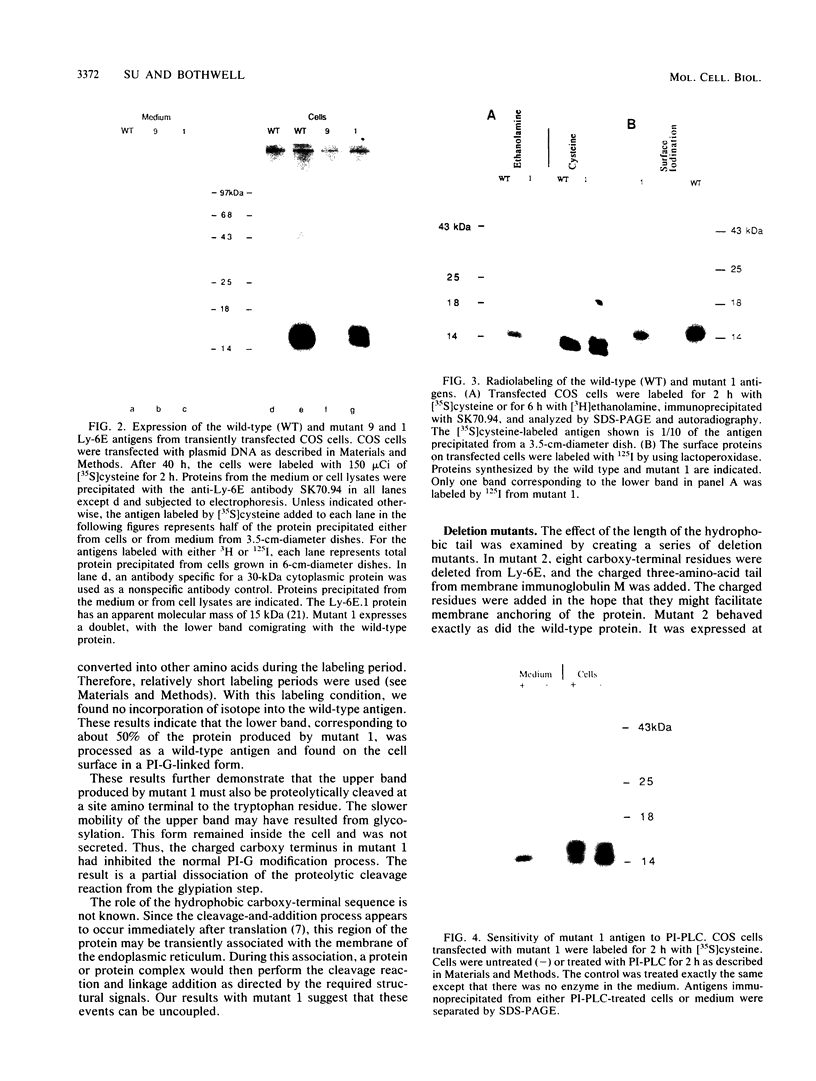
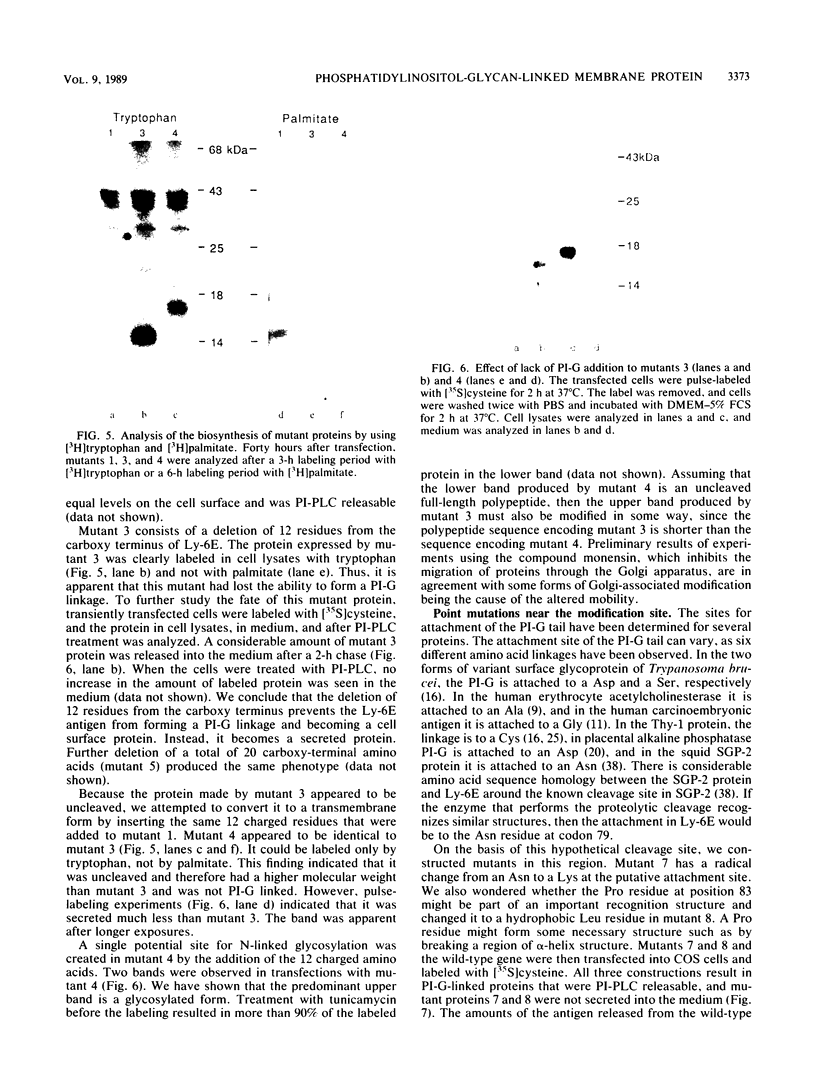
The Ly-6E/A protein is a murine cell surface protein expressed at high levels on activated peripheral T cells. The only linkage known to be responsible for its association with the plasma membrane is a phosphatidylinositol-glycan (PI-G) moiety. To examine the biosynthesis of this structure, we constructed a series of mutants of Ly-6E that were expressed in COS cells by using transient-transfection procedures. When 12 or 20 carboxy-terminal residues were deleted from the primary translation product, the PI-G modification was completely abolished and the mutant proteins became secreted. Addition of the PI-G tail was partially inhibited when the charged 12-amino-acid peptide found as a cytoplasmic tail on the transmembrane form of LFA-3 was added to the COOH terminus of the Ly-6E protein. Proteolytic cleavage occurred on this mutant protein, but the PI-G moiety was added to only 50% of the molecules. Changing an Asn residue to a Lys at the hypothetical cleavage site resulted in a PI-G-linked protein having a detectable alteration in electrophoretic mobility. This finding raises the possibility that proteolytic cleavage at other amino acid sites may occur and that PI-G attachment can occur at this new site. A model identifying two regions that may act as necessary signals for the biosynthesis of the PI-G tail is presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamezai A., Reiser H., Rock K. L. T cell receptor/CD3 negative variants are unresponsive to stimulation through the Ly-6 encoded molecule, TAP. J Immunol. 1988 Sep 1;141(5):1423–1428. [PubMed] [Google Scholar]
- Berger J., Howard A. D., Brink L., Gerber L., Hauber J., Cullen B. R., Udenfriend S. COOH-terminal requirements for the correct processing of a phosphatidylinositol-glycan anchored membrane protein. J Biol Chem. 1988 Jul 15;263(20):10016–10021. [PubMed] [Google Scholar]
- Bothwell A., Pace P. E., LeClair K. P. Isolation and expression of an IFN-responsive Ly-6C chromosomal gene. J Immunol. 1988 Apr 15;140(8):2815–2820. [PubMed] [Google Scholar]
- Caras I. W., Weddell G. N., Davitz M. A., Nussenzweig V., Martin D. W., Jr Signal for attachment of a phospholipid membrane anchor in decay accelerating factor. Science. 1987 Nov 27;238(4831):1280–1283. doi: 10.1126/science.2446389. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Hammelburger J. W., Palfree R. G., Sirlin S., Hämmerling U. Demonstration of phosphatidylinositol anchors on Ly-6 molecules by specific phospholipase C digestion and gel electrophoresis in octylglucoside. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1304–1311. doi: 10.1016/s0006-291x(87)80275-9. [DOI] [PubMed] [Google Scholar]
- Hefta S. A., Hefta L. J., Lee T. D., Paxton R. J., Shively J. E. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeClair K. P., Palfree R. G., Flood P. M., Hammerling U., Bothwell A. Isolation of a murine Ly-6 cDNA reveals a new multigene family. EMBO J. 1986 Dec 1;5(12):3227–3234. doi: 10.1002/j.1460-2075.1986.tb04633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClair K. P., Rabin M., Nesbitt M. N., Pravtcheva D., Ruddle F. H., Palfree R. G., Bothwell A. Murine Ly-6 multigene family is located on chromosome 15. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1638–1642. doi: 10.1073/pnas.84.6.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ortega G., Chan C., Kroczek R. A., Shevach E. M. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986 Sep 1;164(3):709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micanovic R., Bailey C. A., Brink L., Gerber L., Pan Y. C., Hulmes J. D., Udenfriend S. Aspartic acid-484 of nascent placental alkaline phosphatase condenses with a phosphatidylinositol glycan to become the carboxyl terminus of the mature enzyme. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1398–1402. doi: 10.1073/pnas.85.5.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfree R. G., Hämmerling U. Biochemical characterization of the murine activated lymphocyte alloantigen Ly-6E.1 controlled by the Ly-6 locus. J Immunol. 1986 Jan;136(2):594–600. [PubMed] [Google Scholar]
- Palfree R. G., LeClair K. P., Bothwell A., Hämmerling U. cDNA characterization of an Ly-6.2 gene expressed in BW5147 tumor cells. Immunogenetics. 1987;26(6):389–391. doi: 10.1007/BF00343712. [DOI] [PubMed] [Google Scholar]
- Palfree R. G., Sirlin S., Dumont F. J., Hämmerling U. N-terminal and cDNA characterization of murine lymphocyte antigen Ly-6C.2. J Immunol. 1988 Jan 1;140(1):305–310. [PubMed] [Google Scholar]
- Reiser H., Coligan J., Palmer E., Benacerraf B., Rock K. L. Cloning and expression of a cDNA for the T-cell-activating protein TAP. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2255–2259. doi: 10.1073/pnas.85.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser H., Oettgen H., Yeh E. T., Terhorst C., Low M. G., Benacerraf B., Rock K. L. Structural characterization of the TAP molecule: a phosphatidylinositol-linked glycoprotein distinct from the T cell receptor/T3 complex and Thy-1. Cell. 1986 Nov 7;47(3):365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Yeh E. T., Gramm C. F., Haber S. I., Reiser H., Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986 Feb 1;163(2):315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Aihara Y., Weissman I. L., Klein J. The stem cell antigens Sca-1 and Sca-2 subdivide thymic and peripheral T lymphocytes into unique subsets. J Immunol. 1988 Dec 1;141(11):3697–3707. [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. J., Merćep M., Saito T., Germain R. N., Bonvini E., Ashwell J. D. Dissociation of phosphoinositide hydrolysis and Ca2+ fluxes from the biological responses of a T-cell hybridoma. Nature. 1988 Aug 18;334(6183):625–628. doi: 10.1038/334625a0. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Saito T., Shevach E. M., Germain R. N., Ashwell J. D. Thy-1- and Ly-6-mediated lymphokine production and growth inhibition of a T cell hybridoma require co-expression of the T cell antigen receptor complex. J Immunol. 1988 Apr 15;140(8):2520–2526. [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]
- Tykocinski M. L., Shu H. K., Ayers D. J., Walter E. I., Getty R. R., Groger R. K., Hauer C. A., Medof M. E. Glycolipid reanchoring of T-lymphocyte surface antigen CD8 using the 3' end sequence of decay-accelerating factor's mRNA. Proc Natl Acad Sci U S A. 1988 May;85(10):3555–3559. doi: 10.1073/pnas.85.10.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Frey A. Z., Tizard R., Mattaliano R. J., Hession C., Sanders M. E., Dustin M. L., Springer T. A. Primary structure of lymphocyte function-associated antigen 3 (LFA-3). The ligand of the T lymphocyte CD2 glycoprotein. J Exp Med. 1987 Oct 1;166(4):923–932. doi: 10.1084/jem.166.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Sherman D. H., Kincade P. W., Low M. G., Flavell R. A. Molecular mapping of signals in the Qa-2 antigen required for attachment of the phosphatidylinositol membrane anchor. Proc Natl Acad Sci U S A. 1988 Jan;85(2):577–581. doi: 10.1073/pnas.85.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Stein M. E., Flavell R. A. Conversion of a PI-anchored protein to an integral membrane protein by a single amino acid mutation. Science. 1988 Aug 5;241(4866):697–699. doi: 10.1126/science.3399901. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Tse A. G., Gagnon J. Squid glycoproteins with structural similarities to Thy-1 and Ly-6 antigens. Immunogenetics. 1988;27(4):265–272. doi: 10.1007/BF00376121. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Bamezai A., Rock K. L. TAP transcription and phosphatidylinositol linkage mutants are defective in activation through the T cell receptor. Cell. 1988 Mar 11;52(5):665–674. doi: 10.1016/0092-8674(88)90404-7. [DOI] [PubMed] [Google Scholar]
- Yeh E. T., Reiser H., Daley J., Rock K. L. Stimulation of T cells via the TAP molecule, a member in a family of activating proteins encoded in the Ly-6 locus. J Immunol. 1987 Jan 1;138(1):91–97. [PubMed] [Google Scholar]