Abstract
Central nervous system (CNS) metastasis from breast cancer may be characterized as either parenchymal brain metastasis (BM) or leptomeningeal (LM) metastasis. BM are much more common (about 80% of all CNS metastases), and have been more extensively studied than LM. CNS metastasis in breast cancer has been associated with reduced overall survival, with the shortest survival generally observed in cases of LM. Here, we review the epidemiology, prognostic factors, diagnostic tools, currently available treatments, and potential future therapies for LM from breast cancer.
Keywords: Leptomeningeal metastases, intrathecal chemotherapy, breast cancer
Epidemiology
With improved systemic therapies successfully resulting in more long-term survivors with advanced cancers, the incidence of central nervous system (CNS) metastasis is increasing [1,2]. There were an estimated 69,325 cases of brain metastasis (BM) from the 15 most common primary sites in 2007, a projected 5% increase from 2003, with breast cancer BM comprising 15.4% of these [3].
In breast cancer, aggressive chemotherapy has resulted in improved outcomes for individuals with advanced disease [4]. Along with increased survival, late-onset metastatic spread has become an increasing clinical problem. Although less frequent than solid organ and bone metastasis, central nervous system (CNS) metastasis occur somewhat commonly in breast cancer, and may present long after treatment of the primary cancer [5]. Individuals diagnosed with early stage breast cancer have a 5% long-term risk of developing CNS metastasis [6,7]. Although CNS metastasis most commonly occurs in those with known systemic metastasis, the overall risk of CNS recurrence as the initial site of metastatic spread is 1.3% [7]. The median overall survival in those with breast cancer and CNS metastasis is 9 months, with a one-year survival rate of 20% [7]. BM occur most commonly (10%) in the young adults (20-39 years-old), and are more common in African Americans compared to white patients (7.4% versus 4.6%) [6].
Certain breast cancer subtypes have been associated with an increased risk of CNS metastasis. For example, those that are hormone receptor negative are 4 times more likely to have CNS metastasis than those that are hormone receptor positive, and individuals with lung metastasis as a first site of relapse are also 4 times more likely to develop CNS metastasis [8]. A 24-month metastasis-free interval following the diagnosis of breast cancer is associated with a reduced overall risk of CNS metastasis [8].
The majority of CNS metastasis is due to parenchymal BM, with leptomeningeal metastasis (LM) comprising a much smaller number. The precise incidence is difficult to estimate, in part because of the infrequency of LM, and in part because of variability in the detection of LM, and some instances of minimally or asymptomatic disease. There are also regional differences in patient populations, and the potential for referral bias at large centers. As a result, the actual incidence is likely higher than what has been reported. Overall, LM likely comprises about 11-20% of CNS metastasis [9,10]. Prospective studies have found a median overall survival (OS) of 9-30.3 weeks in those with breast cancer following the diagnosis of LM [11-13] (Table 1). Compared to lung cancer LM, studies have shown mixed results, with some favoring a longer survival for breast cancer LM [12] and others demonstrating no difference [13].
Table 1.
Prognostic Factors in Leptomeningeal Breast Cancer
| Prognostic factor | Favorable (reference #) | Unfavorable (reference #) | Nonsignificant (reference #) |
|---|---|---|---|
| Clinical | |||
| Good Initial Performance Status | 14, 36, 37, 56 | 34, 38 | |
| Histology | 56 | ||
| Histological Grade | 56 | ||
| Active systemic disease | 34, 36 | 38 | |
| Concurrent Brain Metastasis | 36 | ||
| Increased ICP at Diagnosis | 14 | ||
| HR Receptor Positivity | 56 | 14, 34, 36 | |
| HER 2 Receptor Positivity | 34, 56 | ||
| Triple Negative Receptor Status | 14 | ||
| Diagnostic | |||
| Positive Initial CSF cytology | 36 | ||
| Normal Initial CSF Protein | 14 | 36, 38 | |
| Low CSF glucose | 36, 38 | ||
| Elevated CSF Cyfra 21-1 level | 56 | ||
| Therapeutic | |||
| Any chemo | 14, 34 | ||
| IT Chemo | 37 | 36 | |
| IV Chemo | 37 | 36 | |
| Combined Modality Tx | 34 | ||
| > 3 prior chemotherapy regimens | 56 | ||
| WBRT | 14, 37 | ||
| Spine RT | 37 | ||
| Response | |||
| Clinical Response | 14, 37 | ||
| CSF Cytologic Clearance | 34, 38 | ||
ICP=intracranial pressure; HR=hormone receptor; HER 2=human epidermal growth factor receptor 2; CSF=cerebrospinal fluid; cyfra=cytokeratin fragment; IT=intrathecal; IV=intravenous; Tx=treatment; WBRT=whole-brain radiation therapy; RT=radiation therapy.
Clinical features
Presenting symptoms in breast cancer LM likely occur at frequencies similar to what has been observed in trials that have included multiple solid tumor histologies, although direct comparisons have not been made. Headache is among the most common symptoms, likely due to infiltration of the meninges by tumor cells, or elevation in intracranial pressure (ICP). Any new headache syndrome in a patient with cancer, and especially headaches that are worse upon awakening or when recumbent, and headaches that awaken a person from sleep should heighten suspicion for LM and potentially elevated ICP. In extreme cases, ICP elevation may result in severe headaches, papilledema or a depressed level of consciousness. In breast cancer LM, up to 46% of cases have confirmed ICP elevation at diagnosis [14]. Cranial neuropathies or back pain due to involvement of spinal nerve roots is also common. Seizures and focal neurological deficits localizing to brain parenchyma are more frequently seen with BM [15]. Up to 1/3 of cases with LM are asymptomatic.
The interval between initial cancer diagnosis and the development of LM is longer for breast cancer than in other solid tumors. Median time from initial breast cancer diagnosis to LM diagnosis is 3 ½ years [14,16,17] compared to one year or less for lung cancer LM [16-18].
Breast cancer LM may occur as an isolated CNS metastatic site, or may occur with concurrent BM at variable frequencies (37-63%) [9,10,19]. Concurrent active cancer outside the CNS occurs in 60-80% at LM diagnosis [19,20].
Diagnostics
Traditionally, the combination of clinical symptomatology and demonstration of malignant cells in cerebrospinal fluid (CSF) have been required to establish a diagnosis of LM. However, the sensitivity of CSF cytology in solid tumors is somewhat limited and may be adversely impacted by limited sample size, or delays in processing [21]. Repeating the CSF cytology up to 3 times increases the sensitivity in solid tumors from 75% to above 90% [21]. Ambiguous CSF cytology, often reported as ‘cytological atypia’ is suggestive of LM, but makes diagnosis of LM and assessment of cytologic response challenging.
Contrast-enhanced magnetic resonance imaging (MRI) has emerged as a reliable diagnostic tool in LM [22]. Leptomeningeal enhancement, nodular enhancement or cranial/spinal nerve enhancement are all characteristic (Figure 1). In the appropriate clinical context, findings suggestive of LM on MRI are adequate to initiate treatment of LM even in the absence of a positive CSF cytology [22]. Survival in solid tumor LM is similar between individuals with positive versus negative CSF cytology [23].
Figure 1.
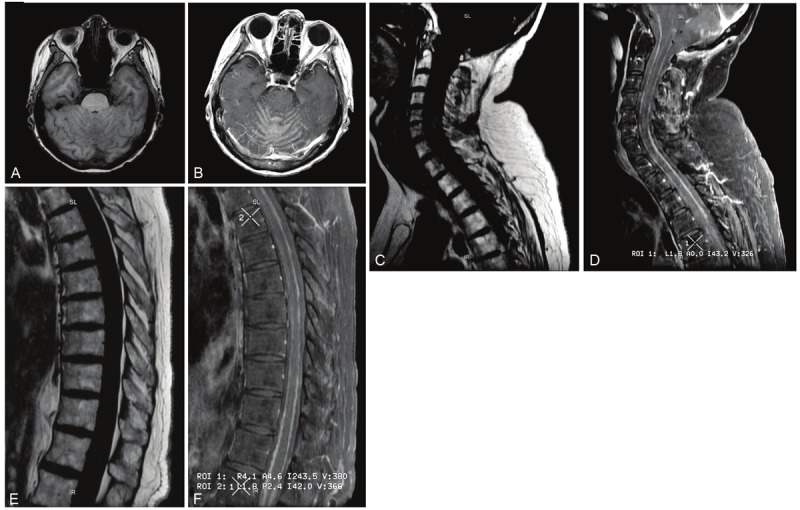
Characteristic Magnetic Resonance Imaging features in leptomeningeal breast cancer. A, B: Axial T1-weighted imaging of the brain pre (left) and post (right) gadolinium administration showing abnormal enhancement of the leptomeninges surrounding the cerebellar folia; C-F: sagittal T1-weighted imaging of; the cervical (C, D) and thoracic (E, F) spine pre (C, E) and post (D, F) gadolinium administration showing abnormal nodular leptomeningeal enhancement along the dorsal and ventral aspect of the spinal cord.
Breast cancer molecular subtypes and LM
Measurement of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) expression levels in breast cancer gives prognostic information, and helps to guide systemic chemotherapy. Overall survival and proclivity to develop metastatic spread including CNS metastasis differs depending on hormone receptor (ER/PR) and HER-2 status at diagnosis.
Overexpression of HER-2 has been associated with an increased incidence of CNS metastasis compared to other molecular subtypes [24-26]. Late occurrence of CNS metastasis in spite of good systemic disease control in HER2 positive breast cancer has led to the postulation that treatment with the anti-HER2 monoclonal antibody trastuzumab may have a causative role. However, the incidence of brain metastasis appears similar between trastuzumab treated and non-treated individuals [27-29], making it more likely that brain metastasis occur more commonly as part of the intrinsic biology of HER2 positive breast cancer. Large studies in HER2 positive breast cancer have demonstrated improved overall survival, longer time to the development of CNS metastasis [30] and improved survival following the diagnosis of BM in those who received trastuzumab [4]. One possible explanation for the benefit with trastuzumab is that even though it does not appear to readily cross the blood-brain barrier (BBB) and produce therapeutic levels in CSF [31,32], the antibody may prevent circulating tumor cells from entering the CNS, or it may reach the tumor regionally in sites where tumor infiltration has disrupted BBB integrity [33].
In long-term follow-up of individuals with early-stage breast cancer, those with ER/PR negative HER-2 positive tumors were most likely to develop CNS metastasis (14.3%), while CNS metastasis were less common in ER/PR/HER-2 positive breast cancers (7.9%) [5]. Luminal A (ER +, low grade) breast cancer was associated with the least frequent development of CNS metastasis (2.2%) [5].
Initial hormone receptor positivity is associated with a higher incidence of bone metastasis and a longer median time to the development of LM when compared to triple-negative breast cancer [34]. Triple negative breast cancer is more likely than receptor positive breast cancer to present with isolated LM and has been associated with a shorter median overall survival following the development of distant metastases. In 3 year median follow-up, of individuals with triple negative breast cancer who experienced a recurrence, 13% recurred initially in the CNS, and 36% had CNS involvement overall in their clinical course [25].
As a prognostic indicator in breast cancer LM, however, investigations looking at hormone receptor and HER-2 status have produced variable results. Some series have found that hormone receptor positivity is associated with longer survival [35], while others have found no association [34,36]. HER-2 status does not appear to impact overall survival from LM, but treatment with trastuzumab was associated with a significantly longer time to the development of LM (15.2 versus 9.9 months) [34,36].
Other prognostic factors
Institutional series have found a variety of factors that impact survival in breast cancer LM beyond hormone receptor status (Table 1). Better initial performance status is generally associated with improved survival both in LM from all cancers [20], and from breast cancer LM specifically [35-37]. The cytologic conversion of CSF from positive to negative during the course of treatment (cytologic response) [34,38] and clinical improvement following treatment (clinical response) [37] have also been associated with improved survival. Histology and histological grade are significant in some studies [36], but not in others [35]. Treatment with radiation and/or chemotherapy is consistently associated with better overall survival [34,35,37]. However, given that these are nonrandomized, retrospectively collected observations, treatment bias cannot be excluded. It is likely that in some cases, those treated with more aggressive chemotherapy and radiation were younger and had a better initial performance status, two factors that also impact prognosis.
Current therapies
There is currently no generally accepted standard of care in the treatment of breast cancer LM. Surgery (for hydrocephalus), radiation therapy (RT), and chemotherapy (systemic or intra-CSF) may be considered. Treatment decisions are influenced by the individual’s functional status, ability and willingness to receive additional treatment, and extent of active systemic disease. In some cases, the diagnosis of LM compels providers and patients to pursue palliative care, especially when LM is accompanied by a dramatic clinical decline.
One caveat to consider when assessing functional status in LM patients is whether or not an individual has elevated ICP. CSF outflow obstruction that occurs when the arachnoid villi are no longer able to effectively reabsorb CSF is often associated with a progressive headache syndrome and depressed level of consciousness. Relief of CSF outflow obstruction by CSF diversion has been shown to improve functional status, and is likely to prolong survival in these cases [39]. A ventriculoperitoneal shunt (VPS) procedure carries a small risk of hemorrhage, infection or shunt malfunction. However, placement of a VPS is a definitive treatment for elevated ICP, and may be combined with a reversible on/off valve to facilitate administration of intrathecal (IT) chemotherapy [39]. For those in whom a surgical procedure is not desired or tolerable, palliative RT is also effective in relieving CSF outflow obstruction, although the duration of benefit is variable [40].
RT is a palliative treatment or adjunctive therapy with IT or IV chemotherapy (see below). A short course of fractionated RT may be delivered that is generally tolerable, and may be useful to relieve pain in sites of nerve root compression. RT is especially important to consider in cases with bulky leptomeningeal disease, as the penetration of IT chemotherapy is poor in these instances [41].
IV chemotherapy with high-dose methotrexate likely improves survival over radiation alone, and has shown a trend toward improved overall survival compared to IT chemotherapy with improved tolerability [11]. The main advantage of IV chemotherapy is that it does not cause chemical meningitis and has a lower risk of leukoencephalopathy compared to IT treatment. However, IV methotrexate may produce systemic side-effects such as mucositis, bone-marrow suppression and nephrotoxicity. IV methotrexate requires inpatient monitoring to ensure adequate clearance, which may adversely impact quality of life.
IT chemotherapy is an alternative to IV methotrexate. One advantage over IV administration is that IT treatments may be given in the ambulatory setting, typically every 2 weeks. Liposomal cytarabine, methotrexate and thiotepa are the most commonly administered IT chemotherapeutic agents. IT chemotherapy is mechanistically attractive, because it circumvents the pharmacologic challenges of drug delivery beyond the blood-brain barrier, and it is less myelotoxic than systemic chemotherapy. This makes it an attractive option for heavily pretreated individuals or those receiving IV chemotherapy for concurrent active systemic disease.
However, IT chemotherapy still has limitations related to distribution and toxicity. The distribution of IT chemotherapy is dependent on normal CSF circulation. As mentioned above, up to 46% of LM patients have evidence of CSF outflow obstruction. Therefore, prior to administration, individuals receiving IT chemotherapy should have no clinical evidence of CSF outflow obstruction or elevated ICP. The most common toxicity of IT chemotherapy is ventriculitis/arachnoiditis, occurring in 10-23% of cases [13,42,43]. This is a non-infectious ‘chemical meningitis’ that occurs in response to IT chemotherapy. It can be extremely uncomfortable, resulting in severe headaches, nausea and vomiting. Pretreatment with dexamethasone substantially reduces the incidence of chemical meningitis. Other rare but serious toxicities of IT therapy include: leukoencephalopathy (7.5%), and bacterial meningitis (3.75%) associated with the presence of an intraventricular reservoir [42].
A handful of randomized clinical trials are available to guide treatment of breast cancer LM. These are summarized in Table 2. The trials that have compared IT treatments (methotrexate versus thiotepa [12], methotrexate versus combination methotrexate plus cytosine arabinoside [13], and methotrexate versus liposomal cytarabine [43]) for LM from multiple different cancers found no significant differences in survival between the treatment arms.
Table 2.
Leptomeningeal Metastasis Randomized Controlled Trial Survival Data
| Study | LM Cancer Types | Treatment Arms | n (Br/Total) | Median OS Breast | Median OS Total |
|---|---|---|---|---|---|
| Boogerd et al. [11] | Breast Only | IT | 17/17 | 18.3 | n/a |
| IV | 18/18 | 30.3 | n/a | ||
| Grossman et al. [12] | Br, Lu, Ly, Other | IT T | 24 | NR | 14.14 |
| IT MTX | 28 | NR | 15.86 | ||
| Both Groups | 25/52 | 15.14 | |||
| Hitchins et al. [13] | Lu, Br, Gl, CUP, | IT MTX | 23 | NR | 12 |
| Ly, Other | IT MTX + Ara C | 20 | NR | 7 | |
| Both Groups | 11/43 | 9 | 8 | ||
| Glantz et al. [43] | Br, Lu, Mel, Gl | IT liposomal cytarabine | 11/31 | NR | 15 |
| IT MTX | 11/30 | NR | 11.14 |
LM=leptomeningeal metastasis; n=number of LM cases; OS=overall survival. Br=breast; Lu=lung; Ly=lymphoma; GI=gastrointestinal; CUP=cancer of unknown primary; Mel=melanoma. Ara C= cytosine arabinoside; T=thiotepa; MTX=methotrexate. NR=not reported.
Future directions
Molecular diagnostic and therapeutic strategies will provide a means for earlier detection of LM, and more effective treatments than are currently in use (Figure 2).
Figure 2.
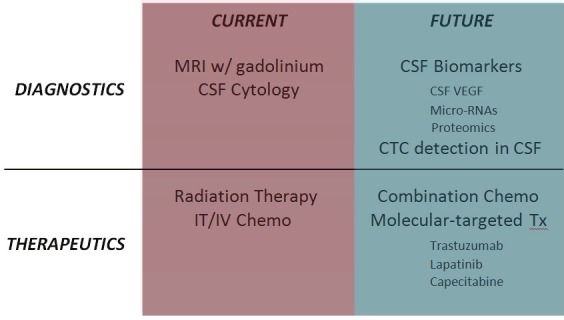
Current and future technologies to advance the diagnosis and treatment of leptomeningeal breast cancer.
Diagnostics
Serum or CSF biomarkers with higher sensitivity than CSF cytology and MRI could allow for earlier and more definitive diagnosis of LM. Abnormalities on MRI or in CSF require a substantial volume of disease, and it is possible that treatment delay due to the insensitivity of current technologies contributes to the poor prognosis in LM. CSF vascular endothelial growth factor (VEGF) has 75% sensitivity, 97% specificity, and 94% negative predictive value in the diagnosis of breast cancer LM using CSF cytology as a gold standard [44].
Validation early in high-risk individuals is required to assess whether elevation in CSF VEGF may be used as a method for early LM detection or screening.
Proteomic analysis of CSF has identified a number of peptides that are differentially expressed in individuals with breast cancer LM compared to breast cancer non-LM individuals and those without breast cancer [45]. Micro-RNA studies have investigated the ability to detect abnormal levels of micro-RNAs in the CSF of cancer versus non-neoplastic conditions. Using 7 micro-RNAs, metastasis versus non-neoplastic controls were correctly identified in 98.9% of cases, and CNS breast versus lung metastasis were discerned correctly in 68.9% [46].
Another potential for early identification is analysis of circulating tumor cells (CTC). Used frequently in serum, CTCs may also be detected in the CSF of individuals with LM [47]. Molecular tumor cell markers may be chosen to identify specific CTCs. Metastatic cells in CSF express epithelial cell adhesion molecule (EPCAM), unlike cells of glial origin that do not. In addition to its diagnostic potential, CTC methods capture individual live tumor cells, which will add to the current understanding of the biology of CNS metastasis and the natural history of LM.
Treatment
Survival following a diagnosis of LM is unacceptably short. Working with currently available therapies, aggressive ICP management and combination IT chemotherapy may afford some survival benefit over previously studied IV or IT monotherapies [48]. Molecular therapeutic strategies are likely to play an increasingly important role in the treatment of breast cancer LM. Individual case reports and case series have shown that IT trastuzumab may have some activity in HER-2 positive breast cancer LM and is potentially well-tolerated [49-52]. Response to treatment following capecitabine [53,54] or lapatinib [55] have also been reported in limited numbers of breast cancer LM.
Trial design
Five prospective randomized trials, 3 of which provide breast cancer specific survival data (Table 2), and a number of institutional retrospective case series (Table 1) provide the evidence base of breast cancer specific LM information to date. Additional prospective clinical trials are required to evaluate the impact of specific treatments on survival and quality of life. Going forward, breast cancer LM trials should be considered in order to answer questions about the impact of treatment interventions and receptor status on survival. Multicenter collaboration is likely to be necessary to execute disease-specific LM trials in a timely manner.
Conclusions
In spite of an increased incidence of breast cancer LM, overall survival with current treatments remains limited to less than 6 months on average. An improved understanding of the mechanisms of CNS metastasis and development of screening and earlier detection methods will lead to more effective therapies. Combination chemotherapy and radiation may be considered in breast cancer LM, especially those without active systemic disease or concurrent brain metastasis. There remain great clinical research opportunities to improve on molecular diagnostic testing and to complete prospective randomized trials. These will undoubtedly lead to innovative therapies for LM and better inform treatment decisions in this challenging and increasing neurological complication of breast cancer.
Acknowledgments
This work was supported in part by grants from NIH (NIH 3P30CA023100-25S8) to S. Kesari. The authors report no conflict of interests.
References
- 1.Frisk G, Svensson T, Backlund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012 May 22;106:1850–3. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin. 2003 Feb;21:25–66. doi: 10.1016/s0733-8619(02)00032-4. [DOI] [PubMed] [Google Scholar]
- 3.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012 Sep;14:1171–7. doi: 10.1093/neuonc/nos152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: Circumventing the blood-brain barrier. Cancer Treat Rev. 2013 May;39:261–9. doi: 10.1016/j.ctrv.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010 Jul 10;28:3271–7. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 6.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004 Jul 15;22:2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 7.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, Crivellari D, Fey MF, Murray E, Pagani O, Simoncini E, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A International Breast Cancer Study Group (IBCSG) Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 8.Slimane K, Andre F, Delaloge S, Dunant A, Perez A, Grenier J, Massard C, Spielmann M. Risk factors for brain relapse in patients with metastatic breast cancer. Ann Oncol. 2004 Nov;15:1640–4. doi: 10.1093/annonc/mdh432. [DOI] [PubMed] [Google Scholar]
- 9.Altundag K, Bondy ML, Mirza NQ, Kau SW, Broglio K, Hortobagyi GN, Rivera E. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer. 2007;110:2640–2647. doi: 10.1002/cncr.23088. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim TM, Oh DY, Kim JH, Lee SH, Chie EK, Han W, Kim DW, Kim TY, Noh DY, Heo DS, Park IA, Bang YJ, Ha SW. Clinical outcome of central nervous system metastases from breast cancer: differences in survival depending on systemic treatment. J Neurooncol. 2012 Jan;106:303–13. doi: 10.1007/s11060-011-0664-8. [DOI] [PubMed] [Google Scholar]
- 11.Boogerd W, van den Bent MJ, Koehler PJ, Heimans JJ, van der Sande JJ, Aaronson NK, Hart AA, Benraadt J, Vecht C. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004 Dec;40:2726–33. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Grossman SA, Finkelstein DM, Ruckdeschel JC, Trump DL, Moynihan T, Ettinger DS. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J. Clin. Oncol. 1993 Mar;11:561–9. doi: 10.1200/JCO.1993.11.3.561. [DOI] [PubMed] [Google Scholar]
- 13.Hitchins RN, Bell DR, Woods RL, Levi JA. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J. Clin. Oncol. 1987 Oct;5:1655–62. doi: 10.1200/JCO.1987.5.10.1655. [DOI] [PubMed] [Google Scholar]
- 14.Zairi FKN, Rodrigues I, Baranzelli M, Andre C, Dubois F, Devos P, Faivre-Pierret M, Assaker R, Bonneterre J, Le Rhun E. Prospective follow-up of a cohort of 112 patients with leptomeningeal metastases of breast cancer recruited from 2007-2011: Prognostic factors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012:30. [Google Scholar]
- 15.Van Breemen MS, Wilms EB, Vecht CJ. Seizure control in brain tumors. Handb Clin Neurol. 2012;104:381–9. doi: 10.1016/B978-0-444-52138-5.00026-8. [DOI] [PubMed] [Google Scholar]
- 16.Bruna J, Gonzalez L, Miro J, Velasco R, Gil M, Tortosa A Neuro-Oncology Unit of the Institute of Biomedical Investigation of Bellvitge. Leptomeningeal carcinomatosis: prognostic implications of clinical and cerebrospinal fluid features. Cancer. 2009;115:381–389. doi: 10.1002/cncr.24041. [DOI] [PubMed] [Google Scholar]
- 17.Oechsle K, Lange-Brock V, Kruell A, Bokemeyer C, de Wit M. Prognostic factors and treatment options in patients with leptomeningeal metastases of different primary tumors: a retrospective analysis. J Cancer Res Clin Oncol. 2010 Nov;136:1729–35. doi: 10.1007/s00432-010-0831-x. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Kim YJ, Lee JO, Lee KW, Kim JH, Bang SM, Chung JH, Kim JS, Lee JS. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012 Jun;76:387–92. doi: 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Kiewe P, Fischer L, Martus P, Thiel E, Korfel A. Meningeal dissemination in primary CNS lymphoma: diagnosis, treatment, and survival in a large monocenter cohort. Neuro Oncol. 2010 Apr;12:409–17. doi: 10.1093/neuonc/nop053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain MC, Johnston SK, Glantz MJ. Neoplastic meningitis-related prognostic significance of the Karnofsky performance status. Arch Neurol. 2009 Jan;66:74–8. doi: 10.1001/archneurol.2008.506. [DOI] [PubMed] [Google Scholar]
- 21.Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, Walters BC, Recht LD. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82:733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Clarke JL, Perez HR, Jacks LM, Panageas KS, Deangelis LM. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chamberlain MC, Johnston SK. Neoplastic meningitis: survival as a function of cerebrospinal fluid cytology. Cancer. 2009;115:1941–1946. doi: 10.1002/cncr.24210. [DOI] [PubMed] [Google Scholar]
- 24.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J. Clin. Oncol. 2006 Dec 20;24:5658–63. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 25.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC. Clinicopathologic Features, Patterns of Recurrence, and Survival Among Women With Triple-Negative Breast Cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musolino A, Ciccolallo L, Panebianco M, Fontana E, Zanoni D, Bozzetti C, Michiara M, Silini EM, Ardizzoni A. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 27.Lai R, Dang CT, Malkin MG, Abrey LE. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101:810–816. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 28.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J. Clin. Oncol. 2009 Nov 1;27:5278–86. doi: 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 29.Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003 Jun;4:114–9. doi: 10.3816/cbc.2003.n.016. [DOI] [PubMed] [Google Scholar]
- 30.Dawood S, Broglio K, Esteva FJ, Ibrahim NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN, Gonzalez-Angulo AM. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008 Jul;19:1242–8. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 31.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J. Clin. Oncol. 2000 Jun;18:2349–51. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 32.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007 Jan;18:23–8. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 33.Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, van Dongen GA, Schroder CP, Lub-de Hooge MN, de Vries EG. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010 May;87:586–92. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Ahn HK, Park YH, Nam do H, Lee JI, Park W, Choi DH, Huh SJ, Park KT, Ahn JS, Im YH. Leptomeningeal metastases from breast cancer: intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011 Oct;129:809–17. doi: 10.1007/s10549-011-1682-0. [DOI] [PubMed] [Google Scholar]
- 35.Gauthier H, Guilhaume MN, Bidard FC, Pierga JY, Girre V, Cottu PH, Laurence V, Livartowski A, Mignot L, Dieras V. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010 Nov;21:2183–7. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 36.de Azevedo CR, Cruz MR, Chinen LT, Peres SV, Peterlevitz MA, de Azevedo Pereira AE, Fanelli MF, Gimenes DL. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011 Sep;104:565–72. doi: 10.1007/s11060-010-0524-y. [DOI] [PubMed] [Google Scholar]
- 37.Rudnicka H, Niwinska A, Murawska M. Breast cancer leptomeningeal metastasis--the role of multimodality treatment. J Neurooncol. 2007 Aug;84:57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 38.Clatot F, Philippin-Lauridant G, Ouvrier MJ, Nakry T, Laberge-Le-Couteulx S, Guillemet C, Veyret C, Blot E. Clinical improvement and survival in breast cancer leptomeningeal metastasis correlate with the cytologic response to intrathecal chemotherapy. J Neurooncol. 2009 Dec;95:421–6. doi: 10.1007/s11060-009-9940-2. [DOI] [PubMed] [Google Scholar]
- 39.Lin N, Dunn IF, Glantz M, Allison DL, Jensen R, Johnson MD, Friedlander RM, Kesari S. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: a retrospective, case-controlled study. J Neurosurg. 2011 Oct;115:730–6. doi: 10.3171/2011.5.JNS101768. [DOI] [PubMed] [Google Scholar]
- 40.Omuro AM, Lallana EC, Bilsky MH, DeAngelis LM. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625–1627. doi: 10.1212/01.WNL.0000160396.69050.DC. [DOI] [PubMed] [Google Scholar]
- 41.Chamberlain MC, Kormanik PA. Prognostic significance of coexistent bulky metastatic central nervous system disease in patients with leptomeningeal metastases. Arch Neurol. 1997 Nov;54:1364–8. doi: 10.1001/archneur.1997.00550230037013. [DOI] [PubMed] [Google Scholar]
- 42.Chamberlain MC. Neurotoxicity of intra-CSF liposomal cytarabine (DepoCyt) administered for the treatment of leptomeningeal metastases: a retrospective case series. J Neurooncol. 2012 Aug;109:143–8. doi: 10.1007/s11060-012-0880-x. [DOI] [PubMed] [Google Scholar]
- 43.Glantz MJ, Jaeckle KA, Chamberlain MC, Phuphanich S, Recht L, Swinnen LJ, Maria B, La-Follette S, Schumann GB, Cole BF, Howell SB. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999 Nov;5:3394–402. [PubMed] [Google Scholar]
- 44.Groves MD, Hess KR, Puduvalli VK, Colman H, Conrad CA, Gilbert MR, Weinberg J, Cristofanilli M, Yung WKA, Liu TJ. Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol. 2009 Sep;94:229–34. doi: 10.1007/s11060-009-9819-2. [DOI] [PubMed] [Google Scholar]
- 45.Rompp A, Dekker L, Taban I, Jenster G, Boogerd W, Bonfrer H, Spengler B, Heeren R, Smitt PS, Luider TM. Identification of leptomeningeal metastasis-related proteins in cerebrospinal fluid of patients with breast cancer by a combination of MALDI-TOF, MALDI-FTICR and nanoLC-FTICR MS. Proteomics. 2007;7:474–481. doi: 10.1002/pmic.200600719. [DOI] [PubMed] [Google Scholar]
- 46.Teplyuk NM, Mollenhauer B, Gabriely G, Giese A, Kim E, Smolsky M, Kim RY, Saria MG, Pastorino S, Kesari S, Krichevsky AM. MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol. 2012 Jun;14:689–700. doi: 10.1093/neuonc/nos074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel AS, Allen JE, Dicker DT, Peters KL, Sheehan JM, Glantz MJ, El-Deiry WS. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget. 2011;2:752–760. doi: 10.18632/oncotarget.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott BJ, Brown T, Van Vught V, Kim R, Fanta PT, Bazhenova L, Kesari S. Concurrent intrathecal methotrexate and liposomal cytarabine for the treatment of leptomeningeal metastasis from solid tumors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012:30. [Google Scholar]
- 49.Mego M, Sycova-Mila Z, Obertova J, Rajec J, Liskova S, Palacka P, Porsok S, Mardiak J. Intrathecal administration of trastuzumab with cytarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. Breast. 2011 Oct;20:478–80. doi: 10.1016/j.breast.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Mir O, Ropert S, Alexandre J, Goldwasser F. Hypertension as a surrogate marker for the activity of anti-VEGF agents. Ann Oncol. 2009 May;20:967–70. doi: 10.1093/annonc/mdp206. [DOI] [PubMed] [Google Scholar]
- 51.Oliveira M, Braga S, Passos-Coelho JL, Fonseca R, Oliveira J. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011 Jun;127:841–4. doi: 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]
- 52.Stemmler HJ, Mengele K, Schmitt M, Harbeck N, Laessig D, Herrmann KA, Schaffer P, Heinemann V. Intrathecal trastuzumab (Herceptin) and methotrexate for meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer: a case report. Anticancer Drugs. 2008 Sep;19:832–6. doi: 10.1097/CAD.0b013e32830b58b0. [DOI] [PubMed] [Google Scholar]
- 53.Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007 Nov;85:223–7. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 54.Shigekawa T, Takeuchi H, Misumi M, Matsuura K, Sano H, Fujiuchi N, Okubo K, Osaki A, Aogi K, Saeki T. Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast Cancer. 2009;16:88–92. doi: 10.1007/s12282-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 55.Onishi H, Morisaki T, Nakafusa Y, Nakashima Y, Yokohata K, Katano M. Objective response with lapatinib in patients with meningitis carcinomatosa derived from HER2/HER1-negative breast cancer. Int J Clin Oncol. 2011 Dec;16:718–21. doi: 10.1007/s10147-011-0195-5. [DOI] [PubMed] [Google Scholar]


